Environmentally Friendly Strategies for Formulating Vegetable Oil-Based Nanoparticles for Anticancer Medicine
Abstract
1. Introduction
2. Green Chemistry: Monomers and Polymers from Renewable Resources
3. Synthesis of Monomers from Vegetable Oils
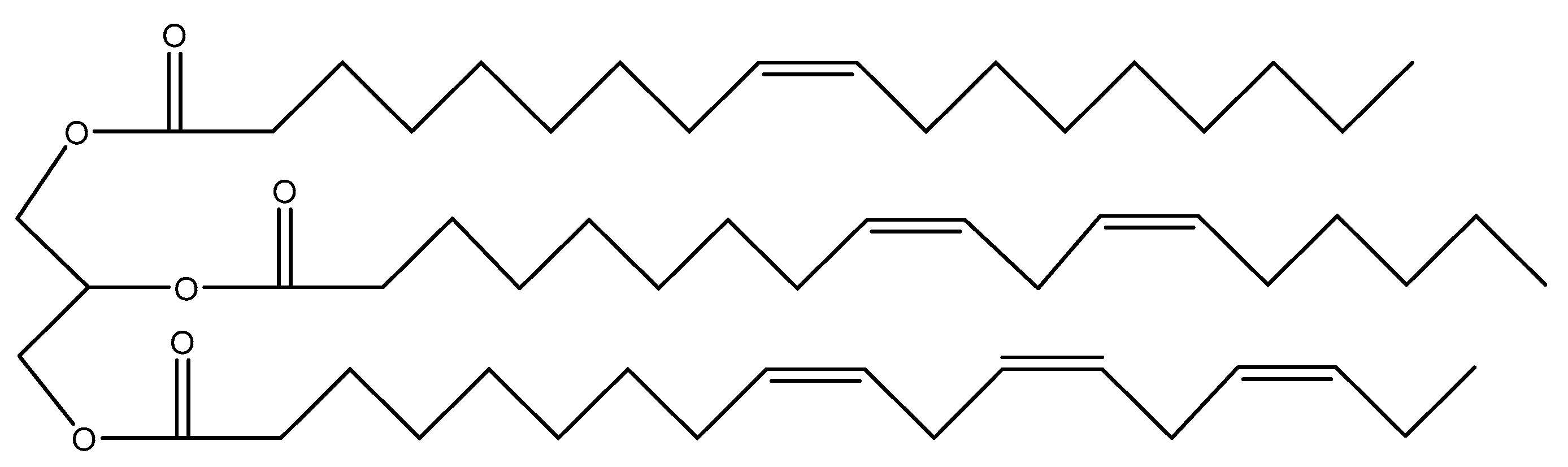
4. Castor Oil as a Renewable Raw Material
5. Polymeric Nanoparticles and Some Production Techniques
5.1. Solvent Evaporation Technique
5.2. Miniemulsion Polymerization
6. Thiol-Ene Polymerization for Nanoparticle Production
7. Application of Polymeric Nanoparticles in Cancer Therapy
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Montero De Espinosa, L.; Meier, M.A.R. Plant Oils: The Perfect Renewable Resource for Polymer Science? Eur. Polym. J. 2011, 47, 837–852. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable Polymers from Renewable Resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Llevot, A.; Meier, M.A.R. Renewability-a Principle of Utmost Importance! Green Chem. 2016, 18, 4800–4803. [Google Scholar] [CrossRef]
- Nurchi, C.; Buonvino, S.; Arciero, I.; Melino, S. Sustainable Vegetable Oil-Based Biomaterials: Synthesis and Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 2153. [Google Scholar] [CrossRef]
- Islam, M.R.; Beg, M.D.H.; Jamari, S.S. Development of Vegetable-Oil-Based Polymers. J. Appl. Polym. Sci. 2014, 131, 9016–9028. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-Ene “Click” Reactions and Recent Applications in Polymer and Materials Synthesis: A First Update. Polym. Chem. 2014, 5, 4820–4870. [Google Scholar] [CrossRef]
- Machado, F.; Lima, E.L.; Pinto, J.C. A Review on Suspension Polymerization Processes. Polimeros 2007, 17, 166–179. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Nguyen, K.T. Targeted Nanoparticles for Cancer Therapy:Promises and Challenges. J. Nanomed. Nanotechnol. 2011, 2, 1000103e. [Google Scholar] [CrossRef]
- Piccolo, M.; Menale, C.; Crispi, S. Combined Anticancer Therapies: An Overview of the Latest Applications. Anticancer. Agents Med. Chem. 2015, 15, 408–422. [Google Scholar] [CrossRef]
- Vaccaro, L. Green Chemistry. Beilstein J. Org. Chem. 2016, 12, 2763–2765. [Google Scholar] [CrossRef]
- Britannica Green Chemistry. Available online: https://www.britannica.com/science/green-chemistry (accessed on 19 May 2020).
- Tschan, M.J.L.; Brulé, E.; Haquette, P.; Thomas, C.M. Synthesis of Biodegradable Polymers from Renewable Resources. Polym. Chem. 2012, 3, 836–851. [Google Scholar] [CrossRef]
- Belgacem, M.; Gandini, A. Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9780080453163. [Google Scholar]
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant Oil Renewable Resources as Green Alternatives in Polymer Science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Biermann, U.; Bornscheuer, U.; Meier, M.A.R.; Metzger, J.O.; Schäfer, H.J. Oils and Fats as Renewable Raw Materials in Chemistry. Angew. Chemie Int. Ed. 2011, 50, 3854–3871. [Google Scholar] [CrossRef]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-Oil-Based Polymers as Future Polymeric Biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef]
- Türünç, O.; Meier, M.A.R. Fatty Acid Derived Monomers and Related Polymers via Thiol-Ene (Click) Additions. Macromol. Rapid Commun. 2010, 31, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, E.; Lligadas, G.; Ronda, J.C.; Galià, M.; Meier, M.A.R.; Cádiz, V. Polyurethanes from Polyols Obtained by ADMET Polymerization of a Castor Oil-Based Diene: Characterization and Shape Memory Properties. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 518–525. [Google Scholar] [CrossRef]
- Mutlu, H.; Meier, M.A.R. Castor Oil as a Renewable Resource for the Chemical Industry. Eur. J. Lipid Sci. Technol. 2010, 112, 10–30. [Google Scholar] [CrossRef]
- Firdaus, M.; Meier, M.A.R.; Biermann, U.; Metzger, J.O. Renewable Co-Polymers Derived from Castor Oil and Limonene. Eur. J. Lipid Sci. Technol. 2014, 116, 31–36. [Google Scholar] [CrossRef]
- Kreye, O.; Tóth, T.; Meier, M.A.R. Poly-α,β-Unsaturated Aldehydes Derived from Castor Oil via ADMET Polymerization. Eur. J. Lipid Sci. Technol. 2011, 113, 31–38. [Google Scholar] [CrossRef]
- Mensah, M.B.; Awudza, J.A.M.; O’Brien, P. Castor Oil: A Suitable Green Source of Capping Agent for Nanoparticle Syntheses and Facile Surface Functionalization. R. Soc. Open Sci. 2018, 5, 180824. [Google Scholar] [CrossRef]
- Rajalakshmi, P.; Marie, J.M.; Maria Xavier, A.J. Castor Oil-Derived Monomer Ricinoleic Acid Based Biodegradable Unsaturated Polyesters. Polym. Degrad. Stab. 2019, 170, 109016. [Google Scholar] [CrossRef]
- Laurentino, L.S.; Medeiros, A.M.M.S.; Machado, F.; Costa, C.; Araújo, P.H.H.; Sayer, C. Synthesis of a Biobased Monomer Derived from Castor Oil and Copolymerization in Aqueous Medium. Chem. Eng. Res. Des. 2018, 137, 213–220. [Google Scholar] [CrossRef]
- Cardoso, P.B.; Machado, T.O.; Feuser, P.E.; Sayer, C.; Meier, M.A.R.; Araújo, P.H.H. Biocompatible Polymeric Nanoparticles From Castor Oil Derivatives via Thiol-Ene Miniemulsion Polymerization. Eur. J. Lipid Sci. Technol. 2018, 120, 1700212. [Google Scholar] [CrossRef]
- Machado, T.O.; Cardoso, P.B.; Feuser, P.E.; Sayer, C.; Araújo, P.H.H. Thiol-Ene Miniemulsion Polymerization of a Biobased Monomer for Biomedical Applications. Colloids Surf. B Biointerfaces 2017, 159, 509–517. [Google Scholar] [CrossRef]
- El-Say, K.M.; El-Sawy, H.S. Polymeric Nanoparticles: Promising Platform for Drug Delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef]
- Chauhan, N.; Chawla, S.; Pundir, C.S.; Jain, U. An Electrochemical Sensor for Detection of Neurotransmitter-Acetylcholine Using Metal Nanoparticles, 2D Material and Conducting Polymer Modified Electrode. Biosens. Bioelectron. 2017, 89, 377–383. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Zhou, T.; Wang, L.; Gao, D.; Zhang, X.; Liu, Y.; Wu, C.; Yuan, Z. A PIID-DTBT Based Semi-Conducting Polymer Dots with Broad and Strong Optical Absorption in the Visible-Light Region: Highly Effective Contrast Agents for Multiscale and Multi-Spectral Photoacoustic Imaging. Nano Res. 2017, 10, 64–76. [Google Scholar] [CrossRef]
- Klepac, D.; Kostková, H.; Petrova, S.; Chytil, P.; Etrych, T.; Kereïche, S.; Raška, I.; Weitz, D.A.; Filippov, S.K. Interaction of Spin-Labeled HPMA-Based Nanoparticles with Human Blood Plasma Proteins-the Introduction of Protein-Corona-Free Polymer Nanomedicine. Nanoscale 2018, 10, 6194–6204. [Google Scholar] [CrossRef]
- Talianov, P.; Fatkhutdinova, L.I.; Timin, A.S.; Milichko, V.A.; Zyuzin, M.V. Adaptive Nanoparticle-Polymer Complexes as Optical Elements: Design and Application in Nanophotonics and Nanomedicine. Laser Photonics Rev. 2021, 15, 2000421. [Google Scholar] [CrossRef]
- Khan, M.M.; Madni, A.; Filipczak, N.; Pan, J.; Rehman, M.; Rai, N.; Attia, S.A.; Torchilin, V.P. Folate Targeted Lipid Chitosan Hybrid Nanoparticles for Enhanced Anti-Tumor Efficacy. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102228. [Google Scholar] [CrossRef] [PubMed]
- Nagavarma, B.V.N.; Yadav, H.K.S.; Ayaz, A.; Vasudha, L.S.; Shivakumar, H.G. Different Techniques for Preparation of Polymeric Nanoparticles—A Review. Asian J. Pharm. Clin. Res. 2012, 5, 16–23. [Google Scholar]
- dos Santos, P.C.M.; Feuser, P.E.; Cardoso, P.B.; Steiner, B.T.; da Córneo, E.S.; Scussel, R.; da Viegas, A.C.; Machado-de-Ávila, R.A.; Sayer, C.; de Araújo, P.H.H. Evaluation of in Vitro Cytotoxicity of Superparamagnetic Poly(Thioether-Ester) Nanoparticles on Erythrocytes, Non-Tumor (NIH3T3), Tumor (HeLa) Cells and Hyperthermia Studies. J. Biomater. Sci. Polym. Ed. 2019, 29, 1935–1948. [Google Scholar] [CrossRef]
- Zhang, Z.; Grijpma, D.W.; Feijen, J. Poly(Trimethylene Carbonate) and Monomethoxy Poly(Ethylene Glycol)-Block-Poly(Trimethylene Carbonate) Nanoparticles for the Controlled Release of Dexamethasone. J. Control. Release 2006, 111, 263–270. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Barakat, N.A.M.; Kanjwal, M.A.; Aryal, S.; Khil, M.S.; Kim, H.Y. Novel Self-Assembled Amphiphilic Poly(ε-Caprolactone)-Grafted- Poly(Vinyl Alcohol) Nanoparticles: Hydrophobic and Hydrophilic Drugs Carrier Nanoparticles. J. Mater. Sci. Mater. Med. 2009, 20, 821–831. [Google Scholar] [CrossRef]
- Mishima, K. Biodegradable Particle Formation for Drug and Gene Delivery Using Supercritical Fluid and Dense Gas. Adv. Drug Deliv. Rev. 2008, 60, 411–432. [Google Scholar] [CrossRef]
- Gurny, R.; Peppas, N.A.; Harrington, D.D.; Banker, G.S. Development of Biodegradable and Injectable Latices for Controlled Release of Potent Drugs. Drug Dev. Ind. Pharm. 1981, 7, 1–25. [Google Scholar] [CrossRef]
- Ahlin Grabnar, P.; Kristl, J. The Manufacturing Techniques of Drug-Loaded Polymeric Nanoparticles from Preformed Polymers. J. Microencapsul. 2011, 28, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Masood, F. Polymeric Nanoparticles for Targeted Drug Delivery System for Cancer Therapy. Mater. Sci. Eng. C 2016, 60, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Quintanar-Guerrero, D.; Allémann, E.; Fessi, H.; Doelker, E. Preparation Techniques and Mechanisms of Formation of Biodegradable Nanoparticles from Preformed Polymers. Drug Dev. Ind. Pharm. 1998, 24, 1113–1128. [Google Scholar] [CrossRef]
- Bagherzadeh-Khajehmarjan, E.; Nikniazi, A.; Olyaeefar, B.; Ahmadi-kandjani, S.; Nunzi, J.-M. Morphology Enhancement of Self-Assembled CH3NH3PbI3 Nanoparticles through Customized Solvent Evaporation Temperatures. J. Cryst. Growth 2023, 601, 126970. [Google Scholar] [CrossRef]
- Ma, W.; Lopez, G.; Ameduri, B.; Takahara, A. Fluoropolymer Nanoparticles Prepared Using Trifluoropropene Telomer Based Fluorosurfactants. Langmuir 2020, 36, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Niyom, Y.; Crespy, D.; Flood, A.E. Compatibility between Drugs and Polymer in Nanoparticles Produced by the Miniemulsion-Solvent Evaporation Technique. Macromol. Mater. Eng. 2021, 306, 2100102. [Google Scholar] [CrossRef]
- Antonietti, M.; Landfester, K. Polyreactions in Miniemulsions. Prog. Polym. Sci. 2002, 27, 689–757. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus Microemulsions: Terminology, Differences, and Similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Ugelstad, J.; El-Aasser, M.S.; Vanderhoff, J. Emulsion Polymeriza-Tion: Initiation of Polymerization in Monomer Droplets. J. Polym. Sci. Polym. Lett. Ed. 1973, 11, 503–2013. [Google Scholar] [CrossRef]
- Asua, J.M. Miniemulsion Polymerisation. Prog. Polym. Sci 2002, 27, 1283–1346. [Google Scholar] [CrossRef]
- Fonseca, L.B.; Nele, M.; Volpato, N.M.; Seiceira, R.C.; Pinto, J.C. Production of PMMA Nanoparticles Loaded with Praziquantel Through “In Situ” Miniemulsion Polymerization. Macromol. React. Eng. 2013, 7, 54–63. [Google Scholar] [CrossRef]
- Landfester, K. Synthesis of Colloidal Particles in Miniemulsions. Annu. Rev. Mater. Res. 2006, 36, 231–279. [Google Scholar] [CrossRef]
- Schork, F.J.; Poehlein, G.W.; Wang, S.; Reimers, J.; Rodrigues, J.; Samer, C. Miniemulsion Polymerization. Colloids Surf. A Physicochem. Eng. Asp. 1999, 153, 39–45. [Google Scholar] [CrossRef]
- Machado, T.O.; Sayer, C.; Araujo, P.H.H. Thiol-Ene Polymerisation: A Promising Technique to Obtain Novel Biomaterials. Eur. Polym. J. 2017, 86, 200–215. [Google Scholar] [CrossRef]
- Türünç, O.; Meier, M.A.R. A Novel Polymerization Approach via Thiol-Yne Addition. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1689–1695. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-Ene “Click” Reactions and Recent Applications in Polymer and Materials Synthesis. Polym. Chem. 2010, 1, 17–36. [Google Scholar] [CrossRef]
- Lluch, C.; Ronda, J.C.; Galiá, M.; Lligadas, G.; Cádiz, V. Rapid Approach to Biobased Telechelics through Two One-Pot Thiol-Ene Click Reactions. Biomacromolecules 2010, 11, 1646–1653. [Google Scholar] [CrossRef]
- Hu, Y.; Deng, M.; Yang, H.; Chen, L.; Xiao, C.; Zhuang, X.; Chen, X. Multi-Responsive Core-Crosslinked Poly (Thiolether Ester) Micelles for Smart Drug Delivery. Polymer 2017, 110, 235–241. [Google Scholar] [CrossRef]
- Chen, C.K.; Law, W.C.; Aalinkeel, R.; Yu, Y.; Nair, B.; Wu, J.; Mahajan, S.; Reynolds, J.L.; Li, Y.; Lai, C.K.; et al. Biodegradable Cationic Polymeric Nanocapsules for Overcoming Multidrug Resistance and Enabling Drug-Gene Co-Delivery to Cancer Cells. Nanoscale 2014, 6, 1567–1572. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol-Ene Click Chemistry. Angew. Chem.—Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Vandenbergh, J.; Peeters, M.; Kretschmer, T.; Wagner, P.; Junkers, T. Cross-Linked Degradable Poly(β-Thioester) Networks via Amine-Catalyzed Thiol-Ene Click Polymerization. Polymer 2014, 55, 3525–3532. [Google Scholar] [CrossRef]
- Vandenbergh, J.; Ranieri, K.; Junkers, T. Synthesis of (Bio)-Degradable Poly( β-Thioester)s via Amine Catalyzed Thiol-Ene Click Polymerization. Macromol. Chem. Phys. 2012, 213, 2611–2617. [Google Scholar] [CrossRef]
- Vivek, R.; Thangam, R.; Nipunbabu, V.; Rejeeth, C.; Sivasubramanian, S.; Gunasekaran, P.; Muthuchelian, K.; Kannan, S. Multifunctional HER2-Antibody Conjugated Polymeric Nanocarrier-Based Drug Delivery System for Multi-Drug-Resistant Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2014, 6, 6469–6480. [Google Scholar] [CrossRef] [PubMed]
- Masood, F.; Chen, P.; Yasin, T.; Fatima, N.; Hasan, F.; Hameed, A. Encapsulation of Ellipticine in Poly-(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Based Nanoparticles and Its in Vitro Application. Mater. Sci. Eng. 2013, 33, 1054–1106. [Google Scholar] [CrossRef] [PubMed]
- Masood, F.; Chen, P.; Yasin, T.; Hasan, F.; Ahmad, B.; Hameed, A. Synthesis of Poly-(3-Hydroxybutyrate-Co-12 Mol % 3-Hydroxyvalerate) by Bacillus Cereus FB11: Its Characterization and Application as a Drug Carrier. J. Mater. Sci. Mater. Med. 2013, 24, 1927–1937. [Google Scholar] [CrossRef]
- Shah, M.; Ullah, N.; Choi, M.H.; Kim, M.O.; Yoon, S.C. Amorphous Amphiphilic P(3HV-Co-4HB)-b-MPEG Block Copolymer Synthesized from Bacterial Copolyester via Melt Transesterification: Nanoparticle Preparation, Cisplatin-Loading for Cancer Therapy and in Vitro Evaluation. Eur. J. Pharm. Biopharm. 2012, 80, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Imran, M.; Hwan, M.; Ok, M.; Chul, S. Amphiphilic PHA—MPEG Copolymeric Nanocontainers for Drug Delivery: Preparation, Characterization and in Vitro Evaluation. Int. J. Pharm. 2010, 400, 165–175. [Google Scholar] [CrossRef]
- Vilos, C.; Morales, F.A.; Solar, P.A.; Herrera, N.S.; Gonzalez-Nilo, F.D.; Aguayo, D.A.; Mendoza, H.L.; Comer, J.; Bravo, M.L.; Gonzalez, P.A.; et al. Paclitaxel-PHBV Nanoparticles and Their Toxicity to Endometrial and Primary Ovarian Cancer Cells. Biomaterials 2013, 34, 4098–4108. [Google Scholar] [CrossRef]
- Lu, X.Y.; Zhang, Y.; Wang, L. Preparation and in Vitro Drug-Release Behavior of 5-Fluorouracil-Loaded Poly(Hydroxybutyrateco-Hydroxyhexanoate) Nanoparticles and Microparticles. J. Appl. Polym. Sci. 2010, 116, 2944–2950. [Google Scholar]
- Kılıçay, E.; Demirbilek, M.; Türk, M.; Güven, E.; Hazer, B.; Denkbas, E.B. Preparation and Characterization of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) (PHBHHX) Based Nanoparticles for Targeted Cancer Therapy. Eur. J. Pharm. Sci. 2011, 44, 310–320. [Google Scholar] [CrossRef]
- Chan, Z.; Zhao, L.; Dong, Y.; Zhang, X.; Lin, J.; Chen, Z. Folate-Mediated Poly(3-Hydroxybutyrate-Co-3-Hydroxyoctanoate) Nanoparticles for Targeting Drug Delivery. Eur. J. Pharm. Biopharm. 2010, 76, 10–16. [Google Scholar]
- Yao, Y.-C.; Zhan, X.-Y.; Zhang, J.; Zou, X.-H.; Wang, Z.-H.; Xiong, Y.-C.; Chen, J.; Chen, G.-Q. A Specific Drug Targeting System Based on Polyhydroxyalkanoate Granule Binding Protein PhaP Fused with Targeted Cell Ligands. Biomaterials 2008, 29, 4823–4830. [Google Scholar] [CrossRef] [PubMed]
- Varan, C.; Bilensoy, E. Development of Implantable Hydroxypropyl-β-Cyclodextrin Coated Polycaprolactone Nanoparticles for the Controlled Delivery of Docetaxel to Solid Tumors. J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 9–15. [Google Scholar] [CrossRef]
- Cirpanli, Y.; Bilensoy, E.; Doğan, A.L.; Caliş, S. Comparative Evaluation of Polymeric and Amphiphilic Cyclodextrin Nanoparticles for Effective Camptothecin Delivery. Eur. J. Pharm. Biopharm. 2009, 73, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Perret, F.; Duffour, M.; Chevalier, Y.; Parrot-Lopez, H. Design, Synthesis, and In Vitro Evaluation of New Amphiphilic Cyclodextrin-Based Nanoparticles for the Incorporation and Controlled Release of Acyclovir. Eur. J. Pharm. Biopharm. 2013, 83, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Li, S.; Han, S.; Wang, Z.; Wu, Y.; Nie, G. Construction of Hydroxypropyl-β-Cyclodextrin Copolymer Nanoparticles and Targeting Delivery of Paclitaxel. J. Nanoparticle Res. 2012, 14, 1043. [Google Scholar] [CrossRef]
- Freire, N.F.; Feuser, P.E.; da Silva Abel, J.; Machado-de-Ávila, R.A.; Lopes Fialho, R.; Cabral Albuquerque, E.; Sayer, C.; Hermes de Araújo, P.H. Zinc Phthalocyanine Encapsulation via Thiol-Ene Miniemulsion Polymerization and in Vitro Photoxicity Studies. Int. J. Polym. Mater. Polym. Biomater. 2020, 71, 349–358. [Google Scholar] [CrossRef]
- Freire, N.; Emílio, P.; Maria, E.; Ambel, T.; Cordani, M.; Zielinski, A.F.; Sayer, C.; De Pieri, E.; Avila, R.A.M.; Henrique, P.; et al. Colloids and Surfaces A: Physicochemical and Engineering Aspects Preparation and Characterization of Full-Spectrum Cannabis Extract Loaded Poly ( Thioether-Ester ) Nanoparticles: In Vitro Evaluation of Their Antitumoral Efficacy. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130676. [Google Scholar] [CrossRef]
- Feuser, P.E.; dos Santos, P.C.M.; Cordeiro, A.P.; Stefanes, N.M.; Walter, L.O.; Maioral, M.F.; Santos-Silva, M.C.; de Araújo, P.H.H.; Sayer, C. Antineoplastic Activity of Free 4-Nitrochalcone and Encapsulated in Poly(Thioether-Ester) Nanoparticles Obtained by Thiol-Ene Polymerization in Two Human Leukemia Cell Lines (Jurkat and K562). J. Drug Deliv. Sci. Technol. 2022, 67, 102924. [Google Scholar] [CrossRef]
- Danhier, F.; Lecouturier, N.; Vroman, B.; Jerome, C.; Marchand-Brynaert, J.; Feron, O.; Préat, V. Paclitaxel-Loaded PEGylated PLGA-Based Nanoparticles: In Vitro and in Vivo Evaluation. J. Control. Release 2009, 133, 11–17. [Google Scholar] [CrossRef]
- Khuroo, T.; Verma, D.; Talegaonkar, S.; Padhi, S.; Panda, A.K.; Iqbal, Z. Topotecan–Tamoxifen Duple PLGA Polymeric Nanoparticles: Investigation of in Vitro, in Vivo and Cellular Uptake Potential. Int. J. Pharm. 2014, 473, 384–394. [Google Scholar] [CrossRef]
- Cháirez-Ramírez, M.; Sánchez-Burgos, J.; Gomes, C.; Moreno-Jiménez, M.; González-Laredo, R.; Bernad-Bernad, M.; Medina-Torres, L.; Ramírez-Mares, M.; Gallegos-Infante, J.; Rocha-Guzmán, N. Morphological and Release Characterization of Nanoparticles Formulated with Poly (Dl-Lactide-Co-Glycolide) (PLGA) and Lupeol: In Vitro Permeability and Modulator Effect on NF-ΚB in Caco-2 Cell System Stimulated with TNF-α. Food Chem. Toxicol. 2015, 85, 2–9. [Google Scholar] [CrossRef]
- Jaidev, L.R.; Krishnan, U.M.; Sethuraman, S. Gemcitabine Loaded Biodegradable PLGA Nanospheres for in Vitro Pancreatic Cancer Therapy. Mater. Sci. Eng. C. Mater. Biol. Appl. 2015, 47, 40–47. [Google Scholar] [CrossRef]
- Derakhshandeh, K.; Erfan, M.; Dadashzadeh, S. Encapsulation of 9-Nitrocamptothecin, a Novel Anticancer Drug, in Biodegradable Nanoparticles: Factorial Design, Characterization and Release Kinetics. Eur. J. Pharm. Biopharm. 2007, 66, 34–41. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Wu, Y.; Hu, Y.-L.; Nan, K.; Nie, G.; Chen, H. Enhanced Anti-Tumor Efficacy by Co-Delivery of Doxorubicin and Paclitaxel with Amphiphilic Methoxy PEG-PLGA Copolymer Nanoparticles. Biomaterials 2011, 32, 8281–8290. [Google Scholar] [CrossRef]
- Le Broc-Ryckewaert, D.; Carpentier, R.; Lipka, E.; Daher, S.; Vaccher, C.; Betbeder, D.; Furman, C. Development of Innovative Paclitaxel-Loaded Small PLGA Nanoparticles: Study of Their Antiproliferative Activity and Their Molecular Interactions on Prostatic Cancer Cells. Int. J. Pharm. 2013, 454, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Mattheolabakis, G.; Taoufik, E.; Haralambous, S.; Roberts, M.L.; Avgoustakis, K. In Vivo Investigation of Tolerance and Antitumor Activity of Cisplatin-Loaded PLGA-MPEG Nanoparticles. Eur. J. Pharm. Biopharm. 2009, 71, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Schleich, N.; Sibret, P.; Danhier, P.; Ucakar, B.; Laurent, S.; Muller; Jérôme, C.; Gallez, B.; Préat, V.; Danhier, F. Dual Anticancer Drug/Superparamagnetic Iron Oxide-Loaded PLGAbased Nanoparticles for Cancer Therapy and Magnetic Resonance Imaging. Int. J. Pharm. 2013, 447, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Thanki, K.; Jain, S. Co-Encapsulation of Tamoxifen and Quercetin in Polymeric Nanoparticles: Implications on Oral Bioavailability, Antitumor Efficacy, and Drug-Induced Toxicity. Mol. Pharm. 2013, 10, 3459–3474. [Google Scholar] [CrossRef]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.W.; Langer, R.; Farokhzad, O.C. PLGA-Lecithin-PEG Core-Shell Nanoparticles for Controlled Drug Delivery. Biomaterials 2009, 30, 1627–1634. [Google Scholar] [CrossRef]
- Martín-Banderas, L.; Muñoz-Rubio, I.; Prados, J.; Álvarez-Fuentes, J.; Calderón-Montaño, J.M.; López-Lázaro, M.; Arias, J.L.; Leiva, M.C.; Holgado, M.A.; Fernández-Arévalo, M. In Vitro and In Vivo Evaluation of Delta9-Tetrahidrocannabinol/PLGA Nanoparticles for Cancer Chemotherapy. Int. J. Pharm. 2015, 487, 205–212. [Google Scholar] [CrossRef]
- Chittasupho, C.; Xie, S.-X.; Baoum, A.; Yakovleva, T.; Siahaan, T.J.; Berkland, C.J. ICAM-1 Targeting of Doxorubicin-Loaded PLGA Nanoparticles to Lung Epithelial Cells. Eur. J. Pharm. Sci. 2009, 37, 141–150. [Google Scholar] [CrossRef]
- Liang, C.; Yang, Y.; Ling, Y.; Huang, Y.; Li, T.; Li, X. Improved Therapeutic Effect of Folate-Decorated PLGA-PEG Nanoparticles for Endometrial Carcinoma. Bioorganic Med. Chem. 2011, 19, 4057–4066. [Google Scholar] [CrossRef]
- Dhas, N.L.; Ige, P.P.; Kudarha, R.R. Design, Optimization and in-Vitro Study of Folic Acid Conjugated-Chitosan Functionalized PLGA Nanoparticle for Delivery of Bicalutamide in Prostate Cancer. Powder Technol. 2015, 283, 234–245. [Google Scholar] [CrossRef]
- Su, W.-C.; Su, W.-P.; Cheng, F.-Y.; Shieh, D.-B.; Yeh, C.-S. PLGA Nanoparticles Codeliver Paclitaxel and Stat3 SiRNA to Overcome Cellular Resistance in Lung Cancer Cells. Int. J. Nanomed. 2012, 7, 4269–4283. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, Q.; Chow, P.K.-H.; Wang, D.; Wang, C.-H. Transferrin-Conjugated Magnetic Silica PLGA Nanoparticles Loaded with Doxorubicin and Paclitaxel for Brain Glioma Treatment. Biomaterials 2013, 34, 8511–8520. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, A.; Garg, N.K.; Tyagi, R.K.; Singh, B.; Katare, O.P.; Webster, T.J.; Soni, V. Surface Engineered Polymeric Nanocarriers Mediate the Delivery of Transferrin-Methotrexate Conjugates for an Improved Understanding of Brain Cancer. Acta Biomater. 2015, 24, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Gu, F.X.; Langer, R.; Farokhzad, O.C.; Lippard, S.J. Targeted Delivery of Cisplatin to Prostate Cancer Cells by Aptamer Functionalized Pt(IV) Prodrug-PLGA-PEG Nanoparticles. Proc. Natl. Acad. Sci. USA 2008, 105, 7356–17361. [Google Scholar] [CrossRef] [PubMed]
- Chittasupho, C.; Lirdprapamongkol, K.; Kewsuwan, P.; Sarisuta, N. Targeted Delivery of Doxorubicin to A549 Lung Cancer Cells by CXCR4 Antagonist Conjugated PLGA Nanoparticles. Eur. J. Pharm. Biopharm. 2014, 88, 529–538. [Google Scholar] [CrossRef]
- Danhier, F.; Pourcelle, V.; Marchand-Brynaert, J.; Jérôme, C.; Feron, O.; Préat, V. Targeting of Tumor Endothelium by RGD-Grafted PLGA-Nanoparticles. Methods Enzym. 2012, 508, 157–175. [Google Scholar]
- Li, L.; Xiang, D.; Shigdar, S.; Yang, W.; Li, Q.; Lin, J.; Liu, K.; Duan, W. Epithelial Cell Adhesion Molecule Aptamer Functionalized PLGA-Lecithincurcumin-PEG Nanoparticles for Targeted Drug Delivery to Human Colorectal Adenocarcinoma Cells. Int. J. Nanomed. 2014, 9, 1083–1096. [Google Scholar]
- Chen, H.; Gao, J.; Lu, Y.; Kou, G.; Zhang, H.; Fan, L.; Sun, Z.; Guo, Y.; Zhong, Y. Preparation and Characterization of PE38KDEL-Loaded Anti-HER2 Nanoparticles for Targeted Cancer Therapy. J. Control. Release 2008, 128, 209–216. [Google Scholar] [CrossRef]
- Aravind, A.; Nair, R.; Raveendran, S.; Veeranarayanan, S.; Nagaoka, Y.; Fukuda, T.; Hasumura, T.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; et al. Aptamer Conjugated Paclitaxel and Magnetic Fluid Loaded Fluorescently Tagged PLGA Nanoparticles for Targeted Cancer Therapy. J. Magn. Magn. Mater. 2013, 344, 116–123. [Google Scholar] [CrossRef]
- Aggarwal, S.; Yadav, S.; Gupta, S. EGFR Targeted PLGA Nanoparticles Using Gemcitabine for Treatment of Pancreatic Cancer. J. Biomed. Nanotechnol. 2011, 7, 137–138. [Google Scholar] [CrossRef]
- Narayanan, S.; Mony, U.; Vijaykumar, D.K.; Koyakutty, M.; Paul-Prasanth, B.; Menon, D. Sequential Release of Epigallocatechin Gallate and Paclitaxel from PLGA-Casein Core/Shell Nanoparticles Sensitizes Drug-Resistant Breast Cancer Cells. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Peng, X.; Zou, F. Folate-Decorated PEG-PLGA Nanoparticles with Silica Shells for Capecitabine Controlled and Targeted Delivery. Int. J. Pharm. 2014, 464, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Vangara, K.K.; Liu, J.L.; Palakurthi, S. Hyaluronic Acid-Decorated PLGAPEG Nanoparticles for Targeted Delivery of SN-38 to Ovarian Cancer. Anticancer Res. 2013, 33, 2425–2434. [Google Scholar] [PubMed]
- Kocbek, P.; Obermajer, N.; Cegnar, M.; Kos, J.; Kristl, J. Targeting Cancer Cells Using PLGA Nanoparticles Surface Modified with Monoclonal Antibody. J. Control. Release 2007, 120, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, P.; Chawla, R.; Dutta, P.K. PH-Responsive Charge-Convertible N-Succinyl Chitosan-Quercetin Coordination Polymer Nanoparticles for Effective NIR Photothermal Cancer Therapy. Macromol. Chem. Phys. 2022, 223, 2200140. [Google Scholar] [CrossRef]
- Gogoi, P.; Dutta, A.; Ramteke, A.; Maji, T.K. Preparation, Characterization and Cytotoxic Applications of Curcumin-(±) α-Lipoic Acid Coloaded Phosphorylated Chitosan Nanoparticles in MDA MB 231 Breast Cancer Cell Line. Polym. Adv. Technol. 2020, 31, 2827–2841. [Google Scholar] [CrossRef]
- Snima, K.S.; Jayakumar, R.; Lakshmanan, V.K. In Vitro and in Vivo Biological Evaluation of O-Carboxymethyl Chitosan Encapsulated Metformin Nanoparticles for Pancreatic Cancer Therapy. Pharm. Res. 2014, 31, 3361–3370. [Google Scholar] [CrossRef]
- Ding, Y.F.; Li, S.; Liang, L.; Huang, Q.; Yuwen, L.; Yang, W.; Wang, R.; Wang, L.H. Highly Biocompatible Chlorin E6-Loaded Chitosan Nanoparticles for Improved Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 9980–9987. [Google Scholar] [CrossRef]
- Kou, C.H.; Han, J.; Han, X.L.; Zhuang, H.J.; Zhao, Z.M. Preparation and Characterization of the Adriamycin-Loaded Amphiphilic Chitosan Nanoparticles and Their Application in the Treatment of Liver Cancer. Oncol. Lett. 2017, 14, 7833–7841. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, Y.; Zeng, X.; Jiang, L.; Chen, H.; Liu, R.; Huang, L.; Mei, L. Novel Docetaxel-Loaded Nanoparticles Based on PCL-Tween 80 Copolymer for Cancer Treatment. Int. J. Nanomed. 2011, 6, 2679–2688. [Google Scholar]
- Chen, L.X.; Ni, X.L.; Zhang, H.; Wu, M.; Liu, J.; Xu, S.; Yang, L.L.; Fu, S.Z.; Wu, J. Preparation, Characterization, in Vitro and in Vivo Anti-Tumor Effect of Thalidomide Nanoparticles on Lung Cancer. Int. J. Nanomed. 2018, 13, 2463–2476. [Google Scholar] [CrossRef] [PubMed]
- Raspantini, G.L.; Luiz, M.T.; Abriata, J.P.; de Eloy, J.O.; Vaidergorn, M.M.; da Emery, F.S.; Marchetti, J.M. PCL-TPGS Polymeric Nanoparticles for Docetaxel Delivery to Prostate Cancer: Development, Physicochemical and Biological Characterization. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127144. [Google Scholar] [CrossRef]
- Lu, Y.; Wen, Q.; Luo, J.; Xiong, K.; Wu, Z.X.; Wang, B.Q.; Chen, Y.; Yang, B.; Fu, S.Z. Self-Assembled Dihydroartemisinin Nanoparticles as a Platform for Cervical Cancer Chemotherapy. Drug Deliv. 2020, 27, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Huang, L.; Yang, J.; Wang, Y.; Yang, M.; Tang, M.; Qiu, T. Preparation and Evaluation of MPEG-PCL Polymeric Nanoparticles Against Gastric Cancer. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2020, 35, 1162–1168. [Google Scholar] [CrossRef]
- Xiong, K.; Zhang, Y.; Wen, Q.; Luo, J.; Lu, Y.; Wu, Z.X.; Wang, B.Q.; Chen, Y.; Zhao, L.; Fu, S.Z. Co-Delivery of Paclitaxel and Curcumin by Biodegradable Polymeric Nanoparticles for Breast Cancer Chemotherapy. Int. J. Pharm. 2020, 589, 119875. [Google Scholar] [CrossRef]
- Rao, S.V.; Kumar, S.S. MPEG-PCL Nanoparticles as New Carriers for Delivery of a Prostae Cancer Drug Fluamide. Res. J. Pharm. Technol. 2021, 14, 3657–3661. [Google Scholar] [CrossRef]
- Badran, M.M.; Mady, M.M.; Ghannam, M.M.; Shakeel, F. Preparation and Characterization of Polymeric Nanoparticles Surface Modified with Chitosan for Target Treatment of Colorectal Cancer. Int. J. Biol. Macromol. 2017, 95, 643–649. [Google Scholar] [CrossRef]
- Patel, P.; Raval, M.; Manvar, A.; Airao, V.; Bhatt, V.; Shah, P. Lung Cancer Targeting Efficiency of Silibinin Loaded Poly Caprolactone /Pluronic F68 Inhalable Nanoparticles: In Vitro and In Vivo Study. PLoS ONE 2022, 17, e0267257. [Google Scholar] [CrossRef]
- Li, M.; Tang, Z.; Lin, J.; Zhang, Y.; Lv, S.; Song, W.; Huang, Y.; Chen, X. Synergistic Antitumor Effects of Doxorubicin-Loaded Carboxymethyl Cellulose Nanoparticle in Combination with Endostar for Effective Treatment of Non-Small-Cell Lung Cancer. Adv. Healthc. Mater. 2014, 3, 1877–1888. [Google Scholar] [CrossRef]
- Yusefi, M.; Lee-Kiun, M.S.; Shameli, K.; Teow, S.Y.; Ali, R.R.; Siew, K.K.; Chan, H.Y.; Wong, M.M.T.; Lim, W.L.; Kuča, K. 5-Fluorouracil Loaded Magnetic Cellulose Bionanocomposites for Potential Colorectal Cancer Treatment. Carbohydr. Polym. 2021, 273, 118523. [Google Scholar] [CrossRef] [PubMed]
- Asabuwa Ngwabebhoh, F.; Ilkar Erdagi, S.; Yildiz, U. Pickering Emulsions Stabilized Nanocellulosic-Based Nanoparticles for Coumarin and Curcumin Nanoencapsulations: In Vitro Release, Anticancer and Antimicrobial Activities. Carbohydr. Polym. 2018, 201, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Ren, Y.; Long, L.; Zhong, Y.; Shen, C.; Pu, P.; Yuan, X.; Kang, C. Inhibition of C6 Glioma in Vivo by Combination Chemotherapy of Implantation of Polymer Wafer and Intracarotid Perfusion of Transferrin-Decorated Nanoparticles. Oncol. Rep. 2012, 27, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Feuser, P.E.; Bubniak, L.D.S.; Bodack, C.D.N.; Valério, A.; Silva, M.C.D.S.; Ricci, E.; Sayer, C.; De Araújo, P.H.H. In Vitro Cytotoxicity of Poly(Methyl Methacrylate) Nanoparticles and Nanocapsules Obtained by Miniemulsion Polymerization for Drug Delivery Application. J. Nanosci. Nanotechnol. 2016, 16, 7669–7676. [Google Scholar] [CrossRef]
- Guo, W.; Wang, T.; Huang, C.; Ning, S.; Guo, Q.; Zhang, W.; Yang, H.; Zhu, D.; Huang, Q.; Qian, H.; et al. Platelet Membrane-Coated C-TiO2 Hollow Nanospheres for Combined Sonodynamic and Alkyl-Radical Cancer Therapy. Nano Res. 2023, 16, 782–791. [Google Scholar] [CrossRef]
- Liu, D.; Dai, X.; Zhang, W.; Zhu, X.; Zha, Z.; Qian, H.; Cheng, L.; Wang, X. Liquid Exfoliation of Ultrasmall Zirconium Carbide Nanodots as a Noninflammatory Photothermal Agent in the Treatment of Glioma. Biomaterials 2023, 292, 121917. [Google Scholar] [CrossRef]
- Ning, S.; Dai, X.; Tang, W.; Guo, Q.; Lyu, M.; Zhu, D.; Zhang, W.; Qian, H.; Yao, X.; Wang, X. Cancer Cell Membrane-Coated C-TiO2 Hollow Nanoshells for Combined Sonodynamic and Hypoxia-Activated Chemotherapy. Acta Biomater. 2022, 152, 562–574. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Yue, Q.; Xu, H.; Zhong, X.; Sun, L.; Li, G.; Gong, Y.; Yang, N.; Wang, Z.; et al. Liquid Exfoliation of TiN Nanodots as Novel Sonosensitizers for Photothermal-Enhanced Sonodynamic Therapy against Cancer. Nano Today 2021, 39, 101170. [Google Scholar] [CrossRef]
- Guo, Q.; Yin, M.; Fan, J.; Yang, Y.; Liu, T.; Qian, H.; Dai, X.; Wang, X. Peroxidase-Mimicking TA-VOx Nanobranches for Enhanced Photothermal/Chemodynamic Therapy of Glioma by Inhibiting the Expression of HSP60. Mater. Des. 2022, 224, 111366. [Google Scholar] [CrossRef]
- Montané, X.; Bajek, A.; Roszkowski, K.; Montornés, J.M.; Giamberini, M.; Roszkowski, S.; Kowalczyk, O.; Garcia-Valls, R.; Tylkowski, B. Encapsulation for Cancer Therapy. Molecules 2020, 25, 1605. [Google Scholar] [CrossRef]
- Kifle, Z.D.; Tadele, M.; Alemu, E.; Gedamu, T.; Ayele, A.G. A Recent Development of New Therapeutic Agents and Novel Drug Targets for Cancer Treatment. SAGE Open Med. 2021, 9, 205031212110670. [Google Scholar] [CrossRef]
- Wadhwa, K.; Kadian, V.; Puri, V.; Bhardwaj, B.Y.; Sharma, A.; Pahwa, R.; Rao, R.; Gupta, M.; Singh, I. New Insights into Quercetin Nanoformulations for Topical Delivery. Phytomed. Plus 2022, 2, 100257. [Google Scholar] [CrossRef]
- Lawson, M.K. Improvement of Therapeutic Value of Quercetin with Chitosan Nanoparticle Delivery Systems and Potential Applications. Int. J. Mol. Sci. 2023, 24, 3293. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gautam, A.; Kundu, P.P. Synthesis of PH-Sensitive Grafted Psyllium: Encapsulation of Quercetin for Colon Cancer Treatment. J. Appl. Polym. Sci. 2022, 139, 51552. [Google Scholar] [CrossRef]
- Chen, L.C.; Chen, Y.C.; Su, C.Y.; Hong, C.S.; Ho, H.O.; Sheu, M.T. Development and Characterization of Self-Assembling Lecithin-Based Mixed Polymeric Micelles Containing Quercetin in Cancer Treatment and an in Vivo Pharmacokinetic Study. Int. J. Nanomed. 2016, 11, 1557–1566. [Google Scholar] [CrossRef]
- de Redín, I.L.; Expósito, F.; Agüeros, M.; Collantes, M.; Peñuelas, I.; Allemandi, D.; Llabot, J.M.; Calvo, A.; Irache, J.M. In Vivo Efficacy of Bevacizumab-Loaded Albumin Nanoparticles in the Treatment of Colorectal Cancer. Drug Deliv. Transl. Res. 2020, 10, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Gallarate, M.; Peira, E.; Chirio, D.; Solazzi, I.; Giordano, S.M.A.; Gigliotti, C.L.; Riganti, C.; Dianzani, C. Bevacizumab Loaded Solid Lipid Nanoparticles Prepared by the Coacervation Technique: Preliminary in Vitro Studies. Nanotechnology 2015, 26, 255102. [Google Scholar] [CrossRef]
- Sousa, F.; Dhaliwal, H.K.; Gattacceca, F.; Sarmento, B.; Amiji, M.M. Enhanced Anti-Angiogenic Effects of Bevacizumab in Glioblastoma Treatment upon Intranasal Administration in Polymeric Nanoparticles. J. Control. Release 2019, 309, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, L.D.; Duarte, J.L.; Azambuja, J.H.; Mancuso, R.I.; Luiz, M.T.; Araújo, V.H.S.; Figueiredo, I.D.; Barretto-de-Souza, L.; Sábio, R.M.; Sasso-Cerri, E.; et al. Glioblastoma Multiforme Targeted Delivery of Docetaxel Using Bevacizumab-Modified Nanostructured Lipid Carriers Impair in Vitro Cell Growth and in Vivo Tumor Progression. Int. J. Pharm. 2022, 618, 121682. [Google Scholar] [CrossRef]
- Siti, Z.S.; Ahmad, N.H.; Hamid, S. Characterization of PLGA-PEG Catharanthus Roseus Nanoparticles and Assessing Its Anticancer Effects in Her2-Overexpressed Breast Cancer Cells. Pharmacogn. Mag. 2022, 18, 273. [Google Scholar]
- Ke, Y.; Al Aboody, M.S.; Alturaiki, W.; Alsagaby, S.A.; Alfaiz, F.A.; Veeraraghavan, V.P.; Mickymaray, S. Photosynthesized Gold Nanoparticles from Catharanthus Roseus Induces Caspase-Mediated Apoptosis in Cervical Cancer Cells (HeLa). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1938–1946. [Google Scholar] [CrossRef]
- Azhar, N.A.; Ghozali, S.Z.; Bakar, S.A.A.; Lim, V.; Ahmad, N.H. Suppressing Growth, Migration, and Invasion of Human Hepatocellular Carcinoma HepG2 Cells by Catharanthus Roseus-silver Nanoparticles. Toxicol. Vitr. 2020, 67, 104910. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.; Cui, H.; Zhang, F.; Zhao, L.; Liu, Y.; Meng, Q. Combined and Targeted Drugs Delivery System for Colorectal Cancer Treatment: Conatumumab Decorated, Reactive Oxygen Species Sensitive Irinotecan Prodrug and Quercetin Co-Loaded Nanostructured Lipid Carriers. Drug Deliv. 2022, 29, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, J.; Chan, R.; Ji, Y.; Lu, J.; Liao, Y.P.; Okene, M.; Lin, J.; Lin, P.; Chang, C.H.; et al. Improved Efficacy and Reduced Toxicity Using a Custom-Designed Irinotecan-Delivering Silicasome for Orthotopic Colon Cancer. ACS Nano 2019, 13, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Feng, Z. Synergic Fabrication of Combination Therapy of Irinotecan and 5-Fluorouracil Encapsulated Polymeric Nanoparticles for the Treatment of Gastric Cancer Therapy. Process Biochem. 2021, 106, 191–198. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I.; Cohen, M.; Delie, F.; Bastida-Ruiz, D.; Yart, L.; Martin-Sabroso, C.; Fernández-Carballido, A. PLGA Nanoparticles for the Intraperitoneal Administration of CBD in the Treatment of Ovarian Cancer: In Vitro and in Ovo Assessment. Pharmaceutics 2020, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- De La Ossa, D.H.P.; Gil-Alegre, M.E.; Ligresti, A.; Aberturas, M.D.R.; Molpeceres, J.; Torres, A.I.; Di Marzo, V. Preparation and Characterization of Delta9-Tetrahydrocannabinol-Loaded Biodegradable Polymeric Microparticles and Their Antitumoral Efficacy on Cancer Cell Lines. J. Drug Target. 2013, 21, 710–718. [Google Scholar] [CrossRef]
- Tangutoori, S.; Korideck, H.; Makrigiorgos, M.; Cormack, R.; Sridhar, S. A Novel Nano-Formulation for Systemic Administration of PARPi-Olaparib (Nano-Olaparib) for Radiosensitization, Chemosensitization, and Combinatorial Therapy in Prostate Cancer. Mol. Cancer Ther. 2013, 12, A81. [Google Scholar] [CrossRef]
- Zhang, S.; Li, E.; Liu, Z.; Shang, H.; Chen, Y.; Jing, H. Anoparticle-Based Olaparib Delivery Enhances Its Effect, and Improves Drug Sensitivity to Cisplatin in Triple Negative Breast Cancer. J. Drug Deliv. Sci. Technol. 2022, 76, 103731. [Google Scholar] [CrossRef]
- Anwer, M.K.; Ali, E.A.; Iqbal, M.; Ahmed, M.M.; Aldawsari, M.F.; Al Saqr, A.; Alalaiwe, A.; Soliman, G.A. Development of Chitosan-Coated PLGA-Based Nanoparticles for Improved Oral Olaparib Delivery: In Vitro Characterization, and In Vivo Pharmacokinetic Studies. Processes 2022, 10, 1329. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Rajesh, M.; Arun, R.; MubarakAli, D.; Sathishkumar, G.; Sivanandhan, G.; Dev, G.K.; Manickavasagam, M.; Premkumar, K.; Thajuddin, N.; et al. An Investigation on the Cytotoxicity and Caspase-Mediated Apoptotic Effect of Biologically Synthesized Silver Nanoparticles Using Podophyllum Hexandrum on Human Cervical Carcinoma Cells. Colloids Surf. B Biointerfaces 2013, 102, 708–717. [Google Scholar] [CrossRef]
- Kumbhar, P.S.; Sakate, A.M.; Patil, O.B.; Manjappa, A.S.; Disouza, J.I. Podophyllotoxin-Polyacrylic Acid Conjugate Micelles: Improved Anticancer Efficacy against Multidrug-Resistant Breast Cancer. J. Egypt. Natl. Canc. Inst. 2020, 32, 42. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, M.; Yao, B.; Lu, X.; Zhang, X.; He, P.; Vasilatos, S.N.; Ren, X.; Bian, W.; Yao, C. Transferrin Receptor-Targeted Redox/PH-Sensitive Podophyllotoxin Prodrug Micelles for Multidrug-Resistant Breast Cancer Therapy. J. Mater. Chem. B 2019, 7, 5814–5824. [Google Scholar] [CrossRef]
- Zhang, P.; Tang, M.; Huang, Q.; Zhao, G.; Huang, N.; Zhang, X.; Tan, Y.; Cheng, Y. Combination of 3-Methyladenine Therapy and Asn-Gly-Arg (NGR)-Modified Mesoporous Silica Nanoparticles Loaded with Temozolomide for Glioma Therapy in Vitro. Biochem. Biophys. Res. Commun. 2019, 509, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Wang, K.; Stephen, Z.R.; Mu, Q.; Kievit, F.M.; Chiu, D.T.; Press, O.W.; Zhang, M. Temozolomide Nanoparticles for Targeted Glioblastoma Therapy. ACS Appl. Mater. Interfaces 2015, 7, 6674–6682. [Google Scholar] [CrossRef]
- Li, K.; Liang, N.; Yang, H.; Liu, H.; Li, S. Temozolomide Encapsulated and Folic Acid Decorated Chitosan Nanoparticles for Lung Tumor Targeting: Improving Therapeutic Efficacy Both in Vitro and in Vivo. Oncotarget 2017, 8, 111318–111332. [Google Scholar] [CrossRef]
- Almajidi, Y.Q.; Maraie, N.K.; Raauf, A.M.R. Modified Solid in Oil Nanodispersion Containing Vemurafenib-Lipid Complex-in Vitro/in Vivo Study. F1000Research 2022, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Saraswat, A.; Wei, Z.; Agrawal, M.Y.; Dukhande, V.V.; Reznik, S.E.; Patel, K. Development of Dual Arv-825 and Nintedanib-Loaded Pegylated Nano-Liposomes for Synergistic Efficacy in Vemurafnib-Resistant Melanoma. Pharmaceutics 2021, 13, 1005. [Google Scholar] [CrossRef]
- Xia, L.; Kong, X.; Liu, X.; Tu, L.; Zhang, Y.; Chang, Y.; Liu, K.; Shen, D.; Zhao, H.; Zhang, H. An Upconversion Nanoparticle—Zinc Phthalocyanine Based Nanophotosensitizer for Photodynamic Therapy. Biomaterials 2014, 35, 4146–4156. [Google Scholar] [CrossRef]
- Yurt, F.; Ocakoglu, K.; Ince, M.; Colak, S.G.; Er, O.; Soylu, H.M.; Gunduz, C.; Biray Avci, C.; Caliskan Kurt, C. Photodynamic Therapy and Nuclear Imaging Activities of Zinc Phthalocyanine-Integrated TiO 2 Nanoparticles in Breast and Cervical Tumors. Chem. Biol. Drug Des. 2018, 91, 789–796. [Google Scholar] [CrossRef]






| Polymeric Nanoparticles | Oncology APIs | Nanoparticle Production | Biological Study | References |
|---|---|---|---|---|
| Polyhydroxyalkanoate (PHA) nanoparticles | Ellipticine | Emulsification/Solvent evaporation | in vitro | [63,64] |
| Cisplatin | Emulsification/Solvent evaporation | in vitro | [65] | |
| Thymoquinone | Emulsification/Solvent evaporation | in vitro | [66] | |
| Paclitaxel | Double emulsification/Solvent evaporation | in vitro | [67] | |
| 5-Fluorouracil | Double emulsification/Solvent evaporation | in vitro | [68] | |
| Etoposide | Solvent evaporation | in vitro | [69] | |
| Doxorubicin | Double emulsification/Solvent evaporation | in vitro | [70] | |
| Rhodamine B isothiocyanate (RBITC) | Emulsification/Solvent evaporation | in vitro | [71] | |
| Cyclodextrin (CD) nanoparticles | Docetaxel | Nanoprecipitation | in vitro | [72] |
| Camptothecin | Nanoprecipitation | in vitro | [73] | |
| Acyclovir | Nanoprecipitation | in vitro | [74] | |
| Paclitaxel | Emulsification/Solvent evaporation method | in vivo | [75] | |
| Poly(thioether-ester) nanoparticles | Zinc phthalocyanine | Thiol-ene miniemulsion polymerization | in vitro | [76] |
| Full-spectrum cannabis extract | Thiol-ene miniemulsion and emulsification/Solvent evaporation | in vitro | [77] | |
| 4-nitrochalcone | Thiol-ene miniemulsion polymerization | in vitro | [78] | |
| Poly (lactic co-glycolic acid) (PLGA) nanoparticles | Paclitaxel | Emulsification and nanopracipitation | pre-clinical (mice) | [79] |
| Topotecan–tamoxifen | Double emulsification/Solvent evaporation | in vitro | [80] | |
| Lupeol | Emulsification/Solvent evaporation | in vitro | [81] | |
| Gemcitabine | Emulsification/Solvent evaporation | in vitro | [82] | |
| 9-nitro-camptothecin | Nanoprecipitation | in vitro | [83] | |
| Paclitaxel, doxorubicin | Double emulsification/Solvent evaporation | in vitro | [84] | |
| Paclitaxel | Nanoprecipitation | in vitro | [85] | |
| Cisplatin | Emulsification/Solvent evaporation | in vitro | [86] | |
| Paclitaxel/superparamagnetic iron oxide | Emulsification/Solvent evaporation | in vitro | [87] | |
| Tamoxifen, quercetin | Emulsification/Solvent evaporation | in vitro | [88] | |
| Docetaxel | Nanoprecipitation | in vitro | [89] | |
| Δ9-Tetrahidrocannabinol | Nanoprecipitation | in vitro | [90] | |
| Doxorubicin | Solvent displacement | in vitro | [91] | |
| Paclitaxel | Nanoprecipitation | pre-clinical | [92] | |
| Bicalutamide | Nanoprecipitation | in vitro | [93] | |
| siRNA, paclitaxel | Emulsification/Solvent evaporation | in vitro | [94] | |
| Paclitaxel, doxorubicin | Double emulsification/Solvent evaporation | in vivo | [95] | |
| Methotrexate | Emulsification and diffusion | in vivo | [96] | |
| cisplatin | nanoprecipitation | -re-clinical | [97] | |
| Poly (lactic co-glycolic acid) (PLGA) nanoparticles | Doxorubicin | Solvent displacement | in vitro | [98] |
| Paclitaxel | Nanoprecipitation | -re-clinical (mice) | [99] | |
| Curcumin | Nanoprecipitation | in vivo | [100] | |
| PE38KDL | Double emulsification/Solvent evaporation | pre-clinical (mice) | [101] | |
| Paclitaxel and magnetic fluid | Emulsification/Solvent evaporation | in vitro | [102] | |
| Gemcitabine | Double emulsification/Solvent evaporation | in vitro | [103] | |
| Paclitaxel | Emulsification/Precipitation | in vitro | [104] | |
| Capecitabine | Emulsification/Solvent evaporation | in vitro | [105] | |
| SN-38 | Emulsification/Solvent evaporation | in vitro | [106] | |
| BSA | Double emulsification/Solvent evaporation | in vitro | [107] | |
| Chitosan nanoparticles | Quercetin | Coordination reaction | in vitro | [108] |
| Curcumin | Ionic gelation method | in vitro | [109] | |
| Metformin | Ionic gelation method | in vitro and in vivo | [110] | |
| Chlorin e6 | Nonsolvent-aided counterion complexation | in vitro | [111] | |
| Adriamycin | Dialysis method | in vitro and in vivo | [112] | |
| Polycaprolactone (PCL) nanoparticles | Docetaxel | Emulsification/Solvent evaporation | in vitro | [113] |
| Thalidomide | Dialysis method | in vitro and in vivo | [114] | |
| Docetaxel | Nanoprecipitation technique | in vitro and in vivo | [115] | |
| Dihydroartemisinin | Self-assembly method | in vitro and in vivo | [116] | |
| Oxymatrine | pH gradient method | in vitro | [117] | |
| Polycaprolactone (PCL) nanoparticles | Paclitaxel and curcumin | Self-assembly method | in vitro and in vivo | [118] |
| Flutamide | Nanoprecipitation method | - | [119] | |
| 5-fluorouracil | Double emulsion technique | in vitro | [120] | |
| Silibinin | Solvent displacement process | in vitro and in vivo | [121] | |
| Cellulose nanoparticles | Doxorubicin | Self-assembly method | in vitro and in vivo | [122] |
| 5-Fluorouracil | Co-precipitation method | in vitro | [123] | |
| coumarin and curcumin | oil in water emulsion technique | in vitro | [124] |
| Oncology (APIs) | Type of Cancer | Biological Study | References |
|---|---|---|---|
| Quercetin | Breast, lung, liver, colon, intestine | in vitro and in vivo | [134,135,136,137] |
| Bevacizumab | Colorectal, glibastoma | in vitro and vitro | [138,139,140,141] |
| Catharanthus roseus extract | Breast, cervical, liver | in vitro | [142,143,144] |
| Irinotecan | Colorectal, colon, gastric | in vitro | [145,146,147] |
| Isolated cannabinoids or full-spectrum cannabis extract | Melanoma, glioma, ovarian, leukemia, adenocarcinoma, lung | in vitro, in ovo and in vivo | [77,90,148,149] |
| Olaparib | Prostate, pancreatic, breast, ovarian | in vitro and vitro | [150,151,152] |
| Podophyllum extract | Carcinoma, breast | in vitro | [153,154,155] |
| Temozolomide | Glioma, gliobastoma, lung | in vitro and vitro | [156,157,158] |
| Vemurafenib | Resistant melanoma | in vitro and vitro | [159,160] |
| Zinc phthalocyanine | Breast, liver, carcinoma, cervical adenocarcinoma | in vitro and in vivo | [76,161,162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freire, N.; Barbosa, R.d.M.; García-Villén, F.; Viseras, C.; Perioli, L.; Fialho, R.; Albuquerque, E. Environmentally Friendly Strategies for Formulating Vegetable Oil-Based Nanoparticles for Anticancer Medicine. Pharmaceutics 2023, 15, 1908. https://doi.org/10.3390/pharmaceutics15071908
Freire N, Barbosa RdM, García-Villén F, Viseras C, Perioli L, Fialho R, Albuquerque E. Environmentally Friendly Strategies for Formulating Vegetable Oil-Based Nanoparticles for Anticancer Medicine. Pharmaceutics. 2023; 15(7):1908. https://doi.org/10.3390/pharmaceutics15071908
Chicago/Turabian StyleFreire, Nathália, Raquel de Melo Barbosa, Fátima García-Villén, César Viseras, Luana Perioli, Rosana Fialho, and Elaine Albuquerque. 2023. "Environmentally Friendly Strategies for Formulating Vegetable Oil-Based Nanoparticles for Anticancer Medicine" Pharmaceutics 15, no. 7: 1908. https://doi.org/10.3390/pharmaceutics15071908
APA StyleFreire, N., Barbosa, R. d. M., García-Villén, F., Viseras, C., Perioli, L., Fialho, R., & Albuquerque, E. (2023). Environmentally Friendly Strategies for Formulating Vegetable Oil-Based Nanoparticles for Anticancer Medicine. Pharmaceutics, 15(7), 1908. https://doi.org/10.3390/pharmaceutics15071908








