Pistacia vera Extract Potentiates the Effect of Melatonin on Human Melatonin MT1 and MT2 Receptors with Functional Selectivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Extracts
2.2. Test of Solubility
2.3. Cell Culture of HEK293 Cells
2.4. 2─[125I]─MLT Radioligand Binding Assay
2.5. Accumulative cAMP Assay
2.6. ERK1/2 Activation Assay
2.7. β-arrestin2 Recruitment Assay
2.8. BRET β-arrestin2 Recruitment Assay
2.9. Determination of Melatonin Content by ELISA
2.10. MTT Assay
3. Results
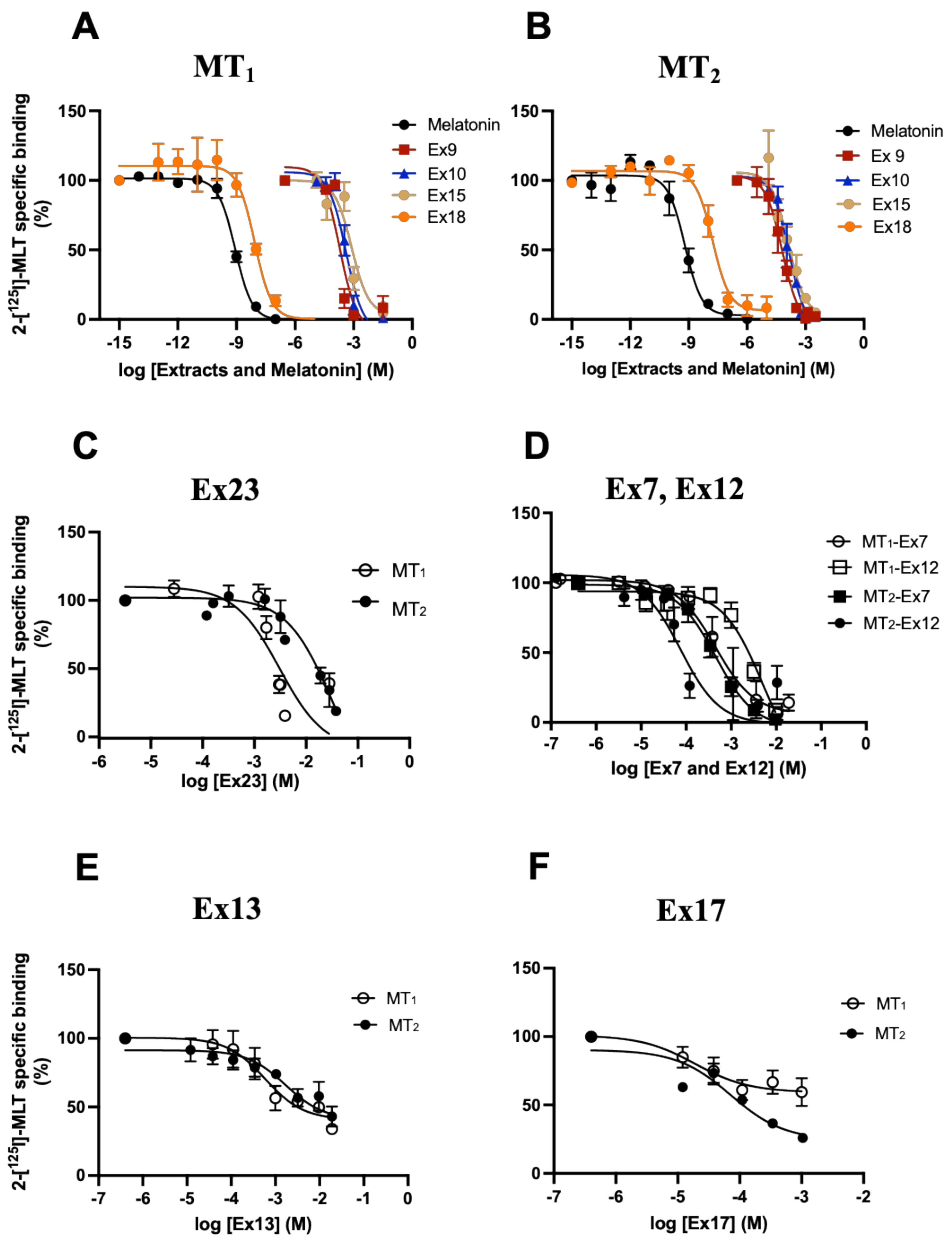
3.1. Identification of Plant Extracts Interfering with 2─[125I]iodomelatonin Binding to Human MT1 and MT2 Receptors
3.2. Characterization of Plant Extracts in Functional Melatonin Receptor Assays
3.3. Ex18 Contains High Amounts of Melatonin
3.4. Ex18 Potentiates the Effect of Exogeneous Melatonin on the cAMP and ERK1/2 Pathways
3.5. Presence of Active Principle Potentiating the Effect of Exogeneous Melatonin in Several Plant Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lundstrom, K.; Pham, H.T.; Dinh, L.D. Interaction of Plant Extracts with Central Nervous System Receptors. Medicines 2017, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.S.; Weng, J.K. Demystifying traditional herbal medicine with modern approach. Nat. Plants 2017, 3, 17109. [Google Scholar] [CrossRef] [PubMed]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Bowery, N.G.; Greengrass, P.M.; Phillipson, J.D. Application of radioligand receptor binding assays in the search for CNS active principles from Chinese medicinal plants. J. Ethnopharmacol. 1996, 54, 153–164. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, S.; Yang, D.; Wu, Y.; Xin, Y.; Cao, H.; Huang, X.P.; Cai, X.; Sun, W.; Ye, N.; et al. A Novel G Protein-Biased and Subtype-Selective Agonist for a G Protein-Coupled Receptor Discovered from Screening Herbal Extracts. ACS Cent. Sci. 2020, 6, 213–225. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Hernandez, M.G.; Ramon, E.; Lupala, C.S.; Tena-Campos, M.; Perez, J.J.; Garriga, P. Flavonoid allosteric modulation of mutated visual rhodopsin associated with retinitis pigmentosa. Sci. Rep. 2017, 7, 11167. [Google Scholar] [CrossRef] [Green Version]
- Muratspahic, E.; Tomasevic, N.; Nasrollahi-Shirazi, S.; Gattringer, J.; Emser, F.S.; Freissmuth, M.; Gruber, C.W. Plant-Derived Cyclotides Modulate kappa-Opioid Receptor Signaling. J. Nat. Prod. 2021, 84, 2238–2248. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sugden, D.; Cardinali, D.P.; Olcese, J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 2010, 62, 343–380. [Google Scholar] [CrossRef] [Green Version]
- Gobbi, G.; Comai, S. Differential Function of Melatonin MT(1) and MT(2) Receptors in REM and NREM Sleep. Front. Endocrinol. 2019, 10, 87. [Google Scholar] [CrossRef] [Green Version]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016, 173, 2702–2725. [Google Scholar] [CrossRef] [Green Version]
- Cecon, E.; Oishi, A.; Jockers, R. Melatonin receptors: Molecular pharmacology and signalling in the context of system bias. Br. J. Pharmacol. 2018, 175, 3263–3280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.H.; Su, H.R.; Boutin, J.A.; Renard, M.P.; Wang, M.W. High-throughput screening assay for new ligands at human melatonin receptors. Acta Pharmacol. Sin. 2008, 29, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Abourashed, E.A.; Koetter, U.; Brattstrom, A. In vitro binding experiments with a Valerian, hops and their fixed combination extract (Ze91019) to selected central nervous system receptors. Phytomedicine 2004, 11, 633–638. [Google Scholar] [CrossRef]
- Kumari, Y.; Choo, B.K.M.; Shaikh, M.F.; Othman, I. Melatonin receptor agonist Piper betle L. ameliorates dexamethasone-induced early life stress in adult zebrafish. Exp. Ther. Med. 2019, 18, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Genovese, S.; Fiorito, S.; Epifano, F.; Witt-Enderby, P.A. Oxyprenylated Phenylpropanoids Bind to MT1 Melatonin Receptors and Inhibit Breast Cancer Cell Proliferation and Migration. J. Nat. Prod. 2017, 80, 3324–3329. [Google Scholar] [CrossRef]
- Chen, S.Y.; Geng, C.A.; Ma, Y.B.; Chen, J.J. Melatonin Receptors Agonistic Activities of Phenols from Gastrodia elata. Nat. Prod. Bioprospect. 2019, 9, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Geng, C.A.; Huang, X.Y.; Ma, Y.B.; Hou, B.; Li, T.Z.; Zhang, X.M.; Chen, J.J. (+/−)-Uncarilins A and B, Dimeric Isoechinulin-Type Alkaloids from Uncaria rhynchophylla. J. Nat. Prod. 2017, 80, 959–964. [Google Scholar] [CrossRef]
- Geng, C.A.; Yang, T.H.; Huang, X.Y.; Ma, Y.B.; Zhang, X.M.; Chen, J.J. Antidepressant potential of Uncaria rhynchophylla and its active flavanol, catechin, targeting melatonin receptors. J. Ethnopharmacol. 2019, 232, 39–46. [Google Scholar] [CrossRef]
- Wang, H.; Geng, C.A.; Xu, H.B.; Huang, X.Y.; Ma, Y.B.; Yang, C.Y.; Zhang, X.M.; Chen, J.J. Lignans from the Fruits of Melia toosendan and Their Agonistic Activities on Melatonin Receptor MT1. Planta Med. 2015, 81, 847–854. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.J.; Huang, X.Y.; Ma, Y.B.; Geng, C.A.; Li, T.Z.; Chen, X.L.; Yang, T.H.; Zhou, J.; Zhang, X.M.; Chen, J.J. Bioactivity-guided synthesis of gramine derivatives as new MT(1) and 5-HT(1A) receptors agonists. J. Asian Nat. Prod. Res. 2017, 19, 610–622. [Google Scholar] [CrossRef]
- Zhang, J.G.; Geng, C.A.; Huang, X.Y.; Chen, X.L.; Ma, Y.B.; Zhang, X.M.; Chen, J.J. Chemical and biological comparison of different sections of Uncaria rhynchophylla (Gou-Teng). Eur. J. Mass Spectrom. 2017, 23, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Fahradpour, M.; Keov, P.; Tognola, C.; Perez-Santamarina, E.; McCormick, P.J.; Ghassempour, A.; Gruber, C.W. Cyclotides Isolated from an Ipecac Root Extract Antagonize the Corticotropin Releasing Factor Type 1 Receptor. Front. Pharmacol. 2017, 8, 616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarris, J.; Panossian, A.; Schweitzer, I.; Stough, C.; Scholey, A. Herbal medicine for depression, anxiety and insomnia: A review of psychopharmacology and clinical evidence. Eur. Neuropsychopharmacol. 2011, 21, 841–860. [Google Scholar] [CrossRef]
- Wheatley, D. Medicinal plants for insomnia: A review of their pharmacology, efficacy and tolerability. J. Psychopharmacol. 2005, 19, 414–421. [Google Scholar] [CrossRef]
- Muller, C.E.; Schumacher, B.; Brattstrom, A.; Abourashed, E.A.; Koetter, U. Interactions of valerian extracts and a fixed valerian-hop extract combination with adenosine receptors. Life Sci. 2002, 71, 1939–1949. [Google Scholar] [CrossRef]
- Kaushik, M.K.; Kaul, S.C.; Wadhwa, R.; Yanagisawa, M.; Urade, Y. Triethylene glycol, an active component of Ashwagandha (Withania somnifera) leaves, is responsible for sleep induction. PLoS ONE 2017, 12, e0172508. [Google Scholar] [CrossRef] [Green Version]
- Rostampour, M.; Hadipour, E.; Oryan, S.; Soltani, B.; Saadat, F. Anxiolytic-like effect of hydroalcoholic extract of ripe pistachio hulls in adult female Wistar rats and its possible mechanisms. Res. Pharm. Sci. 2016, 11, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.M.; Sung, J.Y.; Hebert, T.E. Gbetagamma subunits-Different spaces, different faces. Pharmacol. Res. 2016, 111, 434–441. [Google Scholar] [CrossRef]
- Gbahou, F.; Jockers, R. 2-[(125)I]iodomelatonin and [(3)H]melatonin Binding Assays for Melatonin Receptors. Methods Mol. Biol. 2022, 2550, 141–149. [Google Scholar] [CrossRef]
- Guillaume, J.L.; Daulat, A.M.; Maurice, P.; Levoye, A.; Migaud, M.; Brydon, L.; Malpaux, B.; Borg-Capra, C.; Jockers, R. The PDZ protein mupp1 promotes Gi coupling and signaling of the Mt1 melatonin receptor. J. Biol. Chem. 2008, 283, 16762–16771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cecon, E.; Guillaume, J.L.; Jockers, R. Functional Investigation of Melatonin Receptor Activation by Homogenous cAMP Assay. Methods Mol. Biol. 2022, 2550, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Cecon, E.; Karamitri, A.; Gao, W.; Gerbier, R.; Ahmad, R.; Jockers, R. Melatonin MT(1) and MT(2) receptor ERK signaling is differentially dependent on G(i/o) and G(q/11) proteins. J. Pineal Res. 2020, 68, e12641. [Google Scholar] [CrossRef] [PubMed]
- Karamitri, A.; Plouffe, B.; Bonnefond, A.; Chen, M.; Gallion, J.; Guillaume, J.L.; Hegron, A.; Boissel, M.; Canouil, M.; Langenberg, C.; et al. Type 2 diabetes-associated variants of the MT(2) melatonin receptor affect distinct modes of signaling. Sci. Signal 2018, 11, eaan6622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorli, S.C.; Le Gonidec, S.; Knibiehler, B.; Audigier, Y. Apelin is a potent activator of tumour neoangiogenesis. Oncogene 2007, 26, 7692–7699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iriti, M. Melatonin in grape, not just a myth, maybe a panacea. J. Pineal. Res. 2009, 46, 353. [Google Scholar] [CrossRef]
- Reiter, E.; Ahn, S.; Shukla, A.K.; Lefkowitz, R.J. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 179–197. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atwood, B.K.; Lopez, J.; Wager-Miller, J.; Mackie, K.; Straiker, A. Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genom. 2011, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Kondo, S.; El Omri, A.; Han, J.; Isoda, H. Antidepressant-like effects of rosmarinic acid through mitogen-activated protein kinase phosphatase-1 and brain-derived neurotrophic factor modulation. J. Funct. Foods 2015, 14, 758–766. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Chang, H.S.; Hu, K.L.; Yu, X.; Li, L.N.; Xu, X.Q. Combination of Geniposide and Eleutheroside B Exerts Antidepressant-like Effect on Lipopolysaccharide-Induced Depression Mice Model. Chin. J. Integr. Med. 2021, 27, 534–541. [Google Scholar] [CrossRef]
- Perviz, S.; Khan, H.; Pervaiz, A. Plant Alkaloids as an Emerging Therapeutic Alternative for the Treatment of Depression. Front. Pharmacol. 2016, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimpfel, W.; Schombert, L.; Panossian, A.G. Assessing the Quality and Potential Efficacy of Commercial Extracts of Rhodiola rosea L. by Analyzing the Salidroside and Rosavin Content and the Electrophysiological Activity in Hippocampal Long-Term Potentiation, a Synaptic Model of Memory. Front. Pharmacol. 2018, 9, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, B.; Ribnicky, D.M.; Poulev, A.; Logendra, S.; Cefalu, W.T.; Raskin, I. A natural history of botanical therapeutics. Metabolism 2008, 57, S3–S9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyohara, H.; Matsumoto, T.; Yamada, H. Combination Effects of Herbs in a Multi-herbal Formula: Expression of Juzen-taiho-to’s Immuno-modulatory Activity on the Intestinal Immune System. Evid. Based Complement. Alternat. Med. 2004, 1, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Bozorgi, M.; Memariani, Z.; Mobli, M.; Salehi Surmaghi, M.H.; Shams-Ardekani, M.R.; Rahimi, R. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): A review of their traditional uses, phytochemistry, and pharmacology. Sci. World J. 2013, 2013, 219815. [Google Scholar] [CrossRef] [Green Version]
- Kanwar, M.K.; Yu, J.; Zhou, J. Phytomelatonin: Recent advances and future prospects. J. Pineal. Res. 2018, 65, e12526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Llamas, F.; Hernandez-Ruiz, J.; Cuesta, A.; Zamora, S.; Arnao, M.B. Development of a Phytomelatonin-Rich Extract from Cultured Plants with Excellent Biochemical and Functional Properties as an Alternative to Synthetic Melatonin. Antioxidants 2020, 9, 158. [Google Scholar] [CrossRef] [Green Version]
- Oladi, E.; Mohamadi, M.; Shamspur, T.; Mostafavi, A. Spectrofluorimetric determination of melatonin in kernels of four different Pistacia varieties after ultrasound-assisted solid-liquid extraction. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 132, 326–329. [Google Scholar] [CrossRef]
- Oladi, E.; Mohamadi, M.; Shamspur, T.; Mostafavi, A. “Expression of Concern to Spectrofluorimetric Determination of Melatonin in Kernels of Four Different Pistacia Varieties after Ultrasound-Assisted Solid-Liquid Extraction” [Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 132 (2014) 326-329]. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 217, 322. [Google Scholar] [CrossRef]
- Lautie, E.; Russo, O.; Ducrot, P.; Boutin, J.A. Unraveling Plant Natural Chemical Diversity for Drug Discovery Purposes. Front. Pharmacol. 2020, 11, 397. [Google Scholar] [CrossRef]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamitri, A.; Jockers, R. Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 2019, 15, 105–125. [Google Scholar] [CrossRef] [PubMed]









| Code | Scientific Name | Plant Part | Extraction Method a | Identified Active Ingredients/Extraction Techniques Used a | Provider |
|---|---|---|---|---|---|
| Ex1 | Melissa officinalis | Leaves | EtOH/H2O (30%/70%) v/v | Rosmarinic acid (7.02%)/Ultraviolet-visible-VIS | PLANTEX |
| Ex2 | Passiflora incarnata L. | Aeral part | EtOH/H2O (30%/70%) v/v | Flavonoids; vitexin (4.18%)/Ultraviolet- visible-VIS | PLANTEX |
| Ex3 | Eschscholizia californica cham | Flowering grass | EtOH/H2O | Californidine (0.5%)/- | BERNETT |
| Ex4 | Crocus sativus | Red stigmas | H2O/EtOH (20%/80%) | Flavonoids: vitexin (2.99%)/HPLC | GREEN PLANT EXTRACT |
| Ex5 | Passiflora incarnata L. | Aerial part | H2O (100%) | Flavonoids: vitexin (2.99%)/HPLC | NATUREX |
| Ex6 | Melissa officinalis L. | Leaves | EtOH/H2O (30%/70%) v/v | Rosmarinic acid (7.08%)/Ultraviolet-visible-VIS | PLANTEX |
| Ex7 | Eschscholzia californica cham | Flowering grass | EtOH/H2O | Californidine (0.45%)/- | BERNETT |
| Ex8 | Eleutherococcus senticosus | Roots | EtOH/H2O (30%) | Eleutheroside B and E (0.36%)/- | HEALTH FOOD |
| Ex9 | Hypericum perforatum | Unknown | EtOH | Flavonoids (10.5% rutin); hyperforin (3%)/HPLC | EUROMED |
| Ex10 | Matricaria chamomilla | Flowers | EtOH/H2O (74%/26%) | Apigenin (1.61%)/HPLC | NATUREX |
| Ex11 | Zizyphus jujuba | Seeds | EtOH/H2O (60–70%) | - | HUISONG |
| Ex12 | Humulus lupulus | Flowers | EtOH/H2O (96%) | - | HUISONG |
| Ex13 | Withania somnifera | - | - | Withaferin A (0.29%)/HPLC | NATREON |
| Ex14 | Griffonia simplicifolia | Seeds | EtOH/H2O (40–60%) | 5-HTP or L-5 Hydroxytryptophan (>30%)/HPLC | NATUREX |
| Ex15 | Crataegus monogyna | Leaves and Flower | EtOH/H2O (80%/20%) | Flavonoids: vitexin-2-orhamnoside (1.80–3%)/HPLC | NATUREX |
| Ex16 | Asparagus officinalis | Stems | - | - | AMINOUP |
| Ex17 | Camellia sinensis | - | - | - | - |
| Ex18 | Pistacia vera | Fruits, dry | EtOH/H2O (30%) | - | NETWORK NUTRITION |
| Ex19 | Rhodiola rosea L. | Roots | EtOH/H2O (45–65%)/(35–55%) | Rosavins and salidroside (>1; >3%)/HPLC | NATUREX |
| Ex20 | Mentha x piperita L. | Aerial part | EtOH/H2O (30%/70%) | - | NATUREX |
| Ex21 | Camellia sinensis L. | Leaves | H2O (100%) | - | NATUREX |
| Ex22 | Malvaceae tilia cordata | Flowers | H2O (100%) | - | PLANTEX |
| Ex23 | Verbena officinalis L. | Leaves | H2O (100%) | - | PLANTEX |
| Ex24 | Valeriana officinallis L. | Roots (rhizomes, and stallions) | H2O (100%) | Sesquiterpenic acids (Hydroxyvalerenic and acetoxyvalericic acid) (0.062%)/HPLC | EUROMED |
| Ex25 | Valeriana officinallis L. | Roots (rhizomes, and stallions) | EtOH (70%) | Valerenic acid (0.3%)/HPLC | EUROMED |
| Extract | MT1 | MT2 | MT1/MT2 Ratio |
|---|---|---|---|
| App. Ki ± S.E.M (mg/mL) (µM) | |||
| Ex1 | 0.63 ± 0.39 (1260) | 0.69 ± 0.45 (1380) | 0.91 |
| Ex2 | 0.14 ± 0.07 (280) | 0.09 ± 0.08 (180) | 1.55 |
| Ex3 | 0.20 ± 0.13 (400) | 0.07 ± 0.04 (140) | 2.85 |
| Ex4 | 1.73 ± 0.42 (3460) | 1.99 ± 1.20 (3980) | 0.86 |
| Ex5 | 0.13 ± 0.05 (260) | 0.14 ± 0.41 (280) | 0.92 |
| Ex6 | 0.31 ± 0.13 (620) | 0.16 ± 0.10 (320) | 1.94 |
| Ex7 | 0.49 ± 0.57 (980) | 0.04 ± 0.04 (80) | 12.25 |
| Ex8 | 2.52 ± 2.33 (5040) | 0.42 ± 0.28 (840) | 6.00 |
| Ex9 | 0.05 ± 0.01 (100) | 0.03 ± 0.03 (60) | 1.66 |
| Ex10 | 0.21 ± 0.08 (420) | 0.07 ± 0.04 (140) | 3.00 |
| Ex11 | 0.29 ± 0.08 (580) | 0.36 ± 0.01 (720) | 0.80 |
| Ex12 | 2.04 ± 1.00 (4080) | 0.20 ± 0.13 (400) | 10 |
| Ex13 Ex14 | ~1.47 ± 1.54 (2940) b ~12.54 ± 13.84(25,080) a | ~0.59 ± 0.71 (1180) b 2.90 ± 1.64 (5800) | ~2.49 ~4.32 |
| Ex15 | 0.81 ± 0.51 (1620) | 0.07 ± 0.04 (140) | 11.57 |
| Ex16 | 7.80 ± 1.09 (15,600) | 5.68 ± 2.92 (11,360) | 1.37 |
| Ex17 | ~0.009 ± 0.004 (18) c | ~0.03 ± 0.02 (60) c | ~0.3 |
| Ex18 | 3.31 ± 1.57 10−6 (0.00662) | 9.88 ± 4.76 10−6 (0.0196) | 0.33 |
| Ex19 | 0.08 ± 0.07 (160) | 0.19 ± 0.19 (380) | 0.42 |
| Ex20 | 0.44 ± 0.16 (880) | 0.20 ± 0.11 (400) | 2.2 |
| Ex21 | 0.70 ± 0.44 (1400) | 1.94 ± 2.54(3880) | 0.36 |
| Ex22 | ~9.38 ± 2.84 (18,760) a | ~3.44 ± 4.82 (6880) a | ~2.73 |
| Ex23 | ~0.76 ± 0.25 (1520) a | ~5.07 ± 2.60 (10,140) a | ~0.15 |
| Ex24 | ~1.22 ± 0.70 (2440) d | ~14.94 ± 14.67 (29,880) d | ~0.08 |
| Ex25 | 0.18 ± 0.11 (360) | 0.24 ± 0.06 (480) | 0.75 |
| MLT | 0.11 ± 0.03 10−6 (0.000224) | 0.20 ± 0.19 10−6 (0.00040) | 0.56 |
| Receptor | 2─[125I]─MLT Compet. | cAMP Inh. | ERK Act. | β-ARR | |
|---|---|---|---|---|---|
| pKi ± S.E.M. | pEC50 ± S.E.M. | ||||
| MT1 | Melatonin | 9.27 ± 0.12 | 9.02 ± 0.05 | 8.73 ± 0.65 | 9.12 ± 0.16 |
| Ex18 | * 10.2 ± 0.19 | 10.3 ± 0.96 | ** 10.4 ± 0.64 | 9.60 ± 0.54 | |
| MT2 | Melatonin | 9.27 ± 0.48 | 8.95 ± 0.39 | 8.71 ± 0.17 | 10.0 ± 0.87 |
| Ex18 | 9.79 ± 0.28 | 9.99 ± 0.34 | * 9.38 ± 0.07 | 10.8 ± 0.99 | |
| Code | Scientific Name | Melatonin Content (mg/g of Extract) | Estimated Melatonin Concentration in ERK Assay (Figure 10) (nM) |
|---|---|---|---|
| Ex6 | Melissa officinalis L. | 0.03 | 0.20 |
| Ex8 | Eleutherococcus senticosus | nd | nd |
| Ex19 | Rhodiola rosea L. | nd | nd |
| Ex24 | Valeriana officinallis L. | 0.06 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labani, N.; Gbahou, F.; Noblet, M.; Masri, B.; Broussaud, O.; Liu, J.; Jockers, R. Pistacia vera Extract Potentiates the Effect of Melatonin on Human Melatonin MT1 and MT2 Receptors with Functional Selectivity. Pharmaceutics 2023, 15, 1845. https://doi.org/10.3390/pharmaceutics15071845
Labani N, Gbahou F, Noblet M, Masri B, Broussaud O, Liu J, Jockers R. Pistacia vera Extract Potentiates the Effect of Melatonin on Human Melatonin MT1 and MT2 Receptors with Functional Selectivity. Pharmaceutics. 2023; 15(7):1845. https://doi.org/10.3390/pharmaceutics15071845
Chicago/Turabian StyleLabani, Nedjma, Florence Gbahou, Marc Noblet, Bernard Masri, Olivier Broussaud, Jianfeng Liu, and Ralf Jockers. 2023. "Pistacia vera Extract Potentiates the Effect of Melatonin on Human Melatonin MT1 and MT2 Receptors with Functional Selectivity" Pharmaceutics 15, no. 7: 1845. https://doi.org/10.3390/pharmaceutics15071845





