Low-Frequency Raman Spectroscopy of Pure and Cocrystallized Mycophenolic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Low-Frequency Raman Spectroscopy (LFRS)
2.3. Computational Methods
3. Results and Discussion
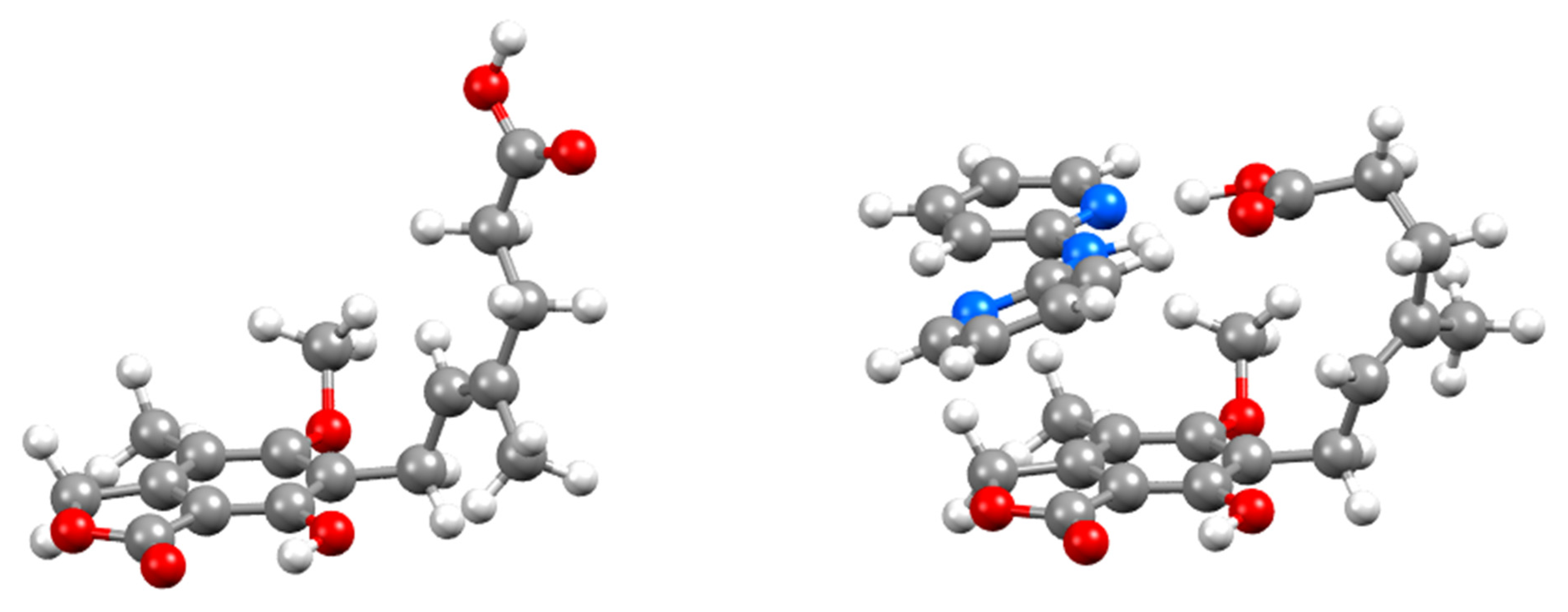
3.1. Crystal Structure Analyses
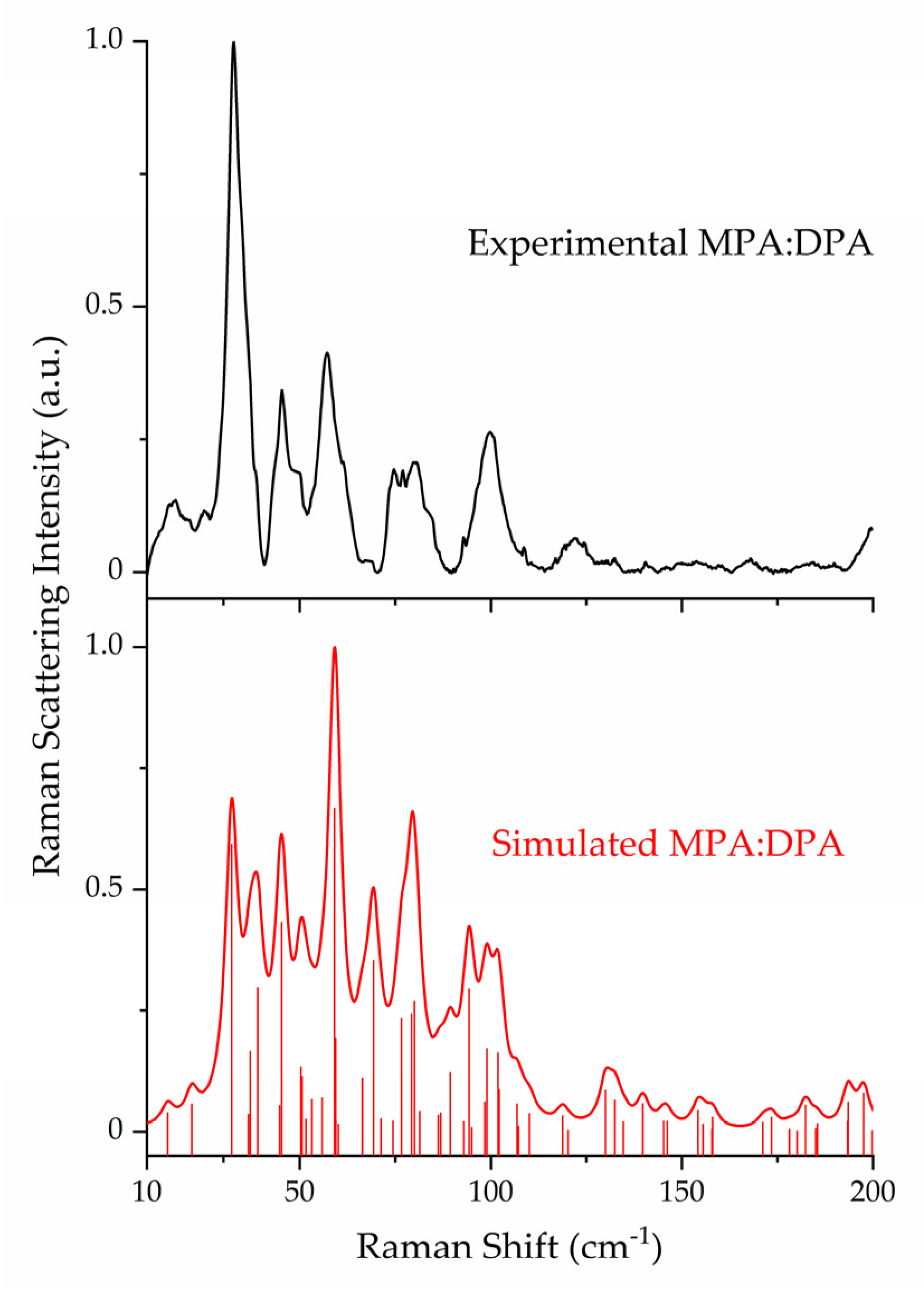
3.2. Raman Spectroscopy and Peak Assignments
3.3. Evaluation of Conformational and Cohesive Energies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shan, N.; Zaworotko, M.J. The role of cocrystals in pharmaceutical science. Drug Discov. Today 2008, 13, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Karagianni, A.; Malamatari, M.; Kachrimanis, K. Pharmaceutical Cocrystals: New Solid Phase Modification Approaches for the Formulation of APIs. Pharmaceutics 2018, 10, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolla, G.; Sarma, B.; Nangia, A.K. Crystal Engineering of Pharmaceutical Cocrystals in the Discovery and Development of Improved Drugs. Chem. Rev. 2022, 122, 11514–11603. [Google Scholar] [CrossRef]
- Qiao, N.; Li, M.; Schlindwein, W.; Malek, N.; Davies, A.; Trappitt, G. Pharmaceutical cocrystals: An overview. Int. J. Pharm. 2011, 419, 1–11. [Google Scholar] [CrossRef]
- Schultheiss, N.; Newman, A. Pharmaceutical Cocrystals and Their Physicochemical Properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, F.G.; Clawson, J.S.; Strohmeier, M.; Edwards, A.J.; Pham, T.N.; Watson, S.A. Solid-State NMR Analysis of Organic Cocrystals and Complexes. Cryst. Growth Des. 2009, 9, 921–937. [Google Scholar] [CrossRef]
- Parrott, E.P.J.; Zeitler, J.A.; Friščić, T.; Pepper, M.; Jones, W.; Day, G.M.; Gladden, L.F. Testing the Sensitivity of Terahertz Spectroscopy to Changes in Molecular and Supramolecular Structure: A Study of Structurally Similar Cocrystals. Cryst. Growth Des. 2009, 9, 1452–1460. [Google Scholar] [CrossRef]
- Zeitler, J.A.; Taday, P.F.; Newnham, D.A.; Pepper, M.; Gordon, K.C.; Rades, T. Terahertz pulsed spectroscopy and imaging in the pharmaceutical setting—A review. J. Pharm. Pharmacol. 2010, 59, 209–223. [Google Scholar] [CrossRef]
- Bērziņš, K.; Fraser-Miller, S.J.; Gordon, K.C. Recent advances in low-frequency Raman spectroscopy for pharmaceutical applications. Int. J. Pharm. 2021, 592, 120034. [Google Scholar] [CrossRef]
- Larkin, P.J.; Dabros, M.; Sarsfield, B.; Chan, E.; Carriere, J.T.; Smith, B.C. Polymorph Characterization of Active Pharmaceutical Ingredients (APIs) Using Low-Frequency Raman Spectroscopy. Appl. Spectrosc. 2014, 68, 758–776. [Google Scholar] [CrossRef]
- Ruggiero, M.T.; Sutton, J.J.; Fraser-Miller, S.J.; Zaczek, A.J.; Korter, T.M.; Gordon, K.C.; Zeitler, J.A. Revisiting the Thermodynamic Stability of Indomethacin Polymorphs with Low-Frequency Vibrational Spectroscopy and Quantum Mechanical Simulations. Cryst. Growth Des. 2018, 18, 6513–6520. [Google Scholar] [CrossRef] [Green Version]
- Dampf, S.J.; Korter, T.M. Crystalline Molecular Standards for Low-Frequency Vibrational Spectroscopies. J. Infrared Millim. Terahertz Waves 2020, 41, 1284–1300. [Google Scholar] [CrossRef]
- Davis, M.P.; Korter, T.M. Low-Frequency Vibrational Spectroscopy and Quantum Mechanical Simulations of the Crystalline Polymorphs of the Antiviral Drug Ribavirin. Mol. Pharm. 2022, 19, 3385–3393. [Google Scholar] [CrossRef]
- Bentley, R. Mycophenolic Acid: A One Hundred Year Odyssey from Antibiotic to Immunosuppressant. Chem. Rev. 2000, 100, 3801–3826. [Google Scholar] [CrossRef]
- Zeng, Q.-Z.; Ouyang, J.; Zhang, S.; Zhang, L. Structural characterization and dissolution profile of mycophenolic acid cocrystals. Eur. J. Pharm. Sci. 2017, 102, 140–146. [Google Scholar] [CrossRef]
- Brogden, D.W.; Berry, J.F. Coordination Chemistry of 2,2′-Dipyridylamine: The Gift That Keeps on Giving. Comments Inorg. Chem. 2016, 36, 17–37. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Menges, F. Spectragryph—Optical Spectroscopy Software, Version 1.2.16.1; 2022. Available online: https://www.effemm2.de/spectragryph/ (accessed on 22 May 2023).
- Dovesi, R.; Erba, A.; Orlando, R.; Zicovich-Wilson, C.M.; Civalleri, B.; Maschio, L.; Rérat, M.; Casassa, S.; Baima, J.; Salustro, S.; et al. Quantum-mechanical condensed matter simulations with CRYSTAL. WIREs Comput. Mol. Sci. 2018, 8, e1360. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 151101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Hansen, A.; Brandenburg, J.G.; Bannwarth, C. Dispersion-Corrected Mean-Field Electronic Structure Methods. Chem. Rev. 2016, 116, 5105–5154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axilrod, B.M.; Teller, E. Interaction of the van der Waals Type Between Three Atoms. J. Chem. Phys. 2004, 11, 299–300. [Google Scholar] [CrossRef]
- Mohara, M.; Davis, M.P.; Korter, T.M.; Shimura, K.; Ono, T.; Aiko, K. Study on Hydration and Dehydration of Ezetimibe by Terahertz Spectroscopy with Humidity-Controlled Measurements and Theoretical Analysis. J. Phys. Chem. A 2022, 126, 2879–2888. [Google Scholar] [CrossRef]
- Pascale, F.; Zicovich-Wilson, C.M.; López Gejo, F.; Civalleri, B.; Orlando, R.; Dovesi, R. The calculation of the vibrational frequencies of crystalline compounds and its implementation in the CRYSTAL code. J. Comput. Chem. 2004, 25, 888–897. [Google Scholar] [CrossRef]
- Zicovich-Wilson, C.M.; Pascale, F.; Roetti, C.; Saunders, V.R.; Orlando, R.; Dovesi, R. Calculation of the vibration frequencies of α-quartz: The effect of Hamiltonian and basis set. J. Comput. Chem. 2004, 25, 1873–1881. [Google Scholar] [CrossRef]
- Maschio, L.; Kirtman, B.; Rérat, M.; Orlando, R.; Dovesi, R. Ab initio analytical Raman intensities for periodic systems through a coupled perturbed Hartree-Fock/Kohn-Sham method in an atomic orbital basis. I. Theory. J. Chem. Phys. 2013, 139, 164101. [Google Scholar] [CrossRef] [Green Version]
- Maschio, L.; Kirtman, B.; Rérat, M.; Orlando, R.; Dovesi, R. Ab initio analytical Raman intensities for periodic systems through a coupled perturbed Hartree-Fock/Kohn-Sham method in an atomic orbital basis. II. Validation and comparison with experiments. J. Chem. Phys. 2013, 139, 164102. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.E.; Jacobson, R.A. The crystal and molecular structure of di(2-pyridyl)amine. Acta Crystallogr. Sect. B 1973, 29, 1669–1674. [Google Scholar] [CrossRef] [Green Version]
- Terayama, K.; Sumita, M.; Katouda, M.; Tsuda, K.; Okuno, Y. Efficient Search for Energetically Favorable Molecular Conformations against Metastable States via Gray-Box Optimization. J. Chem. Theory Comput. 2021, 17, 5419–5427. [Google Scholar] [CrossRef] [PubMed]
- Yathirajan, H.S.; Nagaraj, B.; Gaonkar, S.L.; Narasegowda, R.S.; Nagaraja, P.; Bolte, M. Mycophenolate mofetil. Acta Crystallogr. Sect. E 2004, 60, o2223–o2224. [Google Scholar] [CrossRef]
- Schnieders, M.J.; Baltrusaitis, J.; Shi, Y.; Chattree, G.; Zheng, L.; Yang, W.; Ren, P. The Structure, Thermodynamics, and Solubility of Organic Crystals from Simulation with a Polarizable Force Field. J. Chem. Theory Comput. 2012, 8, 1721–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| MPA | DPA | MPA:DPA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Exp. [15] | ss-DFT | % Error | Exp. [32] | ss-DFT | % Error | Exp. [15] | ss-DFT | % Error | |
| a | 7.3469 | 7.15191 | −2.7 | 18.416 | 18.17738 | −1.3 | 11.6989 | 11.55358 | −1.2 |
| b | 9.5469 | 9.52802 | −0.2 | 12.294 | 12.15401 | −1.1 | 14.8760 | 15.04209 | 1.1 |
| c | 11.6228 | 11.49439 | −1.1 | 7.691 | 7.56011 | −1.7 | 15.019 | 14.78744 | −1.5 |
| α | 102.809 | 101.8443 | −0.9 | 90 | 90 | - | 90 | 90 | - |
| β | 90.902 | 92.3840 | 1.6 | 90 | 90 | - | 107.925 | 108.2683 | 0.3 |
| γ | 90.873 | 88.8070 | −2.3 | 90 | 90 | - | 90 | 90 | - |
| V | 794.69 | 765.87 | −3.6 | 1741.29 | 1670.24 | −4.1 | 2486.93 | 2440.38 | −1.9 |
| MPA | DPA | MPA:DPA | |
|---|---|---|---|
| Bond lengths (Å) | 0.023 | 0.018 | 0.018 |
| Bond angles (°) | 0.650 | 0.619 | 0.477 |
| Torsional angles (°) | 4.916 | 0.972 | 3.864 |
| Atoms | D-H (Å) | H···A (Å) | D-H···A (o) | D···A (Å) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Exp. | ss-DFT | Exp. | ss-DFT | Exp. | ss-DFT | Exp. | ss-DFT | ||
| MPA | O4-H1···O6 | 0.82 | 0.9880 | 2.443 | 2.1901 | 127.7 | 130.11 | 3.015 | 2.9257 |
| O5-H2···O6 | 0.82 | 1.0211 | 1.902 | 1.6300 | 175.3 | 176.14 | 2.720 | 2.6500 | |
| DPA | N2-H3···N1 | 0.87 | 1.0482 | 2.180 | 1.8634 | 172.4 | 172.26 | 3.043 | 2.9055 |
| MPA:DPA | O4-H1···O5 | 0.82 | 0.9862 | 2.422 | 2.2969 | 121.2 | 117.37 | 2.933 | 2.8864 |
| O5-H2···N1 | 0.95 | 1.0915 | 1.700 | 1.5061 | 172.9 | 171.33 | 2.642 | 2.5903 | |
| N2-H3···O6 | 0.85 | 1.0384 | 2.026 | 1.8196 | 179.6 | 179.18 | 2.879 | 2.8579 | |
| Exp. freq | ss-DFT freq | Mode Description | |
|---|---|---|---|
| MPA | 33.6 | 35.80 | ring-chain torsion |
| 40.1 | 33.13 | rotation about a | |
| 53.3 | 51.88 | rotation about c | |
| 63.8 | 61.89 65.36 | ring-chain torsion ring-chain torsion | |
| 77.4 | 74.83 | ring-chain torsion | |
| 85.2 | 87.91 | ring-chain torsion | |
| DPA | 26.4 | 26.41 28.12 | rotation about b translation along b |
| 36.0 | 36.54 | translation along a | |
| 47.9 | 48.78 | rotation about c | |
| 52.7 | 53.37 | rotation about b | |
| 58.5 | 60.17 | rotation about b | |
| 70.7 | 72.43 75.52 | rotation about c rotation about c | |
| 73.7 | 77.83 | ring torsion at amine linkage | |
| 81.5 | 82.89 | rotation about c | |
| 87.6 | 91.95 | ring torsion at amine linkage | |
| 100–115 (broad) | 105.10 108.18 112.71 113.37 | ring torsion at amine linkage ring torsion at amine linkage ring torsion at amine linkage rotation about b | |
| 128.8 | 132.30 | bending at amine linkage |
| Exp. freq | ss-DFT freq | Mode Description |
|---|---|---|
| 32.7 | 32.20 37.09 39.00 | rotation about b rotation about c rotation about b |
| 45.3 | 45.26 | ring-chain torsion (MPA) |
| 49.2 | 50.29 50.60 | rotation about b translation along b |
| 57.2 | 59.07 | rotation about a |
| 75–85 (broad) | 76.64 79.24 79.99 | methoxy torsion (MPA) methoxy torsion (MPA) intra-chain torsion (MPA) |
| 95–105 (broad) | 94.30 98.97 101.86 | ring-ring torsion (DPA) ring-ring torsion (DPA) ring-ring bending (DPA) |
| 122.1 | 118.83 | ring-ring torsion (DPA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallace, C.S.; Davis, M.P.; Korter, T.M. Low-Frequency Raman Spectroscopy of Pure and Cocrystallized Mycophenolic Acid. Pharmaceutics 2023, 15, 1924. https://doi.org/10.3390/pharmaceutics15071924
Wallace CS, Davis MP, Korter TM. Low-Frequency Raman Spectroscopy of Pure and Cocrystallized Mycophenolic Acid. Pharmaceutics. 2023; 15(7):1924. https://doi.org/10.3390/pharmaceutics15071924
Chicago/Turabian StyleWallace, Catherine S., Margaret P. Davis, and Timothy M. Korter. 2023. "Low-Frequency Raman Spectroscopy of Pure and Cocrystallized Mycophenolic Acid" Pharmaceutics 15, no. 7: 1924. https://doi.org/10.3390/pharmaceutics15071924





