Osteoinductive Electrospun Scaffold Based on PCL-Col as a Regenerative Therapy for Peri-Implantitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning Parameters
2.3. Physicochemical Characterization
2.3.1. Mechanical Properties
2.3.2. TGA-DSC
2.3.3. FTIR
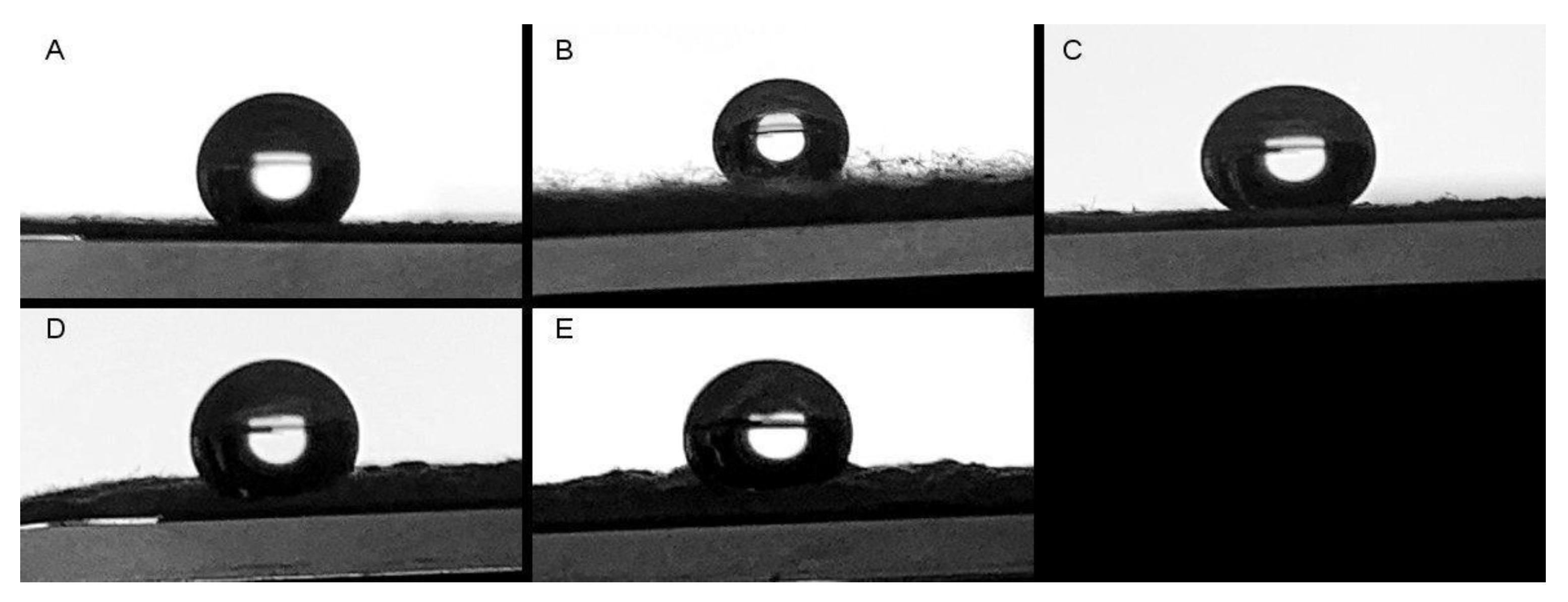
2.3.4. Water Contact Angle
2.4. Biocompatibility Assays
2.4.1. Proliferation Analysis
2.4.2. Cytotoxicity Analysis
2.4.3. Hemocompatibility Evaluation
2.4.4. Bone Matrix Evaluation
2.5. Statistical Analysis
3. Results and Discussion
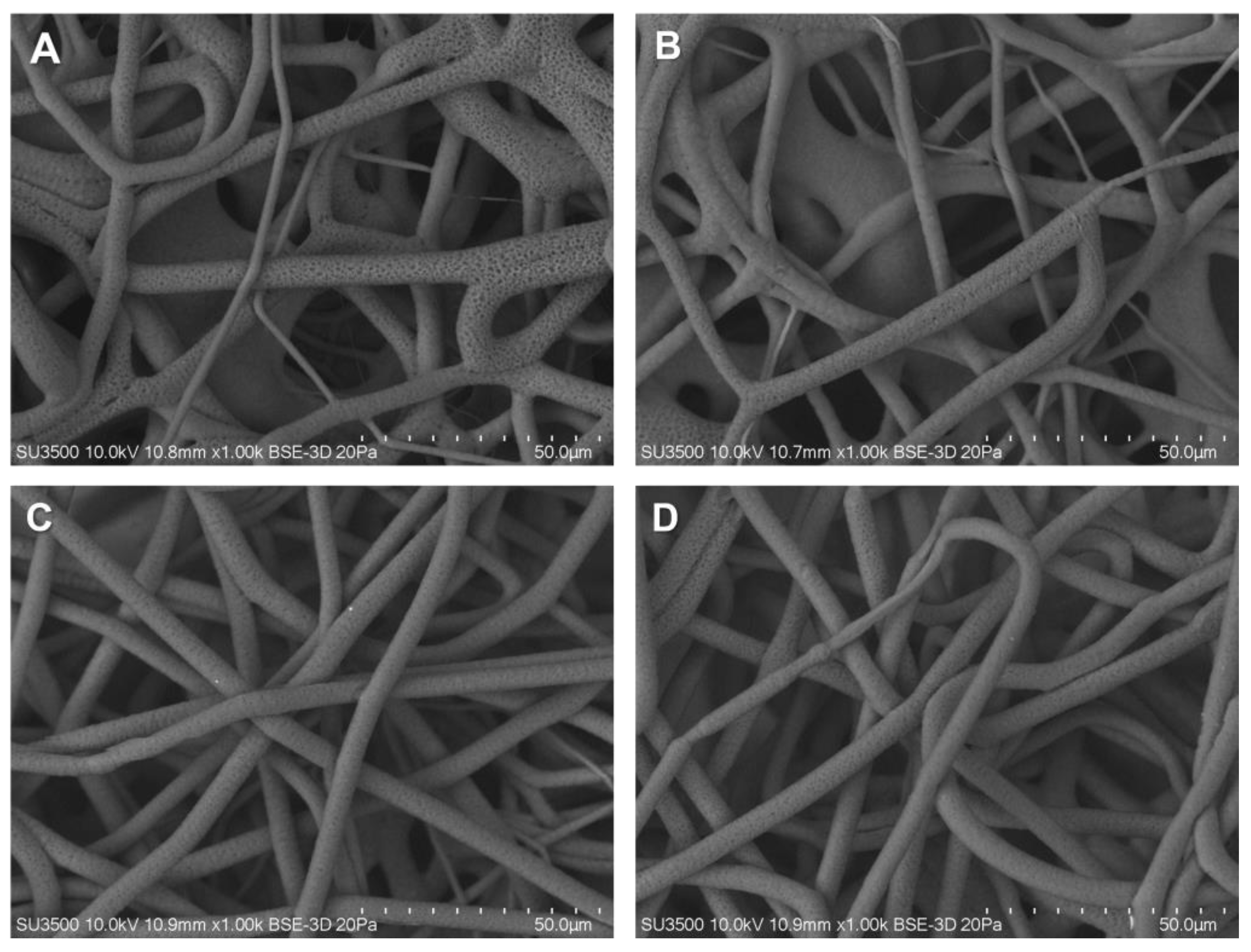
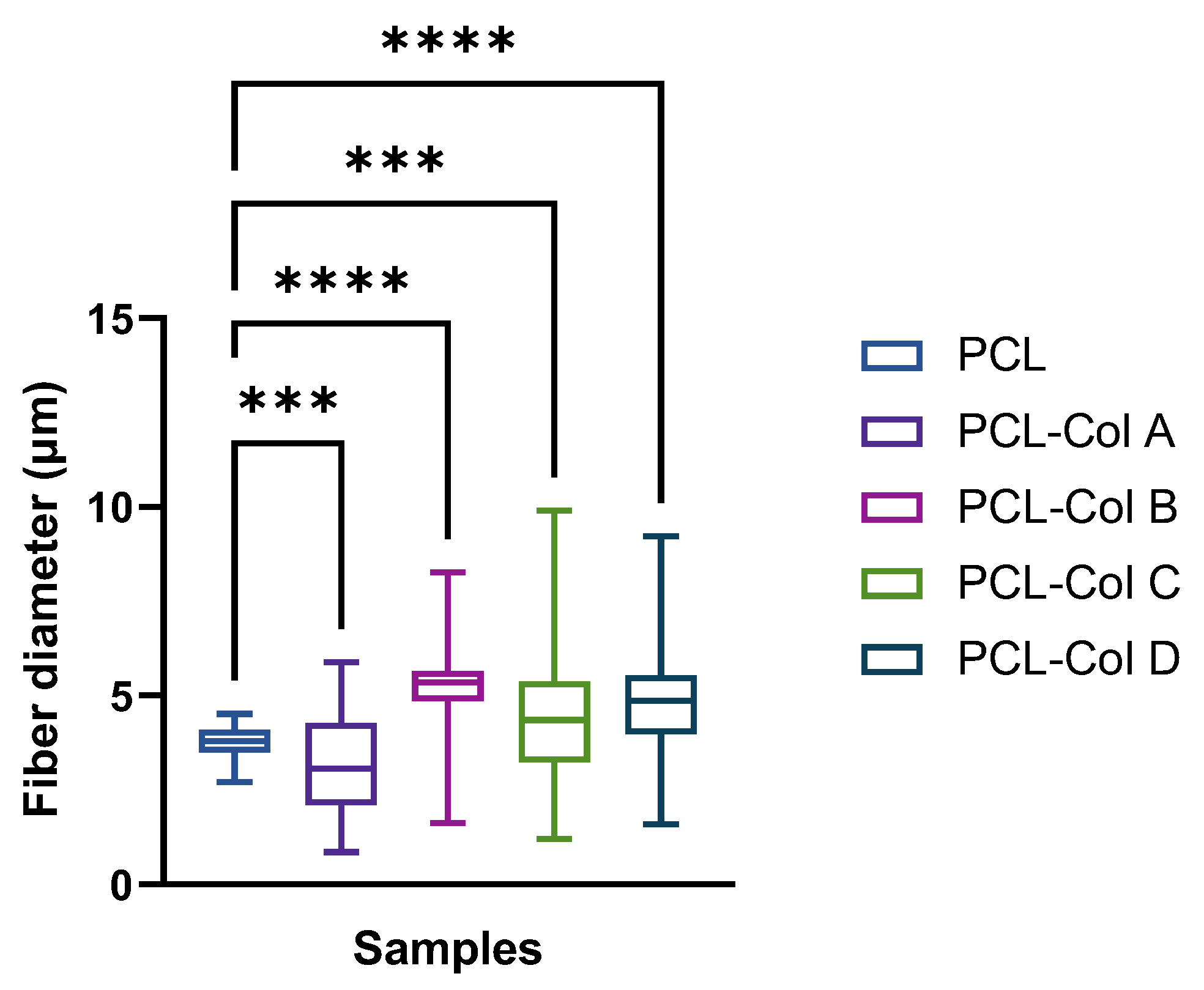
3.1. Fibers Characterization
3.2. Mechanical Characterization
3.3. Thermogravimetric Analysis
3.4. Cell Biocompatibility Evaluation
3.4.1. Cell Proliferation
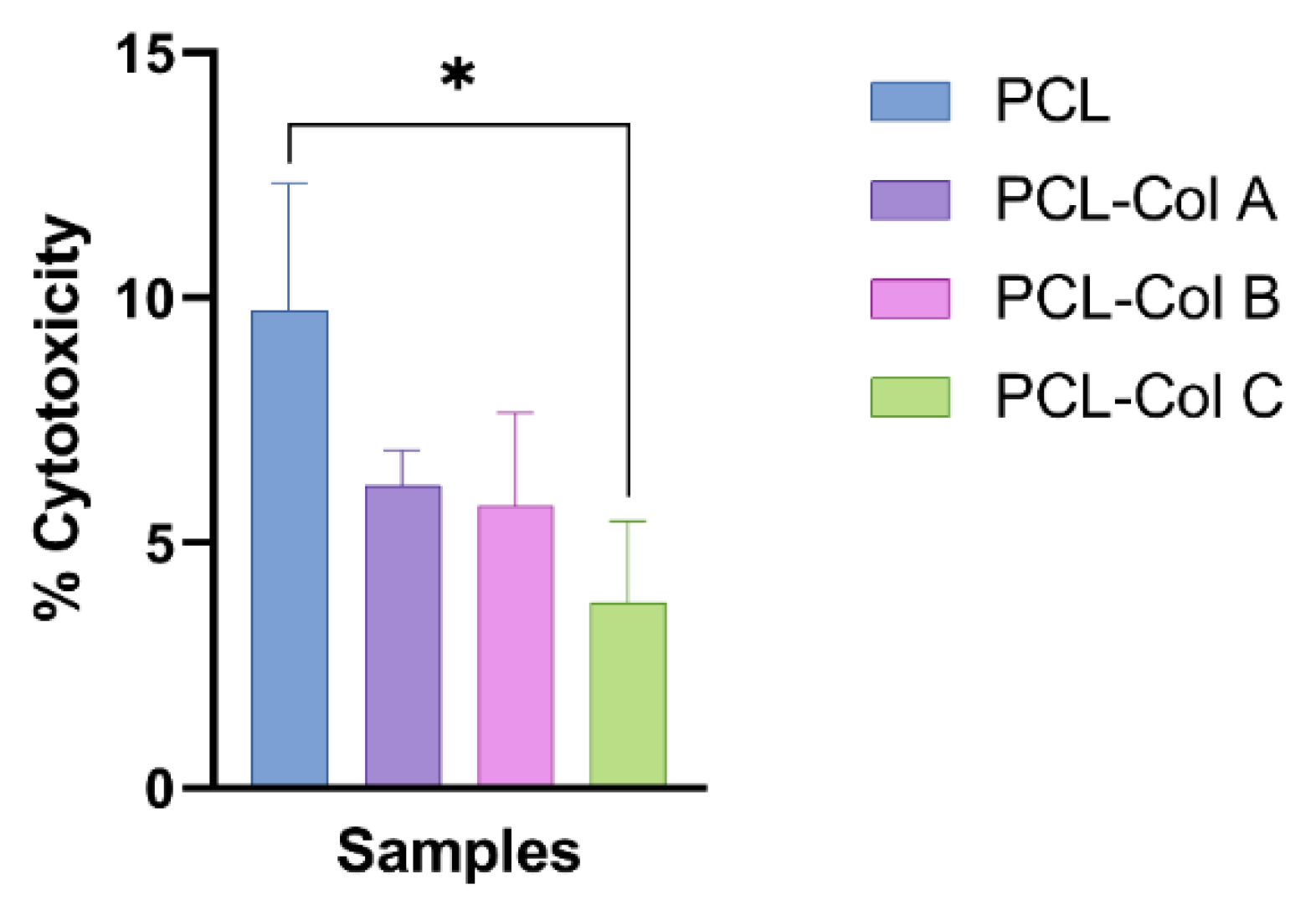
3.4.2. Cell Cytotoxicity
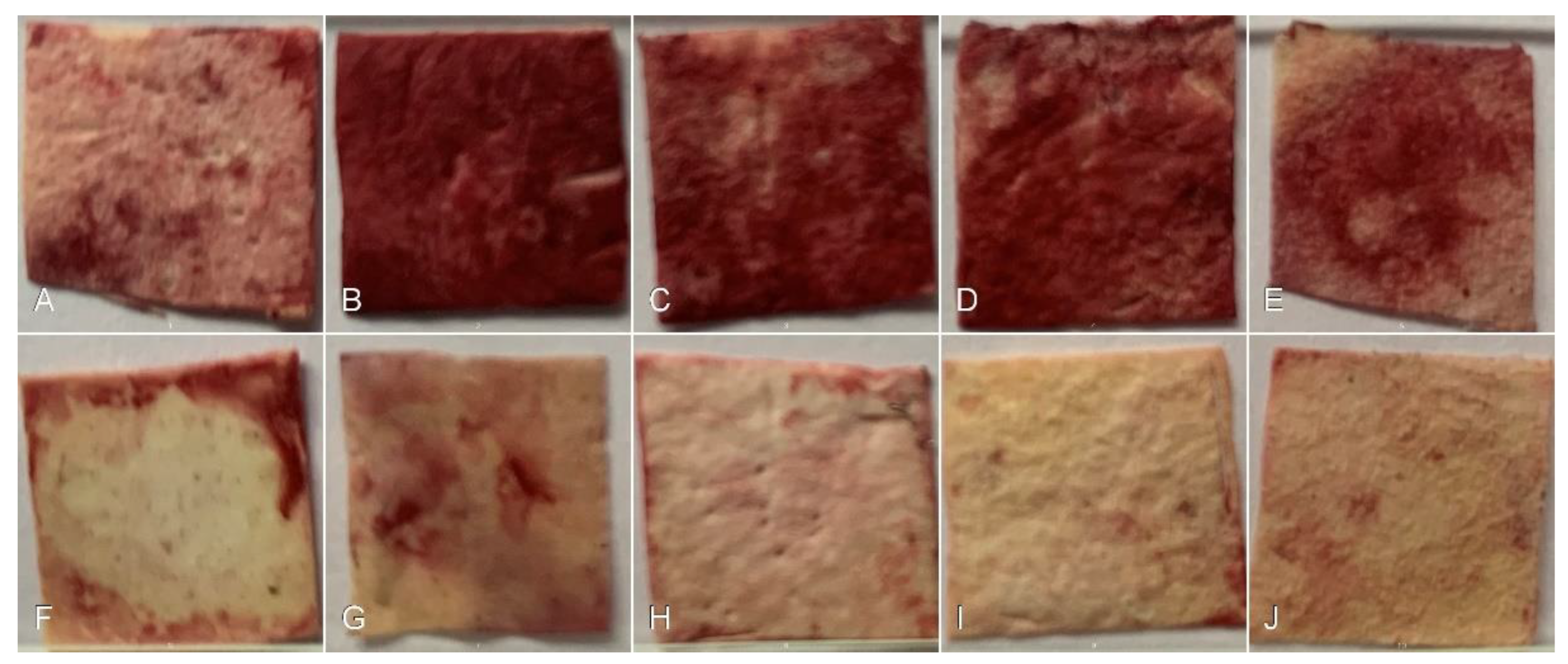
3.4.3. Bone Matrix Evaluation
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dreyer, H.; Grischke, J.; Tiede, C.; Eberhard, J.; Schweitzer, A.; Toikkanen, S.E.; Glöckner, S.; Krause, G.; Stiesch, M. Epidemiology and risk factors of peri-implantitis: A systematic review. J. Periodontal Res. 2018, 53, 657–681. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodontol. 2018, 89, S267–S290. [Google Scholar] [CrossRef] [PubMed]
- Klinge, B.; Klinge, A.; Bertl, K.; Stavropoulos, A. Peri-implant diseases. Eur. J. Oral Sci. 2018, 126, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Avila, E.D.; Oirschot, B.A.; van den Beucken, J.J.J.P. Biomaterial-based possibilities for managing peri-implantitis. J. Periodontal Res. 2020, 55, 165–173. [Google Scholar] [CrossRef]
- Martin, V.; Bettencourt, A. Bone regeneration: Biomaterials as local delivery systems with improved osteoinductive properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 363–371. [Google Scholar] [CrossRef]
- Jin, S.; Li, J.; Wang, J.; Jiang, J.; Zuo, Y.; Li, Y.; Yang, F. Electrospun silver ion-loaded calcium phosphate/ chitosan antibacterial composite fibrous membranes for guided bone regeneration. Int. J. Nanomed. 2018, 13, 4591–4605. [Google Scholar] [CrossRef] [Green Version]
- Bhushani, A.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Kitsara, M.; Agbulut, O.; Kontziampasis, D.; Chen, Y.; Menasché, P. Fibers for hearts: A critical review on electrospinning for cardiac tissue engineering. Acta Biomater. 2017, 48, 20–40. [Google Scholar] [CrossRef]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014, 10, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Nune, S.K.; Rama, K.S.; Dirisala, V.R.; Chavali, M.Y. Chapter 11—Electrospinning of Collagen Nanofiber Scaffolds for Tissue Repair and Regeneration; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780323461429. [Google Scholar]
- Kotcharat, P.; Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. Development of bacterial cellulose and polycaprolactone (PCL) based composite for medical material. Sustain. Chem. Pharm. 2021, 20, 100404. [Google Scholar] [CrossRef]
- Ho, T.T.P.; Doan, V.K.; Tran, N.M.P.; Nguyen, L.K.K.; Le, A.N.M.; Ho, M.H.; Trinh, N.T.; Van Vo, T.; Tran, L.D.; Nguyen, T.H. Fabrication of chitosan oligomer-coated electrospun polycaprolactone membrane for wound dressing application. Mater. Sci. Eng. C 2021, 120, 111724. [Google Scholar] [CrossRef] [PubMed]
- Satué, M.; Córdoba, A.; Ramis, J.M.; Monjo, M. UV-irradiated 7-dehydrocholesterol coating on polystyrene surfaces is converted to active vitamin D by osteoblastic MC3T3-E1 cells. Photochem. Photobiol. Sci. 2013, 12, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza, C.; Diaz-Rodriguez, P.; Villegas, P.; González, Á.; Seeger, M.; Suárez-González, J.; Concheiro, A.; Alvarez-Lorenzo, C.; Acevedo, F. Influence of the carbon source on the properties of poly-(3)-hydroxybutyrate produced by Paraburkholderia xenovorans LB400 and its electrospun fibers. Int. J. Biol. Macromol. 2020, 152, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, F.; Hermosilla, J.; Sanhueza, C.; Mora-Lagos, B.; Fuentes, I.; Rubilar, M.; Concheiro, A.; Alvarez-Lorenzo, C. Gallic acid loaded PEO-core/zein-shell nanofibers for chemopreventive action on gallbladder cancer cells. Eur. J. Pharm. Sci. 2018, 119, 49–61. [Google Scholar] [CrossRef]
- Acevedo, F.; Villegas, P.; Urtuvia, V.; Hermosilla, J.; Navia, R.; Seeger, M. Bacterial polyhydroxybutyrate for electrospun fiber production. Int. J. Biol. Macromol. 2018, 106, 692–697. [Google Scholar] [CrossRef]
- Sanhueza, C.; Hermosilla, J.; Bugallo-Casal, A.; Da Silva-Candal, A.; Taboada, C.; Millán, R.; Concheiro, A.; Alvarez-Lorenzo, C.; Acevedo, F. One-step electrospun scaffold of dual-sized gelatin/poly-3-hydroxybutyrate nano/microfibers for skin regeneration in diabetic wound. Mater. Sci. Eng. C 2021, 119, 111602. [Google Scholar] [CrossRef]
- Pereira, I.H.L.; Ayres, E.; Averous, L.; Schlatter, G.; Hebraud, A.; de Paula, A.C.C.; Viana, P.H.L.; Goes, A.M.; Oréfice, R.L. Differentiation of human adipose-derived stem cells seeded on mineralized electrospun co-axial poly(ε-caprolactone) (PCL)/gelatin nanofibers. J. Mater. Sci. Mater. Med. 2014, 25, 1137–1148. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Van Der Schueren, L.; De Schoenmaker, B.; Kalaoglu, Ö.I.; De Clerck, K. An alternative solvent system for the steady state electrospinning of polycaprolactone. Eur. Polym. J. 2011, 47, 1256–1263. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; He, M.; Liang, Y.; Crawford, A.; Coates, P.; Chen, D.; Shi, R.; Zhang, L. Fabrication and evaluation of electrospun PCL-gelatin micro-/nanofiber membranes for anti-infective GTR implants. J. Mater. Chem. B 2014, 2, 6867–6877. [Google Scholar] [CrossRef]
- Baker, S.; Sigley, J.; Helms, C.C.; Stitzel, J.; Berry, J.; Bonin, K.; Guthold, M. The mechanical properties of dry, electrospun fibrinogen fibers. Mater. Sci. Eng. C 2012, 32, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and properties of electrospun PCL and its composites for medical applications: A mini review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef] [Green Version]
- Aydogdu, M.O.; Ekren, N.; Suleymanoglu, M.; Erdem-Kuruca, S.; Lin, C.-C.; Bulbul, E.; Erdol, M.N.; Oktar, F.N.; Terzi, U.K.; Kilic, O.; et al. Novel electrospun polycaprolactone/graphene oxide/Fe3O4 nanocomposites for biomedical applications. Colloids Surf. B Biointerfaces 2018, 172, 718–727. [Google Scholar] [CrossRef]
- Son, S.-R.; Linh, N.-T.B.; Yang, H.-M.; Lee, B.-T. In vitro and in vivo evaluation of electrospun PCL/PMMA fibrous scaffolds for bone regeneration. Sci. Technol. Adv. Mater. 2013, 14, 015009. [Google Scholar] [CrossRef]
- Pattanashetti, N.A.; Achari, D.D.; Torvi, A.I.; Doddamani, R.V.; Kariduraganavar, M.Y. Development of multilayered nanofibrous scaffolds with PCL and PVA:NaAlg using electrospinning technique for bone tissue regeneration. Materialia 2020, 12, 100826. [Google Scholar] [CrossRef]
- Siddiqui, N.; Kishori, B.; Rao, S.; Anjum, M.; Hemanth, V.; Das, S.; Jabbari, E. Electropsun Polycaprolactone Fibres in Bone Tissue Engineering: A Review. Mol. Biotechnol. 2021, 63, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.G.B.; Diba, M.; Kersten, M.; Jansen, J.A.; van den Beucken, J.J.J.P.; Yang, F. Development of a PCL-silica nanoparticles composite membrane for Guided Bone Regeneration. Mater. Sci. Eng. C 2018, 85, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Feng, B.; Guo, X.; Wang, J.; Zhao, L.; Zhou, G.; Liu, W.; Cao, Y.; Zhang, W.J. Engineering of epidermis skin grafts using electrospun nanofibrous gelatin/polycaprolactone membranes. Int. J. Nanomed. 2013, 8, 2077–2084. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.T.; Hamid, Z.A.; Cheong, K.Y. A Review of Mechanical Properties of Scaffold in Tissue Engineering: Aloe Vera Composites. J. Phys. Conf. Ser. 2018, 1082, 012080. [Google Scholar] [CrossRef]
- Wsoo, M.A.; Razak, S.I.A.; Bohari, S.P.M.; Shahir, S.; Salihu, R.; Kadir, M.R.A.; Nayan, N.H.M. Vitamin D3-loaded electrospun cellulose acetate/polycaprolactone nanofibers: Characterization, in-vitro drug release and cytotoxicity studies. Int. J. Biol. Macromol. 2021, 181, 82–98. [Google Scholar] [CrossRef]
- Vatankhah, E. Rosmarinic acid-loaded electrospun nanofibers: In vitro release kinetic study and bioactivity assessment. Eng. Life Sci. 2018, 18, 732–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosworth, L.A.; Downes, S. Physicochemical characterisation of degrading polycaprolactone scaffolds. Polym. Degrad. Stab. 2010, 95, 2269–2276. [Google Scholar] [CrossRef]
- Tardajos, M.G.; Cama, G.; Dash, M.; Misseeuw, L.; Gheysens, T.; Gorzelanny, C.; Coenye, T.; Dubruel, P. Chitosan functionalized poly-ε-caprolactone electrospun fibers and 3D printed scaffolds as antibacterial materials for tissue engineering applications. Carbohydr. Polym. 2018, 191, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Sattary, M.; Khorasani, M.T.; Rafienia, M.; Rozve, H.S. Incorporation of nanohydroxyapatite and vitamin D3 into electrospun PCL/Gelatin scaffolds: The influence on the physical and chemical properties and cell behavior for bone tissue engineering. Polym. Adv. Technol. 2018, 29, 451–462. [Google Scholar] [CrossRef]
- Asghari, F.; Rabiei Faradonbeh, D.; Malekshahi, Z.V.; Nekounam, H.; Ghaemi, B.; Yousefpoor, Y.; Ghanbari, H.; Faridi-Majidi, R. Hybrid PCL/chitosan-PEO nanofibrous scaffolds incorporated with A. euchroma extract for skin tissue engineering application. Carbohydr. Polym. 2022, 278, 118926. [Google Scholar] [CrossRef]
- Wang, F.; Liu, K.; Xi, Y.; Li, Z. One-step electrospinning PCL/ph-LPSQ nanofibrous membrane with excellent self-cleaning and oil-water separation performance. Polymer 2022, 249, 124858. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, P.; Zhang, X.; Xiang, C.; Li, L. Preparation of hierarchically structured PCL superhydrophobic membrane via alternate electrospinning/electrospraying techniques. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 421–430. [Google Scholar] [CrossRef]
- Zavan, B.; Gardin, C.; Guarino, V.; Rocca, T.; Maya, I.C.; Zanotti, F.; Ferroni, L.; Brunello, G.; Chachques, J.C.; Ambrosio, L.; et al. Electrospun pcl-based vascular grafts: In vitro tests. Nanomaterials 2021, 11, 751. [Google Scholar] [CrossRef]
- Al-Bishari, A.M.; Al-Shaaobi, B.A.; Al-Bishari, A.A.; Al-Baadani, M.A.; Yu, L.; Shen, J.; Cai, L.; Shen, Y.; Deng, Z.; Gao, P. Vitamin D and curcumin-loaded PCL nanofibrous for engineering osteogenesis and immunomodulatory scaffold. Front. Bioeng. Biotechnol. 2022, 10, 975431. [Google Scholar] [CrossRef]
- Belgheisi, G.; Nazarpak, M.H.; Hashjin, M.S. Bone tissue engineering electrospun scaffolds based on layered double hydroxides with the ability to release vitamin D3: Fabrication, characterization and in vitro study. Appl. Clay Sci. 2020, 185, 105434. [Google Scholar] [CrossRef]
- Chen, W.-C.; Huang, B.-Y.; Huang, S.-M.; Liu, S.-M.; Chang, K.-C.; Ko, C.-L.; Lin, C.-L. In vitro evaluation of electrospun polyvinylidene fluoride hybrid nanoparticles as direct piezoelectric membranes for guided bone regeneration. Biomater. Adv. 2023, 144, 213228. [Google Scholar] [CrossRef] [PubMed]
- Sattary, M.; Rafienia, M.; Kazemi, M.; Salehi, H.; Mahmoudzadeh, M. Promoting effect of nano hydroxyapatite and vitamin D3 on the osteogenic differentiation of human adipose-derived stem cells in polycaprolactone/gelatin scaffold for bone tissue engineering. Mater. Sci. Eng. C 2019, 97, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.S.; Kohles, S.S.; Winn, S.R.; Zelick, R.D. Extrahepatic 25-Hydroxylation of Vitamin D 3 in an Engineered Osteoblast Precursor Cell Line Exploring the Influence on Cellular Proliferation and Matrix Maturation during Bone Development. ISRN Biomed. Eng. 2013, 2013, 956362. [Google Scholar] [CrossRef] [PubMed]





| Elongation at Break (%) | Tensile Strength (Mpa) | Young’s Modulus | ||
|---|---|---|---|---|
| PCL | Dry | 665.26 ± 49.10 | 3.10 ± 0.13 | 8.92 ± 0.30 |
| Wet | 524.03 ± 134.26 | 25.21 ± 3.50 | 35.81 ± 18.26 | |
| PCL-Col A | Dry | 616.70 ± 139.15 | 1.35 ± 0.36 | 0.97 ± 0.13 |
| Wet | 364.62 ± 67.58 | 12.69 ± 0.83 | 12.24 ± 0.83 | |
| PCL-Col B | Dry | 514.35 ± 124.76 | 1.11 ± 0.10 | 1.12 ± 0.07 |
| Wet | 420.72 ± 29.78 | 12.79 ± 0.73 | 13.35 ± 0.97 | |
| PCL-Col C | Dry | 1053.30 ± 186.28 * | 2.75 ± 0.13 | 5.04 ± 0.81 |
| Wet | 1024.36 ± 509.51 * | 25.47 ± 2.61 | 24.99 ± 0.99 | |
| PCL-Col D | Dry | 589.38 ± 56.48 | 1.56 ± 0.29 | 2.21 ± 0.93 |
| Wet | 791.93 ± 328.53 | 15.75 ± 0.00 | 14.80 ± 3.31 |
| ΔHm (J/g) | Tm (°C) | Cx (%) | Tb (°C) | Contact Angle (°) | |
|---|---|---|---|---|---|
| PCL | 32.600 | 61.40 | 23.40 | 410.07 | 130.04 ± 3.32 |
| PCL-Col A | 109.705 | 34.65 | 78.78 | 410.05 | 124.22 ± 4.78 |
| PCL-Col B | 56.313 | 37.26 | 40.42 | 404.04 | 130.95 ± 6.26 |
| PCL-Col C | 87.702 | 37.98 | 62.95 | 422.95 | 132.22 ± 13.91 |
| PCL-Col D | 96.045 | 36.72 | 68.94 | 422.14 | 131.77 ± 10.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanhueza, C.; Hermosilla, J.; Klein, C.; Chaparro, A.; Valdivia-Gandur, I.; Beltrán, V.; Acevedo, F. Osteoinductive Electrospun Scaffold Based on PCL-Col as a Regenerative Therapy for Peri-Implantitis. Pharmaceutics 2023, 15, 1939. https://doi.org/10.3390/pharmaceutics15071939
Sanhueza C, Hermosilla J, Klein C, Chaparro A, Valdivia-Gandur I, Beltrán V, Acevedo F. Osteoinductive Electrospun Scaffold Based on PCL-Col as a Regenerative Therapy for Peri-Implantitis. Pharmaceutics. 2023; 15(7):1939. https://doi.org/10.3390/pharmaceutics15071939
Chicago/Turabian StyleSanhueza, Claudia, Jeyson Hermosilla, Catherine Klein, Alejandra Chaparro, Iván Valdivia-Gandur, Víctor Beltrán, and Francisca Acevedo. 2023. "Osteoinductive Electrospun Scaffold Based on PCL-Col as a Regenerative Therapy for Peri-Implantitis" Pharmaceutics 15, no. 7: 1939. https://doi.org/10.3390/pharmaceutics15071939






