Virus-Based Biological Systems as Next-Generation Carriers for the Therapy of Central Nervous System Diseases
Abstract
:1. Introduction
2. Limitations of Currently Used Drug Delivery Systems to the Central Nervous System
2.1. Conventional Drug Delivery Systems
2.2. Nanoformulations
2.3. Gene Therapy and Drug Delivery Systems
3. Advantages of Virus-Based Biological Systems as Next-Generation Carriers for the Therapy of Central Nervous System Diseases
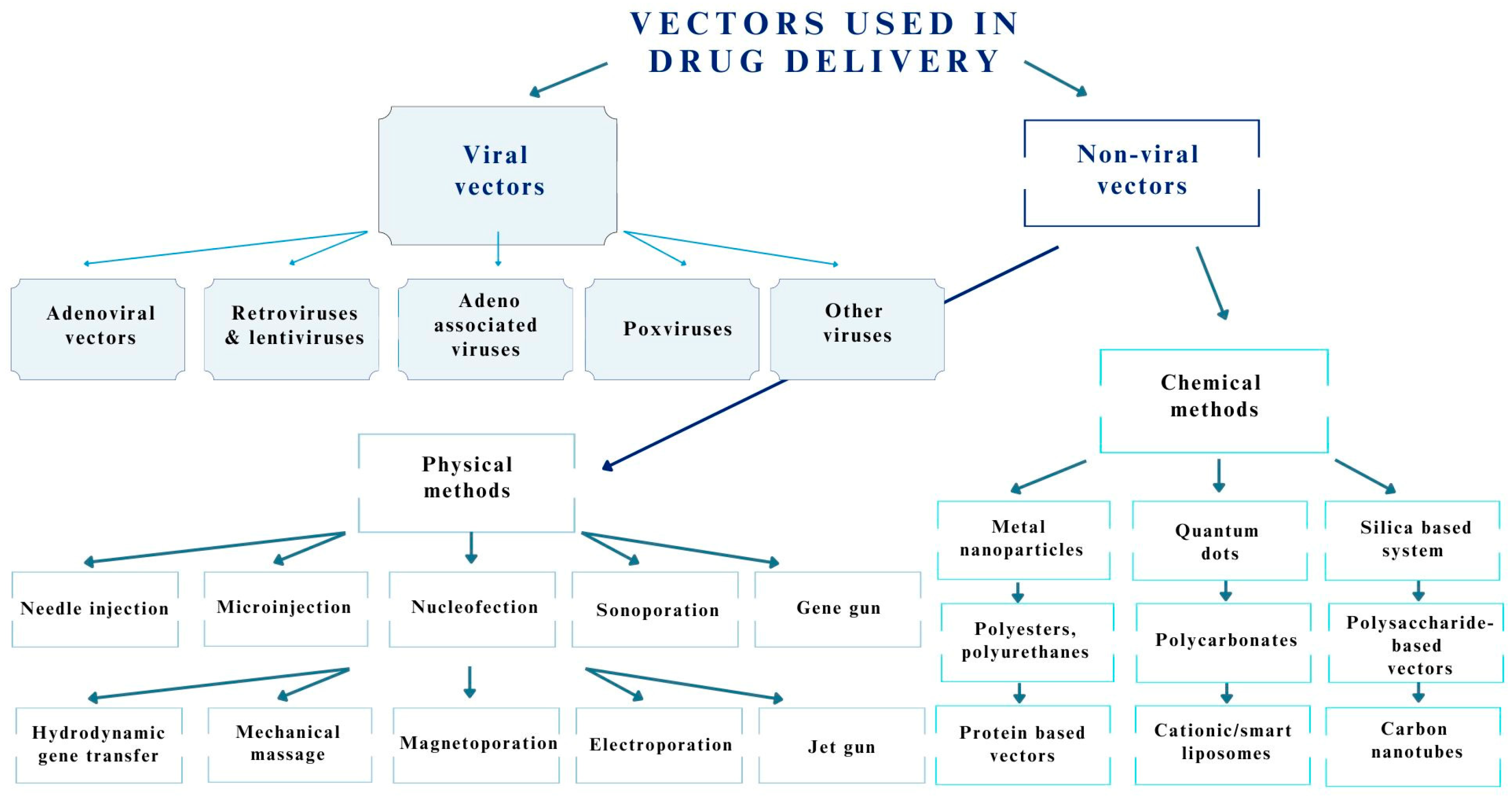
3.1. Viruses Used as Drug Delivery Systems for the Central Nervous System
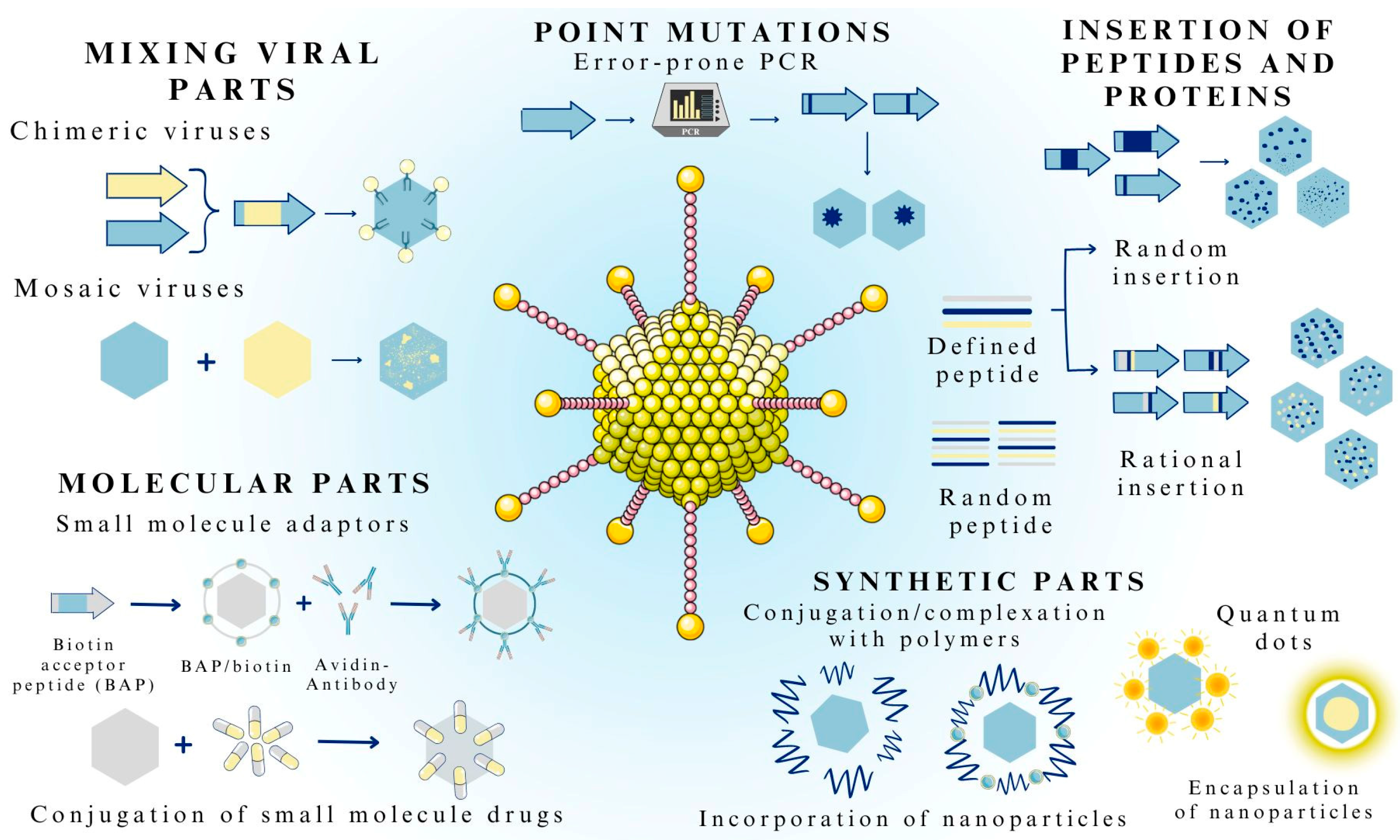
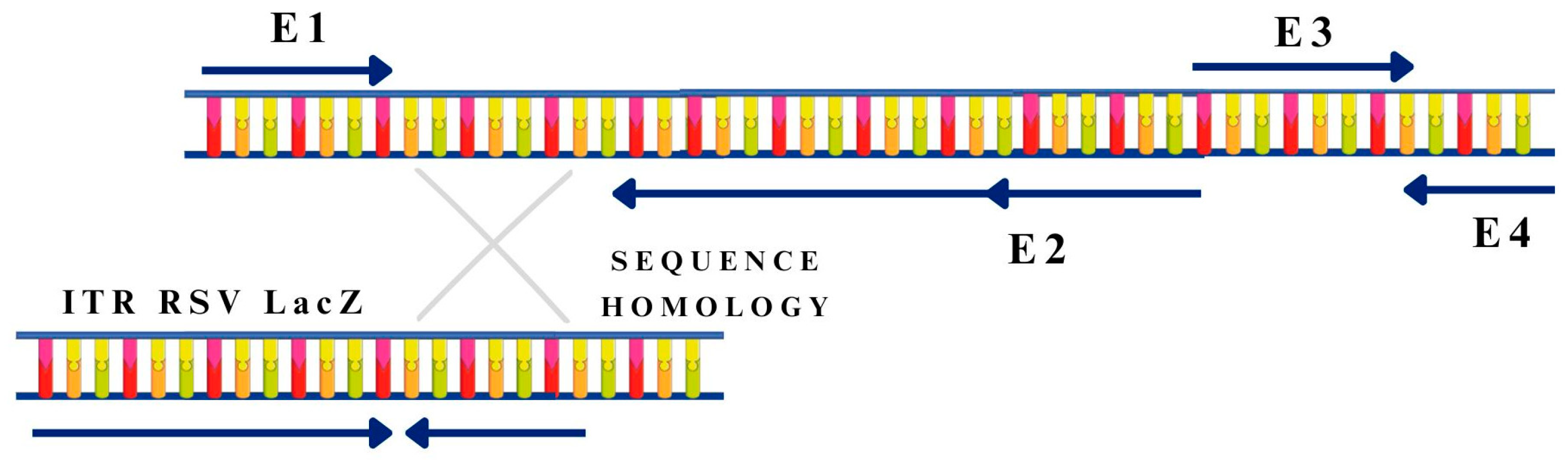
3.2. Methods of Viral Vector Formulation
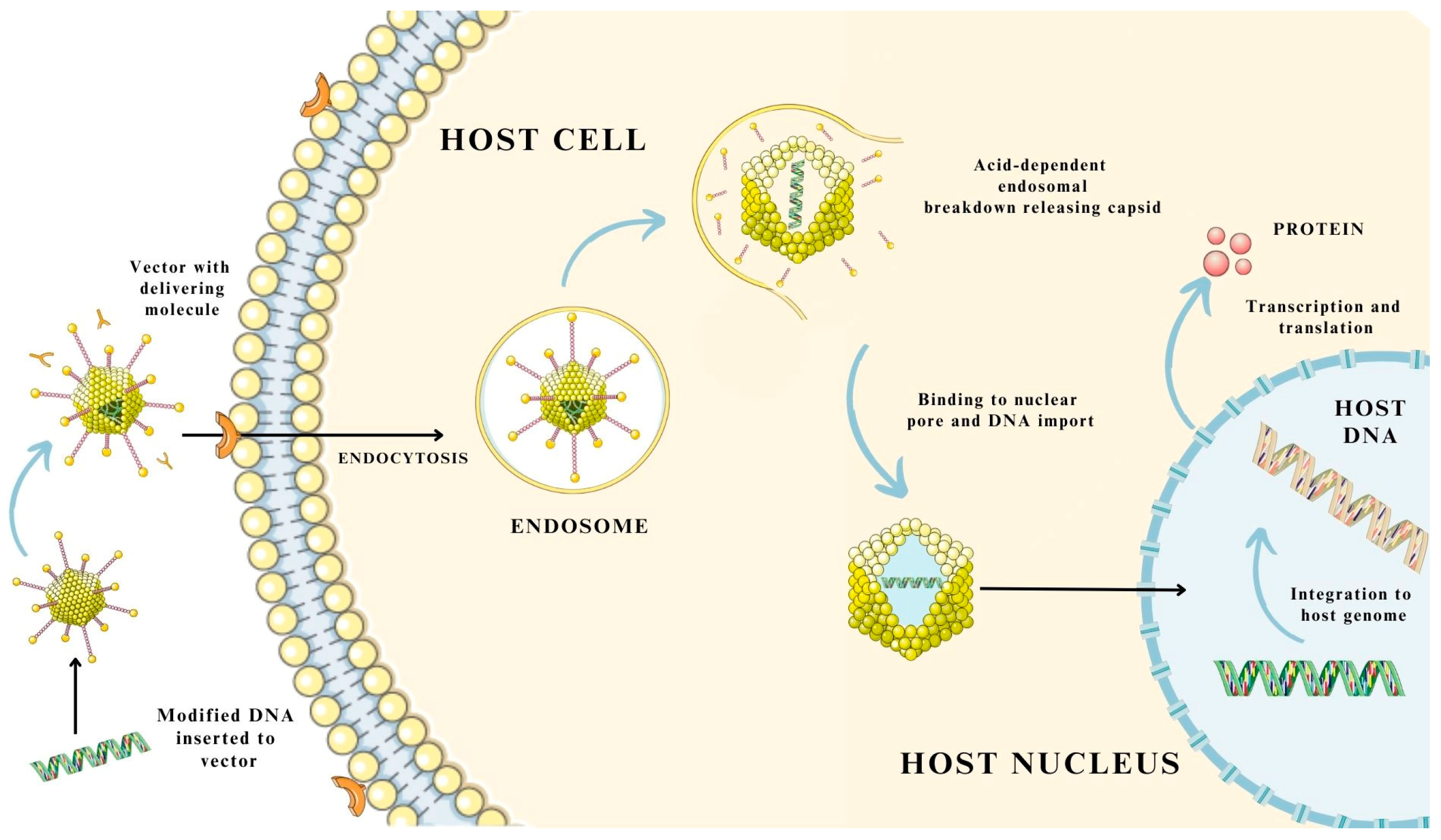
3.3. Mechanism of Gene and Drug Delivery Using Virus-Based Nanosystems
4. Viruses as Drug Delivery Systems in Selected Central Nervous System Diseases
4.1. Alzheimer’s Disease
4.2. Parkinson’s Disease
4.3. Multiple Sclerosis
4.4. Glioblastoma Multiforme
4.5. Canavan Disease
5. Virus-like Particles in the Treatment of Central Nervous System Diseases
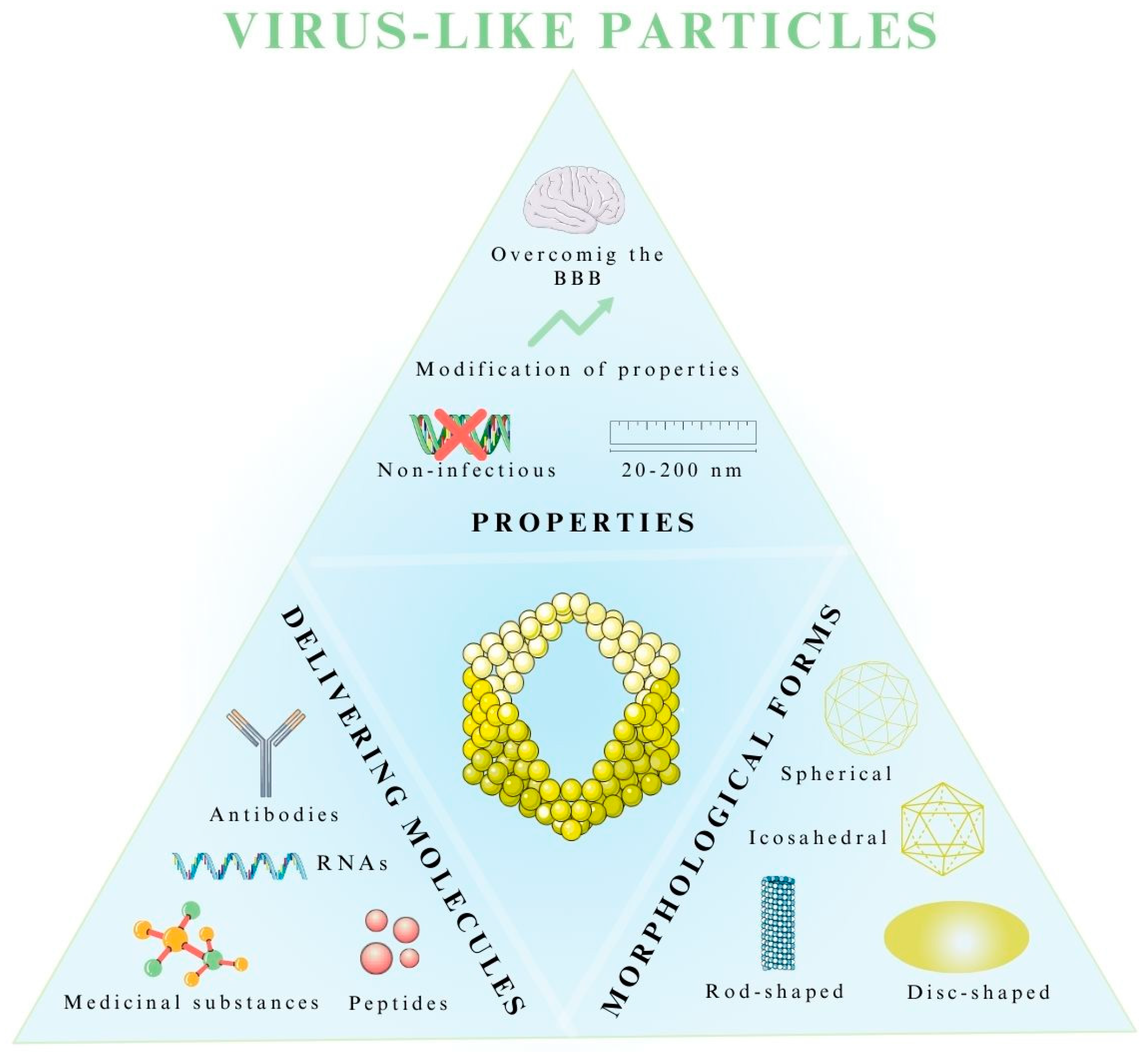
5.1. Properties of Virus-like Particles
5.2. Morphological Forms of Virus-like Particles
5.3. Expression Platforms Used in the Production of Virus-like Particles
5.4. Application of VLPs as Drug Delivery Systems in the Treatment of CNS Diseases
6. Industrial Production, Regulatory Requirement and Limitations of Using Virus-Based Biological Systems as Carriers for Therapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vatansever, S.; Schlessinger, A.; Wacker, D.; Kaniskan, H.Ü.; Jin, J.; Zhou, M.M.; Zhang, B. Artificial intelligence and machine learning-aided drug discovery in central nervous system diseases: State-of-the-arts and future directions. Med. Res. Rev. 2021, 41, 1427–1473. [Google Scholar] [CrossRef] [PubMed]
- Puris, E.; Fricker, G.; Gynther, M. Targeting transporters for drug delivery to the brain: Can we do better? Pharm. Res. 2022, 39, 1415–1455. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Chhibber, T.; Lahooti, B.; Verma, A.; Borse, V.; Jayant, R.D. In-vitro blood-brain barrier models for drug screening and permeation studies: An overview. Drug Des. Devel. Ther. 2019, 13, 3591–3605. [Google Scholar] [CrossRef] [PubMed]
- Rufino-Ramos, D.; Albuquerque, P.R.; Carmona, V.; Perfeito, R.; Nobre, R.J.; Pereira de Almeida, L. Extracellular vesicles: Novel promising delivery systems for therapy of brain diseases. J. Control. Release 2017, 262, 247–258. [Google Scholar] [CrossRef]
- Furtado, D.; Björnmalm, M.; Ayton, S.; Bush, A.I.; Kempe, K.; Caruso, F. Overcoming the blood-brain barrier: The role of nanomaterials in treating neurological diseases. Adv. Mater. 2018, 30, e1801362. [Google Scholar] [CrossRef]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-brain barrier: From physiology to disease and back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Alahmari, A. Blood-brain barrier overview: Structural and functional correlation. Neural Plast. 2021, 2021, 6564585. [Google Scholar] [CrossRef]
- Galea, I. The blood-brain barrier in systemic infection and inflammation. Cell. Mol. Immunol. 2021, 18, 2489–2501. [Google Scholar] [CrossRef]
- Langen, U.H.; Ayloo, S.; Gu, C. Development and cell biology of the blood-brain barrier. Annu. Rev. Cell Dev. Biol. 2019, 35, 591–613. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gan, L.; Ren, L.; Lin, Y.; Ma, C.; Lin, X. Factors influencing the blood-brain barrier permeability. Brain Res. 2022, 1788, 147937. [Google Scholar] [CrossRef] [PubMed]
- Madej, M.; Kurowska, N.; Strzalka-Mrozik, B. Polymeric nanoparticles—Tools in a drug delivery system in selected cancer therapies. Appl. Sci. 2022, 12, 9479. [Google Scholar] [CrossRef]
- Helms, H.C.C.; Kristensen, M.; Saaby, L.; Fricker, G.; Brodin, B. Drug delivery strategies to overcome the blood-brain barrier (BBB). Handb. Exp. Pharmacol. 2022, 273, 151–183. [Google Scholar]
- Nguyen, T.T.; Maeng, H.J. Pharmacokinetics and pharmacodynamics of intranasal solid lipid nanoparticles and nanostructured lipid carriers for nose-to-brain delivery. Pharmaceutics 2022, 14, 572. [Google Scholar] [CrossRef]
- Constantinescu, T.; Lungu, C.N.; Lung, I. Lipophilicity as a central component of drug-like properties of chalchones and flavonoid derivatives. Molecules 2019, 24, 1505. [Google Scholar] [CrossRef]
- Park, H.; Otte, A.; Park, K. Evolution of drug delivery systems: From 1950 to 2020 and beyond. J. Control. Release 2022, 342, 53–65. [Google Scholar] [CrossRef]
- Ceña, V.; Játiva, P. Nanoparticle crossing of blood-brain barrier: A road to new therapeutic approaches to central nervous system diseases. Nanomedicine 2018, 13, 1513–1516. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnology 2021, 19, 59. [Google Scholar] [CrossRef]
- Chen, W.; Yao, S.; Wan, J.; Tian, Y.; Huang, L.; Wang, S.; Akter, F.; Wu, Y.; Yao, Y.; Zhang, X. BBB-crossing adeno-associated virus vector: An excellent gene delivery tool for CNS disease treatment. J. Control. Release 2021, 333, 129–138. [Google Scholar] [CrossRef]
- Ingusci, S.; Verlengia, G.; Soukupova, M.; Zucchini, S.; Simonato, M. Gene therapy tools for brain diseases. Front. Pharmacol. 2019, 10, 724. [Google Scholar] [CrossRef] [PubMed]
- Tekade, A.R.; Mittha, P.S.; Pisal, C.S. Nanostructured lipid carriers for nose to brain delivery targeting CNS: Diversified role of liquid lipids for synergistic action. Adv. Pharm. Bull. 2022, 12, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tsibouklis, J.; Weng, T.; Zhang, B.; Yin, G.; Feng, G.; Cui, Y.; Savina, I.N.; Mikhalovska, L.I.; Sandeman, S.R.; et al. Nano carriers for drug transport across the blood-brain barrier. J. Drug Target. 2017, 25, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S.; Alzarea, S.I.; Almalki, W.H.; Tariq, A.; Mubeen, B.; Murtaza, B.N.; Iftikhar, S.; Riaz, N.; et al. Nanoparticles in drug delivery: From history to therapeutic applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Shen, Z.; Anraku, Y.; Kataoka, K.; Chen, X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials 2019, 224, 119491. [Google Scholar] [CrossRef]
- Turek, A.; Rech, J.; Borecka, A.; Wilińska, J.; Kobielarz, M.; Janeczek, H.; Kasperczyk, J. The role of the mechanical, structural, and thermal properties of poly(l-lactide-co-glycolide-co-trimethylene carbonate) in the development of rods with aripiprazole. Polymers 2021, 13, 3556. [Google Scholar] [CrossRef]
- Olakowska, E.; Wlaszczuk, A.; Turek, A.; Borecka, A.; Liskiewicz, A.; Wawro, D.; Kasperczyk, J.; Jedrzejowska-Szypulka, H. Effects of 17-β-estradiol released from shape-memory terpolymer rods on sciatic nerve regeneration after injury and repair with chitosan nerve conduit in female rats. J. Appl. Biomed. 2022, 20, 87–97. [Google Scholar] [CrossRef]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef]
- Mittal, P.; Saharan, A.; Verma, R.; Altalbawy, F.M.A.; Alfaidi, M.A.; Batiha, G.E.; Akter, W.; Gautam, R.K.; Uddin, M.S.; Rahman, M.S. Dendrimers: A new race of pharmaceutical nanocarriers. Biomed. Res. Int. 2021, 2021, 8844030. [Google Scholar] [CrossRef]
- Negri, V.; Pacheco-Torres, J.; Calle, D.; López-Larrubia, P. Carbon nanotubes in biomedicine. Top Curr. Chem. 2020, 378, 15. [Google Scholar] [CrossRef]
- Ahmad, J.; Akhter, S.; Rizwanullah, M.; Khan, M.A.; Pigeon, L.; Addo, R.T.; Greig, N.H.; Midoux, P.; Pichon, C.; Kamal, M.A. Nanotechnology based theranostic approaches in Alzheimer’s disease management: Current status and future perspective. Curr. Alzheimer Res. 2017, 14, 1164–1181. [Google Scholar] [CrossRef]
- Zhou, X.; Smith, Q.R.; Liu, X. Brain penetrating peptides and peptide-drug conjugates to overcome the blood-brain barrier and target CNS diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1695. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.H.; Zaman, M.; Ahmad, A.; Khan, R.; Mallhi, T.H.; Hasan, M.M.; Khan, Y.H.; Hafeez, S.; Massoud, E.E.S.; Rahman, M.H.; et al. Appraisal for the potential of viral and nonviral vectors in gene therapy: A review. Genes 2022, 13, 1370. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Tom, V.J. The use of viral vectors to promote repair after spinal cord injury. Exp. Neurol. 2022, 354, 114102. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zheng, M.; Zhang, Z.; Tang, X.; Chen, Y.; Peng, A.; Peng, X.; Tong, A.; Zhou, L. Interleukin-7-loaded oncolytic adenovirus improves CAR-T cell therapy for glioblastoma. Cancer Immunol. Immunother. 2021, 70, 2453–2465. [Google Scholar] [CrossRef]
- Chen, P.; Yan, Q.; Wang, S.; Wang, C.; Zhao, P. Transfer of three transcription factors via a lentiviral vector ameliorates spatial learning and memory impairment in a mouse model of Alzheimer’s disease. Gene 2016, 587, 59–63. [Google Scholar] [CrossRef]
- GuhaSarkar, D.; Su, Q.; Gao, G.; Sena-Esteves, M. Systemic AAV9-IFNβ gene delivery treats highly invasive glioblastoma. Neuro-oncology 2016, 18, 1508–1518. [Google Scholar] [CrossRef]
- Jeon, S.G.; Kang, M.; Kim, Y.S.; Kim, D.H.; Nam, D.W.; Song, E.J.; Mook-Jung, I.; Moon, M. Intrahippocampal injection of a lentiviral vector expressing neurogranin enhances cognitive function in 5XFAD mice. Exp. Mol. Med. 2018, 50, e461. [Google Scholar] [CrossRef]
- Wei, X.; Lv, T.; Chen, D.; Guan, J. Lentiviral vector mediated delivery of RHBDD1 shRNA down regulated the proliferation of human glioblastoma cells. Technol. Cancer Res. Treat. 2014, 13, 87–93. [Google Scholar] [CrossRef]
- Kim, J.W.; Kane, J.R.; Panek, W.K.; Young, J.S.; Rashidi, A.; Yu, D.; Kanojia, D.; Hasan, T.; Miska, J.; Gómez-Lim, M.A.; et al. A dendritic cell-targeted adenoviral vector facilitates adaptive immune response against human glioma antigen (CMV-IE) and prolongs survival in a human glioma tumor model. Neurotherapeutics 2018, 15, 1127–1138. [Google Scholar] [CrossRef]
- Liu, X.L.; Zhao, D.; Sun, D.P.; Wang, Y.; Li, Y.; Qiu, F.Q.; Ma, P. Adenovirus-mediated delivery of CALR and MAGE-A3 inhibits invasion and angiogenesis of glioblastoma cell line U87. J. Exp. Clin. Cancer Res. 2012, 31, 8. [Google Scholar] [CrossRef] [PubMed]
- Trinh, D.; Nash, J.; Goertz, D.; Hynynen, K.; Bulner, S.; Iqbal, U.; Keenan, J. Microbubble drug conjugate and focused ultrasound blood brain barrier delivery of AAV-2 SIRT-3. Drug Deliv. 2022, 29, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.B.; Kaplitt, M.G.; De, B.P.; Chen, A.; Flagiello, T.; Salami, C.; Pey, E.; Zhao, L.; Ricart Arbona, R.J.; Monette, S.; et al. AAVrh.10-mediated APOE2 central nervous system gene therapy for APOE4-associated Alzheimer’s disease. Hum. Gene Ther. Clin. Dev. 2018, 29, 24–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Bai, L.; Lu, Z.; Gu, R.; Zhao, D.; Yan, F.; Bai, J. TRPV4 contributes to ER stress and inflammation: Implications for Parkinson’s disease. J. Neuroinflamm. 2022, 19, 26. [Google Scholar] [CrossRef]
- van Kan-Davelaar, H.E.; van Hest, J.C.; Cornelissen, J.J.; Koay, M.S. Using viruses as nanomedicines. Br. J. Pharmacol. 2014, 171, 4001–4009. [Google Scholar] [CrossRef]
- Guenther, C.M.; Kuypers, B.E.; Lam, M.T.; Robinson, T.M.; Zhao, J.; Suh, J. Synthetic virology: Engineering viruses for gene delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 548–558. [Google Scholar] [CrossRef]
- Wei, F.; McConnell, K.I.; Yu, T.K.; Suh, J. Conjugation of paclitaxel on adeno-associated virus (AAV) nanoparticles for co-delivery of genes and drugs. Eur. J. Pharm. Sci. 2012, 46, 167–172. [Google Scholar] [CrossRef]
- Wu, W.; Hsiao, S.C.; Carrico, Z.M.; Francis, M.B. Genome-free viral capsids as multivalent carriers for taxol delivery. Angew. Chem. Int. Ed. Engl. 2009, 48, 9493–9497. [Google Scholar] [CrossRef]
- Mohan, K.; Weiss, G.A. Chemically modifying viruses for diverse applications. ACS. Chem. Biol. 2016, 11, 1167–1179. [Google Scholar] [CrossRef]
- El Andari, J.; Grimm, D. Production, processing, and characterization of synthetic AAV gene therapy vectors. Biotechnol. J. 2021, 16, e2000025. [Google Scholar] [CrossRef]
- Duvergé, A.; Negroni, M. Pseudotyping lentiviral vectors: When the clothes make the virus. Viruses 2020, 12, 1311. [Google Scholar] [CrossRef] [PubMed]
- Schwartze, J.T.; Havenga, M.; Bakker, W.A.M.; Bradshaw, A.C.; Nicklin, S.A. Adenoviral vectors for cardiovascular gene therapy applications: A clinical and industry perspective. J. Mol. Med. 2022, 100, 875–901. [Google Scholar]
- Labbé, R.P.; Vessillier, S.; Rafiq, Q.A. Lentiviral vectors for T cell engineering: Clinical applications, bioprocessing and future perspectives. Viruses 2021, 13, 1528. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hong, W.; Ren, W.; Xu, T.; Qian, Z.; He, Z. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct. Target. Ther. 2021, 6, 225. [Google Scholar] [PubMed]
- Tosolini, A.P.; Sleigh, J.N. Intramuscular delivery of gene therapy for targeting the nervous system. Front. Mol. Neurosci. 2020, 13, 129. [Google Scholar] [CrossRef]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent advancements in pathogenesis, diagnostics and treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive review on Alzheimer’s disease: Causes and treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, F1000 Faculty Rev-1161. [Google Scholar] [CrossRef]
- Qu, Y.; Liu, Y.; Noor, A.F.; Tran, J.; Li, R. Characteristics and advantages of adeno-associated virus vector-mediated gene therapy for neurodegenerative diseases. Neural Regen. Res. 2019, 14, 931–938. [Google Scholar]
- Raikwar, S.P.; Thangavel, R.; Dubova, I.; Selvakumar, G.P.; Ahmed, M.E.; Kempuraj, D.; Zaheer, S.A.; Iyer, S.S.; Zaheer, A. Targeted gene editing of glia maturation factor in microglia: A novel Alzheimer’s disease therapeutic target. Mol. Neurobiol. 2019, 56, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Sasmita, A.O. Current viral-mediated gene transfer research for treatment of Alzheimer’s disease. Biotechnol. Genet. Eng. Rev. 2019, 35, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Martinez, B.; Peplow, P.V. Neuroprotection by immunomodulatory agents in animal models of Parkinson’s disease. Neural Regen. Res. 2018, 13, 1493–1506. [Google Scholar] [PubMed]
- Church, F.C. Treatment options for motor and non-motor symptoms of Parkinson’s disease. Biomolecules 2021, 11, 612. [Google Scholar] [CrossRef]
- Wang, S.; Nie, L.; Song, Y.; Zhang, F.; Chen, X.; Shi, W.; Yang, Z.; Sun, Y.; Dang, Q.; Gao, A. Neurturin promotes tumor cell motility and angiogenesis in colorectal cancer. Exp. Cell Res. 2022, 413, 113049. [Google Scholar] [CrossRef]
- Pearson, T.S.; Gupta, N.; San Sebastian, W.; Imamura-Ching, J.; Viehoever, A.; Grijalvo-Perez, A.; Fay, A.J.; Seth, N.; Lundy, S.M.; Seo, Y.; et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons. Nat. Commun. 2021, 12, 4251. [Google Scholar] [CrossRef]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and treatment of multiple sclerosis: A review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef]
- Doshi, A.; Chataway, J. Multiple sclerosis, a treatable disease. Clin. Med. 2016, 16, 53–59. [Google Scholar] [CrossRef]
- Zhong, C.; Chen, Z.; Xia, Y.; Wu, J.; Zhang, F.; Cheng, C.; Wu, X.; Zhuang, Y.; Xiao, X. Treatment of experimental autoimmune encephalomyelitis using AAV gene therapy by blocking T cell costimulatory pathways. Mol. Ther. Methods Clin. Dev. 2022, 25, 461–475. [Google Scholar] [CrossRef]
- Keeler, G.D.; Kumar, S.; Palaschak, B.; Silverberg, E.L.; Markusic, D.M.; Jones, N.T.; Hoffman, B.E. Gene therapy-induced antigen-specific Tregs inhibit neuro-inflammation and reverse disease in a mouse model of multiple sclerosis. Mol. Ther. 2018, 26, 173–183. [Google Scholar] [CrossRef]
- Vasquez, M.; Consuegra-Fernández, M.; Aranda, F.; Jimenez, A.; Tenesaca, S.; Fernandez-Sendin, M.; Gomar, C.; Ardaiz, N.; Di Trani, C.A.; Casares, N.; et al. Treatment of experimental autoimmune encephalomyelitis by sustained delivery of low-dose IFN-α. J. Immunol. 2019, 203, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.F.; Gatica, S.; Castillo, A.; Kalergis, A.M.; Bueno, S.M.; Riedel, C.A.; González, P.A. Is there a role for herpes simplex virus type 1 in multiple sclerosis? Microbes Infect. 2022, 25, 105084. [Google Scholar] [CrossRef]
- Milone, M.C.; O’Doherty, U. Clinical use of lentiviral vectors. Leukemia 2018, 32, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef] [PubMed]
- Aldoghachi, A.F.; Aldoghachi, A.F.; Breyne, K.; Ling, K.H.; Cheah, P.S. Recent advances in the therapeutic strategies of glioblastoma multiforme. Neuroscience 2022, 491, 240–270. [Google Scholar] [CrossRef]
- Przybylowski, C.J.; Hervey-Jumper, S.L.; Sanai, N. Surgical strategy for insular glioma. J. Neurooncol. 2021, 151, 491–497. [Google Scholar] [CrossRef]
- Jia, J.L.; Alshamsan, B.; Ng, T.L. Temozolomide chronotherapy in glioma: A systematic review. Curr. Oncol. 2023, 30, 1893–1902. [Google Scholar]
- Kushiya, H.; Hiraoka, K.; Suzuki, T.; Inoko, K.; Inagaki, A.; Niwa, H.; Sasaki, K.; Nakamura, T.; Tsuchikawa, T.; Shichinohe, T.; et al. Retroviral replicating vector Toca 511 (vocimagene amiretrorepvec) for prodrug activator gene therapy of lung cancer. Cancers 2022, 14, 5820. [Google Scholar] [CrossRef]
- Collins, S.A.; Shah, A.H.; Ostertag, D.; Kasahara, N.; Jolly, D.J. Clinical development of retroviral replicating vector Toca 511 for gene therapy of cancer. Expert Opin. Biol. Ther. 2021, 21, 1199–1214. [Google Scholar] [CrossRef]
- Rech, J.; Getinger-Panek, A.; Gałka, S.; Bednarek, I. Origin and composition of exosomes as crucial factors in designing drug delivery systems. Appl. Sci. 2022, 12, 12259. [Google Scholar] [CrossRef]
- Thakur, A.; Faujdar, C.; Sharma, R.; Sharma, S.; Malik, B.; Nepali, K.; Liou, J.P. Glioblastoma: Current status, emerging targets, and recent advances. J. Med. Chem. 2022, 65, 8596–8685. [Google Scholar] [CrossRef] [PubMed]
- Philbrick, B.D.; Adamson, D.C. Early clinical trials of Toca 511 and Toca FC show a promising novel treatment for recurrent malignant glioma. Expert Opin. Investig. Drugs. 2019, 28, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, D.; Kalotay, E.; von Jonquieres, G.; Bongers, A.; Lee, B.; Suchowerska, A.K.; Housley, G.D.; Klugmann, M. Dual-function AAV gene therapy reverses late-stage Canavan disease pathology in mice. Front. Mol. Neurosci. 2022, 15, 1061257. [Google Scholar] [CrossRef] [PubMed]
- Janson, C.; McPhee, S.; Bilaniuk, L.; Haselgrove, J.; Testaiuti, M.; Freese, A.; Wang, D.J.; Shera, D.; Hurh, P.; Rupin, J.; et al. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum. Gene Ther. 2002, 13, 1391–1412. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.L.; Gøtzsche, C.R.; Woldbye, D.P.D. Current and future prospects for gene therapy for rare genetic diseases affecting the brain and spinal cord. Front. Mol. Neurosci. 2021, 14, 695937. [Google Scholar] [CrossRef]
- Kantor, B.; McCown, T.; Leone, P.; Gray, S.J. Clinical applications involving CNS gene transfer. Adv. Genet. 2014, 87, 71–124. [Google Scholar]
- Chao, J.; Feng, L.; Ye, P.; Chen, X.; Cui, Q.; Sun, G.; Zhou, T.; Tian, E.; Li, W.; Hu, W.; et al. Therapeutic development for Canavan disease using patient iPSCs introduced with the wild-type ASPA gene. iScience 2022, 25, 104391. [Google Scholar] [CrossRef]
- Gessler, D.J.; Gao, G. Gene therapy for the treatment of neurological disorders: Metabolic disorders. Methods Mol. Biol. 2016, 1382, 429–465. [Google Scholar]
- Leone, P.; Shera, D.; McPhee, S.W.; Francis, J.S.; Kolodny, E.H.; Bilaniuk, L.T.; Wang, D.J.; Assadi, M.; Goldfarb, O.; Goldman, H.W.; et al. Long-term follow-up after gene therapy for canavan disease. Sci. Transl. Med. 2012, 4, 165ra163. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.A.; Rodino-Klapac, L.R.; Goodspeed, K.; Gray, S.J.; Kay, C.N.; Boye, S.L.; Boye, S.E.; George, L.A.; Salabarria, S.; et al. Current clinical applications of in vivo gene therapy with AAVs. Mol. Ther. 2021, 29, 464–488. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Li, H.; Cao, C.; Sikoglu, E.M.; Denninger, A.R.; Su, Q.; Eaton, S.; Liso Navarro, A.A.; Xie, J.; Szucs, S.; et al. A single intravenous rAAV injection as late as P20 achieves efficacious and sustained CNS Gene therapy in Canavan mice. Mol. Ther. 2013, 21, 2136–2147. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chao, J.; Tian, E.; Li, L.; Ye, P.; Zhang, M.; Chen, X.; Cui, Q.; Sun, G.; Zhou, T.; et al. Cell-based therapy for Canavan disease using human iPSC-derived NPCs and OPCs. Adv. Sci. 2020, 7, 2002155. [Google Scholar] [CrossRef] [PubMed]
- Hajebi, S.; Yousefiasl, S.; Rahimmanesh, I.; Dahim, A.; Ahmadi, S.; Kadumudi, F.B.; Rahgozar, N.; Amani, S.; Kumar, A.; Kamrani, E.; et al. Genetically engineered viral vectors and organic-based non-viral nanocarriers for drug delivery applications. Adv. Healthc. Mater. 2022, 11, e2201583. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Dung Nguyen, T.T.; Vo, T.K.; Tran, N.M.; Nguyen, M.K.; Van Vo, T.; Van Vo, G. Nanotechnology-based drug delivery for central nervous system disorders. Biomed. Pharmacother. 2021, 143, 112117. [Google Scholar] [CrossRef]
- Mittal, K.R.; Pharasi, N.; Sarna, B.; Singh, M.; Rachana Haider, S.; Singh, S.K.; Dua, K.; Jha, S.K.; Dey, A.; Ojha, S.; et al. Nanotechnology-based drug delivery for the treatment of CNS disorders. Transl. Neurosci. 2022, 13, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Méndez, J.L.; Vazquez-Duhalt, R.; Hernández, L.R.; Sánchez-Arreola, E.; Bach, H. Virus-like particles: Fundamentals and biomedical applications. Int. J. Mol. Sci. 2022, 23, 8579. [Google Scholar] [CrossRef]
- De Sá Magalhães, S.; De Santis, E.; Hussein-Gore, S.; Colomb-Delsuc, M.; Keshavarz-Moore, E. Quality assessment of virus-like particle: A new transmission electron microscopy approach. Front. Mol. Biosci. 2022, 9, 975054. [Google Scholar] [CrossRef]
- He, J.; Yu, L.; Lin, X.; Liu, X.; Zhang, Y.; Yang, F.; Deng, W. Virus-like particles as nanocarriers for intracellular delivery of biomolecules and compounds. Viruses 2022, 14, 1905. [Google Scholar] [CrossRef]
- Harypursat, V.; Zhou, Y.; Tang, S.; Chen, Y. JC Polyomavirus, progressive multifocal leukoencephalopathy and immune reconstitution inflammatory syndrome: A review. AIDS Res. Ther. 2020, 17, 37. [Google Scholar] [CrossRef]
- Zepeda-Cervantes, J.; Ramírez-Jarquín, J.O.; Vaca, L. Interaction between virus-like particles (VLPs) and pattern recognition receptors (PRRs) from dendritic cells (DCs): Toward better engineering of VLPs. Front. Immunol. 2020, 11, 1100. [Google Scholar] [CrossRef]
- Finbloom, J.A.; Aanei, I.L.; Bernard, J.M.; Klass, S.H.; Elledge, S.K.; Han, K.; Ozawa, T.; Nicolaides, T.P.; Berger, M.S.; Francis, M.B. Evaluation of three morphologically distinct virus-like particles as nanocarriers for convection-enhanced drug delivery to glioblastoma. Nanomaterials 2018, 8, 1007. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chi, Y.; Bao, J.; Zhao, X.; Zhang, J.; Wang, L. Virus-like particles for TEM regulation and antitumor therapy. J. Funct. Biomater. 2022, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Besson, S.; Boucher, E.; Laurin, D.; Manches, O.; Aspord, C.; Hannani, D.; Fender, P. Stimulation of the immune system by a tumor antigen-bearing adenovirus-inspired VLP allows control of melanoma growth. Mol. Ther. Methods Clin. Dev. 2022, 28, 76–89. [Google Scholar] [CrossRef]
- Fuenmayor, J.; Gòdia, F.; Cervera, L. Production of virus-like particles for vaccines. N. Biotechnol. 2017, 39, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Tariq, H.; Batool, S.; Asif, S.; Ali, M.; Abbasi, B.H. Virus-like particles: Revolutionary platforms for developing vaccines against emerging infectious diseases. Front. Microbiol. 2022, 12, 790121. [Google Scholar] [CrossRef]
- Ikwuagwu, B.; Tullman-Ercek, D. Virus-like particles for drug delivery: A review of methods and applications. Curr. Opin. Biotechnol. 2022, 78, 102785. [Google Scholar] [CrossRef]
- Gupta, R.; Arora, K.; Roy, S.S.; Joseph, A.; Rastogi, R.; Arora, N.M.; Kundu, P.K. Platforms, advances, and technical challenges in virus-like particles-based vaccines. Front. Immunol. 2023, 14, 1123805. [Google Scholar] [CrossRef]
- Grataitong, K.; Huault, S.; Chotwiwatthanakun, C.; Jariyapong, P.; Thongsum, O.; Chawiwithaya, C.; Chakrabandhu, K.; Hueber, A.O.; Weerachatyanukul, W. Chimeric virus-like particles (VLPs) designed from shrimp nodavirus (MrNV) capsid protein specifically target EGFR-positive human colorectal cancer cells. Sci. Rep. 2021, 11, 16579. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Tagliamonte, M.; Buonaguro, F.M.; Tornesello, M.L.; Buonaguro, L. Virus-like particles as preventive and therapeutic cancer vaccines. Vaccines 2022, 10, 227. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, W.; Ma, X.; Sun, X.; Fan, J.; Wang, Y. Virus-like particles as antiviral vaccine: Mechanism, design, and application. Biotechnol. Bioprocess Eng. 2023, 6, 1–16. [Google Scholar] [CrossRef]
- Aljabali, A.A.; Hassan, S.S.; Pabari, R.M.; Shahcheraghi, S.H.; Mishra, V.; Charbe, N.B.; Chellappan, D.K.; Dureja, H.; Gupta, G.; Almutary, A.G.; et al. The viral capsid as novel nanomaterials for drug delivery. Future Sci. OA 2021, 7, FSO744. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Xie, X.X.; Liu, D.Q.; Yu, X.L.; Zhang, Y.; Zhang, L.X.; Wang, S.W.; Huang, Y.R.; Liu, R.T. Hepatitis B core VLP-based mis-disordered tau vaccine elicits strong immune response and alleviates cognitive deficits and neuropathology progression in Tau.P301S mouse model of Alzheimer’s disease and frontotemporal dementia. Alzheimers Res. Ther. 2018, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Maphis, N.M.; Peabody, J.; Crossey, E.; Jiang, S.; Jamaleddin Ahmad, F.A.; Alvarez, M.; Mansoor, S.K.; Yaney, A.; Yang, Y.; Sillerud, L.O.; et al. Qß virus-like particle-based vaccine induces robust immunity and protects against tauopathy. NPJ Vaccines 2019, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Castro, R.; Acero Galindo, G.; García Salcedo, Y.; Uribe Campero, L.; Vazquez Perez, V.; Carrillo-Tripp, M.; Gevorkian, G.; Gomez Lim, M.A. Plant-based chimeric HPV-virus-like particles bearing amyloid-β epitopes elicit antibodies able to recognize amyloid plaques in APP-tg mouse and Alzheimer’s disease brains. Inflammopharmacology 2018, 26, 817–827. [Google Scholar] [CrossRef]
- Zeltins, A.; West, J.; Zabel, F.; El Turabi, A.; Balke, I.; Haas, S.; Maudrich, M.; Storni, F.; Engeroff, P.; Jennings, G.T.; et al. Incorporation of tetanus-epitope into virus-like particles achieves vaccine responses even in older recipients in models of psoriasis, Alzheimer’s and cat allergy. NPJ Vaccines 2017, 2, 30. [Google Scholar] [CrossRef]
- Daddacha, W.; Monroe, D.; Carver, K.; Usoro, E.R.; Alptekin, A.; Xu, H.; Osuka, S.; Arbab, A.S.; Sakamuro, D. Viral particle-mediated SAMHD1 depletion sensitizes refractory glioblastoma to DNA-damaging therapeutics by impairing homologous recombination. Cancers 2022, 14, 4490. [Google Scholar] [CrossRef]
- Chao, C.N.; Yang, Y.H.; Wu, M.S.; Chou, M.C.; Fang, C.Y.; Lin, M.C.; Tai, C.K.; Shen, C.H.; Chen, P.L.; Chang, D.; et al. Gene therapy for human glioblastoma using neurotropic JC virus-like particles as a gene delivery vector. Sci. Rep. 2018, 8, 2213. [Google Scholar] [CrossRef]
- Pang, H.H.; Huang, C.Y.; Chou, Y.W.; Lin, C.J.; Zhou, Z.L.; Shiue, Y.L.; Wei, K.C.; Yang, H.W. Bioengineering fluorescent virus-like particle/RNAi nanocomplexes act synergistically with temozolomide to eradicate brain tumors. Nanoscale 2019, 11, 8102–8109. [Google Scholar] [CrossRef]
- Thuenemann, E.C.; Le, D.H.T.; Lomonossoff, G.P.; Steinmetz, N.F. Bluetongue virus particles as nanoreactors for enzyme delivery and cancer therapy. Mol. Pharm. 2021, 18, 1150–1156. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Liu, Y.; Zhang, X.; Shan, W.; Ye, S.; Zhou, X.; Ge, Y.; Wang, X.; Ren, L. Nanoparticle-based co-delivery of siRNA and paclitaxel for dual-targeting of glioblastoma. Nanomedicine 2020, 15, 1391–1409. [Google Scholar] [CrossRef]
- Doucet, M.; El-Turabi, A.; Zabel, F.; Hunn, B.H.M.; Bengoa-Vergniory, N.; Cioroch, M.; Ramm, M.; Smith, A.M.; Gomes, A.C.; Cabral de Miranda, G.; et al. Preclinical development of a vaccine against oligomeric alpha-synuclein based on virus-like particles. PLoS ONE 2017, 12, e0181844. [Google Scholar] [CrossRef] [PubMed]
- Lothert, K.; Eilts, F.; Wolff, M.W. Quantification methods for viruses and virus-like particles applied in biopharmaceutical production processes. Expert Rev. Vaccines 2022, 21, 1029–1044. [Google Scholar] [CrossRef] [PubMed]
| Viral Drug Delivery System | Type of Virus | Tissue Targeting | Molecule | In vitro/In vivo | Disease | Ref. |
|---|---|---|---|---|---|---|
| pLentiM1.2-hNRGN | LV | Hippocampus cells | Ng | In vivo—C57BL/6 mice; 5XFAD mice | AD | [38] |
| Recombinant adenovirus | AV | Malignant glioma cells | shRNA RHBDD1 | In vitro—U87MG; U251 | GBM | [39] |
| Ad5scFvDEC205FF | AV | Dendritic cells | scFv | In vitro—GL261; GL261CMV-IE In vivo—C57BL/6 mice | GBM | [40] |
| Ad-CALR/MAGE-A3 | AV | Glioma cells | CALR; MAGE-A3 | In vitro—U87MG | GBM | [41] |
| AAV.SIRT3-myc | AAV | Stratum | SIRT3 | In vivo—Sprague Dawley rats | PD | [42] |
| AAVrh.10hAPOE2-HA | AAV | Hippocampus cells | APOE2 | In vivo—adult Chlorocebus aethiops sabaeus NHPs | AD | [43] |
| Modified AAV | AAV | Cells of the substantia nigra | TRPV4 shRNAi | In vivo—C57BL/6J mice | PD | [44] |
| Research Title | Drug/Molecule | Status | Application Route | NCT Number | Phase/Disease | Participants |
|---|---|---|---|---|---|---|
| Randomized, Controlled Study Evaluating CERE-110 in Subjects with Mild to Moderate Alzheimer’s Disease | CERE-110, (AAV2-NGF) | Non recruiting | Injection into brain | NCT00876863 | II/AD | 49 |
| Gene Therapy for APOE4 Homozygote of Alzheimer’s Disease | LX1001(AAVrh.10hAPOE2) | Recruiting | n/d | NCT03634007 | I and II/AD | 15 |
| AAV2-GDNF for Advanced Parkinson’s Disease | AAV2-GDNF | Non recruiting | Injection into single arm | NCT01621581 | I/PD | 25 |
| A Study of AAV-hAADC-2 in Subjects with Parkinson’s Disease | AAV-hAADC-2 | Non recruiting | Injection into the striatum | NCT00229736 | I/PD | 10 |
| Study of AAV-GAD Gene Transfer into the Subthalamic Nucleus for Parkinson’s Disease | AAV-GAD | Terminated (due to financial reasons) | Infusion into the subthalamic nucleus region of the brain | NCT00643890 | II/PD | 44 |
| Research Title | Drug/Molecule | Status | Application Route | NCT Number | Phase | Participants |
|---|---|---|---|---|---|---|
| Study of a Retroviral Replicating Vector Combined with a Prodrug to Treat Patients Undergoing Surgery for a Recurrent Malignant Brain Tumor | Toca 511; Toca FC | Non recruiting | Injections into resection cavity wall; orally | NCT01470794 | I | 56 |
| The Toca 5 Trial: Toca 511 & Toca FC Versus Standard of Care in Patients with Recurrent High Grade Glioma (Toca5) | Toca 511; Toca FC | Non recruiting | Injections into resection cavity wall; orally | NCT02414165 | II/III | 403 |
| Viral Therapy in Treating Patients with Recurrent Glioblastoma Multiforme | MV-CEA | Completed | Injection into resection cavity or around tumor bed | NCT00390299 | I | 23 |
| DNX-2440 Oncolytic Adenovirus for Recurrent Glioblastoma | DNX-2440 | Completed | Injection stereotactically | NCT03714334 | I | 16 |
| Genetically Engineered HSV-1 Phase 1 Study for the Treatment of Recurrent Malignant Glioma (M032-HSV-1) | M032 | Active, not recruiting | Infusion through catheters into regions of tumor | NCT02062827 | I | 24 |
| PVSRIPO for Recurrent Glioblastoma (GBM) (PVSRIPO) | PVSRIPO | Completed | Infusion into the tumor | NCT01491893 | I | 60 |
| Research Title | Drug/Molecule | Status | Application Route | NCT Number | Phase | Participants |
|---|---|---|---|---|---|---|
| A Study of AAV9 Gene Therapy in Participants with Canavan Disease (CANaspire) | AAV9 BBP-812 | Recruiting | Intravenous infusion | NCT04998396 | I and II | 18 |
| rAAV-Olig001-ASPA Gene Therapy for Treatment of Children with Typical Canavan Disease (CAN-GT) | rAAV-Olig001-ASPA; levetiracetam; prednisone | Active non recruiting | Intraventricular; administered orally or by gavage | NCT04833907 | I and II | 24 |
| Canavan-Single Patient IND | rAAV9-CB6-AspA | Available | Single intravenous and intraventricular | NCT05317780 | n/d | 1 |
| Virus-Like Particle | Virus | Molecule | Expression Platform | In vitro/In vivo | Disease | Ref. |
|---|---|---|---|---|---|---|
| HBc VLPs | Hepatitis B virus | tau294–305 protein | Escherichia coli (BL21) | In vivo—Tau.P301S mice | AD | [112] |
| Qβ VLPs | Qβ bacteriophage | pT181 | Escherichia coli | In vivo—bitransgenic rTg4510 mice | AD | [113] |
| HPV16 L1a and L1b VLPs | Human papilloma virus | Aβ11–28 epitope | Plants | In vivo—C57BL/6J mice | AD | [114] |
| CMV VLPs | Cucumber mosaic virus | N-terminus of Aβ1–42 epitope | n/d | In vivo—C57BL/6J mice; BALB/c mice | AD | [115] |
| HIV-2 and SIV VLPs | Human immunodeficiency virus type 2 and simian immunodeficiency virus | Vpx | 293T cells | In vitro—H4; LN-229; U87 MG In vivo—female athymic nude mice | GBM | [116] |
| JCPyV VLPs | JC polyomavirus | GFP and thymidine kinase suicide gene | Escherichia coli (JM109) | In vitro—U87 MG In vivo—nu/nu mice | GBM | [117] |
| Qβ VLPs with surface modification by CCP and ApoE | Qβ bacteriophage | RNAic-met | Escherichia coli | In vitro—U87 MG | GBM | [118] |
| BTV VLPs | Bluetongue virus | HSV1-TK | Nicotiana benthamiana | In vitro—U87 MG | GBM | [119] |
| TGN/RGD-HBc VLPs | Hepatitis B virus | Co-delivery: Paclitaxel and YAP siRNA | Escherichia coli (BL21) | In vitro—U87 MG In vivo—BALB/c mice | GBM | [120] |
| Qβ VLPs with human a-syn | Qβ bacteriophage | Synthetic peptides:
| Escherichia coli (JM109) | In vivo—C57BL/6 mice | PD | [121] |
| Patent Title | Patent Number | Viral Vector | Molecule/Drug | Disease | Brief Description |
|---|---|---|---|---|---|
| Compositions and methods for the treatment of neurological disorders related to glucosylceramidase beta deficiency | WO2023091949A2 | AAV particle | Gene encoding GCase | PD | Increased delivery of the gene encoding GCase to enhance alleviation of loss of function and intracellular lipid transport. Improved lysosomal glycolipid metabolism results in reduction, arrest or reversal of PD symptoms |
| Compositions and methods for the treatment of tau-related disorders | WO2023092004A1 | AAV particle | Antibody molecule binding to tau | AD | Delivery of antibodies targeting tau proteins via AAV viral particles, which have a modified genome. Such modification enables expression of anti-tau antibodies by the virus, contributing to the treatment of diseases associated with tau protein pathology |
| AADC/GDNF polynucleotide, and use thereof in treating Parkinson’s disease | WO2023093905A1 | AAV virus | AADC/GDNF polynucleotide | PD | Utilization and optimization of AAV vector to deliver genes encoding AADC and GDNF in the form of polynucleotides for the treatment of Parkinson’s disease patients |
| A method for providing a VLP derived from John Cunningham virus | CA3121689A1 | JCV VLP | Proteins | Brain-related diseases | The invention relates to methods for the delivery of JCV VLPs containing a protein cargo, in particular, the development of a step for the disassembly of VLPs, their re-aggregation and the formation of VLPs. This is aimed at developing an optimal method of drug delivery to areas where the BBB is an obstacle |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, I.; Madej, M.; Secemska, J.; Sarna, R.; Strzalka-Mrozik, B. Virus-Based Biological Systems as Next-Generation Carriers for the Therapy of Central Nervous System Diseases. Pharmaceutics 2023, 15, 1931. https://doi.org/10.3390/pharmaceutics15071931
Nowak I, Madej M, Secemska J, Sarna R, Strzalka-Mrozik B. Virus-Based Biological Systems as Next-Generation Carriers for the Therapy of Central Nervous System Diseases. Pharmaceutics. 2023; 15(7):1931. https://doi.org/10.3390/pharmaceutics15071931
Chicago/Turabian StyleNowak, Ilona, Marcel Madej, Julia Secemska, Robert Sarna, and Barbara Strzalka-Mrozik. 2023. "Virus-Based Biological Systems as Next-Generation Carriers for the Therapy of Central Nervous System Diseases" Pharmaceutics 15, no. 7: 1931. https://doi.org/10.3390/pharmaceutics15071931
APA StyleNowak, I., Madej, M., Secemska, J., Sarna, R., & Strzalka-Mrozik, B. (2023). Virus-Based Biological Systems as Next-Generation Carriers for the Therapy of Central Nervous System Diseases. Pharmaceutics, 15(7), 1931. https://doi.org/10.3390/pharmaceutics15071931








