Comparison of Bovine- and Porcine-Derived Decellularized Biomaterials: Promising Platforms for Tissue Engineering Applications
Abstract
1. Introduction
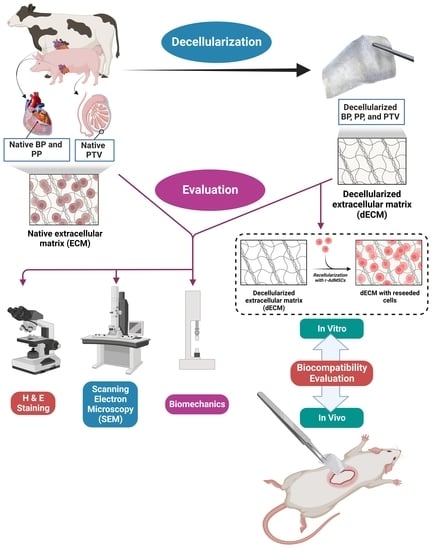
2. Materials and Methods
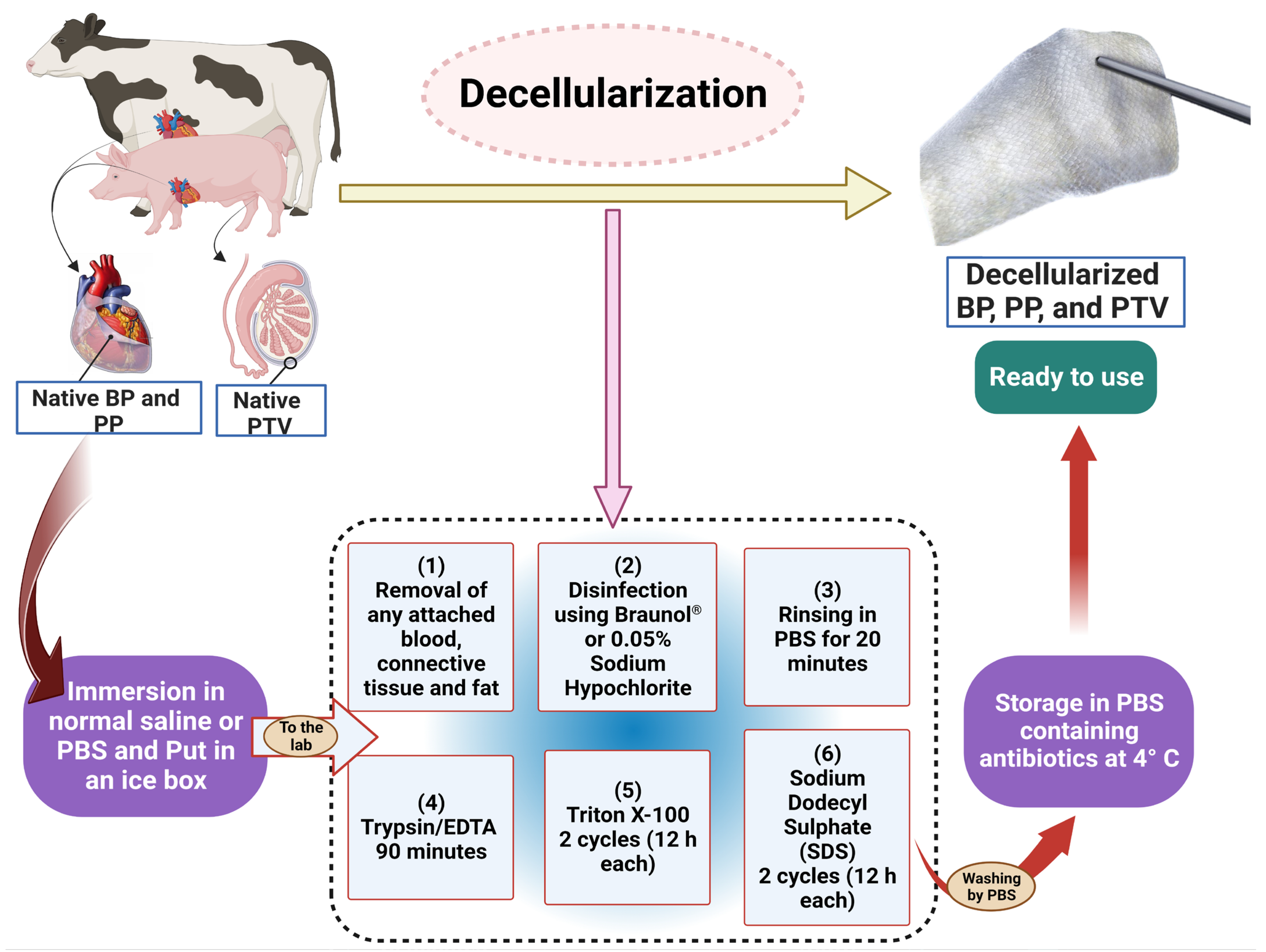
2.1. Decellularization Protocol
2.2. In Vitro Evaluation of the Decellularization Process
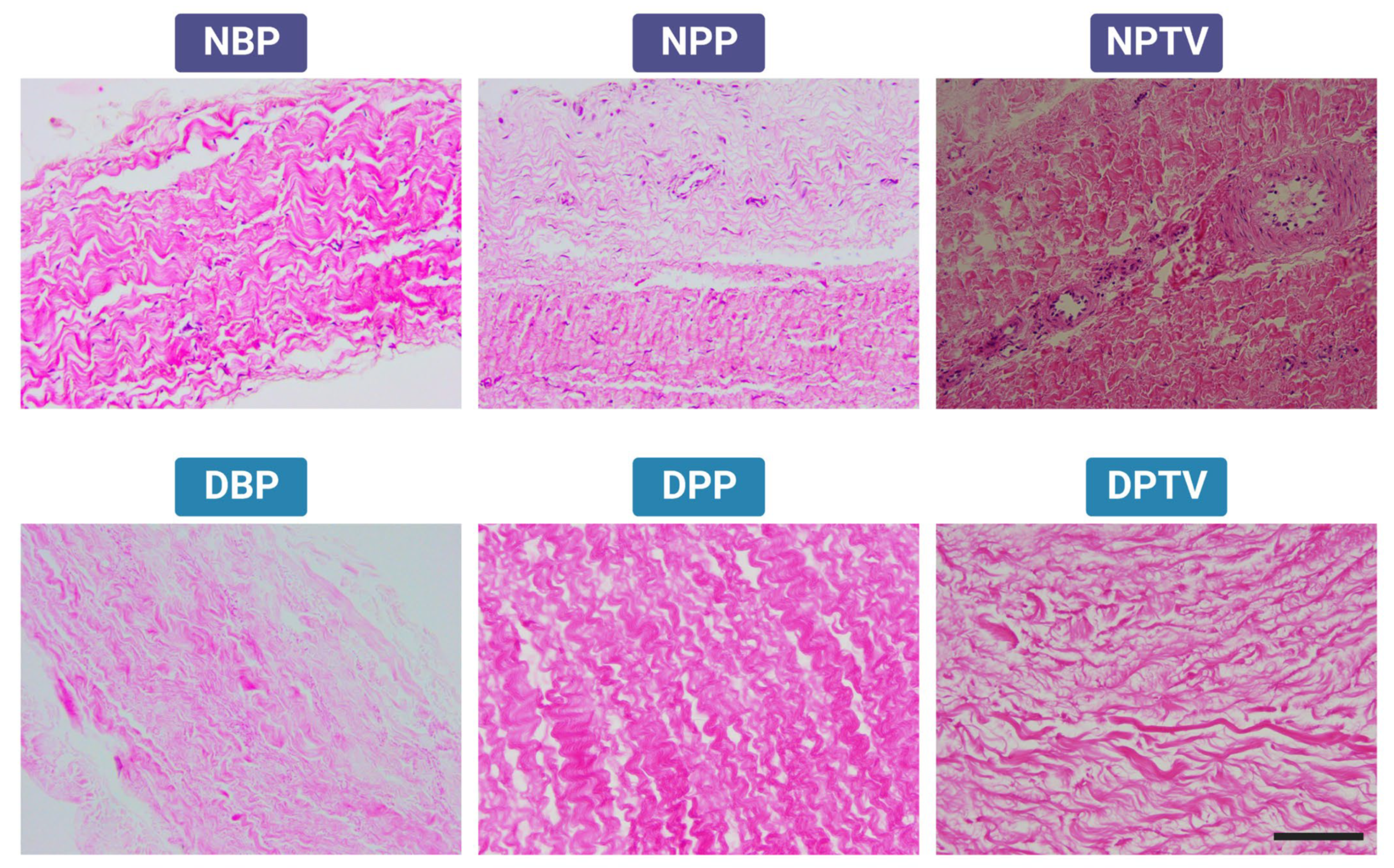
2.2.1. Histology
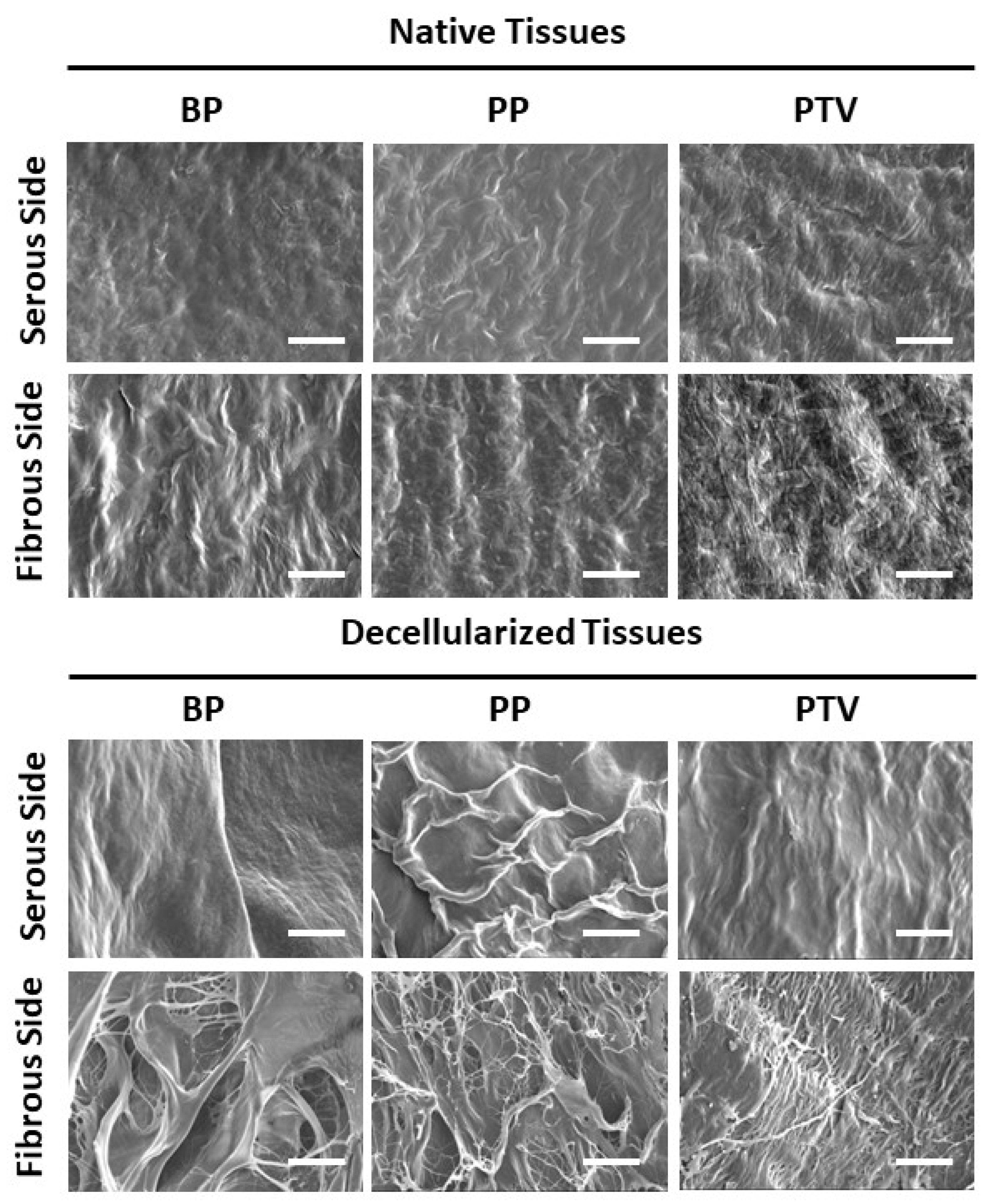
2.2.2. Scanning Electron Microscope (SEM)
2.2.3. Assessment of the Weight Loss
2.2.4. Biomechanics (Tensiometric Evaluation)
2.3. Isolation and Characterization of Rat-Adipose-Derived Mesenchymal Stem Cells (r-ADMSCs)
2.4. In Vitro Assessment of the Cytocompatibility
2.5. In Vivo Assessment of Biocompatibility
2.6. Statistical Analysis
3. Results
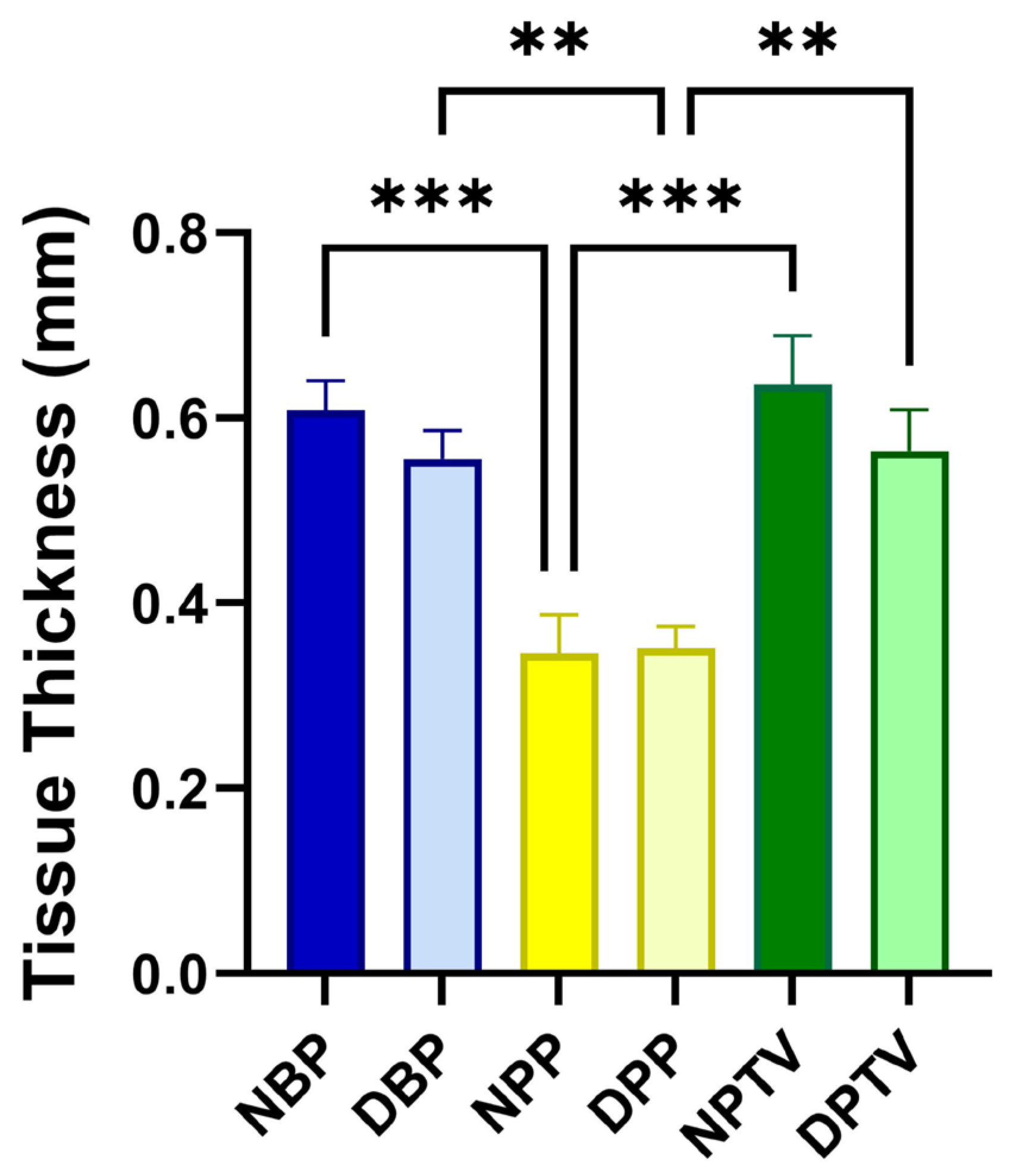
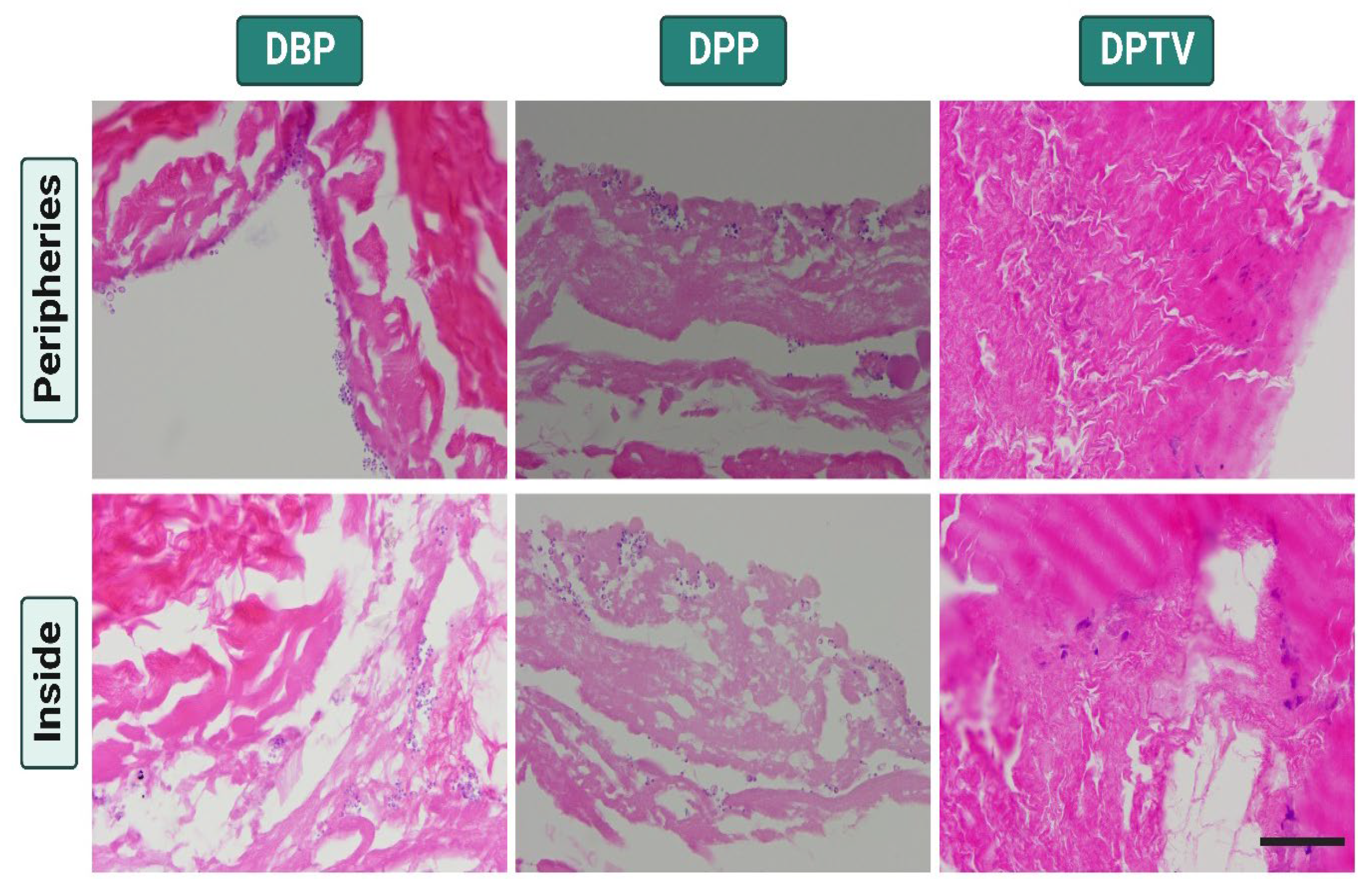
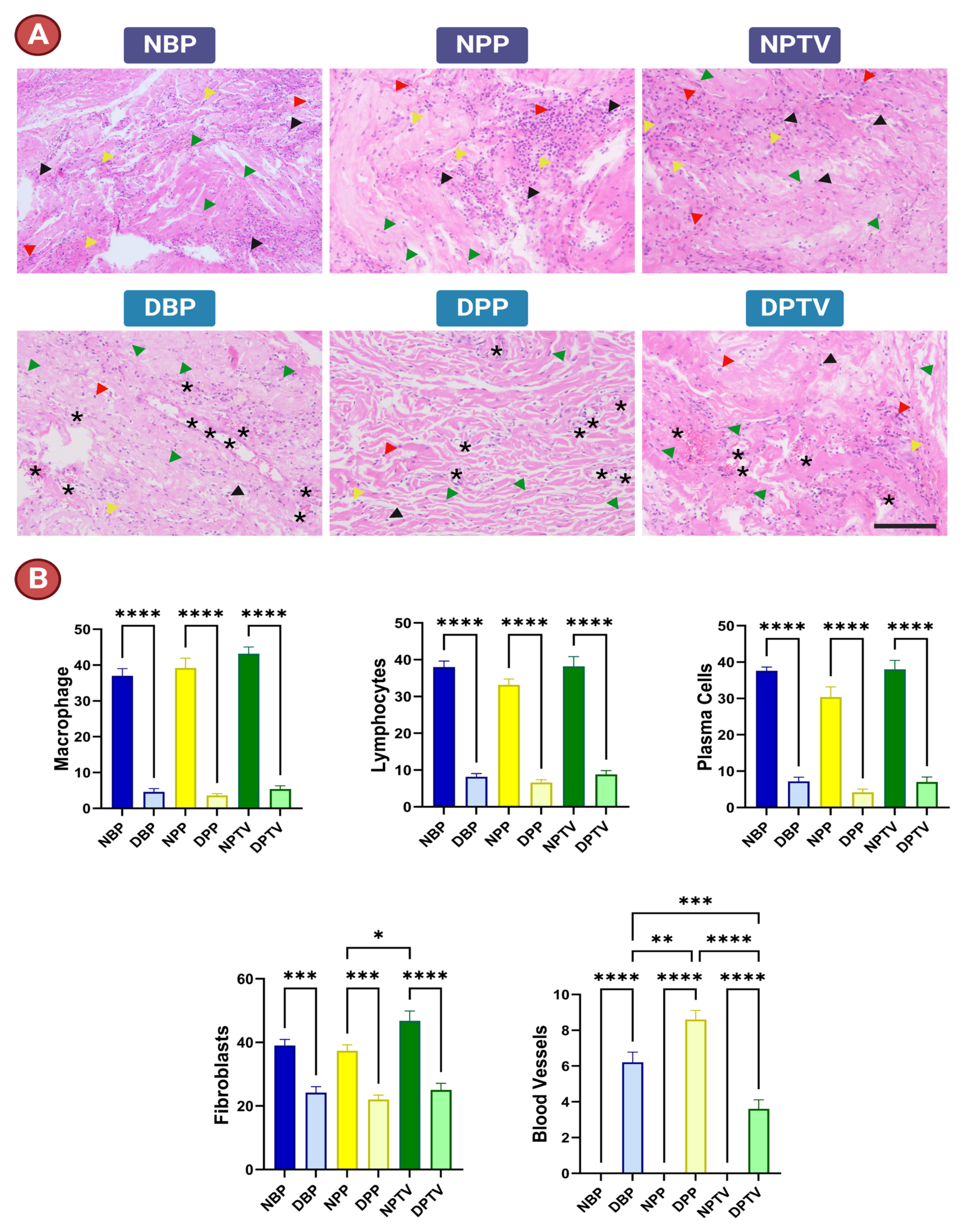
3.1. Histological Assessment
3.2. Scanning Electron Microscopy
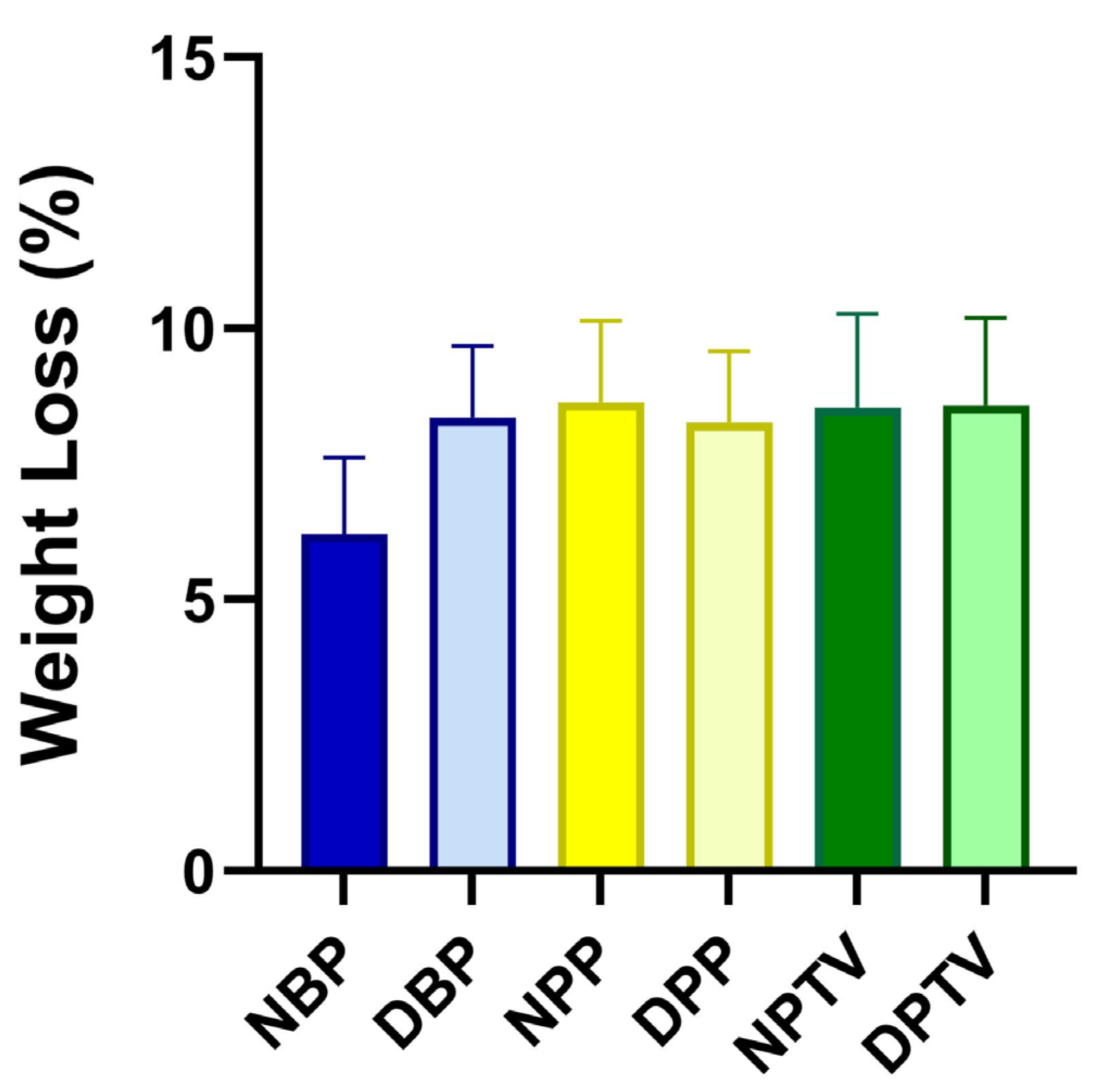
3.3. Weight Loss (%)
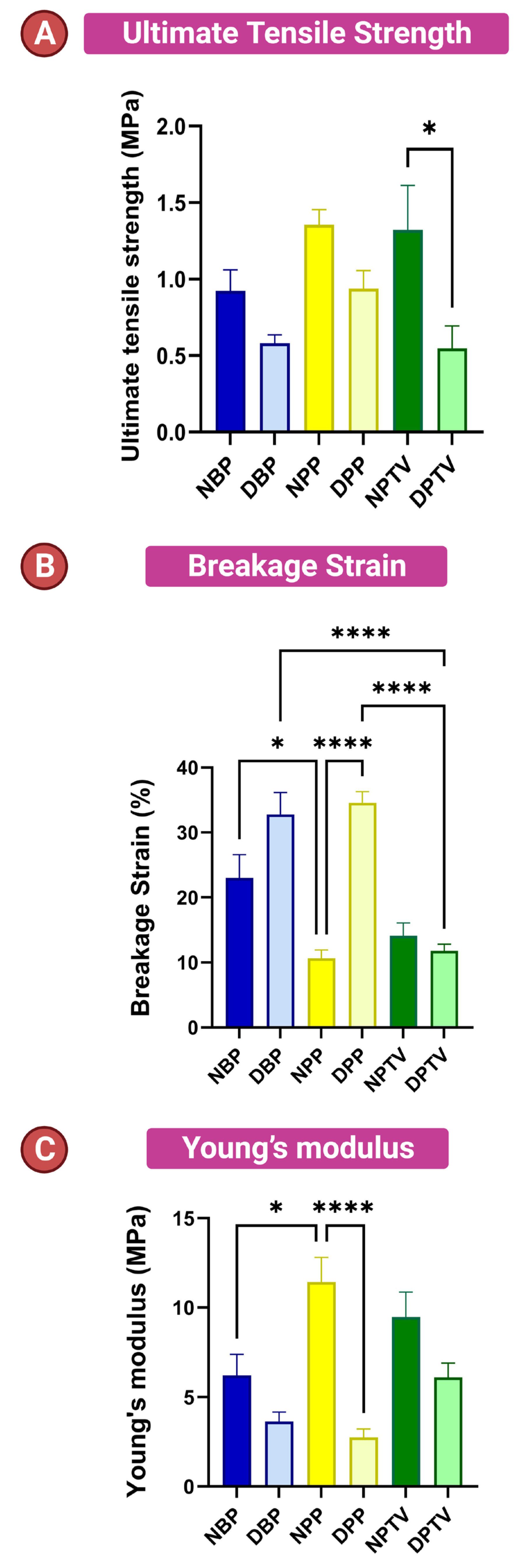
3.4. Biomechanical Characters
3.5. In Vitro Cytocompatibility
3.6. In Vivo Assessment of Biocompatibility
4. Discussion
5. Limitations of the Present Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levitt, M. Could the organ shortage ever be met? Life Sci. Soc. Policy 2015, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; El-Dakroury, W.A.; Doghish, A.S.; Tanaka, R. Stimuli-Responsive Hydrogels: Smart State of-the-Art Platforms for Cardiac Tissue Engineering. 2022. Available online: https://www.researchsquare.com/article/rs-2011475/v1 (accessed on 10 September 2022).
- El-Husseiny, H.M.; Mady, E.A.; El-Dakroury, W.A.; Zewail, M.B.; Noshy, M.; Abdelfatah, A.M.; Doghish, A.S. Smart/stimuli-responsive hydrogels: State-of-the-art platforms for bone tissue engineering. Appl. Mater. Today 2022, 29, 101560. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Radwan, Y.; Nagy, M.; Abugomaa, A.; Elbadawy, M.; Tanaka, R. Hybrid Biodegradable Polymeric Scaffolds for Cardiac Tissue Engineering. In Handbook of Biodegradable Materials; Ali, G.A.M., Makhlouf, A.S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–48. [Google Scholar] [CrossRef]
- Abd Elkodous, M.; El-Husseiny, H.M.; El-Sayyad, G.S.; Hashem, A.H.; Doghish, A.S.; Elfadil, D.; Radwan, Y.; El-Zeiny, H.M.; Bedair, H.; Ikhdair, O.A.; et al. Recent advances in waste-recycled nanomaterials for biomedical applications: Waste-to-wealth. Nanotechnol. Rev. 2021, 10, 1662–1739. [Google Scholar] [CrossRef]
- Hunsberger, J.; Neubert, J.; Wertheim, J.A.; Allickson, J.; Atala, A. Bioengineering Priorities on a Path to Ending Organ Shortage. Curr. Stem Cell Rep. 2016, 2, 118–127. [Google Scholar] [CrossRef]
- Doghish, A.S.; El-Husseiny, A.A.; Abdelmaksoud, N.M.; El-Mahdy, H.A.; Elsakka, E.G.E.; Abdel Mageed, S.S.; Mahmoud, A.M.A.; Raouf, A.A.; Elballal, M.S.; El-Dakroury, W.A.; et al. The interplay of signaling pathways and miRNAs in the pathogenesis and targeted therapy of esophageal cancer. Pathol.—Res. Pract. 2023, 246, 154529. [Google Scholar] [CrossRef]
- Doghish, A.S.; Ismail, A.; El-Mahdy, H.A.; Elkhawaga, S.Y.; Elsakka, E.G.E.; Mady, E.A.; Elrebehy, M.A.; Khalil, M.A.F.; El-Husseiny, H.M. miRNAs insights into rheumatoid arthritis: Favorable and detrimental aspects of key performers. Life Sci. 2023, 314, 121321. [Google Scholar] [CrossRef]
- El-Mahdy, H.A.; Mohamadin, A.M.; Abulsoud, A.I.; Khidr, E.G.; El-Husseiny, A.A.; Ismail, A.; Elsakka, E.G.E.; Mokhlis, H.A.; El-Husseiny, H.M.; Doghish, A.S. miRNAs as potential game-changers in head and neck cancer: Future clinical and medicinal uses. Pathol.—Res. Pract. 2023, 245, 154457. [Google Scholar] [CrossRef]
- Mady, E.A.; Doghish, A.S.; El-Dakroury, W.A.; Elkhawaga, S.Y.; Ismail, A.; El-Mahdy, H.A.; Elsakka, E.G.E.; El-Husseiny, H.M. Impact of the mother’s gut microbiota on infant microbiome and brain development. Neurosci. Biobehav. Rev. 2023, 150, 105195. [Google Scholar] [CrossRef]
- Perkel, J.M. Xenotransplantation makes a comeback. Nat. Biotechnol. 2016, 34, 3–4. [Google Scholar] [CrossRef]
- Mohiuddin, M.M.; Singh, A.K.; Corcoran, P.C.; Hoyt, R.F.; Thomas, M.L.; Ayares, D.; Horvath, K.A. Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation. J. Thorac. Cardiovasc. Surg. 2014, 148, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H. Evaluation of Some Prosthetic Implants for Surgical Management of Different Varieties of Hernias in Domestic Animals; Department of Surgery, Anesthesiology, and Radiology, Faculty of Veterinary Medicine, Benha University: Banha, Egypt, 2017; pp. 42–43. [Google Scholar]
- El-Husseiny, M. Platelet rich fibrin augmented versus non-augmented glycerolized bovine pericardium and polypropylene mesh for repairing of large abdominal wall defects. Eur. J. Med. Nat. Sci. 2019, 3, 33–48. [Google Scholar] [CrossRef]
- Ramm, R.; Goecke, T.; Theodoridis, K.; Hoeffler, K.; Sarikouch, S.; Findeisen, K.; Ciubotaru, A.; Cebotari, S.; Tudorache, I.; Haverich, A.; et al. Decellularization combined with enzymatic removal of N-linked glycans and residual DNA reduces inflammatory response and improves performance of porcine xenogeneic pulmonary heart valves in an ovine in vivo model. Xenotransplantation 2020, 27, e12571. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Parmaksiz, M.; Dogan, A.; Odabas, S.; Elçin, A.E.; Elçin, Y.M. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed. Mater. 2016, 11, 022003. [Google Scholar] [CrossRef]
- Vorotnikova, E.; McIntosh, D.; Dewilde, A.; Zhang, J.; Reing, J.E.; Zhang, L.; Cordero, K.; Bedelbaeva, K.; Gourevitch, D.; Heber-Katz, E.; et al. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010, 29, 690–700. [Google Scholar] [CrossRef]
- Xu, C.C.; Chan, R.W.; Weinberger, D.G.; Efune, G.; Pawlowski, K.S. A bovine acellular scaffold for vocal fold reconstruction in a rat model. J. Biomed. Mater. Res. Part A 2010, 92A, 18–32. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Kaneda, M.; Mady, E.A.; Yoshida, T.; Doghish, A.S.; Tanaka, R. Impact of Adipose Tissue Depot Harvesting Site on the Multilineage Induction Capacity of Male Rat Adipose-Derived Mesenchymal Stem Cells: An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 7513. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Helal, M.A.; Tanaka, R. The Pivotal Role of Stem Cells in Veterinary Regenerative Medicine and Tissue Engineering. Vet. Sci. 2022, 9, 648. [Google Scholar] [CrossRef]
- Hendawy, H.; Uemura, A.; Ma, D.; Namiki, R.; Samir, H.; Ahmed, M.F.; Elfadadny, A.; El-Husseiny, H.M.; Chieh-Jen, C.; Tanaka, R. Tissue Harvesting Site Effect on the Canine Adipose Stromal Vascular Fraction Quantity and Quality. Animals 2021, 11, 460. [Google Scholar] [CrossRef]
- Sharun, K.; Chandran, D.; Manjusha, K.M.; Mankuzhy, P.D.; Kumar, R.; Pawde, A.M.; Dhama, K.; El-Husseiny, H.M. Amarpal. Advances and prospects of platelet-rich plasma therapy in veterinary ophthalmology. Vet. Res. Commun. 2023. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Freund, J.M.; Badylak, S.F. Quantification of DNA in Biologic Scaffold Materials. J. Surg. Res. 2009, 152, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Aguiari, P.; Iop, L.; Favaretto, F.; Fidalgo, C.M.L.; Naso, F.; Milan, G.; Vindigni, V.; Spina, M.; Bassetto, F.; Bagno, A.; et al. In vitro comparative assessment of decellularized bovine pericardial patches and commercial bioprosthetic heart valves. Biomed. Mater. 2017, 12, 015021. [Google Scholar] [CrossRef] [PubMed]
- Keane, T.J.; Londono, R.; Turner, N.J.; Badylak, S.F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 2012, 33, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- McDade, J.K.; Brennan-Pierce, E.P.; Ariganello, M.B.; Labow, R.S.; Michael Lee, J. Interactions of U937 macrophage-like cells with decellularized pericardial matrix materials: Influence of crosslinking treatment. Acta Biomater. 2013, 9, 7191–7199. [Google Scholar] [CrossRef]
- Gauvin, R.; Marinov, G.; Mehri, Y.; Klein, J.; Li, B.; Larouche, D.; Guzman, R.; Zhang, Z.; Germain, L.; Guidoin, R. A comparative study of bovine and porcine pericardium to highlight their potential advantages to manufacture percutaneous cardiovascular implants. J. Biomater. Appl. 2013, 28, 552–565. [Google Scholar] [CrossRef]
- Manji, R.A.; Zhu, L.F.; Nijjar, N.K.; Rayner, D.C.; Korbutt, G.S.; Churchill, T.A.; Rajotte, R.V.; Koshal, A.; Ross, D.B. Glutaraldehyde-Fixed Bioprosthetic Heart Valve Conduits Calcify and Fail From Xenograft Rejection. Circulation 2006, 114, 318–327. [Google Scholar] [CrossRef]
- Schoen, F.J.; Levy, R.J. Calcification of Tissue Heart Valve Substitutes: Progress Toward Understanding and Prevention. Ann. Thorac. Surg. 2005, 79, 1072–1080. [Google Scholar] [CrossRef]
- Mendoza-Novelo, B.; Avila, E.E.; Cauich-Rodríguez, J.V.; Jorge-Herrero, E.; Rojo, F.J.; Guinea, G.V.; Mata-Mata, J.L. Decellularization of pericardial tissue and its impact on tensile viscoelasticity and glycosaminoglycan content. Acta Biomater 2011, 7, 1241–1248. [Google Scholar] [CrossRef]
- Fentie, I.H.; Allen, D.J.; Schenck, M.H.; Didio, L.J. Comparative electron microscopic study of bovine, porcine and human parietal pericardium, as materials for cardiac valve bioprostheses. J. Submicrosc. Cytol. 1986, 18, 53–65. [Google Scholar]
- Huen, K.H.; Macaraeg, A.; Davis-Dao, C.A.; Williamson, S.H.; Boswell, T.C.; Chuang, K.-w.; Stephany, H.A.; Wehbi, E.J.; Khoury, A.E. Single-Layer Acellular Porcine Bladder Matrix as Graft in Corporoplasty for Ventral Curvature in Pediatric Proximal Hypospadias Repair: An Initial Experience. Urology 2022, 169, 196–201. [Google Scholar] [CrossRef]
- Huen, K.H.; Macaraeg, A.; Khoury, A.E. Ventral penile lengthening using tunica vaginalis flap for correction of curvature in proximal hypospadias repair: Technical aspects. Urol. Video J. 2022, 14, 100145. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Heuschkel, M.A.; Leitolis, A.; Roderjan, J.G.; Suss, P.H.; Luzia, C.A.O.; da Costa, F.D.A.; Correa, A.; Stimamiglio, M.A. In vitro evaluation of bovine pericardium after a soft decellularization approach for use in tissue engineering. Xenotransplantation 2019, 26, e12464. [Google Scholar] [CrossRef] [PubMed]
- Mallis, P.; Michalopoulos, E.; Dimitriou, C.; Kostomitsopoulos, N.; Stavropoulos-Giokas, C. Histological and biomechanical characterization of decellularized porcine pericardium as a potential scaffold for tissue engineering applications. Bio-Med. Mater. Eng. 2017, 28, 477–488. [Google Scholar] [CrossRef]
- Chantawong, P.; Tanaka, T.; Uemura, A.; Shimada, K.; Higuchi, A.; Tajiri, H.; Sakura, K.; Murakami, T.; Nakazawa, Y.; Tanaka, R. Silk fibroin-Pellethane® cardiovascular patches: Effect of silk fibroin concentration on vascular remodeling in rat model. J. Mater. Sci. Mater. Med. 2017, 28, 191. [Google Scholar] [CrossRef]
- Koyanagi, E.; Tara, S.; Sakata, C.; Shimada, K.; Kato, K.; Miyachi, H.; Tanaka, R.; Nakazawa, Y. A novel gradient and multilayered sheet with a silk fibroin/polyvinyl alcohol core–shell structure for bioabsorbable arterial grafts. J. Biomed. Mater. Res. Part A 2022, 110, 576–584. [Google Scholar] [CrossRef]
- Choe, J.A.; Jana, S.; Tefft, B.J.; Hennessy, R.S.; Go, J.; Morse, D.; Lerman, A.; Young, M.D. Biomaterial characterization of off-the-shelf decellularized porcine pericardial tissue for use in prosthetic valvular applications. J. Tissue Eng. Regen. Med. 2018, 12, 1608–1620. [Google Scholar] [CrossRef]
- Soares, L.G.; de Oliveira, F.S.; Queiroz, A.; de Medeiros, A.; Bariani Junior, A.F.; Fechis, A.D.S.; Rocha, T. Biomechanics of the fresh and conserved bovine pericardium. Anat. Histol. Embryol. 2021, 50, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Rezakhani, L.; Khodaei, M.; Soleimannejad, M.; Alizadeh, A. Evaluating the effects of vacuum on the microstructure and biocompatibility of bovine decellularized pericardium. J. Tissue Eng. Regen. Med. 2021, 15, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Shan, X.; Xu, L.; Yu, W.; Zhang, M.; Lei, C.; Xu, N.; Lin, J.; Wang, B. Adipose-derived stem cells combined with platelet-rich plasma enhance wound healing in a rat model of full-thickness skin defects. Stem Cell Res. Ther. 2021, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Iop, L.; Palmosi, T.; Dal Sasso, E.; Gerosa, G. Bioengineered tissue solutions for repair, correction and reconstruction in cardiovascular surgery. J. Thorac. Dis. 2018, 10, S2390–S2411. [Google Scholar] [CrossRef] [PubMed]
- Topuz, B.; Günal, G.; Guler, S.; Aydin, H.M. Chapter 4—Use of supercritical CO2 in soft tissue decellularization. In Methods in Cell Biology; Caballero, D., Kundu, S.C., Reis, R.L., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 157, pp. 49–79. [Google Scholar]
- Fernández-Pérez, J.; Ahearne, M. The impact of decellularization methods on extracellular matrix derived hydrogels. Sci. Rep. 2019, 9, 14933. [Google Scholar] [CrossRef]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef]
- White, L.J.; Taylor, A.J.; Faulk, D.M.; Keane, T.J.; Saldin, L.T.; Reing, J.E.; Swinehart, I.T.; Turner, N.J.; Ratner, B.D.; Badylak, S.F. The impact of detergents on the tissue decellularization process: A ToF-SIMS study. Acta Biomater. 2017, 50, 207–219. [Google Scholar] [CrossRef]
- Filippi, R.; Schwarz, M.; Voth, D.; Reisch, R.; Grunert, P.; Perneczky, A. Bovine pericardium for duraplasty: Clinical results in 32 patients. Neurosurg. Rev. 2001, 24, 103–107. [Google Scholar] [CrossRef]
- Zouhair, S.; Dal Sasso, E.; Tuladhar, S.R.; Fidalgo, C.; Vedovelli, L.; Filippi, A.; Borile, G.; Bagno, A.; Marchesan, M.; De Rossi, G.; et al. A Comprehensive Comparison of Bovine and Porcine Decellularized Pericardia: New Insights for Surgical Applications. Biomolecules 2020, 10, 371. [Google Scholar] [CrossRef]
- Bel, A.; Kachatryan, L.; Bruneval, P.; Peyrard, S.; Gagnieu, C.; Fabiani, J.-N.; Menasché, P. A new absorbable collagen membrane to reduce adhesions in cardiac surgery. Interact. CardioVascular Thorac. Surg. 2010, 10, 213–216. [Google Scholar] [CrossRef]
- Gubitosi, A.; Docimo, G.; Parmeggiani, D.; Pirozzi, R.; Vitiello, C.; Schettino, P.; Avellino, M.; Casalino, G.; Amato, M.; Ruggiero, R.; et al. Acellular bovine pericardium dermal matrix in immediate breast reconstruction after Skin Sparing Mastectomy. Int. J. Surg. 2014, 12, S205–S208. [Google Scholar] [CrossRef]
- Le, B.; Borzabadi-Farahani, A.; Nielsen, B. Treatment of Labial Soft Tissue Recession Around Dental Implants in the Esthetic Zone Using Guided Bone Regeneration With Mineralized Allograft: A Retrospective Clinical Case Series. J. Oral Maxillofac. Surg. 2016, 74, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Wang, C.; Fan, Y.; Li, X. Biomimetic SIS-based biocomposites with improved biodegradability, antibacterial activity and angiogenesis for abdominal wall repair. Mater. Sci. Eng. C 2020, 109, 110538. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liu, X.; Qian, Y.; Maitusong, M.; Yu, K.; Cao, N.; Fang, J.; Liu, F.; Chen, J.; Xu, D.; et al. Double-Network Hydrogel Armored Decellularized Porcine Pericardium as Durable Bioprosthetic Heart Valves. Adv. Healthc. Mater. 2022, 11, 2102059. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. Challenges With the Development of Biomaterials for Sustainable Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 127. [Google Scholar] [CrossRef]
- Spina, M.; Naso, F.; Zancan, I.; Iop, L.; Dettin, M.; Gerosa, G. Biocompatibility issues of next generation decellularized bioprosthetic devices. In Conference Papers in Science; Hindawi Publishing Corporation: London, UK, 2014. [Google Scholar]
- Tran, H.L.B.; Dinh, T.T.H.; Nguyen, M.T.N.; To, Q.M.; Pham, A.T.T. Preparation and characterization of acellular porcine pericardium for cardiovascular surgery. Turk. J. Biol. 2016, 40, 1243–1250. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Wong, M.L.; Griffiths, L.G. Effect of bovine pericardial extracellular matrix scaffold niche on seeded human mesenchymal stem cell function. Sci. Rep. 2016, 6, 37089. [Google Scholar] [CrossRef]
- Kagan, H.M.; Crombie, G.D.; Jordan, R.E.; Lewis, W.; Franzblau, C. Proteolysis of elastin-ligand complexes. Stimulation of elastase digestion of insoluble elastin by sodium dodecyl sulfate. Biochemistry 1972, 11, 3412–3418. [Google Scholar] [CrossRef]
- Lakshmanan, R.; Krishnan, U.M.; Sethuraman, S. Living cardiac patch: The elixir for cardiac regeneration. Expert Opin. Biol. Ther. 2012, 12, 1623–1640. [Google Scholar] [CrossRef]
- Wei, H.J.; Chen, S.C.; Chang, Y.; Hwang, S.M.; Lin, W.W.; Lai, P.H.; Chiang, H.K.; Hsu, L.F.; Yang, H.H.; Sung, H.W. Porous acellular bovine pericardia seeded with mesenchymal stem cells as a patch to repair a myocardial defect in a syngeneic rat model. Biomaterials 2006, 27, 5409–5419. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Lepedda, A.J.; Nieddu, G.; Formato, M.; Baker, M.B.; Fernández-Pérez, J.; Moroni, L. Glycosaminoglycans: From Vascular Physiology to Tissue Engineering Applications. Front. Chem. 2021, 9, 680836. [Google Scholar] [CrossRef]
- Kim, H.D.; Hong, X.; An, Y.-H.; Park, M.J.; Kim, D.-G.; Greene, A.K.; Padwa, B.L.; Hwang, N.S.; Lin, R.-Z.; Melero-Martin, J.M. A Biphasic Osteovascular Biomimetic Scaffold for Rapid and Self-Sustained Endochondral Ossification. Adv. Healthc. Mater. 2021, 10, 2100070. [Google Scholar] [CrossRef] [PubMed]
- Zouhair, S.; Aguiari, P.; Iop, L.; Vásquez-Rivera, A.; Filippi, A.; Romanato, F.; Korossis, S.; Wolkers, W.F.; Gerosa, G. Preservation strategies for decellularized pericardial scaffolds for off-the-shelf availability. Acta Biomater. 2019, 84, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, C.; Iop, L.; Sciro, M.; Harder, M.; Mavrilas, D.; Korossis, S.; Bagno, A.; Palù, G.; Aguiari, P.; Gerosa, G. A sterilization method for decellularized xenogeneic cardiovascular scaffolds. Acta Biomater. 2018, 67, 282–294. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Husseiny, H.M.; Mady, E.A.; Kaneda, M.; Shimada, K.; Nakazawa, Y.; Usui, T.; Elbadawy, M.; Ishihara, Y.; Hirose, M.; Kamei, Y.; et al. Comparison of Bovine- and Porcine-Derived Decellularized Biomaterials: Promising Platforms for Tissue Engineering Applications. Pharmaceutics 2023, 15, 1906. https://doi.org/10.3390/pharmaceutics15071906
El-Husseiny HM, Mady EA, Kaneda M, Shimada K, Nakazawa Y, Usui T, Elbadawy M, Ishihara Y, Hirose M, Kamei Y, et al. Comparison of Bovine- and Porcine-Derived Decellularized Biomaterials: Promising Platforms for Tissue Engineering Applications. Pharmaceutics. 2023; 15(7):1906. https://doi.org/10.3390/pharmaceutics15071906
Chicago/Turabian StyleEl-Husseiny, Hussein M., Eman A. Mady, Masahiro Kaneda, Kazumi Shimada, Yasumoto Nakazawa, Tatsuya Usui, Mohamed Elbadawy, Yusuke Ishihara, Moeko Hirose, Yohei Kamei, and et al. 2023. "Comparison of Bovine- and Porcine-Derived Decellularized Biomaterials: Promising Platforms for Tissue Engineering Applications" Pharmaceutics 15, no. 7: 1906. https://doi.org/10.3390/pharmaceutics15071906
APA StyleEl-Husseiny, H. M., Mady, E. A., Kaneda, M., Shimada, K., Nakazawa, Y., Usui, T., Elbadawy, M., Ishihara, Y., Hirose, M., Kamei, Y., Doghish, A. S., El-Mahdy, H. A., El-Dakroury, W. A., & Tanaka, R. (2023). Comparison of Bovine- and Porcine-Derived Decellularized Biomaterials: Promising Platforms for Tissue Engineering Applications. Pharmaceutics, 15(7), 1906. https://doi.org/10.3390/pharmaceutics15071906