Recent Advances in Zein-Based Nanocarriers for Precise Cancer Therapy
Abstract
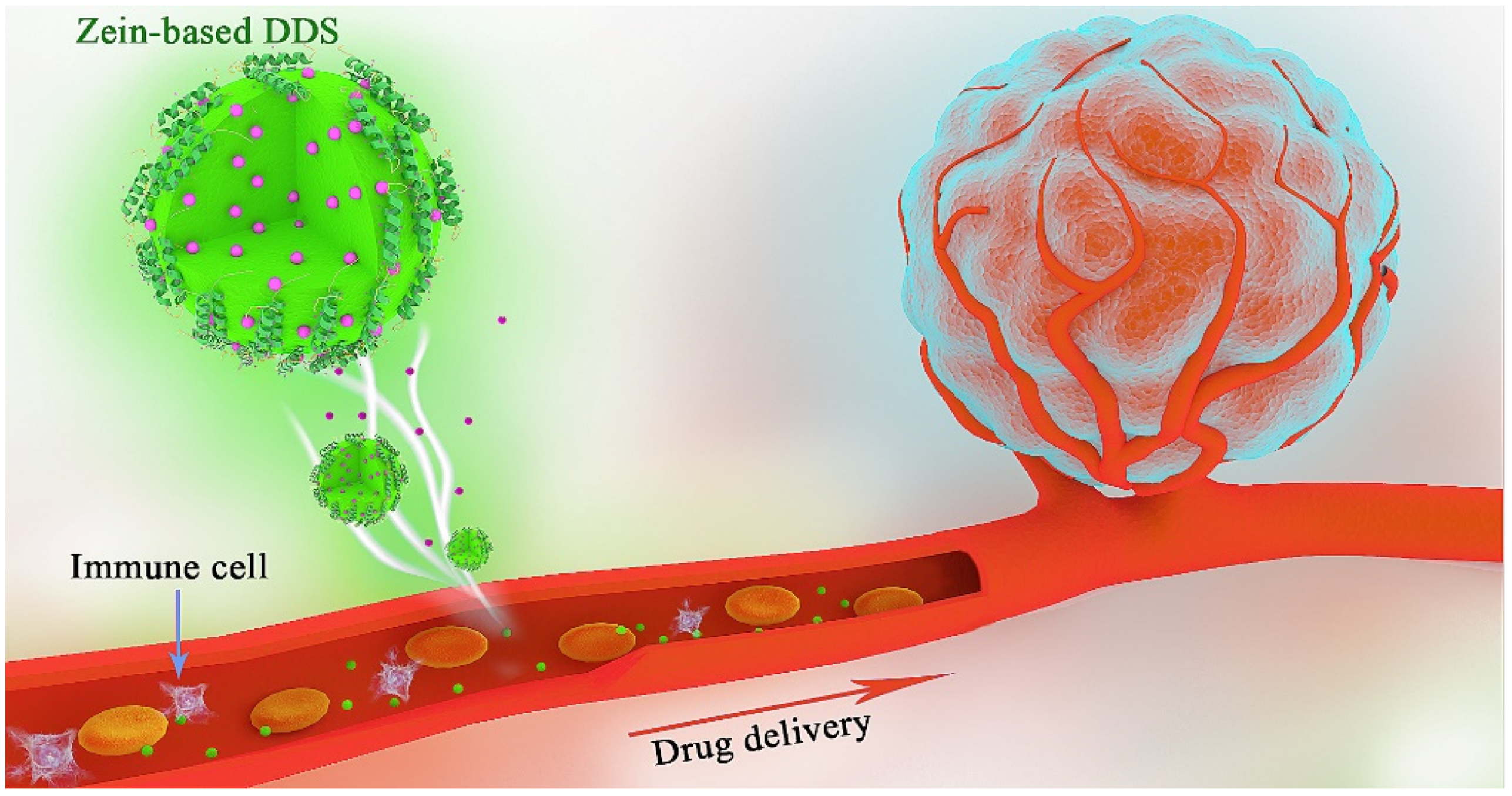
1. Introduction
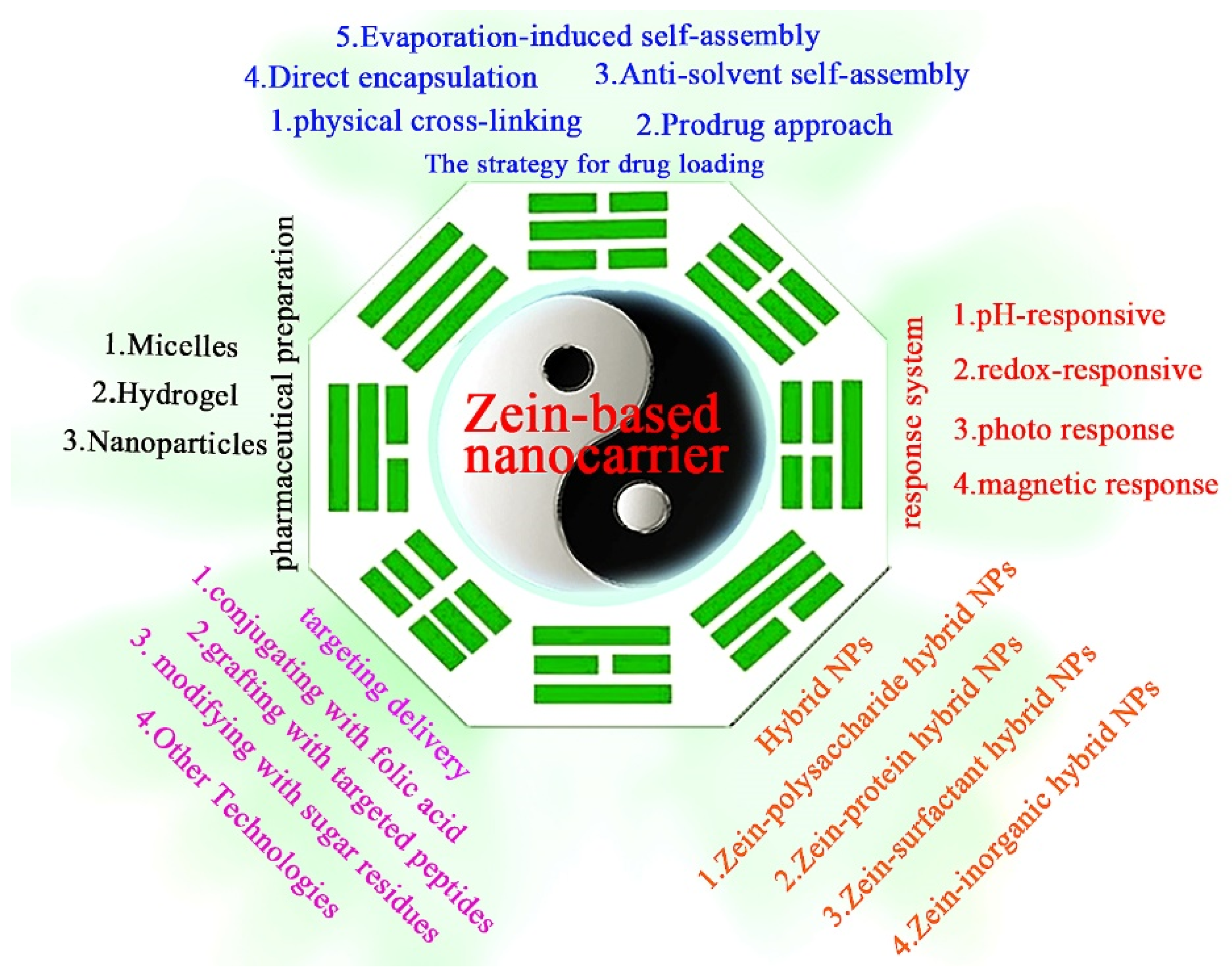
2. Preparation of Zein-Based Nanocarriers for Anticancer Drug Delivery
2.1. The Strategy for Drug Loading
2.1.1. Physical Cross-Linking
2.1.2. Evaporation-Induced Self-Assembly
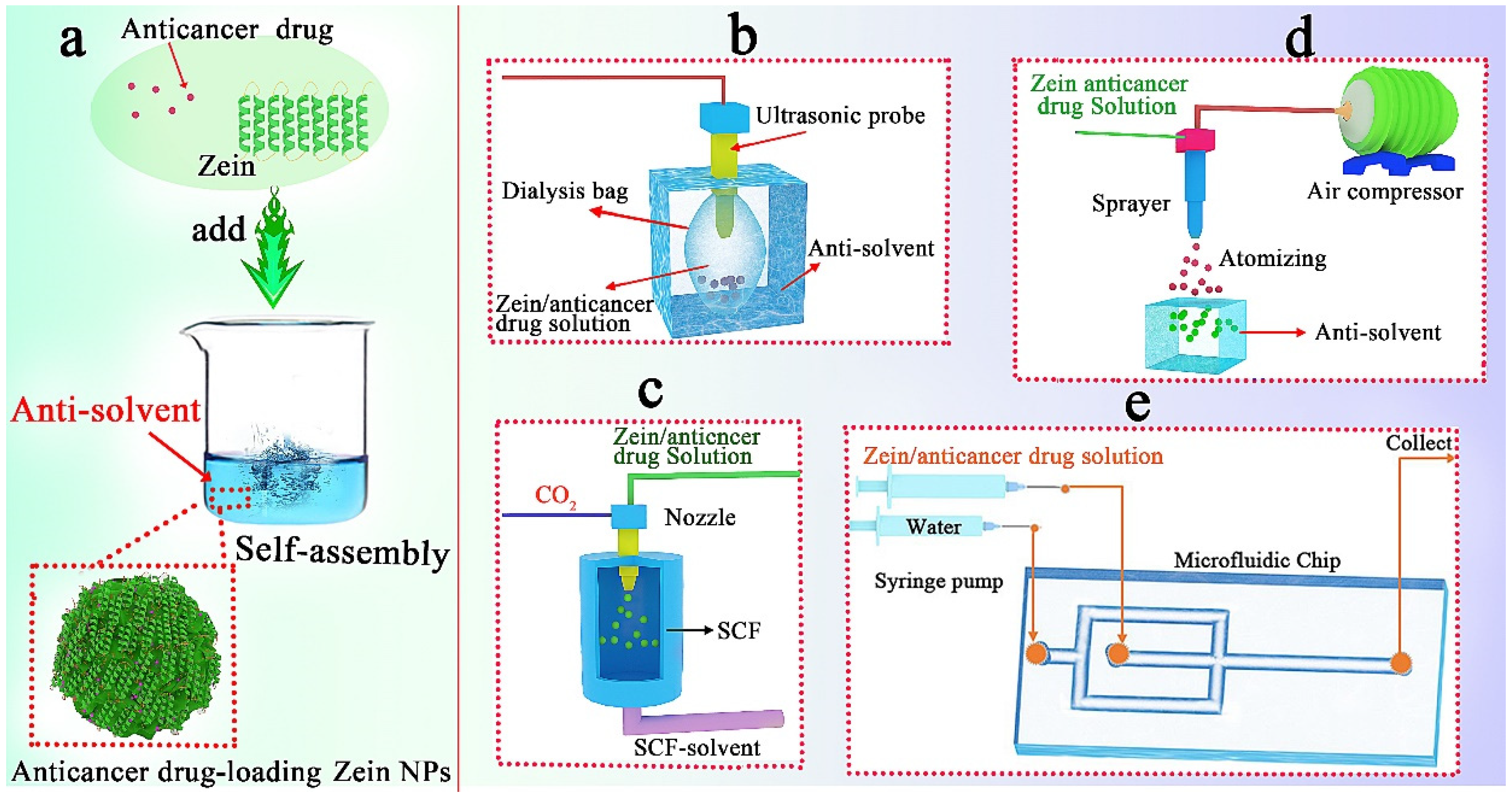
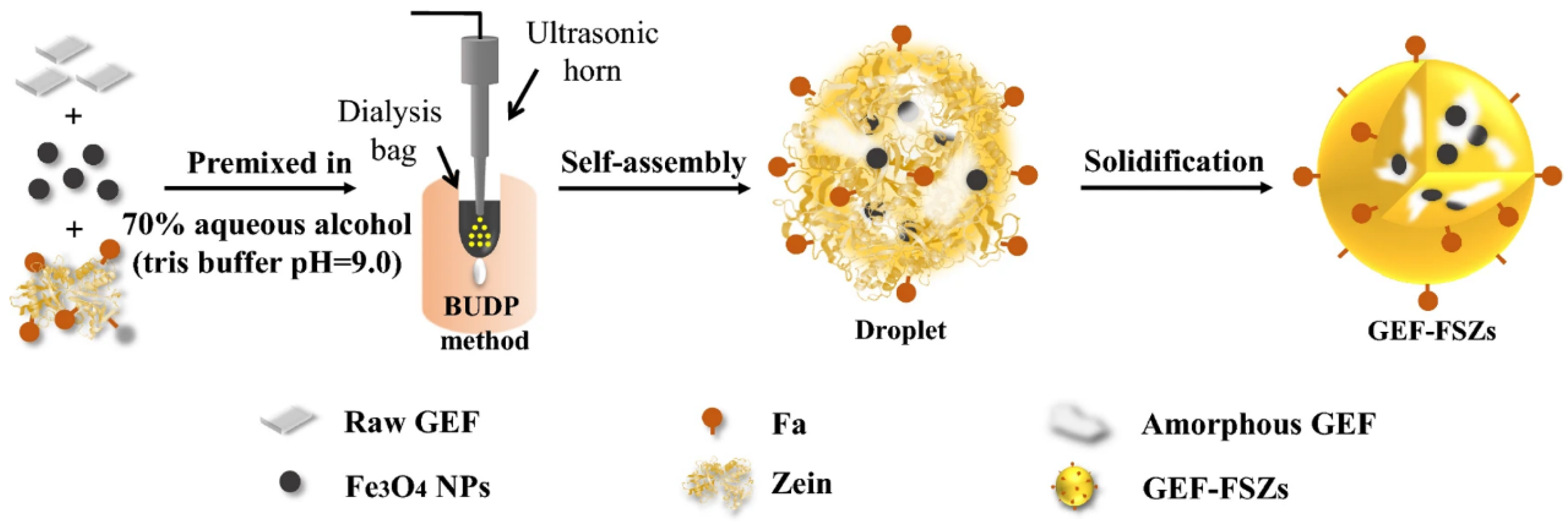
2.1.3. Anti-Solvent Self-Assembly
- (1)
- Ultrasonic dialysis process
- (2)
- Self-assembly in supercritical fluid
- (3)
- Atomizing/antisolvent precipitation process
- (4)
- Microfluidic technology
2.1.4. Chemical Synthesis of Prodrugs
2.2. Type of Pharmaceutical Preparation
2.2.1. Micelles
2.2.2. Hydrogel
2.2.3. Nanoparticles
3. The Challenges and Solutions of Zein-Based Nanocarriers for Precise Cancer Therapy
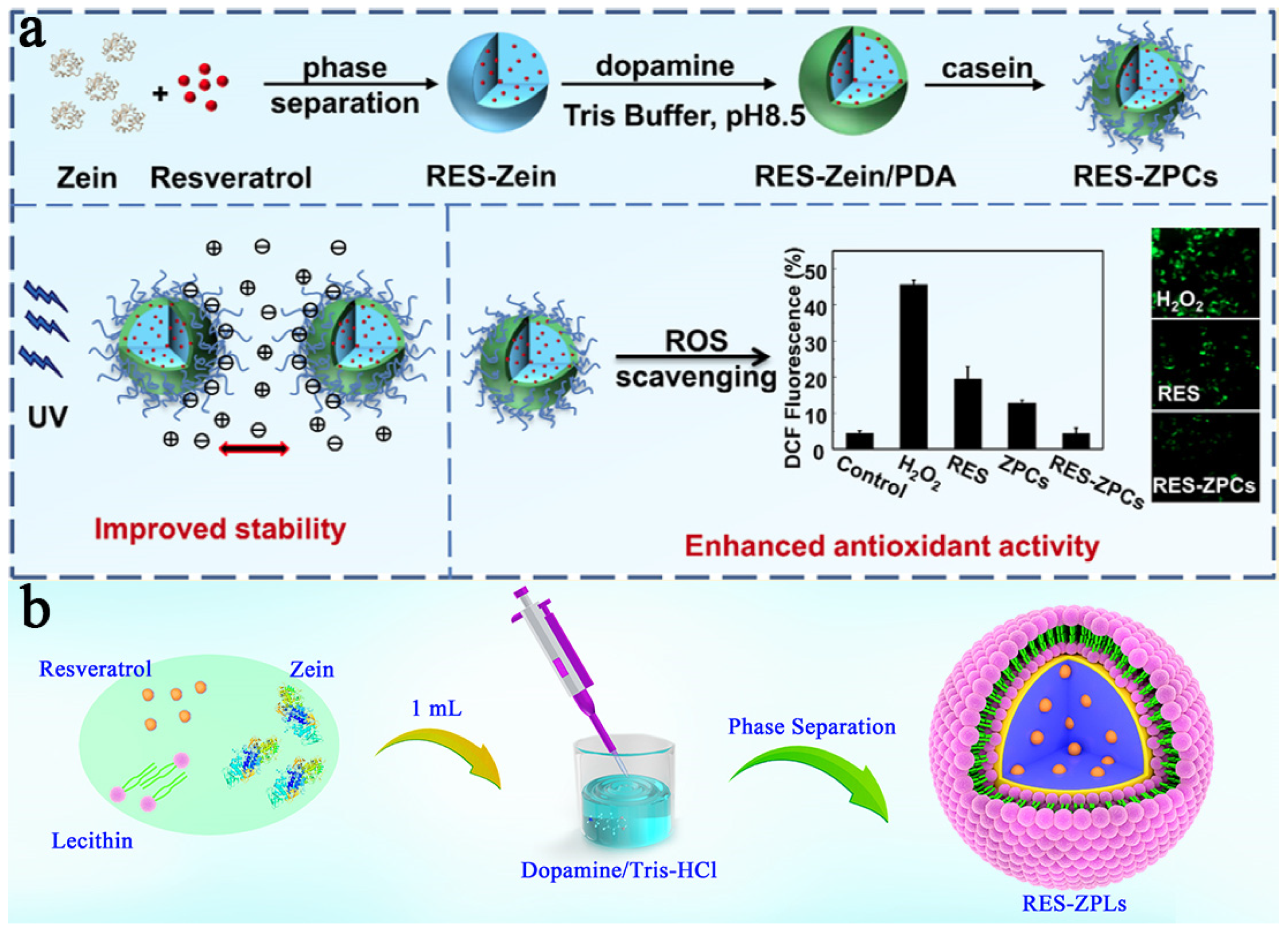
3.1. Stable Zein-Based DDSs
3.2. Targeting Zein-Based DDSs
3.2.1. Conjugating with Folic Acid
3.2.2. Grafting with Peptides
3.2.3. Modifying with Sugar Residues
3.2.4. Others
3.3. Responsive Zein-Based DDSs
3.3.1. pH Response
3.3.2. Redox Response
3.3.3. Photo Response
3.3.4. Magnetic Response
3.4. Hybrid Zein-Based DDSs
3.4.1. Zein–Polysaccharide Hybrid NPs
3.4.2. Zein–Protein Hybrid NPs
3.4.3. Zein–Surfactant Hybrid NPs
3.4.4. Zein–Inorganic Hybrid NPs
4. Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tai, Z.; Cui, Z.; Chai, R.; Zhu, Q.; Chen, Z. Nano-engineered immune cells as “guided missiles” for cancer therapy. J. Control. Release 2022, 341, 60–79. [Google Scholar] [CrossRef]
- Qi, L.; Luo, Q.; Zhang, Y.; Jia, F.; Zhao, Y.; Wang, F. Advances in toxicological research of the anticancer drug cisplatin. Chem. Res. Toxicol. 2019, 32, 1469–1486. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Genotoxicity and in vitro investigation of Gefitinib-loaded polycaprolactone fabricated nanoparticles for anticancer activity against NCI-H460 cell lines. J. Exp. Nanosci. 2022, 17, 214–246. [Google Scholar] [CrossRef]
- Qiao, H.; Chen, Z.; Fu, S.; Yu, X.; Sun, M.; Zhai, Y.; Sun, J. Emerging platinum(0) nanotherapeutics for efficient cancer therapy. J. Control. Release 2022, 352, 276–287. [Google Scholar] [CrossRef]
- Fang, C.Y.; Lou, D.Y.; Zhou, L.Q.; Wang, J.C.; Yang, B.; He, Q.J.; Wang, J.J.; Weng, Q.J. Natural products: Potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol. Sin. 2021, 42, 1951–1969. [Google Scholar] [CrossRef]
- Huang, W.Q.; Li, Z.Q.; Liu, W.Q.; Liu, S.Y.; Zhou, R.J.; Jiang, Y.B. Preparation of B16 cancer cell membrane coated α-zein biomimetic drug delivery system for the enhancement of homotypic target ability. Ind. Crops Prod. 2023, 194, 116301–116312. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Arunkumar, P.; Prasad, R.; Mishra, S.K.; Reddy, B.P.K.; De, A.; Srivastava, R. Facile synthesis of plasmonic zein nanoshells for imaging-guided photothermal cancer therapy. Mater. Sci. Eng. C 2018, 90, 539–548. [Google Scholar] [CrossRef]
- Huang, W.Q.; Deng, Y.H.; Ye, L.P.; Xie, Q.L.; Jiang, Y.B. Enhancing hemocompatibility and the performance of Au@silica nanoparticles by coating with cRGD functionalized zein. Mater. Sci. Eng. C 2021, 125, 112064–112076. [Google Scholar] [CrossRef]
- Jiang, L.; Li, L.; He, X.; Yi, Q.; He, B.; Cao, J.; Pan, W.; Gu, Z. Overcoming drug-resistant lung cancer by paclitaxel loaded dual-functional liposomes with mitochondria targeting and pH-response. Biomaterials 2015, 52, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Gaber, M.; Elhasany, K.A.; Sabra, S.; Helmy, M.W.; Fang, J.Y.; Khattab, S.N.; Bekhit, A.A.; Teleb, M.; Elkodairy, K.A.; Elzoghby, A.O. Co-administration of tretinoin enhances the anti-cancer efficacy of etoposide via tumor-targeted green nano-micelles. Colloids Surf. B Biointerfaces 2020, 192, 110997–111007. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Zhang, Y.; Wu, Y.C.; Ma, Y.; Li, H.J. Co-encapsulation of curcumin and resveratrol in zein-bovine serum albumin nanoparticles using a pH-driven method. Food Funct. 2023, 14, 3169–3178. [Google Scholar] [CrossRef]
- Dong, P.; Rakesh, K.P.; Manukumar, H.M.; Mohammed, Y.H.E.; Karthik, C.S.; Sumathi, S.; Mallu, P.; Qin, H.L. Innovative nano-carriers in anticancer drug delivery—A comprehensive review. Bioorg. Chem. 2019, 85, 325–336. [Google Scholar] [CrossRef]
- Yu, X.; Han, N.; Dong, Z.; Dang, Y.; Zhang, Q.; Hu, W.; Wang, C.; Du, S.; Lu, Y. Combined chemo-immuno-photothermal therapy for effective cancer treatment via an all-in-one and one-for-all nanoplatform. ACS Appl. Mater. Inter. 2022, 14, 42988–43009. [Google Scholar] [CrossRef]
- Jin, J.; Krishnamachary, B.; Barnett, J.D.; Chatterjee, S.; Chang, D.; Mironchik, Y.; Wildes, F.; Jaffee, E.M.; Nimmagadda, S.; Bhujwalla, Z.M. Human cancer cell membrane-coated biomimetic nanoparticles reduce fibroblast-mediated invasion and metastasis and induce T-cells. ACS Appl. Mater. Inter. 2019, 11, 7850–7861. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Preat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Wang, H.D.; Zhu, W.; Huang, Y.N.; Li, Z.X.; Jiang, Y.B.; Xie, Q.L. Facile encapsulation of hydroxycamptothecin nanocrystals into zein-based nanocomplexes for active targeting in drug delivery and cell imaging. Acta Biomater. 2017, 61, 88–100. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug delivery: Is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Hassan, E.A.; Hathout, R.M.; Gad, H.A.; Sammour, O.A. Multi-purpose zein nanoparticles for battling hepatocellular carcinoma: A green approach. Eur. Polym. J. 2022, 176, 111396–111408. [Google Scholar] [CrossRef]
- Caro, C.; Pozo, D. Polysaccharide colloids as smart vehicles in cancer therapy. Curr. Pharm. Des. 2015, 21, 4822–4836. [Google Scholar] [CrossRef]
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and fallacy. J. Control. Release 2014, 190, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Caro, C.; Avasthi, A.; Paez-Munoz, J.M.; Pernia Leal, M.; Garcia-Martin, M.L. Passive targeting of high-grade gliomas via the EPR effect: A closed path for metallic nanoparticles? Biomater. Sci. 2021, 9, 7984–7995. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, W.; Sun, C.; Wei, X.; Liu, S.; Jiang, Y. Gastrointestinal pH-sensitive pickering emulsions stabilized by zein nanoparticles coated with bioactive glycyrrhizic acid for improving oral bioaccessibility of curcumin. ACS Appl. Mater. Inter. 2023, 15, 14678–14689. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.T.; Zhang, D.; Lin, J.W.; Zhang, Y.Y.; Li, C.Y.; Wang, Z.; Ren, J.Y.; Yao, M.J.; Wong, K.H.; Wang, Y. Zein-paclitaxel prodrug nanoparticles for redox-triggered drug delivery and enhanced therapeutic efficiency. J. Agric. Food Chem. 2018, 66, 11812–11822. [Google Scholar] [CrossRef]
- Huang, W.; Li, S.; Li, Z.; Zhu, W.; Lu, S.; Jiang, Y. Development of a resveratrol–zein–dopamine–lecithin delivery system with enhanced stability and mucus permeation. J. Mater. Sci. 2019, 54, 8591–8601. [Google Scholar] [CrossRef]
- van Ballegooie, C.; Wretham, N.; Ren, T.; Popescu, I.M.; Yapp, D.T.; Bally, M.B. PEG conjugated zein nanoparticles for in vivo use. Pharmaceutics 2022, 14, 1831–1850. [Google Scholar] [CrossRef]
- Kaushik, P.; Priyadarshini, E.; Rawat, K.; Rajamani, P.; Bohidar, H.B. pH responsive doxorubucin loaded zein nanoparticle crosslinked pectin hydrogel as effective site-specific anticancer substrates. Int. J. Biol. Macromol. 2020, 152, 1027–1037. [Google Scholar] [CrossRef]
- Wang, H.D.; Zhang, X.T.; Zhu, W.; Jiang, Y.B.; Zhang, Z.B. Self-assembly of zein-based microcarrier system for colon-targeted oral drug delivery. Ind. Eng. Chem. Res. 2018, 57, 12689–12699. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, W.; Ye, L.; Deng, Y.; Xie, Q.; Jiang, Y. Facile preparation of succinylated-zein-ZIF-8 hybrid for enhanced stability and pH-responsive drug delivery. Chem. Eng. Sci. 2020, 228, 115981–115992. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, Y.; Wang, J.; Zhai, G. New progress and prospects: The application of nanogel in drug delivery. Mater. Sci. Eng. C 2016, 60, 560–568. [Google Scholar] [CrossRef]
- Seok, H.Y.; Rejinold, N.S.; Lekshmi, K.M.; Cherukula, K.; Park, I.K.; Kim, Y.C. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: In vitro and in vivo evaluation. J. Control. Release 2018, 280, 20–30. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; El-Lakany, S.A.; Helmy, M.W.; Abu-Serie, M.M.; Elgindy, N.A. Shell-crosslinked zein nanocapsules for oral codelivery of exemestane and resveratrol in breast cancer therapy. Nanomedicine 2017, 12, 2785–2805. [Google Scholar] [CrossRef]
- von Baeckmann, C.; Rubio, G.; Kahlig, H.; Kurzbach, D.; Reithofer, M.R.; Kleitz, F. Evaporation-induced self-assembly of small peptide-conjugated silica nanoparticles. Angew. Chem. Int. Ed. Engl. 2021, 60, 22700–22705. [Google Scholar] [CrossRef]
- Wang, Y.; Padua, G.W. Nanoscale characterization of zein self-assembly. Langmuir 2012, 28, 2429–2435. [Google Scholar] [CrossRef]
- Cai, T.; Xiao, P.; Yu, N.; Zhou, Y.; Mao, J.; Peng, H.; Deng, S. A novel pectin from Akebia trifoliata var. australis fruit peel and its use as a wall-material to coat curcumin-loaded zein nanoparticle. Int. J. Biol. Macromol. 2020, 152, 40–49. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Xiao, M.; Ganesan, K.; Gao, F.; Liu, Q.; Ye, Z.; Sui, Y.; Zhang, F.; Wei, K.; et al. A tumor-targeted delivery of oral isoliquiritigenin through encapsulated zein phosphatidylcholine hybrid nanoparticles prevents triple-negative breast cancer. J. Drug Deliv. Sci. Technol. 2023, 79, 103922–103938. [Google Scholar] [CrossRef]
- Song, J.; Sun, C.; Gul, K.; Mata, A.; Fang, Y. Prolamin-based complexes: Structure design and food-related applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1120–1149. [Google Scholar] [CrossRef]
- Jiang, F.Y.; Yang, L.L.; Wang, S.Y.; Ying, X.G.; Ling, J.H.; Ouyang, X.K. Fabrication and characterization of zein-alginate oligosaccharide complex nanoparticles as delivery vehicles of curcumin. J. Mol. Liq. 2021, 342, 116937–116942. [Google Scholar] [CrossRef]
- Liu, G.J.; Wei, D.W.; Wang, H.D.; Hu, Y.T.; Jiang, Y.B. Self-assembly of zein microspheres with controllable particle size and narrow distribution using a novel built-in ultrasonic dialysis process. Chem. Eng. J. 2016, 284, 1094–1105. [Google Scholar] [CrossRef]
- Ye, L.; Huang, W.; Deng, Y.; Li, Z.; Jiang, Y.; Xie, Q. Development of a pluronic-zein-curcumin drug delivery system with effective improvement of hydrophilicity, stability and sustained-release. J. Cereal Sci. 2022, 104, 103412–103420. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.S.; Kim, S.; Ha, E.S.; Kim, M.S.; Hwang, S.J. Pharmaceutical applications of supercritical fluid extraction of emulsions for micro-/nanoparticle formation. Pharmaceutics 2021, 13, 1928–1957. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Walker, G.M.; Ryan, K.M.; Padrela, L. Controlling polymorphism of carbamazepine nanoparticles in a continuous supercritical-CO2-assisted spray drying process. Cryst. Growth Des. 2019, 19, 3755–3767. [Google Scholar] [CrossRef]
- Palazzo, I.; Campardelli, R.; Scognamiglio, M.; Reverchon, E. Zein/luteolin microparticles formation using a supercritical fluids assisted technique. Powder Technol. 2019, 356, 899–908. [Google Scholar] [CrossRef]
- Liu, G.J.; Li, S.M.; Huang, Y.X.; Wang, H.D.; Jiang, Y.B. Incorporation of 10-hydroxycamptothecin nanocrystals into zein microspheres. Chem. Eng. Sci. 2016, 155, 405–414. [Google Scholar] [CrossRef]
- Wu, Z.; Li, J.; Zhang, X.; Li, Y.; Wei, D.; Tang, L.; Deng, S.; Liu, G. Rational fabrication of folate-conjugated zein/soy lecithin/carboxymethyl chitosan core-shell nanoparticles for delivery of docetaxel. ACS Omega 2022, 7, 13371–13381. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Wu, Z.; Li, J.; Li, J.; Deng, S.; Liu, G. Development of carboxymethyl chitosan-coated zein/soy lecithin nanoparticles for the delivery of resveratrol. Food Funct. 2023, 14, 1636–1647. [Google Scholar] [CrossRef]
- Jain, V.; Patel, V.B.; Singh, B.; Varade, D. Microfluidic device based molecular Self-Assembly structures. J. Mol. Liq. 2022, 362, 119760–119774. [Google Scholar] [CrossRef]
- Staufer, O.; Antona, S.; Zhang, D.; Csatari, J.; Schroter, M.; Janiesch, J.W.; Fabritz, S.; Berger, I.; Platzman, I.; Spatz, J.P. Microfluidic production and characterization of biofunctionalized giant unilamellar vesicles for targeted intracellular cargo delivery. Biomaterials 2021, 264, 120203–120216. [Google Scholar] [CrossRef]
- Guo, H.; Feng, Y.; Deng, Y.; Yan, T.; Liang, Z.; Zhou, Y.; Zhang, W.; Xu, E.; Liu, D.; Wang, W. Continuous flow modulates zein nanoprecipitation solvent environment to obtain colloidal particles with high curcumin loading. Food Hydrocoll. 2023, 134, 108089–108099. [Google Scholar] [CrossRef]
- Meewan, J.; Somani, S.; Almowalad, J.; Laskar, P.; Mullin, M.; MacKenzie, G.; Khadke, S.; Perrie, Y.; Dufes, C. Preparation of zein-based nanoparticles: Nanoprecipitation versus microfluidic-assisted manufacture, effects of PEGylation on nanoparticle characteristics and cellular uptake by melanoma cells. Int. J. Nanomed. 2022, 17, 2809–2822. [Google Scholar] [CrossRef]
- Olenskyj, A.G.; Feng, Y.; Lee, Y. Continuous microfluidic production of zein nanoparticles and correlation of particle size with physical parameters determined using CFD simulation. J. Food Eng. 2017, 211, 50–59. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, L.; Zhang, H.; Shang, L.; Zhao, Y. Microfluidics for drug development: From synthesis to evaluation. Chem. Rev. 2021, 121, 7468–7529. [Google Scholar] [CrossRef]
- Tran, P.; Pyo, Y.C.; Kim, D.H.; Lee, S.E.; Kim, J.K.; Park, J.S. Overview of the manufacturing methods of solid dispersion technology for improving the solubility of poorly water-soluble drugs and application to anticancer Drugs. Pharmaceutics 2019, 11, 132–157. [Google Scholar] [CrossRef]
- Vig, B.S.; Huttunen, K.M.; Laine, K.; Rautio, J. Amino acids as promoieties in prodrug design and development. Adv. Drug Deliv. Rev. 2013, 65, 1370–1385. [Google Scholar] [CrossRef]
- Podaralla, S.; Averineni, R.; Alqahtani, M.; Perumal, O. Synthesis of novel biodegradable methoxy poly(ethylene glycol)-zein micelles for effective delivery of curcumin. Mol. Pharm. 2012, 9, 2778–2786. [Google Scholar] [CrossRef]
- Sabra, S.A.; Elzoghby, A.O.; Sheweita, S.A.; Haroun, M.; Helmy, M.W.; Eldemellawy, M.A.; Xia, Y.; Goodale, D.; Allan, A.L.; Rohani, S. Self-assembled amphiphilic zein-lactoferrin micelles for tumor targeted co-delivery of rapamycin and wogonin to breast cancer. Eur. J. Pharm. Biopharm. 2018, 128, 156–169. [Google Scholar] [CrossRef]
- Song, R.; Zhou, Y.; Li, Y.; Yang, Z.; Li, F.; Huang, Q.; Shi, T.; Zhang, G. Preparation and characterization of mPEG-g-α-zein biohybrid micelles as a nano-carrier. J. Appl. Polym. Sci. 2015, 132, 42555–42560. [Google Scholar] [CrossRef]
- Kunjiappan, S.; Theivendran, P.; Baskararaj, S.; Sankaranarayanan, B.; Palanisamy, P.; Saravanan, G.; Arunachalam, S.; Sankaranarayanan, M.; Natarajan, J.; Somasundaram, B.; et al. Modeling a pH-sensitive zein-co-acrylic acid hybrid hydrogels loaded 5-fluorouracil and rutin for enhanced anticancer efficacy by oral delivery. 3 Biotech 2019, 9, 185–204. [Google Scholar] [CrossRef]
- Shinde, P.; Agraval, H.; Srivastav, A.K.; Yadav, U.C.S.; Kumar, U. Physico-chemical characterization of carvacrol loaded zein nanoparticles for enhanced anticancer activity and investigation of molecular interactions between them by molecular docking. Int. J. Pharm. 2020, 588, 119795–119806. [Google Scholar] [CrossRef]
- Dong, F.; Dong, X.; Zhou, L.; Xiao, H.; Ho, P.Y.; Wong, M.S.; Wang, Y. Doxorubicin-loaded biodegradable self-assembly zein nanoparticle and its anti-cancer effect: Preparation, in vitro evaluation, and cellular uptake. Colloids Surf. B Biointerfaces 2016, 140, 324–331. [Google Scholar] [CrossRef]
- Yu, X.; Wu, H.; Hu, H.; Dong, Z.; Dang, Y.; Qi, Q.; Wang, Y.; Du, S.; Lu, Y. Zein nanoparticles as nontoxic delivery system for maytansine in the treatment of non-small cell lung cancer. Drug Deliv. 2020, 27, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, J.; Liu, D.; Zhu, W.; Guan, S.; Fan, L.; Cai, D. Targeted delivery of honokiol by zein/hyaluronic acid core-shell nanoparticles to suppress breast cancer growth and metastasis. Carbohydr. Polym. 2020, 240, 116325–116337. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Micelles structure development as a strategy to improve smart cancer therapy. Cancers 2018, 10, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Vashist, A.; Vashist, A.; Gupta, Y.K.; Ahmad, S. Recent advances in hydrogel based drug delivery systems for the human body. J. Mater. Chem. B 2014, 2, 147–166. [Google Scholar] [CrossRef]
- Paliwal, R.; Palakurthi, S. Zein in controlled drug delivery and tissue engineering. J. Control. Release 2014, 189, 108–122. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Sun, X.; Wang, S.; Wang, J.; Zhu, J.; Wang, D.; Zhao, L. Stability, bioactivity, and bioaccessibility of fucoxanthin in zein-caseinate composite nanoparticles fabricated at neutral pH by antisolvent precipitation. Food Hydrocoll. 2018, 84, 379–388. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Pang, J.; Chen, J.; Wang, H.; Xie, Q.; Jiang, Y. Polydopamine-mediated carrier with stabilizing and self-antioxidative properties for polyphenol delivery systems. Ind. Eng. Chem. Res. 2018, 57, 590–599. [Google Scholar] [CrossRef]
- Huang, W.Q.; Liu, S.Y.; Li, Z.Q.; Liu, Y.Y.; Xie, Q.L.; Jiang, Y.B. Analysis of the differences in self-assembly behaviour, molecular structure and drug delivery performance between alpha and beta-Zein. Ind. Crops Prod. 2022, 181, 114822–114836. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
- Poon, W.; Kingston, B.R.; Ouyang, B.; Ngo, W.; Chan, W.C.W. A framework for designing delivery systems. Nat. Nanotechnol. 2020, 15, 819–829. [Google Scholar] [CrossRef]
- Jee, J.-P.; Na, J.H.; Lee, S.; Kim, S.H.; Choi, K.; Yeo, Y.; Kwon, I.C. Cancer targeting strategies in nanomedicine: Design and application of chitosan nanoparticles. Curr. Opin. Solid State Mater. Sci. 2012, 16, 333–342. [Google Scholar] [CrossRef]
- Frohlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Shinde, V.R.; Revi, N.; Murugappan, S.; Singh, S.P.; Rengan, A.K. Enhanced permeability and retention effect: A key facilitator for solid tumor targeting by nanoparticles. Photodiag. Photodyn. Ther. 2022, 39, 102915–102926. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent advances in tumor targeting via EPR effect for cancer treatment. J. Pers. Med. 2021, 11, 571–597. [Google Scholar] [CrossRef]
- Liu, G.; Pang, J.; Huang, Y.; Xie, Q.; Guan, G.; Jiang, Y. Self-assembled nanospheres of folate-decorated zein for the targeted delivery of 10-Hydroxycamptothecin. Ind. Eng. Chem. Res. 2017, 56, 8517–8527. [Google Scholar] [CrossRef]
- Agwa, M.M.; Sabra, S. Lactoferrin coated or conjugated nanomaterials as an active targeting approach in nanomedicine. Int. J. Biol. Macromol. 2021, 167, 1527–1543. [Google Scholar] [CrossRef]
- Zhang, D.; Kong, J.; Huang, X.; Zeng, J.; Du, Q.; Yang, T.; Yue, H.; Bao, Q.; Miao, Y.; Xu, Y.; et al. Targeted glioblastoma therapy by integrating brain-targeting peptides and corn-derived cancer cell-penetrating proteins into nanoparticles to cross blood-brain tumor barriers. Mater. Today Nano 2023, 23, 100347–100365. [Google Scholar] [CrossRef]
- Lee, H.S.; Kang, N.W.; Kim, H.; Kim, D.H.; Chae, J.W.; Lee, W.; Song, G.Y.; Cho, C.W.; Kim, D.D.; Lee, J.Y. Chondroitin sulfate-hybridized zein nanoparticles for tumor-targeted delivery of docetaxel. Carbohydr. Polym. 2021, 253, 117187–117199. [Google Scholar] [CrossRef]
- McCord, E.; Pawar, S.; Koneru, T.; Tatiparti, K.; Sau, S.; Iyer, A.K. Folate receptors’ expression in gliomas may possess potential nanoparticle-based drug delivery opportunities. ACS Omega 2021, 6, 4111–4118. [Google Scholar] [CrossRef]
- Soe, Z.C.; Ou, W.; Gautam, M.; Poudel, K.; Kim, B.K.; Pham, L.M.; Phung, C.D.; Jeong, J.H.; Jin, S.G.; Choi, H.G.; et al. Development of folate-functionalized PEGylated zein nanoparticles for ligand-directed delivery of paclitaxel. Pharmaceutics 2019, 11, 562–578. [Google Scholar] [CrossRef]
- Hoppenz, P.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide-drug conjugates and their targets in advanced cancer therapies. Front. Chem. 2020, 8, 571–594. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Chadar, R.; Sheikh, A.; Rizg, W.Y.; Safhi, A.Y. CD44-targeted nanocarrier for cancer therapy. Front Pharmacol. 2021, 12, 800481–800513. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Du, G.; He, C.; Jiang, M.; Mou, X.; Xue, J.; Sun, X. Tumor cell membrane enveloped aluminum phosphate nanoparticles for enhanced cancer vaccination. J. Control. Release 2020, 326, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Yu, G.T.; Meng, Q.F.; Bu, L.L.; Tian, R.; Lin, L.S.; Deng, H.Z.; Yang, W.J.; Zan, M.H.; Ding, J.X.; et al. Cancer cell membrane-coated nanoparticles for personalized therapy in patient-derived xenograft models. Adv. Funct. Mater. 2019, 29, 1905671–1905680. [Google Scholar] [CrossRef]
- Nie, D.; Dai, Z.; Li, J.; Yang, Y.; Xi, Z.; Wang, J.; Zhang, W.; Qian, K.; Guo, S.; Zhu, C.; et al. Cancer-cell-membrane-coated nanoparticles with a yolk-shell structure augment cancer chemotherapy. Nano Lett. 2020, 20, 936–946. [Google Scholar] [CrossRef]
- Rahmanian, M.; Seyfoori, A.; Ghasemi, M.; Shamsi, M.; Kolahchi, A.R.; Modarres, H.P.; Sanati-Nezhad, A.; Majidzadeh, A.K. In-vitro tumor microenvironment models containing physical and biological barriers for modelling multidrug resistance mechanisms and multidrug delivery strategies. J. Control. Release 2021, 334, 164–177. [Google Scholar] [CrossRef]
- Jing, X.; Hu, H.; Sun, Y.; Yu, B.; Cong, H.; Shen, Y. The intracellular and extracellular microenvironment of tumor site: The trigger of stimuli-responsive drug delivery systems. Small Methods 2022, 6, 2101437–2101460. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Shi, Q.; Zhao, Q.; Ma, H. Tumor microenvironment-responsive polypeptide nanogels for controlled antitumor drug delivery. Front. Pharmacol. 2021, 12, 748102–748121. [Google Scholar] [CrossRef]
- Zhu, L.; Torchilin, V.P. Stimulus-responsive nanopreparations for tumor targeting. Integr. Biol. 2013, 5, 96–107. [Google Scholar] [CrossRef]
- Morales-Cruz, M.; Delgado, Y.; Castillo, B.; Figueroa, C.M.; Molina, A.M.; Torres, A.; Milian, M.; Griebenow, K. Smart targeting to improve cancer therapeutics. Drug Des. Dev. Ther. 2019, 13, 3753–3772. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Lin, R.; Li, H.J.; He, W.L.; Du, J.Z.; Wang, J. Strategies to improve tumor penetration of nanomedicines through nanoparticle design. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, 1519–1530. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Yang, J.; Zhou, C.; Sun, J. pH-sensitive polymeric micelles triggered drug release for extracellular and intracellular drug targeting delivery. Asian J. Pharm. Sci. 2013, 8, 159–167. [Google Scholar] [CrossRef]
- Lee, E.S.; Gao, Z.; Bae, Y.H. Recent progress in tumor pH targeting nanotechnology. J. Control. Release 2008, 132, 164–170. [Google Scholar] [CrossRef]
- Liang, H.; Zhou, B.; Li, J.; Xu, W.; Liu, S.; Li, Y.; Chen, Y.; Li, B. Supramolecular design of coordination bonding architecture on zein nanoparticles for pH-responsive anticancer drug delivery. Colloids Surf. B Biointerfaces 2015, 136, 1224–1233. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Bini, B.S.; Manjusha, V. Glycyrrhetinic acid conjugated zein capped aminated mesoporous silica nanoparticle-based dual drug delivery system for liver: A pH-dependent triggered release. J. Mol. Liq. 2021, 340, 116852–116862. [Google Scholar] [CrossRef]
- Kennedy, L.; Sandhu, J.K.; Harper, M.E.; Cuperlovic-Culf, M. Role of glutathione in cancer: From mechanisms to therapies. Biomolecules 2020, 10, 1429–1456. [Google Scholar] [CrossRef]
- Sun, B.; Luo, C.; Yu, H.; Zhang, X.; Chen, Q.; Yang, W.; Wang, M.; Kan, Q.; Zhang, H.; Wang, Y.; et al. Disulfide bond-driven oxidation- and reduction-responsive prodrug nanoassemblies for cancer therapy. Nano Lett. 2018, 18, 3643–3650. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Fu, L.H.; Wan, Y.; Qi, C.; He, J.; Li, C.; Yang, C.; Xu, H.; Lin, J.; Huang, P. Nanocatalytic theranostics with glutathione depletion and enhanced reactive oxygen species generation for efficient cancer Therapy. Adv. Mater. 2021, 33, 2006892–2006902. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827–119853. [Google Scholar] [CrossRef]
- Senthilkumar, T.; Zhou, L.; Gu, Q.; Liu, L.; Lv, F.; Wang, S. Conjugated polymer nanoparticles with appended photo-responsive units for controlled drug delivery, release, and imaging. Angew. Chem. Int. Ed. Engl. 2018, 57, 13114–13119. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Lee, M.K.; Lim, S.J. Enhanced stability of indocyanine green by encapsulation in zein-phosphatidylcholine hybrid nanoparticles for use in the phototherapy of cancer. Pharmaceutics 2021, 13, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Banobre-Lopez, M.; Martins, P.; Lanceros-Mendez, S. Advances in magnetic nanoparticles for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1700845–1700879. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Li, F.; Baik, S.; Shao, W.; Ling, D.; Hyeon, T. Surface design of magnetic nanoparticles for stimuli-responsive cancer imaging and therapy. Biomaterials 2017, 136, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.F.; Li, Z.X.; Li, S.M.; Lin, S.Y.; Wang, H.D.; Xie, Q.L.; Jiang, Y.B. Folate-conjugated zein/Fe3O4 nanocomplexes for the enhancement of cellular uptake and cytotoxicity of gefitinib. J. Mater. Sci. 2018, 53, 14907–14921. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, Q.; Reddy, N.; Yang, Y. Hollow nanoparticles from zein for potential medical applications. J. Mater. Chem. 2011, 21, 18227–18235. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Zein-based micro- and nano-particles for drug and nutrient delivery: A review. J. Appl. Polym. Sci. 2014, 131, 40696–40707. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Synthesis, drying process and medical application of polysaccharide-based aerogels. Int. J. Biol. Macromol. 2020, 145, 1115–1128. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Y.; Zhang, W.; Zhang, S.; Zhang, S. Multiple-therapy strategies via polysaccharides-based nano-systems in fighting cancer. Carbohydr. Polym. 2021, 269, 118323–118341. [Google Scholar] [CrossRef]
- Liu, L.; Yang, S.; Chen, F.; Cheng, K.W. Polysaccharide-zein composite nanoparticles for enhancing cellular uptake and oral bioavailability of curcumin: Characterization, anti-colorectal cancer effect, and pharmacokinetics. Front. Nutr. 2022, 9, 846282–846292. [Google Scholar] [CrossRef]
- Ye, W.; Zhang, G.; Liu, X.; Ren, Q.; Huang, F.; Yan, Y. Fabrication of polysaccharide-stabilized zein nanoparticles by flash nanoprecipitation for doxorubicin sustained release. J. Drug Deliv. Sci. Tecnol. 2022, 70, 103183–103193. [Google Scholar] [CrossRef]
- Lu, M.; Wu, M.; Huang, Y.; Yao, J.; Shao, Z.; Chen, X. Animal protein-plant protein composite nanospheres for dual-drug loading and synergistic cancer therapy. J. Mater. Chem. B 2022, 10, 3798–3807. [Google Scholar] [CrossRef]
- Kamel, N.M.; Helmy, M.W.; Abdelfattah, E.Z.; Khattab, S.N.; Ragab, D.; Samaha, M.W.; Fang, J.Y.; Elzoghby, A.O. Inhalable dual-targeted hybrid lipid nanocore-protein shell composites for combined delivery of genistein and all-trans retinoic acid to lung cancer cells. ACS Biomater. Sci. Eng. 2020, 6, 71–87. [Google Scholar] [CrossRef]
- Kaur, P.; Garg, T.; Rath, G.; Murthy, R.S.; Goyal, A.K. Surfactant-based drug delivery systems for treating drug-resistant lung cancer. Drug Deliv. 2016, 23, 727–738. [Google Scholar] [CrossRef]
- Gagliardi, A.; Paolino, D.; Iannone, M.; Palma, E.; Fresta, M.; Cosco, D. Sodium deoxycholate-decorated zein nanoparticles for a stable colloidal drug delivery system. Int. J. Nanomed. 2018, 13, 601–614. [Google Scholar] [CrossRef]
- Gagliardi, A.; Bonacci, S.; Paolino, D.; Celia, C.; Procopio, A.; Fresta, M.; Cosco, D. Paclitaxel-loaded sodium deoxycholate-stabilized zein nanoparticles: Characterization and in vitro cytotoxicity. Heliyon 2019, 5, 02422–02431. [Google Scholar] [CrossRef]
- Liu, Q.; Kim, Y.J.; Im, G.B.; Zhu, J.; Wu, Y.; Liu, Y.; Bhang, S.H. Inorganic nanoparticles applied as functional therapeutics. Adv. Funct. Mater. 2020, 31, 2008171–2008195. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Hemasa, A.L.; Freag, M.S. Hybrid protein-inorganic nanoparticles: From tumor-targeted drug delivery to cancer imaging. J. Control. Release 2016, 243, 303–322. [Google Scholar] [CrossRef]
- Bahmani, E.; Dizaji, B.F.; Talaei, S.; Koushkbaghi, S.; Yazdani, H.; Abadi, P.G.S.; Akrami, M.; Shahrousvand, M.; Jazi, F.S.; Irani, M. Fabrication of poly(ϵ-caprolactone)/paclitaxel (core)/chitosan/zein/multi-walled carbon nanotubes/doxorubicin (shell) nanofibers against MCF-7 breast cancer. Polym. Adv. Technol. 2022, 34, 789–799. [Google Scholar] [CrossRef]





| Carrier | Drug | Loading Strategy | Formulation | Characterization | Cancer Cell | Administration Route | Reference |
|---|---|---|---|---|---|---|---|
| hyaluronic acid–zein | curcumin | PCL | Nanogels | in vitro/in vivo | CT26, NIH3T3 | injection | [31] |
| Zein–Lipoid-S75 | exemestane and resveratrol | PCL | NPs | in vitro/in vivo | MCF-7, 4T1 | oral gavage | [32] |
| Phosphatidylcholine–zein | isoliquiritigenin | EISA | NPs | in vitro/in vivo | 4T1, MIHA | oral gavage | [36] |
| Pluronic–zein | curcumin | ASP | NPs | in vitro | A549 | - | [40] |
| AuNPS–zein | HCPT | ASP | NPs | in vitro/in vivo | KB, Hela, A549 | injection | [17] |
| Folate–zein | docetaxel | ASP | NPs | in vitro | MCF-7, SKOV-3 | - | [45] |
| PEG–zein | coumarin-6 | ASP | NPs | in vitro | B16-F10-luc-G5 | - | [50] |
| mPEG–zein | curcumin | ASP | Micelles | in vitro/in vivo | NCI/ADR-RES | injection | [55] |
| chondroitin sulfate–zein | etoposide | ASP | Micelles | in vitro/in vivo | MCF-7 | injection | [11] |
| Zein–lactoferrin | rapamycin and wogonin | ASP | Micelles | in vitro/in vivo | MCF-7 | injection | [56] |
| mPEG–zein | curcumin | ASP | Micelles | in vitro | HepG2 | - | [57] |
| Pectin–zein | DOX | PCL | Hydrogels | in vitro | Cervical cells | - | [27] |
| Zein–co-acrylic acid | 5-fluorouracil and rutin | PCL | Hydrogels | in vitro | MDA-MB-231, MCF-7 | - | [58] |
| Zein–lecithin | carvacrol | ASP | NPs | in vitro | SW480 | - | [59] |
| zein | DOX | ASP | NPs | in vitro | HeLa | - | [60] |
| zein | maytansine | ASP | NPs | in vitro | A549 | - | [61] |
| zein/hyaluronic acid | honokiol | ASP | NPs | in vitro/in vivo | 4T1 | injection | [62] |
| Class | Ligand | Drug | Target | Reference |
|---|---|---|---|---|
| Folic acid | Folic acid | HCPT | Folate receptor | [75] |
| Transferrin | Lactoferrin | Rapamycin and wolfsbane | Lactoferrin receptor | [76] |
| Peptides Peptides | cRGD | PTX | Integrin | [9] |
| RVG29 | Dactolisib | Nicotinic acetylcholine receptor | [77] | |
| Sugar residues Sugar residues | Hyaluronic acid | Honokiol | CD44 receptor | [62] |
| Chondroitin sulfate | DOX | CD44 receptor | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Yao, F.; Tian, S.; Liu, M.; Liu, G.; Jiang, Y. Recent Advances in Zein-Based Nanocarriers for Precise Cancer Therapy. Pharmaceutics 2023, 15, 1820. https://doi.org/10.3390/pharmaceutics15071820
Huang W, Yao F, Tian S, Liu M, Liu G, Jiang Y. Recent Advances in Zein-Based Nanocarriers for Precise Cancer Therapy. Pharmaceutics. 2023; 15(7):1820. https://doi.org/10.3390/pharmaceutics15071820
Chicago/Turabian StyleHuang, Wenquan, Fei Yao, Shuangyan Tian, Mohao Liu, Guijin Liu, and Yanbin Jiang. 2023. "Recent Advances in Zein-Based Nanocarriers for Precise Cancer Therapy" Pharmaceutics 15, no. 7: 1820. https://doi.org/10.3390/pharmaceutics15071820
APA StyleHuang, W., Yao, F., Tian, S., Liu, M., Liu, G., & Jiang, Y. (2023). Recent Advances in Zein-Based Nanocarriers for Precise Cancer Therapy. Pharmaceutics, 15(7), 1820. https://doi.org/10.3390/pharmaceutics15071820






