Responsive Sensory Evaluation to Develop Flexible Taste-Masked Paediatric Primaquine Tablets against Malaria for Low-Resource Settings
Abstract
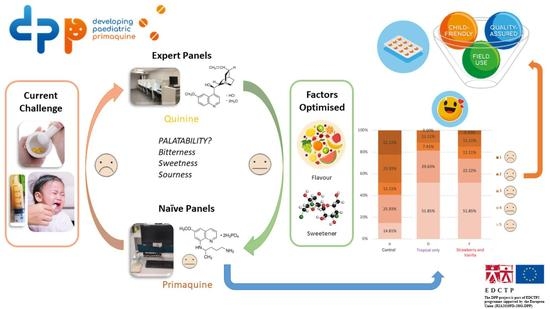
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. End-User Survey to Identify Flavour Preferences
2.3. Overview of Sensory Panel Studies
2.4. Training and Selection of Sensory Assessors
2.4.1. Expert Assessors, EBI
2.4.2. Naïve Assessors, UCL
2.5. Sample Evaluation
2.6. Statistical Analysis
3. Results
3.1. End-User Survey
3.2. Panel I: DoE for Screening Flavours
3.3. Panel II: Verification of Preliminary Flavour Blends
3.4. Panel III: Optimisation of Flavour Blends
3.5. Panel IV: Validation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO/UCN/GMP/2023.01; WHO Guidelines for Malaria. World Health Organization: Geneva, Switzerland, 2023.
- World Health Organization. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Taylor, W.R.; Olupot-Olupot, P.; Onyamboko, M.A.; Peerawaranun, P.; Weere, W.; Namayanja, C.; Onyas, P.; Titin, H.; Baseke, J.; Muhindo, R.; et al. Safety of age-dosed, single low-dose primaquine in children with glucose-6-phosphate dehydrogenase deficiency who are infected with Plasmodium falciparum in Uganda and the Democratic Republic of the Congo: A randomised, double-blind, placebo-controlled, non-inferiority trial. Lancet Infect. Dis. 2023, 23, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.R.; Hoglund, R.M.; Peerawaranun, P.; Nguyen, T.N.; Hien, T.T.; Tarantola, A.; von Seidlein, L.; Tripura, R.; Peto, T.J.; Dondorp, A.M.; et al. Development of weight and age-based dosing of daily primaquine for radical cure of vivax malaria. Malar. J. 2021, 20, 366. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 21st Invitation to Manufacturers of Antimalarial Medicines to Submit an Expression of Interest (EOI) for Product Evaluation to the WHO Prequalification Unit (PQT). Available online: https://extranet.who.int/pqweb/sites/default/files/documents/EOI-MalariaV21_0.pdf (accessed on 5 March 2023).
- EMA/CHMP/QWP/805880/2012 Rev. 2; Guideline on Pharmaceutical Development of Medicines for Paediatric Use. European Medicines Agency: Amsterdam, The Netherlands, 2013.
- Chen, I.; Poirot, E.; Newman, M.; Kandula, D.; Shah, R.; Hwang, J.; Cohen, J.M.; Gosling, R.; Rooney, L. An assessment of the supply, programmatic use, and regulatory issues of single low-dose primaquine as a Plasmodium falciparum gametocytocide for sub-Saharan Africa. Malar. J. 2015, 14, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haraguchi, T.; Okuno, T.; Nishikawa, H.; Kojima, H.; Ikegami, S.; Yoshida, M.; Habara, M.; Ikezaki, H.; Uchida, T. The Relationship between Bitter Taste Sensor Response and Physicochemical Properties of 47 Pediatric Medicines and Their Biopharmaceutics Classification. Chem. Pharm. Bull. 2019, 67, 1271–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, H.; Kurihara, T.; Yoshida, M.; Haraguchi, T.; Nishikawa, H.; Ikegami, S.; Okuno, T.; Yamashita, T.; Nishikawa, J.; Tsujino, H.; et al. A New Bitterness Evaluation Index Obtained Using the Taste Sensor for 48 Active Pharmaceutical Ingredients of Pediatric Medicines. Chem. Pharm. Bull. 2021, 69, 537–547. [Google Scholar] [CrossRef]
- Shah, P.P.; Mashru, R.C. Formulation and evaluation of taste masked oral reconstitutable suspension of primaquine phosphate. AAPS PharmSciTech 2008, 9, 1025–1030. [Google Scholar] [CrossRef] [Green Version]
- Mosha, D.; Kakolwa, M.A.; Mahende, M.K.; Masanja, H.; Abdulla, S.; Drakeley, C.; Gosling, R.; Wamoyi, J. Safety monitoring experience of single-low dose primaquine co-administered with artemether-lumefantrine among providers and patients in routine healthcare practice: A qualitative study in Eastern Tanzania. Malar. J. 2021, 20, 392. [Google Scholar] [CrossRef]
- Walsh, J.; Schaufelberger, D.; Iurian, S.; Klein, S.; Batchelor, H.; Turner, R.; Gizurarson, S.; Boltri, L.; Alessandrini, E.; Tuleu, C. Path towards efficient paediatric formulation development based on partnering with clinical pharmacologists and clinicians, a conect4children expert group white paper. Br. J. Clin. Pharmacol. 2022, 88, 5034–5051. [Google Scholar] [CrossRef]
- Ruiz, F.; Vallet, T.; Pensé-Lhéritier, A.M.; Aoussat, A. Standardized method to assess medicines’ acceptability: Focus on paediatric population. J. Pharm. Pharmacol. 2017, 69, 406–416. [Google Scholar] [CrossRef]
- Pensé-Lhéritier, A.M. Recent developments in the sensorial assessment of cosmetic products: A review. Int. J. Cosmet. Sci. 2015, 37, 465–473. [Google Scholar] [CrossRef]
- ISO 8586:2012; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO 5492:2008/AMD 1:2016; Sensory Analysis—Vocabulary—Amendment 1. International Organization for Standardization: Geneva, Switzerland, 2016.
- Lawless, H.T.; Heymann, H. Discrimination Testing. In Sensory Evaluation of Food: Principles and Practices; Springer: New York, NY, USA, 2010; pp. 79–100. [Google Scholar] [CrossRef]
- Ares, G.; Varela, P. Trained vs. consumer panels for analytical testing: Fueling a long lasting debate in the field. Food Qual. Prefer. 2017, 61, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.M.; Delahunty, C.M.; Baxter, I.A. Descriptive sensory analysis: Past, present and future. Food Res. Int. 2001, 34, 461–471. [Google Scholar] [CrossRef]
- Mammasse, N.; Schlich, P. Adequate number of consumers in a liking test. Insights from resampling in seven studies. Food Qual. Prefer. 2014, 31, 124–128. [Google Scholar] [CrossRef]
- ISO 8589:2007/Amd 1:2014; Sensory analysis—General guidance for the design of test rooms—Amendment 1. International Organization for Standardization: Geneva, Switzerland, 2014.
- ISO 8587:2006/Amd 1:2013; Sensory Analysis—Methodology—Ranking—Amendment 1. International Organization for Standardization: Geneva, Switzerland, 2013.
- Jones, R. Design and Analysis of Experiments (fifth edition), Douglas Montgomery, John Wiley and Sons, 2001, 684 pages, £33.95. Qual. Reliab. Eng. Int. 2002, 18, 163. [Google Scholar] [CrossRef]
- ISO 13299:2016; Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. International Organization for Standardization: Geneva, Switzerland, 2016.
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Taylor and Francis: Abingdon, UK, 2013. [Google Scholar] [CrossRef]
- Clapham, D.; Bennett, J.; Cram, A.; Discihnger, A.; Inghelbrecht, S.; Pense-Lheriter, A.M.; Ruiz, F.; Salunke, S.; Schiele, J.; Soto, J.; et al. Proposed Tool to Compare and Assess the Applicability of Taste Assessment Techniques for Pharmaceuticals. J. Pharm. Sci. 2021, 111, 1219–1223. [Google Scholar] [CrossRef]
- Mennella, J.A.; Mathew, P.S.; Lowenthal, E.D. Use of Adult Sensory Panel to Study Individual Differences in the Palatability of a Pediatric HIV Treatment Drug. Clin. Ther. 2017, 39, 2038–2048. [Google Scholar] [CrossRef]
- Orubu, S.; Kendall, R.A.; Sheng, Y.; Tuleu, C. Evaluating the Taste Masking Ability of Two Novel Dispersible Tablet Platforms Containing Zinc Sulfate and Paracetamol Reconstituted in a Breast Milk Substitute. Pharmaceutics 2022, 14, 420. [Google Scholar] [CrossRef]
- Lopalco, A.; Manni, A.; Keeley, A.; Haider, S.; Li, W.; Lopedota, A.; Altomare, C.D.; Denora, N.; Tuleu, C. In Vivo Investigation of (2-Hydroxypropyl)-β-cyclodextrin-Based Formulation of Spironolactone in Aqueous Solution for Paediatric Use. Pharmaceutics 2022, 14, 780. [Google Scholar] [CrossRef]
- Muoka, L.C.; Ross, S.A.; Mithu, M.S.H.; Nandi, U.; Douroumis, D. Comparative taste-masking evaluation of microencapsulated bitter drugs using Smartseal 30D and ReadyMix for paediatric dosage forms. AAPS PharmSciTech 2021, 22, 141. [Google Scholar] [CrossRef]
- Diezi, L.; Dao, K.; Jullien, V.; Roussel-Maupetit, C.; Burton, I.; André, P.; Bardinet, C.; Rothuizen, L.E.; Chtioui, H.; Manso-Silvan, M.A.; et al. An innovative ethosuximide granule formulation designed for pediatric use: Comparative pharmacokinetics, safety, tolerability, and palatability profile versus reference syrup. Pharmacol. Res. Perspect. 2023, 11, e01032. [Google Scholar] [CrossRef]
- Walsh, J.; Cram, A.; Woertz, K.; Breitkreutz, J.; Winzenburg, G.; Turner, R.; Tuleu, C.; Initiative, E.F. Playing hide and seek with poorly tasting paediatric medicines: Do not forget the excipients. Adv. Drug. Deliv. Rev. 2014, 73, 14–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Liu, X.; Zhang, S.; Quan, D. An Overview of Taste-Masking Technologies: Approaches, Application, and Assessment Methods. AAPS PharmSciTech 2023, 24, 67. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.P.; Mashru, R.C. Formulation and evaluation of primaquine phosphate taste-masked rapidly disintegrating tablet. J. Pharm. Pharmacol. 2008, 60, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Molinary, S.V.; Quinlan, M.E. Sucralose. In Sweeteners and Sugar Alternatives in Food Technology; Wiley: Hoboken, NJ, USA, 2012; pp. 167–183. [Google Scholar]
- Mennella, J.A.; Finkbeiner, S.; Lipchock, S.V.; Hwang, L.D.; Reed, D.R. Preferences for salty and sweet tastes are elevated and related to each other during childhood. PLoS ONE 2014, 9, e92201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mennella, J.A.; Bobowski, N.K. The sweetness and bitterness of childhood: Insights from basic research on taste preferences. Physiol. Behav. 2015, 152, 502–507. [Google Scholar] [CrossRef] [Green Version]
- Bobowski, N.; Mennella, J.A. Personal Variation in Preference for Sweetness: Effects of Age and Obesity. Child. Obes. 2017, 13, 369–376. [Google Scholar] [CrossRef]
- Petty, S.; Salame, C.; Mennella, J.A.; Pepino, M.Y. Relationship between Sucrose Taste Detection Thresholds and Preferences in Children, Adolescents, and Adults. Nutrients 2020, 12, 1918. [Google Scholar] [CrossRef]
- Ayenew, Z.; Puri, V.; Kumar, L.; Bansal, A.K. Trends in pharmaceutical taste masking technologies: A patent review. Recent. Pat. Drug. Deliv. Formul. 2009, 3, 26–39. [Google Scholar] [CrossRef]
- Banek, K.; DiLiberto, D.D.; Webb, E.L.; Smith, S.J.; Chandramohan, D.; Staedke, S.G. Exploring Barriers and Facilitators of Adherence to Artemisinin-Based Combination Therapies for the Treatment of Uncomplicated Malaria in Children in Freetown, Sierra Leone. Healthcare 2021, 9, 1233. [Google Scholar] [CrossRef]
- Beer, N.; Ali, A.S.; Rotllant, G.; Abass, A.K.; Omari, R.S.; Al-mafazy, A.W.; Björkman, A.; Källander, K. Adherence to artesunate-amodiaquine combination therapy for uncomplicated malaria in children in Zanzibar, Tanzania. Trop. Med. Int. Health 2009, 14, 766–774. [Google Scholar] [CrossRef]
- Ewing, V.L.; Terlouw, D.J.; Kapinda, A.; Pace, C.; Richards, E.; Tolhurst, R.; Lalloo, D.G. Perceptions and utilization of the anti-malarials artemether-lumefantrine and dihydroartemisinin-piperaquine in young children in the Chikhwawa District of Malawi: A mixed methods study. Malar. J. 2015, 14, 13. [Google Scholar] [CrossRef] [Green Version]






| Attribute | Targets | Comments |
|---|---|---|
| Target population (age) | Entire range 6 months–<16 years | Different dosing regimens are used, depending on the endemic area and national recommendations, based on mg per body weight or by age or weight dosing bands. |
| Route of administration | Oral | As per the WHO EOI requirement for prequalification [5]. |
| Target release profile | Immediate release | As per the WHO EOI requirement for prequalification [5]. |
| Dosage form | Tablets | At the start of DPP, the 17th WHO EOI invitation in 2019 called for PQ “tablets” only, while the latest 2023 EOI adds “(preferably dispersible for paediatric use)”. Hard tablets are suitable for use in the target population; however, the tablets would need to be scored and/or crushed to be administered to children unable to swallow them whole. |
| Dose and dose flexibility | Paediatric dosage strengths of primaquine base: 2.5 mg 3.75 mg 5 mg (scored) 7.5 mg (scored) 15 mg (scored) | At the start of DPP, the 17th WHO EOI invitation called for prequalification of five tablet strengths; however, the latest EOI no longer lists the 3.75 mg tablet. Five strengths will continue to be developed, providing more flexibility for clinical supplies. Scorelines allow further dosing flexibility. Dose regimens require further investigation and optimisation. Clinical trials are ongoing or planned. Dosing range recommendations are developed according to the patient’s age or weight. For fixed dose combinations, if any, the ratio of active ingredients may change across age groups. |
| Excipients and manufacturing | Excipients: no safety concerns for the proposed patient population. All dosage strengths are to be homothetic. Low-cost manufacturing | All excipients are Generally Recognised as Safe (GRAS) and have approval for use in pharma applications in accordance with EU and African regulations. The safety of excipients for the selected age group has been considered on a risk-benefit basis, on top of regulatory acceptance and precedence. Lower strengths will be produced proportionally to the higher strengths to get a biowaiver for prequalification (i.e., a bioequivalence study is not required for the line extension). Tablets are simple to manufacture. All prices are taken in consideration to keep the finished product low-cost. |
| Patient acceptability | Acceptable for the proposed patient population/caregiver, and disease state. | The flavoured tablets aim to mask the bitter taste of PQ, a key strategy to improve acceptability. Sensory evaluation has been used to guide formulation development and determine palatability. Acceptability studies of the flavoured tablets versus the basic formulation will be nested in two validation field trials conducted in Ethiopia and Burkina Faso using the ClinSearch Acceptability Score Test (CAST) [13]. |
| Administration considerations | Ease of preparation and accurate administration with low risk of dosing errors, a minimum of manipulations (e.g., mixing with water), and no device requirements. | Easy crushing is a target criterion with the aim of using a minimum of water and avoid mixing with other vehicles. The Administration protocol will be confirmed in the following field trials. No specific dosing device is needed for the dose ranges, as the various strengths ensure ease and accuracy of dosing. This mitigates the cost of a device. |
| Panel I: Screening | Panel II: Verification | Panel III: Optimisation | Panel IV: Validation | |
|---|---|---|---|---|
| Aim | Two-level factorial Design of Experiment (DoE) to screen combinations of flavouring agents, sweeteners, and excipient blends for bitter taste masking | Evaluation of the three leading flavour blends resulting from the DoE | Optimisation of the flavour blends and sensory profiling to quantify bitterness and other sensory attributes | Evaluation of the optimised flavoured blends, including hedonic to select two to pursue for clinical field studies. |
| Active Substance | QHCl | PQ | QHCl | PQ |
| Assessors | Expert, n = 26 | Naïve, n = 17 | Expert, n = 27 | Naïve, n = 27 |
| Selection and Recruitment | Formal selection and training as per ISO 8586 [15] | Simple bitterness sensitivity screening using taste strips | Formal selection and training as per ISO 8586 [15] | Simple bitterness sensitivity screening using taste strips. |
| Sample Assessment | Swirl and spit 10 mL of liquid samples for 10 s. | |||
| Outcome Measures | Intensity of bitter, sweet, and sour taste attributes rated on an 11-point scale. | Intensity of bitter taste and aftertaste rated on an 11-point scale. Acceptability rated on a 5-point facial hedonic scale. | Intensity of bitter, sweet, and sour taste attributes rated on an 11-point scale. | Intensity of bitter, sweet, and sour taste and bitter aftertaste rated on an 11-point scale. Acceptability rated on a 5-point facial hedonic scale. |
| Setting | Sensory analysis test rooms, EBI, France | Dispensary skills lab UCL, UK | Sensory analysis test rooms, EBI, France | Dispensary skills lab UCL, UK |
| Factor 1 | Factor 2 | Factor 3 | Factor Levels | |||
|---|---|---|---|---|---|---|
| Std | A: VaniliftTM | B: Sucralose | C: Citric acid | Factor (units %) | Low (−1) | High (+1) |
| 1 | −1 | −1 | −1 | A: VaniliftTM | 1.1 | 2.2 |
| 2 | 1 | −1 | −1 | B: Sucralose | 0.5 | 1.0 |
| 3 | −1 | 1 | −1 | C: Citric acid | 2.0 | 4.0 |
| 4 | 1 | 1 | −1 | |||
| 5 | −1 | −1 | 1 | |||
| 6 | 1 | −1 | 1 | |||
| 7 | −1 | 1 | 1 | |||
| 8 | 1 | 1 | 1 | |||
| 9 | 0 | 0 | 0 | |||
| Formulation | Bitterness Mean | Sweetness Mean | Sourness Mean | Bitter aftertaste Mean |
|---|---|---|---|---|
| A: Control: PQ tablet core | 7.04 | 0.56 | 2.56 | 3.74 |
| B: Tropical with Vanilift™ | 2.81 | 5.37 | 1.19 | 1.44 |
| C: Tropical with Vanilift™ + CA 1 | 2.78 | 5.15 | 2.00 | 1.07 |
| D: Tropical | 2.74 | 5.26 | 1.74 | 1.04 |
| E: Tropical + CA 1 | 2.93 | 5.15 | 2.22 | 1.56 |
| F: Strawberry and Vanilla | 3.70 | 5.30 | 1.81 | 1.15 |
| G: Strawberry and Vanilla + CA 1 | 3.52 | 5.33 | 2.41 | 1.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranmal, S.R.; Lavarde, M.; Wallon, E.; Issa, S.; Taylor, W.R.; Nguyen Ngoc Pouplin, J.L.A.; Tuleu, C.; Pensé-Lhéritier, A.-M. Responsive Sensory Evaluation to Develop Flexible Taste-Masked Paediatric Primaquine Tablets against Malaria for Low-Resource Settings. Pharmaceutics 2023, 15, 1879. https://doi.org/10.3390/pharmaceutics15071879
Ranmal SR, Lavarde M, Wallon E, Issa S, Taylor WR, Nguyen Ngoc Pouplin JLA, Tuleu C, Pensé-Lhéritier A-M. Responsive Sensory Evaluation to Develop Flexible Taste-Masked Paediatric Primaquine Tablets against Malaria for Low-Resource Settings. Pharmaceutics. 2023; 15(7):1879. https://doi.org/10.3390/pharmaceutics15071879
Chicago/Turabian StyleRanmal, Sejal R., Marc Lavarde, Elodie Wallon, Samar Issa, Walter R. Taylor, Julie L. A. Nguyen Ngoc Pouplin, Catherine Tuleu, and Anne-Marie Pensé-Lhéritier. 2023. "Responsive Sensory Evaluation to Develop Flexible Taste-Masked Paediatric Primaquine Tablets against Malaria for Low-Resource Settings" Pharmaceutics 15, no. 7: 1879. https://doi.org/10.3390/pharmaceutics15071879