Impact of Intensive Glycemic Treatment on Diabetes Complications—A Systematic Review
Abstract
:1. Introduction
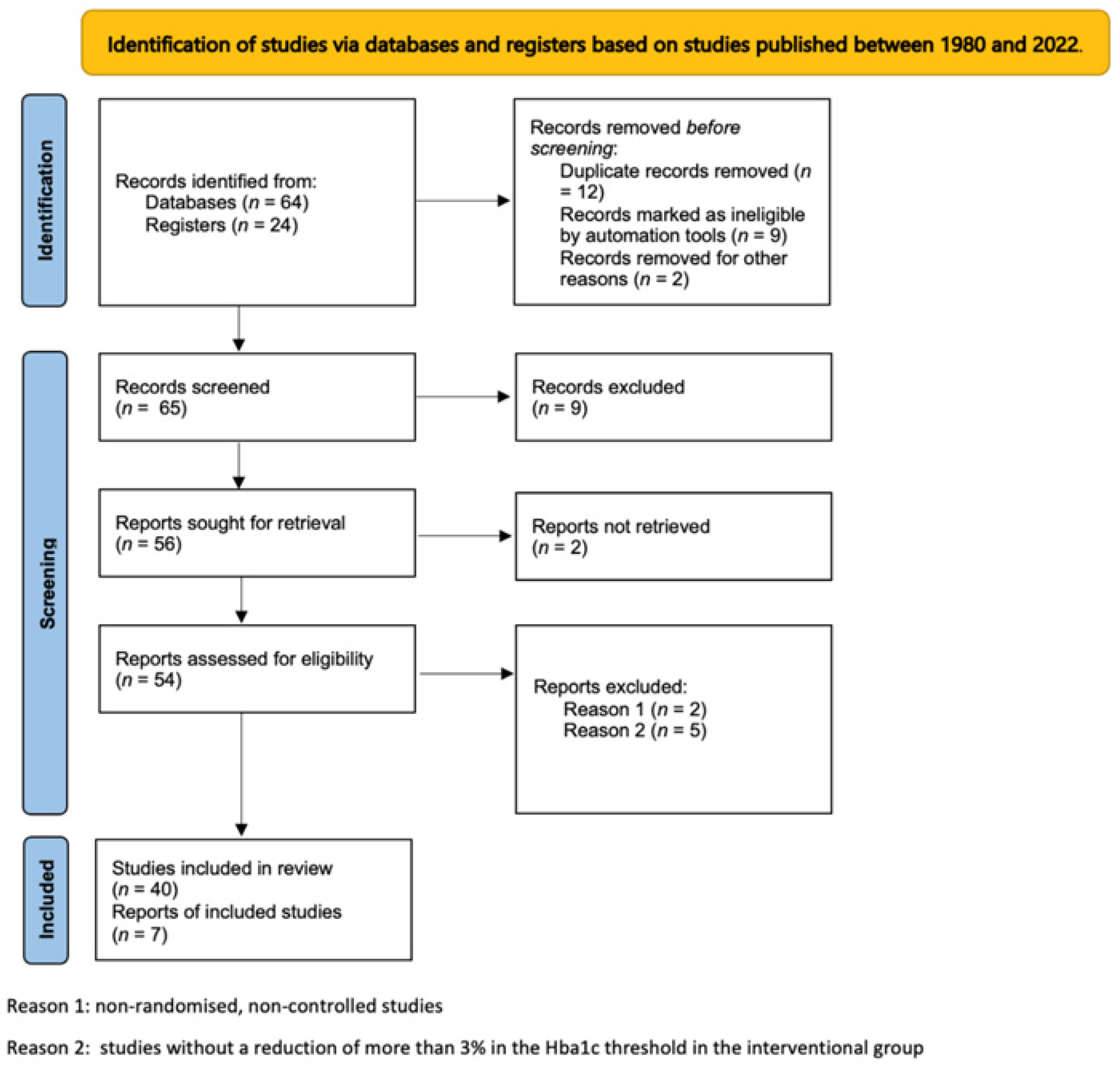
2. Methods
3. Results
3.1. Macroangiopathy
3.1.1. Stroke
3.1.2. Myocardial Infarction
3.1.3. Cardiovascular Mortality
3.1.4. Arteriopathy of Lower Limbs
3.2. Microangiopathy
3.3. Treatment-Induced Neuropathy in Diabetes
3.4. Microvascular Complications Associated with TIDN
3.4.1. Retinopathy
3.4.2. Renal Dysfunction
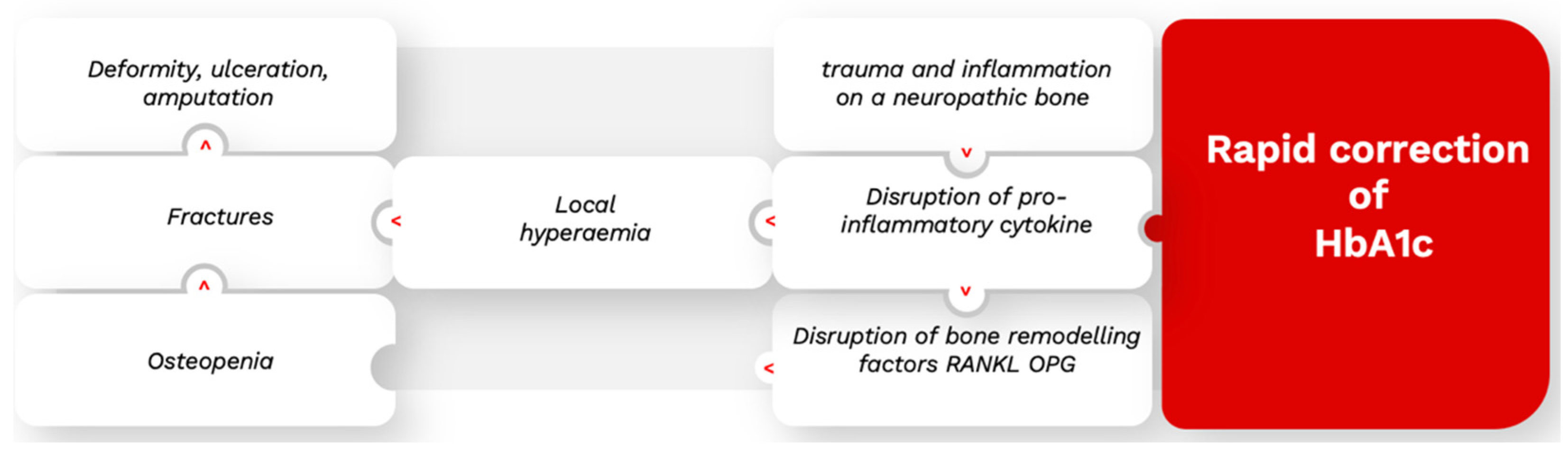
3.4.3. Charcot’s Neuroarthropathy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Schlesinger, S.; Neuenschwander, M.; Barbaresko, J.; Lang, A.; Maalmi, H.; Rathmann, W.; Roden, M.; Herder, C. Prediabetes and risk of mortality, diabetes-related complications and comorbidities: Umbrella review of meta-analyses of prospective studies. Diabetologia 2022, 65, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.A.; Eid, M.A.; Creager, M.A.; Goodney, P.P. Epidemiology and risk of amputation in patients with diabetes mellitus and peripheral artery disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Maida, C.; Pinto, A. Diabetic foot syndrome: Immune-inflammatory features as possible cardiovascular markers in diabetes. World J. Orthop. 2015, 6, 62–76. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goff, D.C., Jr.; Gerstein, H.C.; Ginsberg, H.N.; Cushman, W.C.; Margolis, K.L.; Byington, R.P.; Buse, J.B.; Genuth, S.; Probstfield, J.L.; Simons-Morton, D.G.; et al. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: Current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am. J. Cardiol. 2007, 99, S4–S20. [Google Scholar] [CrossRef] [PubMed]
- ORIGIN Trial Investigators; Gerstein, H.C.; Bosch, J.; Dagenais, G.R.; Díaz, R.; Jung, H.; Maggioni, A.P.; Pogue, J.; Probstfield, J.; Ramachandran, A.; et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N. Engl. J. Med. 2012, 367, 319–328. [Google Scholar]
- Ray, K.K.; Seshasai, S.R.; Wijesuriya, S.; Sivakumaran, R.; Nethercott, S.; Preiss, D.; Erqou, S.; Sattar, N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: A meta-analysis of randomised controlled trials. Lancet 2009, 373, 1765–1772. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222, Erratum in Lancet 2010, 376, 958. [Google Scholar] [CrossRef] [Green Version]
- ACCORD Study Group; Buse, J.B.; Bigger, J.T.; Byington, R.P.; Cooper, L.S.; Cushman, W.C.; Friedewald, W.T.; Genuth, S.; Gerstein, H.C.; Ginsberg, H.N.; et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: Design and methods’. Am. J. Cardiol. 2007, 99, 21i–33i. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865, Erratum in Lancet 1998, 352, 1558. [Google Scholar] [CrossRef]
- Dormandy, J.A.; Charbonnel, B.; Eckland, D.J.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefèbvre, P.J.; Murray, G.D.; et al. PROactive Investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- ADVANCE Collaborative Group; Patel, A.; MacMahon, S.; Chalmers, J.; Neal, B.; Billot, L.; Woodward, M.; Marre, M.; Cooper, M.; Glasziou, P.; et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar] [PubMed] [Green Version]
- Duckworth, W.; Abraira, C.; Moritz, T.; Reda, D.; Emanuele, N.; Reaven, P.D.; Zieve, F.J.; Marks, J.; Davis, S.N.; Hayward, R.; et al. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 2009, 360, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Rigalleau, V.; Larroumet, A.; Ducos, C.; Rigo, M.; Barbet-Massin, M.A.; Majchrzak, C.; Mohammedi, K.; Baillet-Blanco, L.; Monlun, M.; Rami-Arab, L.; et al. Cardiovascular events after a dramatic reduction of HbA1c in hospitalized subjects with type 2 diabetes and high long-term glucose exposure. J. Diabetes Complicat. 2022, 36, 108234. [Google Scholar] [CrossRef] [PubMed]
- Azad, N.; Agrawal, L.; Bahn, G.; Emanuele, N.V.; Reaven, P.D.; Hayward, R.; Reda, D.; VADT Study Group. Eye outcomes in Veteran Affairs Diabetes Trial (VADT) after 17 years. Diabetes Care 2021, 44, 2397–2402. [Google Scholar] [CrossRef]
- Schaan, B.D.; de Figueiredo Neto, J.A.; Moreira, L.B.; Ledur, P.; Mattos, L.A.P.; Magnoni, D.; Precoma, D.B.; Machado, C.A.; da Silva Brasileiro, A.L.; Pena, F.M.; et al. REACT Investigators. Diabetes and cardiovascular events in high-risk patients: Insights from a multicenter registry in a middle-income country. Diabetes Res. Clin. Pract. 2017, 127, 275–284. [Google Scholar] [CrossRef]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Hemmingsen, B.; Lund, S.S.; Gluud, C.; Vaag, A.; Almdal, T.; Hemmingsen, C.; Wetterslev, J. Intensive glycaemic control for patients with type 2 diabetes: Systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. Br. Med. J. 2011, 343, d6898. [Google Scholar] [CrossRef] [Green Version]
- Hasan, R.; Firwana, B.; Elraiyah, T.; Domecq, J.P.; Prutsky, G.; Nabhan, M.; Prokop, L.J.; Henke, P.; Tsapas, A.; Montori, V.M.; et al. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J. Vasc. Surg. 2016, 63 (Suppl. S2), 22S–28S.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldman, M.P.; Corriere, M.A.; Craven, T.; Davis, R.P.; Sheehan, M.; Hurie, J.B.; Velazquez, G.; Williams, T.K.; Chang, K.; Edwards, M.S. Risk factors for incident lower-extremity amputation in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial. J. Am. Coll. Surg. 2018, 227, S295. [Google Scholar]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. Br. Med. J. 2000, 321, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Fisher, V.L.; Tahrani, A.A. Cardiac autonomic neuropathy in patients with diabetes mellitus: Current perspectives. Diabetes Metab. Syndr. Obes. Targets Ther. 2017, 10, 419–434. [Google Scholar] [CrossRef] [Green Version]
- Caravati, C.M. Insulin neuritis: A case report. Va Med Mon. 1993, 59, 745–746. [Google Scholar]
- Kihara, M.; Zollman, P.J.; Smithson, I.L.; Lagerlund, T.D.; Low, P.A. Hypoxic effect of exogenous insulin on normal and diabetic peripheral nerve. Am. J. Physiol. 1994, 266 Pt 1, e980–e985. [Google Scholar] [CrossRef]
- Carstensen, B.; Kristensen, J.K.; Ottosen, P.; Borch-Johnsen, K.; Steering Group of the National Diabetes Register. The Danish National Diabetes Register: Trends in incidence, prevalence and mortality. Diabetologia 2008, 51, 2187–2196. [Google Scholar] [CrossRef] [Green Version]
- Tesfaye, S.; Malik, R.; Harris, N.; Jakubowski, J.J.; Mody, C.; Rennie, I.G.; Ward, J.D. Arterio-venous shunting and proliferating new vessels in acute painful neuropathy of rapid glycaemic control insulin neuritis. Diabetologia 1996, 39, 329–335. [Google Scholar] [CrossRef]
- Dabby, R.; Sadeh, M.; Lampl, Y.; Gilad, R.; Watemberg, N. Acute painful neuropathy induced by rapid correction of serum glucose levels in diabetic patients. Biomed. Pharm. 2009, 63, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Llewelyn, J.G.; Thomas, P.K.; Fonseca, V.; Dandona, P. Acute painful diabetic neuropathy. Diabetes Care 1988, 11, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.H.; Adler, G.K.; Bonyhay, I.; Freeman, R. Experimental hypoglycemia is a human model of stress-induced hyperalgesia. Pain 2012, 153, 2204–2209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llewelyn, J.G.; Thomas, P.K.; Fonseca, V.; King, R.H.; Dandona, P. Acute painful diabetic neuropathy precipitated by strict glycemic control. Acta Neuropathol. 1986, 72, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Nicodemus, J.M.; Enriquez, C.; Marquez, A.; Anaya, C.J.; Jolivalt, C.G. Murine model and mechanisms of treatment-induced painful diabetic neuropath. Neuroscience 2017, 354, 136–145. [Google Scholar] [CrossRef]
- Gibbons, C.H.; Freeman, R. Treatment-induced neuropathy of diabetes: An acute, iatrogenic complication of diabetes. Brain 2015, 138 Pt 1, 43–52. [Google Scholar] [CrossRef]
- Gibbons, C.H.; Freeman, R. Treatment-induced diabetic neuropathy: A reversible painful autonomic neuropathy. Ann. Neurol. 2010, 67, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Lauritzen, T.; Frost-Larsen, K.; Larsen, H.W.; Deckert, T. Effect of 1 year of near-normal blood glucose levels on retinopathy in insulin-dependent diabetics. Lancet 1983, 1, 200–204. [Google Scholar] [CrossRef]
- Kroc Collaborative Study Group. Blood glucose control and the evolution of diabetic retinopathy and albuminuria. A preliminary multicenter trial. N. Engl. J. Med. 1984, 311, 365–372. [Google Scholar] [CrossRef]
- Dahl-Jorgensen, K.; Brinchman-Hansen, O.; Hanssen, K.F.; Sandvik, L.; Aagenaes, O. Rapid tightening of blood glucose control leads to transient deterioration of retinopathy in insulin dependent diabetes mellitus: The Oslo study. Br. Med. J. (Clin. Res. Ed.) 1985, 290, 811–815. [Google Scholar] [CrossRef] [Green Version]
- Chantelau, E.; Meyer-Schwickerath, R. Reversion of ‘early worsening’ of diabetic retinopathy by deliberate restoration of poor metabolic control. Ophthalmologica 2003, 217, 373–377. [Google Scholar] [CrossRef]
- Feldman-Billard, S.; Larger, É.; Massin, P. Standards for screening and surveillance of ocular complications in people with diabetes SFD study group. Early worsening of diabetic retinopathy after rapid improvement of blood glucose control in patients with diabetes. Diabetes Metab. 2018, 44, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Hooymans, J.M.; Ballegooie, E.V.; Schweitzer, N.M.; Doorebos, H.; Reitsma, W.D.; Slutter, W.J. Worsening of diabetic retinopathy with strict control of blood sugar. Lancet 1982, 2, 438. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Mills, J.L.; Metzger, B.E.; Remaley, N.A.; Jovanovic-Peterson, L.; Knopp, R.H.; Conley, M.; Rand, L.; Simpson, J.L.; Holmes, L.B.; et al. Metabolic control and progression of retinopathy. The Diabetes in Early Pregnancy Study. National Institute of Child Health and Human Development Diabetes in Early Pregnancy Study. Diabetes Care 1995, 18, 631–637. [Google Scholar] [CrossRef]
- DCCT Group. Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch. Ophthalmol. 1998, 116, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.; Switzer, N.J.; Ehmann, D.; Rudnisky, C.; Shi, X.; Karmali, S. The impact of bariatric surgery on diabetic retinopathy: A systematic review and meta-analysis. Obes. Surg. 2015, 25, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Shurter, A.; Genter, P.; Ouyang, D.; Ipp, E. Euglycemic progression: Worsening of diabetic retinopathy in poorly controlled type 2 diabetes in minorities. Diabetes Res. Clin. Pract. 2013, 100, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilsbøll, T.; Bain, S.C.; Leiter, L.A.; Lingvay, I.; Matthews, D.; Simó, R.; Helmark, I.C.; Wijayasinghe, N.; Larsen, M. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes. Metab. 2018, 20, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Bain, S.C.; Klufas, M.A.; Ho, A.; Matthews, D.R. Worsening diabetic retinopathy with rapid improvement in systemic glucose control: A review. Diabetes Obes. Metab. 2018, 21, 454–466. [Google Scholar] [CrossRef] [Green Version]
- Cundy, T.; Holden, A.; Stallworthy, E. Early worsening of diabetic nephropathy in type 2 diabetes after rapid improvement in chronic sever hyperglycemia. Diabetes Care 2021, 44, e55–e56. [Google Scholar] [CrossRef]
- Dardari, D. An overview of Charcot’s neuroarthropathy. J. Clin. Transl. Endocrinol. 2020, 22, 100239. [Google Scholar] [CrossRef]
- Matricali, G.A.; Bammens, B.; Kuypers, D.; Flour, M.; Mathieu, C. High rate of Charcot foot attacks early after simultaneous pancreas-kidney transplantation. Transplantation 2007, 83, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Anthony, M.; Cravey, K.S.; Atway, S.A. Development of Charcot neuroarthropathy in diabetic patients who received kidney or kidney-pancreas transplants. J. Foot Ankle Surg. 2019, 58, 475–479. [Google Scholar] [CrossRef] [PubMed]
- García Barrado, F.; Kuypers, D.R.; Matricali, G.A. Charcot neuroarthropathy after simultaneous pancreas-kidney transplantation: Risk factors, prevalence, and outcome. Clin. Transpl. 2015, 29, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Dardari, D.; Ha Van, G.; M’Bemba, J.; Laborne, F.X.; Bourron, O.; Davaine, J.M.; Phan, F.; Foufelle, F.; Jaisser, F.; Penfornis, A.; et al. Rapid glycemic regulation in poorly controlled patients living with diabetes, a new associated factor in the pathophysiology of Charcot’s acute neuroarthropathy. PLoS ONE 2020, 15, e0233168. [Google Scholar] [CrossRef]
- Dardari, D.; Schuldiner, S.; Julien, C.A.; Ha Van, G.; M’Bemba, J.; Bourgeon, M.; Sultan, A.; Lepeut, M.; Grandperret-Vauthier, S.; Baudoux, F.; et al. Trends in the relation between hyperglycemia correction and active Charcot neuroarthropathy: Results from the EPICHAR study. Br. Med. J. Open Diabetes Res. Care 2022, 10, e002380. [Google Scholar] [CrossRef]
- Dardari, D.; Dardari, R. Why the risk of developing neuroarthropathy is higher after simultaneous kidney and pancreatic transplantation compared to kidney transplantation only: The role of euglycemia. Ann. Transpl. 2021, 26, e928449. [Google Scholar] [CrossRef]
- Xiang, G.D.; Sun, H.L.; Zhao, L.S.; Hou, J.; Yue, L.; Xu, L. Changes of osteoprotegerin before and after insulin therapy in type 1 diabetic patients. Zhonghua Yi Xue Za Zhi 2007, 87, 1234–1237. [Google Scholar] [CrossRef]
- Tkác, I. Effect of intensive glycemic control on cardiovascular outcomes and all-cause mortality in type 2 diabetes: Overview and metaanalysis of five trials. Diabetes Res. Clin. Pract. 2009, 86 (Suppl. S1), S57–S62. [Google Scholar] [CrossRef]
- Benhamou, P.Y.; Franc, S.; Reznik, Y.; Thivolet, C.; Schaepelynck, P.; Renard, E.; Guerci, B.; Chaillous, L.; Lukas-Croisier, C.; Jeandidier, N.; et al. DIABELOOP WP7 Trial Investigators. Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: A 12-week multicentre, open-label randomised controlled crossover trial. Lancet Digit. Health 2019, 1, e17–e25. [Google Scholar] [CrossRef] [Green Version]
- Fralick, M.; Colacci, M.; Odutayo, A.; Siemieniuk, R.; Glynn, R.J. Lowering of hemoglobin A1C and risk of cardiovascular outcomes and all-cause mortality, a meta-regression analysis. J. Diabetes Complicat. 2020, 34, 107704. [Google Scholar] [CrossRef] [PubMed]
- Boussageon, R.; Bejan-Angoulvant, T.; Saadatian-Elahi, M.; Lafont, S.; Bergeonneau, C.; Kassaï, B.; Erpeldinger, S.; Wright, J.M.; Gueyffier, F.; Cornu, C. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: Meta-analysis of randomised controlled trials. Br. Med. J. 2011, 343, d4169. [Google Scholar] [CrossRef] [Green Version]
- Nathan, D.M.; Cleary, P.A.; Backlund, J.Y.; Genuth, S.M.; Lachin, J.M.; Orchard, T.J.; Raskin, P.; Zinman, B. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N. Engl. J. Med. 2005, 353, 2643–2653. [Google Scholar] [PubMed]
- Aede, P.; Lund-Andersen, H.; Parving, H.H.; Pedersen, O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 2008, 358, 580–591. [Google Scholar]
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C., Jr.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The diabetes mellitus-atherosclerosis connection: The role of lipid and glucose metabolism and chronic inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef] [Green Version]
- Henricsson, M.; Berntorp, K.; Berntorp, E.; Fernlund, P.; Sundkvist, G. Progression of retinopathy after improved metabolic control in type 2 diabetic patients. Relation to IGF-1 and hemostatic variables. Diabetes Care 1999, 22, 1944–1949. [Google Scholar] [CrossRef]
- Varadhan, L.; Humphreys, T.; Hariman, C.; Walker, A.B.; Varughese, G.I. GLP-1 agonist treatment: Implications for diabetic retinopathy screening. Diabetes Res. Clin. Pract. 2011, 94, e68–e71. [Google Scholar] [CrossRef]
- Chantelau, E.; Meyer-Schwickerath, R.; Klabe, K. Downregulation of serum IGF-1 for treatment of early worsening of diabetic retinopathy: A long-term follow-up of two cases. Ophthalmologica 2010, 224, 243–246. [Google Scholar] [CrossRef]
- Amiel, S.A.; Sherwin, R.S.; Hintz, R.L.; Gertner, J.M.; Press, C.M.; Tamborlane, W.V. Effect of diabetes and its control on insulin-like growth factors in the young subject with type I diabetes. Diabetes 1984, 33, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Chantelau, E.; Kohner, E.M. Why some cases of retinopathy worsen when diabetic control improves. Br. Med. J. 1997, 315, 1105–1106. [Google Scholar] [CrossRef] [Green Version]
- Attia, N.; Caprio, S.; Jones, T.W.; Heptulla, R.; Holcombe, J.; Silver, D.; Sherwin, R.S.; Tamborlane, W.V. Changes in free insulin-like growth factor-1 and leptin concentrations during acute metabolic decompensation in insulin withdrawn patients with type 1 diabetes. J. Clin. Endocrinol. Metab. 1999, 84, 2324–2328. [Google Scholar]
- Fukuda, R.; Hirota, K.; Fan, F.; Jung, Y.D.; Ellis, L.M.; Semenza, G.L. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J. Biol. Chem. 2002, 277, 38205–38211. [Google Scholar] [CrossRef] [Green Version]
- Grant, M.B.; Mames, R.N.; Fitzgerald, C.; Ellis, E.A.; Caballero, S.; Chegini, N.; Guy, J. Insulin-like growth factor I as an angiogenic agent. in vivo and in vitro studies. Ann. N. Y. Acad. Sci. 1993, 692, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Danis, R.P.; Bingaman, D.P. Insulin-like growth factor-1 retinal microangiopathy in the pig eye. Ophthalmology 1997, 104, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Collyns, O.J.; Meier, R.A.; Betts, Z.L.; Chan, D.S.H.; Frampton, C.; Frewen, C.M.; Hewapathirana, N.M.; Jones, S.D.; Roy, A.; Grosman, B.; et al. Improved glycemic outcomes with medtronic minimed advanced hybrid closed-loop delivery: Results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes Care 2021, 44, 969–975. [Google Scholar] [CrossRef]
| Impact of the Intensive Management of Hyperglycemia | Reference | Stroke | Myocardial Infarction | Cardiovascular Mortality | Arteriopathy of Lower Limbs |
|---|---|---|---|---|---|
| UKPDS | [11] | No Impact | Reduction | No Impact | Not evaluated |
| PRO active | [12] | No Impact | No Impact | No Impact | Not evaluated |
| ADVANCE | [13] | Reduction | No Impact | No Impact | Not evaluated |
| ACCORD | [10] | No Impact | Reduction | Reduction | Reduction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poonoosamy, J.; Lopes, P.; Huret, P.; Dardari, R.; Penfornis, A.; Thomas, C.; Dardari, D. Impact of Intensive Glycemic Treatment on Diabetes Complications—A Systematic Review. Pharmaceutics 2023, 15, 1791. https://doi.org/10.3390/pharmaceutics15071791
Poonoosamy J, Lopes P, Huret P, Dardari R, Penfornis A, Thomas C, Dardari D. Impact of Intensive Glycemic Treatment on Diabetes Complications—A Systematic Review. Pharmaceutics. 2023; 15(7):1791. https://doi.org/10.3390/pharmaceutics15071791
Chicago/Turabian StylePoonoosamy, Juliana, Philippe Lopes, Priscille Huret, Randa Dardari, Alfred Penfornis, Claire Thomas, and Dured Dardari. 2023. "Impact of Intensive Glycemic Treatment on Diabetes Complications—A Systematic Review" Pharmaceutics 15, no. 7: 1791. https://doi.org/10.3390/pharmaceutics15071791
APA StylePoonoosamy, J., Lopes, P., Huret, P., Dardari, R., Penfornis, A., Thomas, C., & Dardari, D. (2023). Impact of Intensive Glycemic Treatment on Diabetes Complications—A Systematic Review. Pharmaceutics, 15(7), 1791. https://doi.org/10.3390/pharmaceutics15071791





