Interaction of Near-Infrared (NIR)-Light Responsive Probes with Lipid Membranes: A Combined Simulation and Experimental Study
Abstract
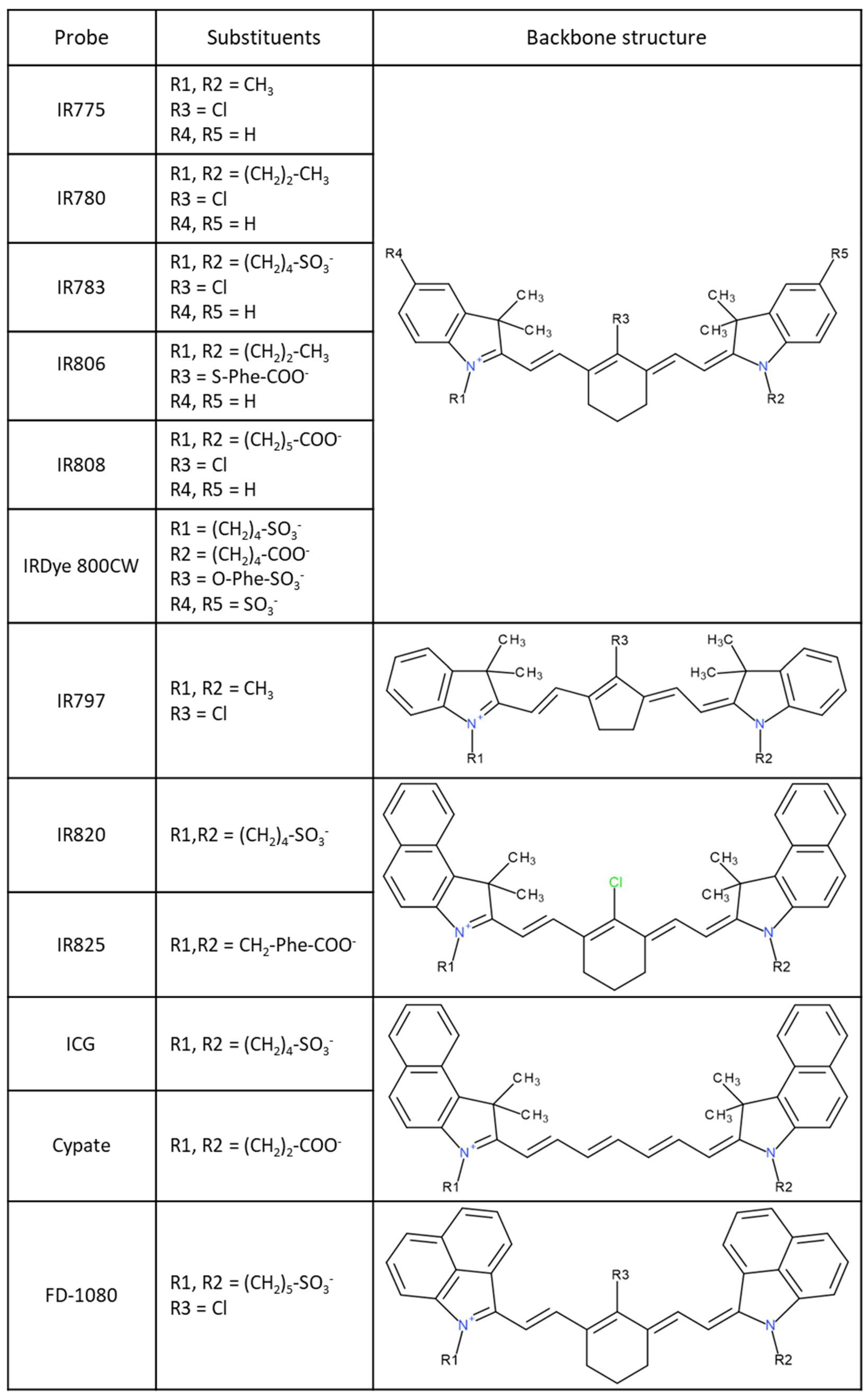
:1. Introduction
2. Materials and Methods
2.1. All-Atom MD Simulation
2.2. Experimental Measurement of the CBF Release from Lipid Vesicles
2.2.1. Preparation of CBF-Loaded Red Blood Cell (RBC)-Membrane-Derived Vesicles
2.2.2. Characterization of the CBF Release in the Presence of IR780 and IR820
3. Results and Discussion
3.1. Detailed Characterization by MD Simulations
3.1.1. Location of the NIR Probes on the Lipid Membrane
3.1.2. Orientation of the NIR Probes on the Lipid Membrane
3.1.3. Increase in the Membrane Surface
3.1.4. Hydrogen Bonding
3.2. Experimental Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA-Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.; Rodrigues, C.F.; Moreira, A.F.; Correia, I.J. Overview of the application of inorganic nanomaterials in cancer photothermal therapy. Biomater. Sci. 2020, 8, 2990–3020. [Google Scholar] [CrossRef]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef]
- Song, X.; Chen, Q.; Liu, Z. Recent advances in the development of organic photothermal nano-agents. Nano Res. 2015, 8, 340–354. [Google Scholar] [CrossRef]
- Alves, C.G.; Lima-Sousa, R.; de Melo-Diogo, D.; Louro, R.O.; Correia, I.J. IR780 based nanomaterials for cancer imaging and photothermal, photodynamic and combinatorial therapies. Int. J. Pharm. 2018, 542, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- Gonçalves, A.S.C.; Rodrigues, C.F.; Fernandes, N.; de Melo-Diogo, D.; Ferreira, P.; Moreira, A.F.; Correia, I.J. IR780 loaded gelatin-PEG coated gold core silica shell nanorods for cancer-targeted photothermal/photodynamic therapy. Biotechnol. Bioeng. 2022, 119, 644–656. [Google Scholar] [CrossRef]
- Cheng, Z.; Cheng, Y.; Chen, Q.; Li, M.; Wang, J.; Liu, H.; Li, M.; Ning, Y.; Yu, Z.; Wang, Y.; et al. Self-assembly of pentapeptides into morphology-adaptable nanomedicines for enhanced combinatorial chemo-photodynamic therapy. Nano Today 2020, 33, 100878. [Google Scholar] [CrossRef]
- Meng, X.; Wang, K.; Lv, L.; Zhao, Y.; Sun, C.; Ma, L.; Zhang, B. Photothermal/Photodynamic Therapy with Immune-Adjuvant Liposomal Complexes for Effective Gastric Cancer Therapy. Part. Part. Syst. Charact. 2019, 36, 1900015. [Google Scholar] [CrossRef]
- Leitão, M.M.; de Melo-Diogo, D.; Alves, C.G.; Lima-Sousa, R.; Correia, I.J. Prototypic Heptamethine Cyanine Incorporating Nanomaterials for Cancer Phototheragnostic. Adv. Healthc. Mater. 2020, 9, 1901665. [Google Scholar] [CrossRef]
- Yi, X.; Yan, F.; Wang, F.; Qin, W.; Wu, G.; Yang, X.; Shao, C.; Chung, L.W.; Yuan, J. IR-780 dye for near-infrared fluorescence imaging in prostate cancer. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 511. [Google Scholar]
- Wang, J.; Sun, C.; Ji, M.; Wang, B.; Wang, P.; Zhou, G.; Dong, B.; Du, W.; Huang, L.; Wang, H.; et al. Design, synthesis and application of near-infrared fluorescence probe IR-780-Crizotinib in detection of ALK positive tumors. Protein Expr. Purif. 2021, 187, 105952. [Google Scholar] [CrossRef]
- Kraft, J.C.; Ho, R.J.Y. Interactions of Indocyanine Green and Lipid in Enhancing Near-Infrared Fluorescence Properties: The Basis for Near-Infrared Imaging In Vivo. Biochemistry 2014, 53, 1275–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Si, W.; Huang, W.; Chen, P.; Shao, J.; Dong, X. Organic Dye Based Nanoparticles for Cancer Phototheranostics. Small 2018, 14, 1704247. [Google Scholar] [CrossRef]
- Zhu, S.; Tian, R.; Antaris, A.L.; Chen, X.; Dai, H. Near-Infrared-II Molecular Dyes for Cancer Imaging and Surgery. Adv. Mater. 2019, 31, 1900321. [Google Scholar] [CrossRef] [PubMed]
- Sneider, A.; VanDyke, D.; Paliwal, S.; Rai, P. Remotely Triggered Nano-Theranostics for Cancer Applications. Nanotheranostics 2017, 1, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Hu, C.; Ran, W.; Meng, J.; Yin, Q.; Li, Y. Recent Progress in Light-Triggered Nanotheranostics for Cancer Treatment. Theranostics 2016, 6, 948–968. [Google Scholar] [CrossRef]
- de Melo-Diogo, D.; Pais-Silva, C.; Dias, D.R.; Moreira, A.F.; Correia, I.J. Strategies to Improve Cancer Photothermal Therapy Mediated by Nanomaterials. Adv. Healthc. Mater. 2017, 6, 1700073. [Google Scholar] [CrossRef]
- Xiang, S.; Zhang, K.; Yang, G.; Gao, D.; Zeng, C.; He, M. Mitochondria-Targeted and Resveratrol-Loaded Dual-Function Titanium Disulfide Nanosheets for Photothermal-Triggered Tumor Chemotherapy. Nanoscale Res. Lett. 2019, 14, 211. [Google Scholar] [CrossRef]
- Yang, Q.; Xiao, Y.; Yin, Y.; Li, G.; Peng, J. Erythrocyte Membrane-Camouflaged IR780 and DTX Coloading Polymeric Nanoparticles for Imaging-Guided Cancer Photo–Chemo Combination Therapy. Mol. Pharm. 2019, 16, 3208–3220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Luo, S.; Tan, X.; Shi, C. Mechanistic study of IR-780 dye as a potential tumor targeting and drug delivery agent. Biomaterials 2014, 35, 771–778. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Yang, K.; Sheng, D.; Tan, B.; Wang, Z.; Ran, H.; Yi, H.; Zhong, Y.; Lin, H.; et al. Mitochondria-Targeted Artificial “Nano-RBCs” for Amplified Synergistic Cancer Phototherapy by a Single NIR Irradiation. Adv. Sci. 2018, 5, 1800049. [Google Scholar] [CrossRef]
- James, N.S.; Chen, Y.; Joshi, P.; Ohulchanskyy, T.Y.; Ethirajan, M.; Henary, M.; Strekowsk, L.; Pandey, R.K. Evaluation of Polymethine Dyes as Potential Probes for Near Infrared Fluorescence Imaging of Tumors: Part-1. Theranostics 2013, 3, 692–702. [Google Scholar] [CrossRef]
- Yang, X.; Shi, C.; Tong, R.; Qian, W.; Zhau, H.E.; Wang, R.; Zhu, G.; Cheng, J.; Yang, V.W.; Cheng, T.; et al. Near IR Heptamethine Cyanine Dye–Mediated Cancer Imaging. Clin. Cancer Res. 2010, 16, 2833–2844. [Google Scholar] [CrossRef] [Green Version]
- Jing, T.; Fu, L.; Liu, L.; Yan, L. A reduction-responsive polypeptide nanogel encapsulating NIR photosensitizer for imaging guided photodynamic therapy. Polym. Chem. 2016, 7, 951–957. [Google Scholar] [CrossRef]
- Li, S.; Sun, Z.; Deng, G.; Meng, X.; Li, W.; Ni, D.; Zhang, J.; Gong, P.; Cai, L. Dual-modal imaging-guided highly efficient photothermal therapy using heptamethine cyanine-conjugated hyaluronic acid micelles. Biomater. Sci. 2017, 5, 1122–1129. [Google Scholar] [CrossRef]
- Hu, X.; Tian, H.; Jiang, W.; Song, A.; Li, Z.; Luan, Y. Rational Design of IR820- and Ce6-Based Versatile Micelle for Single NIR Laser–Induced Imaging and Dual-Modal Phototherapy. Small 2018, 14, 1802994. [Google Scholar] [CrossRef]
- Huang, P.; Rong, P.; Jin, A.; Yan, X.; Zhang, M.G.; Lin, J.; Hu, H.; Wang, Z.; Yue, X.; Li, W.; et al. Dye-Loaded Ferritin Nanocages for Multimodal Imaging and Photothermal Therapy. Adv. Mater. 2014, 26, 6401–6408. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.V.; Draney, D.; Sevick-Muraca, E.M.; Olive, D.M. Single-Dose Intravenous Toxicity Study of IRDye 800CW in Sprague-Dawley Rats. Mol. Imaging Biol. 2010, 12, 583–594. [Google Scholar] [CrossRef] [Green Version]
- Bernhard, W.; Barreto, K.; El-Sayed, A.; Gonzalez, C.; Viswas, R.S.; Toledo, D.; Casaco, A.; DeCoteau, J.; Fonge, H.; Geyer, C.R. Pre-clinical study of IRDye800CW-nimotuzumab formulation, stability, pharmacokinetics, and safety. BMC Cancer 2021, 21, 270. [Google Scholar] [CrossRef] [PubMed]
- Lirussi, F.; Pyrshev, K.; Yesylevskyy, S.; Rivel, T.; Lopez, T.; Coppens, E.; Mura, S.; Couvreur, P.; Ramseyer, C. Plasma membrane lipid bilayer is druggable: Selective delivery of gemcitabine-squalene nano-medicine to cancer cells. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2023, 1869, 166614. [Google Scholar] [CrossRef] [PubMed]
- GROMACS Development Team; GROMACS 2021.3 Source Code. Available online: https://zenodo.org/record/5053201 (accessed on 24 May 2023).
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; MacKerell, A.D.; Pastor, R.W. Update of the CHARMM All-Atom Additive Force Field for Lipids: Validation on Six Lipid Types. J. Phys. Chem. B 2010, 114, 7830–7843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beglov, D.; Roux, B. Finite representation of an infinite bulk system: Solvent boundary potential for computer simulations. J. Chem. Phys. 1994, 100, 9050–9063. [Google Scholar] [CrossRef] [Green Version]
- Noskov, S.Y.; Roux, B. Control of Ion Selectivity in LeuT: Two Na+ Binding Sites with Two Different Mechanisms. J. Mol. Biol. 2008, 377, 804–818. [Google Scholar] [CrossRef] [Green Version]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, W.L. Quantum and statistical mechanical studies of liquids. 10. Transferable intermolecular potential functions for water, alcohols, and ethers. Application to liquid water. J. Am. Chem. Soc. 1981, 103, 335–340. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- MacKerell, A.D.; Bashford, D.; Bellott, M.; Dunbrack, R.L.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; et al. All-Atom Empirical Potential for Molecular Modeling and Dynamics Studies of Proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Jo, S.; Brooks III, C.L.; Lee, H.S.; Im, W. CHARMM-GUI ligand reader and modeler for CHARMM force field generation of small molecules. J. Comput. Chem. 2017, 38, 1879–1886. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Wu, E.L.; Cheng, X.; Jo, S.; Rui, H.; Song, K.C.; Dávila-Contreras, E.M.; Qi, Y.; Lee, J.; Monje-Galvan, V.; Venable, R.M.; et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem. 2014, 35, 1997–2004. [Google Scholar] [CrossRef] [Green Version]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Filipe, H.A.L.; Moreno, M.J.; Loura, L.M.S. Interaction of 7-Nitrobenz-2-oxa-1,3-diazol-4-yl-Labeled Fatty Amines with 1-Palmitoyl, 2-Oleoyl-sn-glycero-3-phosphocholine Bilayers: A Molecular Dynamics Study. J. Phys. Chem. B 2011, 115, 10109–10119. [Google Scholar] [CrossRef]
- Filipe, H.A.L.; Santos, L.S.; Prates Ramalho, J.P.; Moreno, M.J.; Loura, L.M.S. Behaviour of NBD-head group labelled phosphatidylethanolamines in POPC bilayers: A molecular dynamics study. Phys. Chem. Chem. Phys. 2015, 17, 20066–20079. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Filipe, H.A.L.; Loura, L.M.S. Fluorescent Probes cis- and trans-Parinaric Acids in Fluid and Gel Lipid Bilayers: A Molecular Dynamics Study. Molecules 2023, 28, 2241. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef] [Green Version]
- Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 1984, 52, 255–268. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [Green Version]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Gao, W.; Hu, C.-M.J.; Fang, R.H.; Luk, B.T.; Su, J.; Zhang, L. Surface Functionalization of Gold Nanoparticles with Red Blood Cell Membranes. Adv. Mater. 2013, 25, 3549–3553. [Google Scholar] [CrossRef] [Green Version]
- Bennett, W.F.D.; Tieleman, D.P. The Importance of Membrane Defects—Lessons from Simulations. Acc. Chem. Res. 2014, 47, 2244–2251. [Google Scholar] [CrossRef]
- Kirsch, S.A.; Böckmann, R.A. Membrane pore formation in atomistic and coarse-grained simulations. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2266–2277. [Google Scholar] [CrossRef]
- Daison, F.A.; Kumar, N.; Balakrishnan, S.; Venugopal, K.; Elango, S.; Sokkar, P. Molecular Dynamics Studies on the Bacterial Membrane Pore Formation by Small Molecule Antimicrobial Agents. J. Chem. Inf. Model. 2022, 62, 40–48. [Google Scholar] [CrossRef]
- Gurtovenko, A.A.; Onike, O.I.; Anwar, J. Chemically Induced Phospholipid Translocation Across Biological Membranes. Langmuir 2008, 24, 9656–9660. [Google Scholar] [CrossRef]
- Karami, L. Interaction of neutral and protonated Tamoxifen with the DPPC lipid bilayer using molecular dynamics simulation. Steroids 2023, 194, 109225. [Google Scholar] [CrossRef]
- Gullapalli, R.R.; Demirel, M.C.; Butler, P.J. Molecular dynamics simulations of DiI-C-18(3) in a DPPC lipid bilayer. Phys. Chem. Chem. Phys. 2008, 10, 3548–3560. [Google Scholar] [CrossRef] [Green Version]
- Ackerman, D.G.; Heberle, F.A.; Feigenson, G.W. Limited Perturbation of a DPPC Bilayer by Fluorescent Lipid Probes: A Molecular Dynamics Study. J. Phys. Chem. B 2013, 117, 4844–4852. [Google Scholar] [CrossRef] [Green Version]
- Timr, Š.; Brabec, J.; Bondar, A.; Ryba, T.; Železný, M.; Lazar, J.; Jungwirth, P. Nonlinear Optical Properties of Fluorescent Dyes Allow for Accurate Determination of Their Molecular Orientations in Phospholipid Membranes. J. Phys. Chem. B 2015, 119, 9706–9716. [Google Scholar] [CrossRef]
- Paloncýová, M.; Aniander, G.; Larsson, E.; Knippenberg, S. Cyanine dyes with tail length asymmetry enhance photoselection: A multiscale study on DiD probes in a liquid disordered membrane. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117329. [Google Scholar] [CrossRef]
- Filipe, H.A.L.; Bowman, D.; Palmeira, T.; Cardoso, R.M.S.; Loura, L.M.S.; Moreno, M.J. Interaction of NBD-labelled fatty amines with liquid-ordered membranes: A combined molecular dynamics simulation and fluorescence spectroscopy study. Phys. Chem. Chem. Phys. 2015, 17, 27534–27547. [Google Scholar] [CrossRef]
- Filipe, H.A.L.; Moreno, M.J.; Loura, L.M.S. The Secret Lives of Fluorescent Membrane Probes as Revealed by Molecular Dynamics Simulations. Molecules 2020, 25, 3424. [Google Scholar] [CrossRef]
- Filipe, H.A.L.; Pokorná, Š.; Hof, M.; Amaro, M.; Loura, L.M.S. Orientation of nitro-group governs the fluorescence lifetime of nitrobenzoxadiazole (NBD)-labeled lipids in lipid bilayers. Phys. Chem. Chem. Phys. 2019, 21, 1682–1688. [Google Scholar] [CrossRef]
- Neves, M.C.; Filipe, H.A.L.; Reis, R.L.; Prates Ramalho, J.P.; Coreta-Gomes, F.; Moreno, M.J.; Loura, L.M.S. Interaction of Bile Salts With Lipid Bilayers: An Atomistic Molecular Dynamics Study. Front. Physiol. 2019, 10, 393. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, N.; Simões, G.M.; Ramos, C.; Samelo, J.; Oliveira, A.C.; Filipe, H.A.L.; Ramalho, J.P.P.; Moreno, M.J.; Loura, L.M.S. Interactions between Rhodamine Dyes and Model Membrane Systems—Insights from Molecular Dynamics Simulations. Molecules 2022, 27, 1420. [Google Scholar] [CrossRef]
- Knippenberg, S.; Fabre, G.; Osella, S.; Di Meo, F.; Paloncýová, M.; Ameloot, M.; Trouillas, P. Atomistic Picture of Fluorescent Probes with Hydrocarbon Tails in Lipid Bilayer Membranes: An Investigation of Selective Affinities and Fluorescent Anisotropies in Different Environmental Phases. Langmuir 2018, 34, 9072–9084. [Google Scholar] [CrossRef]
- Xu, X.; Yang, G.; Xue, X.; Lu, H.; Wu, H.; Huang, Y.; Jing, D.; Xiao, W.; Tian, J.; Yao, W.; et al. A polymer-free, biomimicry drug self-delivery system fabricated via a synergistic combination of bottom-up and top-down approaches. J. Mater. Chem. B 2018, 6, 7842–7853. [Google Scholar] [CrossRef]
- Deák, R.; Mihály, J.; Szigyártó, I.C.; Wacha, A.; Lelkes, G.; Bóta, A. Physicochemical characterization of artificial nanoerythrosomes derived from erythrocyte ghost membranes. Colloids Surf. B 2015, 135, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Jung, H.; You, G.; Mok, H. Cancer-Cell-Derived Hybrid Vesicles from MCF-7 and HeLa Cells for Dual-Homotypic Targeting of Anticancer Drugs. Macromol. Biosci. 2021, 21, 2100067. [Google Scholar] [CrossRef]
- Marasini, R.; Aryal, S. Indocyanine-type Infrared-820 Encapsulated Polymeric Nanoparticle-Assisted Photothermal Therapy of Cancer. ACS Omega 2022, 7, 12056–12065. [Google Scholar] [CrossRef]
- Yue, C.; Liu, P.; Zheng, M.; Zhao, P.; Wang, Y.; Ma, Y.; Cai, L. IR-780 dye loaded tumor targeting theranostic nanoparticles for NIR imaging and photothermal therapy. Biomaterials 2013, 34, 6853–6861. [Google Scholar] [CrossRef]
- Tan, Y.-N.; Li, Y.-P.; Huang, J.-D.; Luo, M.; Li, S.-S.; Lee, A.W.-M.; Hu, F.-Q.; Guan, X.-Y. Thermal-sensitive lipid nanoparticles potentiate anti-PD therapy through enhancing drug penetration and T lymphocytes infiltration in metastatic tumor. Cancer Lett. 2021, 522, 238–254. [Google Scholar] [CrossRef]
- Bar, L.; Losada-Pérez, P.; Troncoso, J. Effect of diphenylalanine on model phospholipid membrane organization. J. Mol. Liq. 2023, 384, 122196. [Google Scholar] [CrossRef]
- Brown, R.E.; Brockman, H.L. Using Monomolecular Films to Characterize Lipid Lateral Interactions. In Lipid Rafts; McIntosh, T.J., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 41–58. [Google Scholar]
- Redondo-Morata, L.; Losada-Pérez, P.; Giannotti, M.I. Chapter One—Lipid bilayers: Phase behavior and nanomechanics. In Current Topics in Membranes; Levitan, I., Trache, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 86, pp. 1–55. [Google Scholar]
- Neupane, S.; De Smet, Y.; Renner, F.U.; Losada-Pérez, P. Quartz Crystal Microbalance with Dissipation Monitoring: A Versatile Tool to Monitor Phase Transitions in Biomimetic Membranes. Front. Mater. 2018, 5, 46. [Google Scholar] [CrossRef] [Green Version]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipe, H.A.L.; Moreira, A.F.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P. Interaction of Near-Infrared (NIR)-Light Responsive Probes with Lipid Membranes: A Combined Simulation and Experimental Study. Pharmaceutics 2023, 15, 1853. https://doi.org/10.3390/pharmaceutics15071853
Filipe HAL, Moreira AF, Miguel SP, Ribeiro MP, Coutinho P. Interaction of Near-Infrared (NIR)-Light Responsive Probes with Lipid Membranes: A Combined Simulation and Experimental Study. Pharmaceutics. 2023; 15(7):1853. https://doi.org/10.3390/pharmaceutics15071853
Chicago/Turabian StyleFilipe, Hugo A. L., André F. Moreira, Sónia P. Miguel, Maximiano P. Ribeiro, and Paula Coutinho. 2023. "Interaction of Near-Infrared (NIR)-Light Responsive Probes with Lipid Membranes: A Combined Simulation and Experimental Study" Pharmaceutics 15, no. 7: 1853. https://doi.org/10.3390/pharmaceutics15071853




