Nanomedicine and Hyperthermia for the Treatment of Gastrointestinal Cancer: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Eligibility
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Sources
2.5. Study Selection
2.6. Data Extraction
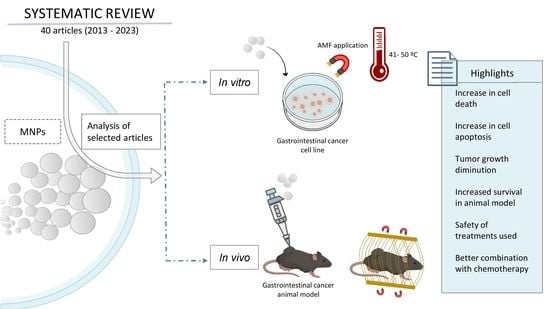
3. Results and Discussion
3.1. Study Description
3.2. Characteristics of Magnetic Nanoformulations
3.3. Biocompatibility of Hyperthermia Assays
3.4. In Vitro Assays
3.5. In Vivo Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.L.; Yu, S.J. Esophageal Cancer: Risk Factors, Genetic Association, and Treatment. Asian J. Surg. 2018, 41, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef]
- Sexton, R.E.; Al Hallak, M.N.; Diab, M.; Azmi, A.S. Gastric Cancer: A Comprehensive Review of Current and Future Treatment Strategies. Cancer Metastasis Rev. 2020, 39, 1179–1203. [Google Scholar] [CrossRef]
- Watanabe, M.; Otake, R.; Kozuki, R.; Toihata, T.; Takahashi, K.; Okamura, A.; Imamura, Y. Recent Progress in Multidisciplinary Treatment for Patients with Esophageal Cancer. Surg. Today 2020, 50, 12–20. [Google Scholar] [CrossRef]
- Knowlton, C.A.; Mackay, M.K.; Speer, T.W.; Vera, R.B.; Arthur, D.W.; Wazer, D.E.; Lanciano, R.; Brashears, J.H.; Knowlton, C.A.; Mackay, M.K.; et al. Cancer Colon. In Encyclopedia of Radiation Oncology; Springer: Berlin/Heidelberg, Germany, 2013; p. 77. [Google Scholar]
- Shiga, T.; Hiraide, M. Cardiotoxicities of 5-Fluorouracil and Other Fluoropyrimidines. Curr. Treat. Options Oncol. 2020, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahayri, Z.N.; AlAhmad, M.M.; Ali, B.R. Current Opinion on the Pharmacogenomics of Paclitaxel-Induced Toxicity. Expert. Opin. Drug Metab. Toxicol. 2021, 17, 785–801. [Google Scholar] [CrossRef]
- Garbayo, E.; Pascual-Gil, S.; Rodríguez-Nogales, C.; Saludas, L.; Estella-Hermoso de Mendoza, A.; Blanco-Prieto, M.J. Nanomedicine and Drug Delivery Systems in Cancer and Regenerative Medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1637. [Google Scholar] [CrossRef]
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and Cancer Therapy: Perspectives for Application of Nanoparticles in the Treatment of Cancers. J. Cell Physiol. 2020, 235, 1962–1972. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Kumar, D.N.; Shaik, R.A.; Eid, B.G.; Abdel-Naim, A.B.; Md, S.; Ahmad, A.; Agrawal, A.K. Lipid-Based Nanoparticles as a Pivotal Delivery Approach in Triple Negative Breast Cancer (TNBC) Therapy. Int. J. Mol. Sci. 2022, 23, 10068. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Valdivieso, J.; Girotti, A.; Schneider, J.; Arias, F.J. Advanced Nanomedicine and Cancer: Challenges and Opportunities in Clinical Translation. Int. J. Pharm. 2021, 599, 120438. [Google Scholar] [CrossRef] [PubMed]
- Milano, G.; Innocenti, F.; Minami, H. Liposomal Irinotecan (Onivyde): Exemplifying the Benefits of Nanotherapeutic Drugs. Cancer Sci. 2022, 113, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Vurro, F.; Jabalera, Y.; Mannucci, S.; Glorani, G.; Sola-Leyva, A.; Gerosa, M.; Romeo, A.; Romanelli, M.G.; Malatesta, M.; Calderan, L.; et al. Improving the Cellular Uptake of Biomimetic Magnetic Nanoparticles. Nanomaterials 2021, 11, 766. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.-P. Magnetic Nanoparticles in Nanomedicine: A Review of Recent Advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef]
- Farzin, A.; Etesami, S.A.; Quint, J.; Memic, A.; Tamayol, A. Magnetic Nanoparticles in Cancer Therapy and Diagnosis. Adv. Heal. Healthc. Mater. 2020, 9, 1901058. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xin, J.; Sun, Y.; Han, T.; Zhang, H.; An, F. Magnetic Resonance Imaging-Guided and Targeted Theranostics of Colorectal Cancer. Cancer Biol. Med. 2020, 17, 307–327. [Google Scholar] [CrossRef]
- Jose, J.; Kumar, R.; Harilal, S.; Mathew, G.E.; Parambi, D.G.T.; Prabhu, A.; Uddin, M.S.; Aleya, L.; Kim, H.; Mathew, B. Magnetic Nanoparticles for Hyperthermia in Cancer Treatment: An Emerging Tool. Environ. Sci. Pollut. Res. 2020, 27, 19214–19225. [Google Scholar] [CrossRef]
- Acar, M.; Solak, K.; Yildiz, S.; Unver, Y.; Mavi, A. Comparative Heating Efficiency and Cytotoxicity of Magnetic Silica Nanoparticles for Magnetic Hyperthermia Treatment on Human Breast Cancer Cells. 3 Biotech 2022, 12, 313. [Google Scholar] [CrossRef]
- Minaei, S.E.; Khoei, S.; Khoee, S.; Mahdavi, S.R. Sensitization of Glioblastoma Cancer Cells to Radiotherapy and Magnetic Hyperthermia by Targeted Temozolomide-Loaded Magnetite Tri-Block Copolymer Nanoparticles as a Nanotheranostic Agent. Life Sci. 2022, 306, 120729. [Google Scholar] [CrossRef]
- Narayanaswamy, V.; Jagal, J.; Khurshid, H.; Al-Omari, I.A.; Haider, M.; Kamzin, A.S.; Obaidat, I.M.; Issa, B. Hyperthermia of Magnetically Soft-Soft Core-Shell Ferrite Nanoparticles. Int. J. Mol. Sci. 2022, 23, 14825. [Google Scholar] [CrossRef]
- Piehler, S.; Dähring, H.; Grandke, J.; Göring, J.; Couleaud, P.; Aires, A.; Cortajarena, A.L.; Courty, J.; Latorre, A.; Somoza, Á.; et al. Iron Oxide Nanoparticles as Carriers for DOX and Magnetic Hyperthermia after Intratumoral Application into Breast Cancer in Mice: Impact and Future Perspectives. Nanomaterials 2020, 10, 1016. [Google Scholar] [CrossRef]
- Rego, G.; Nucci, M.; Mamani, J.; Oliveira, F.; Marti, L.; Filgueiras, I.; Ferreira, J.; Real, C.; Faria, D.; Espinha, P.; et al. Therapeutic Efficiency of Multiple Applications of Magnetic Hyperthermia Technique in Glioblastoma Using Aminosilane Coated Iron Oxide Nanoparticles: In Vitro and In Vivo Study. Int. J. Mol. Sci. 2020, 21, 958. [Google Scholar] [CrossRef]
- Dabaghi, M.; Rasa, S.M.M.; Cirri, E.; Ori, A.; Neri, F.; Quaas, R.; Hilger, I. Iron Oxide Nanoparticles Carrying 5-Fluorouracil in Combination with Magnetic Hyperthermia Induce Thrombogenic Collagen Fibers, Cellular Stress, and Immune Responses in Heterotopic Human Colon Cancer in Mice. Pharmaceutics 2021, 13, 1625. [Google Scholar] [CrossRef] [PubMed]
- Muka, T.; Glisic, M.; Milic, J.; Verhoog, S.; Bohlius, J.; Bramer, W.; Chowdhury, R.; Franco, O.H. A 24-Step Guide on How to Design, Conduct, and Successfully Publish a Systematic Review and Meta-Analysis in Medical Research. Eur. J. Epidemiol. 2020, 35, 49–60. [Google Scholar] [CrossRef]
- Száva-Kováts, E. Unfounded Attribution of the “Half-Life” Index-Number of Literature Obsolescence to Burton and Kebler: A Literature Science Study. J. Am. Soc. Inf. Sci. Technol. 2002, 53, 1098–1105. [Google Scholar] [CrossRef]
- Wanden-Berghe, C.; Sanz-Valero, J. Systematic Reviews in Nutrition: Standardized Methodology. Br. J. Nutr. 2012, 107, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Faruque, H.A.; Kee, H.; Kim, E.; Park, S. Exosome-Based Hybrid Nanostructures for Enhanced Tumor Targeting and Hyperthermia Therapy. Colloids Surf. B Biointerfaces 2021, 205, 111915. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-C.; Lin, F.-H.; Lin, J.-C. In Vitro Characterization of Magnetic Electrospun IDA-Grafted Chitosan Nanofiber Composite for Hyperthermic Tumor Cell Treatment. J. Biomater. Sci. Polym. Ed. 2013, 24, 1152–1163. [Google Scholar] [CrossRef]
- Park, J.; Jin, C.; Lee, S.; Kim, J.; Choi, H. Magnetically Actuated Degradable Microrobots for Actively Controlled Drug Release and Hyperthermia Therapy. Adv. Healthc. Mater. 2019, 8, 1900213. [Google Scholar] [CrossRef]
- Li, C.; Ruan, J.; Yang, M.; Pan, F.; Gao, G.; Qu, S.; Shen, Y.L.; Dang, Y.J.; Wang, K.; Jin, W.L.; et al. Human Induced Pluripotent Stem Cells Labeled with Fluorescent Magnetic Nanoparticles for Targeted Imaging and Hyperthermia Therapy for Gastric Cancer. Cancer Biol. Med. 2015, 12, 163. [Google Scholar] [CrossRef]
- Yang, S.-J.; Tseng, S.-Y.; Wang, C.-H.; Young, T.-H.; Chen, K.-C.; Shieh, M.-J. Magnetic Nanomedicine for CD133-Expressing Cancer Therapy Using Locoregional Hyperthermia Combined with Chemotherapy. Nanomedicine 2020, 15, 2543–2561. [Google Scholar] [CrossRef]
- Kagawa, T.; Matsumi, Y.; Aono, H.; Ohara, T.; Tazawa, H.; Shigeyasu, K.; Yano, S.; Takeda, S.; Komatsu, Y.; Hoffman, R.M.; et al. Immuno-Hyperthermia Effected by Antibody-Conjugated Nanoparticles Selectively Targets and Eradicates Individual Cancer Cells. Cell Cycle 2021, 20, 1221–1230. [Google Scholar] [CrossRef]
- Stanković, A.; Mihailović, J.; Mirković, M.; Radović, M.; Milanović, Z.; Ognjanović, M.; Janković, D.; Antić, B.; Mijović, M.; Vranješ-Đurić, S.; et al. Aminosilanized Flower-Structured Superparamagnetic Iron Oxide Nanoparticles Coupled to 131I-Labeled CC49 Antibody for Combined Radionuclide and Hyperthermia Therapy of Cancer. Int. J. Pharm. 2020, 587, 119628. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Liu, T.Y.; Chan, T.Y.; Tsai, S.C.; Hardiansyah, A.; Huang, L.Y.; Yang, M.C.; Lu, R.H.; Jiang, J.K.; Yang, C.Y.; et al. Magnetically Triggered Nanovehicles for Controlled Drug Release as a Colorectal Cancer Therapy. Colloids Surf. B Biointerfaces 2016, 140, 567–573. [Google Scholar] [CrossRef]
- Fang, Y.; He, Y.; Wu, C.; Zhang, M.; Gu, Z.; Zhang, J.; Liu, E.; Xu, Q.; Asrorov, A.M.; Huang, Y. Magnetism-Mediated Targeting Hyperthermia-Immunotherapy in “Cold” Tumor with CSF1R Inhibitor. Theranostics 2021, 11, 6860–6872. [Google Scholar] [CrossRef]
- Jahangiri, S.; Khoei, S.; Khoee, S.; Safa, M.; Shirvalilou, S.; Pirhajati Mahabadi, V. Potential Anti-Tumor Activity of 13.56 MHz Alternating Magnetic Hyperthermia and Chemotherapy on the Induction of Apoptosis in Human Colon Cancer Cell Lines HT29 and HCT116 by up-Regulation of Bax, Cleaved Caspase 3&9, and Cleaved PARP Proteins. Cancer Nanotechnol. 2021, 12, 34. [Google Scholar] [CrossRef]
- Ha, P.T.; Le, T.T.H.; Ung, T.D.T.; Do, H.D.; Doan, B.T.; Mai, T.T.T.; Pham, H.N.; Hoang, T.M.N.; Phan, K.S.; Bui, T.Q. Properties and Bioeffects of Magneto–near Infrared Nanoparticles on Cancer Diagnosis and Treatment. New J. Chem. 2020, 44, 17277–17288. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Lin, P.-Y.; Hsieh, S.-L.; Kirankumar, R.; Lin, H.-Y.; Li, J.-H.; Chen, Y.-T.; Wu, H.-M.; Hsieh, S. Utilizing Edible Agar as a Carrier for Dual Functional Doxorubicin-Fe3O4 Nanotherapy Drugs. Materials 2021, 14, 1824. [Google Scholar] [CrossRef]
- Liu, J.; Li, N.; Li, L.; Li, D.; Liu, K.; Zhao, L.; Tang, J.; Li, L. Local Hyperthermia for Esophageal Cancer in a Rabbit Tumor Model: Magnetic Stent Hyperthermia versus Magnetic Fluid Hyperthermia. Oncol. Lett. 2013, 6, 1550–1558. [Google Scholar] [CrossRef]
- Fernández-Álvarez, F.; Caro, C.; García-García, G.; García-Martín, M.L.; Arias, J.L. Engineering of Stealth (Maghemite/PLGA)/Chitosan (Core/Shell)/Shell Nanocomposites with Potential Applications for Combined MRI and Hyperthermia against Cancer. J. Mater. Chem. B 2021, 9, 4963–4980. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, G.K.; Cherukula, K.; Lee, H.; Jeong, Y.Y.; Park, I.-K.; Lee, J.Y. Magnetic Field-Inducible Drug-Eluting Nanoparticles for Image-Guided Thermo-Chemotherapy. Biomaterials 2018, 180, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, S.; Ghin, L.; Conti, G.; Tambalo, S.; Lascialfari, A.; Orlando, T.; Benati, D.; Bernardi, P.; Betterle, N.; Bassi, R.; et al. Magnetic Nanoparticles from Magnetospirillum Gryphiswaldense Increase the Efficacy of Thermotherapy in a Model of Colon Carcinoma. PLoS ONE 2014, 9, e108959. [Google Scholar] [CrossRef] [PubMed]
- Muñoz de Escalona, M.; Sáez-Fernández, E.; Prados, J.C.; Melguizo, C.; Arias, J.L. Magnetic Solid Lipid Nanoparticles in Hyperthermia against Colon Cancer. Int. J. Pharm. 2016, 504, 11–19. [Google Scholar] [CrossRef]
- Clares, B.; Biedma-Ortiz, R.A.; Sáez-Fernández, E.; Prados, J.C.; Melguizo, C.; Cabeza, L.; Ortiz, R.; Arias, J.L. Nano-Engineering of 5-Fluorouracil-Loaded Magnetoliposomes for Combined Hyperthermia and Chemotherapy against Colon Cancer. Eur. J. Pharm. Biopharm. 2013, 85, 329–338. [Google Scholar] [CrossRef]
- Jabalera, Y.; Garcia-Pinel, B.; Ortiz, R.; Iglesias, G.; Cabeza, L.; Prados, J.; Jimenez-Lopez, C.; Melguizo, C. Oxaliplatin–Biomimetic Magnetic Nanoparticle Assemblies for Colon Cancer-Targeted Chemotherapy: An In Vitro Study. Pharmaceutics 2019, 11, 395. [Google Scholar] [CrossRef]
- Garanina, A.S.; Naumenko, V.A.; Nikitin, A.A.; Myrovali, E.; Petukhova, A.Y.; Klimyuk, S.V.; Nalench, Y.A.; Ilyasov, A.R.; Vodopyanov, S.S.; Erofeev, A.S.; et al. Temperature-Controlled Magnetic Nanoparticles Hyperthermia Inhibits Primary Tumor Growth and Metastases Dissemination. Nanomedicine 2020, 25, 102171. [Google Scholar] [CrossRef]
- Torres-Lugo, M.; Castillo, A.; Mendez, J.; Rinaldi, C.; Soto, O.; Alvarez-Berrios, M.P. Hyperthermic Potentiation of Cisplatin by Magnetic Nanoparticle Heaters Is Correlated with an Increase in Cell Membrane Fluidity. Int. J. Nanomed. 2013, 8, 1003–1013. [Google Scholar] [CrossRef]
- Mirzaghavami, P.S.; Khoei, S.; Khoee, S.; Shirvalilou, S.; Mahdavi, S.R.; Pirhajati Mahabadi, V. Radio-Sensitivity Enhancement in HT29 Cells through Magnetic Hyperthermia in Combination with Targeted Nano-Carrier of 5-Flourouracil. Mater. Sci. Eng. C 2021, 124, 112043. [Google Scholar] [CrossRef]
- Pawlik, P.; Blasiak, B.; Depciuch, J.; Pruba, M.; Kitala, D.; Vorobyova, S.; Stec, M.; Bushinsky, M.; Konakov, A.; Baran, J.; et al. Application of Iron-Based Magnetic Nanoparticles Stabilized with Triethanolammonium Oleate for Theranostics. J. Mater. Sci. 2022, 57, 4716–4737. [Google Scholar] [CrossRef]
- Fernandes, S.; Fernandez, T.; Metze, S.; Balakrishnan, P.B.; Mai, B.T.; Conteh, J.; De Mei, C.; Turdo, A.; Di Franco, S.; Stassi, G.; et al. Magnetic Nanoparticle-Based Hyperthermia Mediates Drug Delivery and Impairs the Tumorigenic Capacity of Quiescent Colorectal Cancer Stem Cells. ACS Appl. Mater. Interfaces 2021, 13, 15959–15972. [Google Scholar] [CrossRef]
- Beyk, J.; Tavakoli, H. Selective Radiofrequency Ablation of Tumor by Magnetically Targeting of Multifunctional Iron Oxide–Gold Nanohybrid. J. Cancer Res. Clin. Oncol. 2019, 145, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Guo, Y.; Yu, Z.; Hu, P.; Shi, J. Nanocatalytic Bacteria Disintegration Reverses Immunosuppression of Colorectal Cancer. Natl. Sci. Rev. 2022, 9, nwac169. [Google Scholar] [CrossRef]
- Ahmad, A.; Gupta, A.; Ansari, M.M.; Vyawahare, A.; Jayamurugan, G.; Khan, R. Hyperbranched Polymer-Functionalized Magnetic Nanoparticle-Mediated Hyperthermia and Niclosamide Bimodal Therapy of Colorectal Cancer Cells. ACS Biomater. Sci. Eng. 2020, 6, 1102–1111. [Google Scholar] [CrossRef]
- Shen, M.Y.; Liu, T.I.; Yu, T.W.; Kv, R.; Chiang, W.H.; Tsai, Y.C.; Chen, H.H.; Lin, S.C.; Chiu, H.C. Hierarchically Targetable Polysaccharide-Coated Solid Lipid Nanoparticles as an Oral Chemo/Thermotherapy Delivery System for Local Treatment of Colon Cancer. Biomaterials 2019, 197, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.; Oliva, J.; Cordova-Fraga, T.; Basurto-Islas, G.; Benal-Alvarado, J.J.; Mtz-Enriquez, A.I.; Quintana, M.; Gomez-Solis, C. High Heating Efficiency of Magnetite Nanoparticles Synthesized with Citric Acid: Application for Hyperthermia Treatment. J. Electron. Mater. 2022, 51, 4425–4436. [Google Scholar] [CrossRef]
- Castellanos-Rubio, I.; Rodrigo, I.; Olazagoitia-Garmendia, A.; Arriortua, O.; Gil de Muro, I.; Garitaonandia, J.S.; Bilbao, J.R.; Fdez-Gubieda, M.L.; Plazaola, F.; Orue, I.; et al. Highly Reproducible Hyperthermia Response in Water, Agar, and Cellular Environment by Discretely PEGylated Magnetite Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 27917–27929. [Google Scholar] [CrossRef] [PubMed]
- Teo, P.; Wang, X.; Chen, B.; Zhang, H.; Yang, X.; Huang, Y.; Tang, J. Complex of TNF-α and Modified Fe3O4 Nanoparticles Suppresses Tumor Growth by Magnetic Induction Hyperthermia. Cancer Biother. Radiopharm. 2017, 32, 379–386. [Google Scholar] [CrossRef]
- Matsumi, Y.; Kagawa, T.; Yano, S.; Tazawa, H.; Shigeyasu, K.; Takeda, S.; Ohara, T.; Aono, H.; Hoffman, R.M.; Fujiwara, T.; et al. Hyperthermia Generated by Magnetic Nanoparticles for Effective Treatment of Disseminated Peritoneal Cancer in an Orthotopic Nude-Mouse Model. Cell Cycle 2021, 20, 1122–1133. [Google Scholar] [CrossRef]
- Alvarez-Berríos, M.P.; Castillo, A.; Rinaldi, C.; Torres-Lugo, M. Magnetic Fluid Hyperthermia Enhances Cytotoxicity of Bortezomib in Sensitive and Resistant Cancer Cell Lines. Int. J. Nanomed. 2014, 9, 145–153. [Google Scholar] [CrossRef]
- Hardiansyah, A.; Huang, L.Y.; Yang, M.C.; Liu, T.Y.; Tsai, S.C.; Yang, C.Y.; Kuo, C.Y.; Chan, T.Y.; Zou, H.M.; Lian, W.N.; et al. Magnetic Liposomes for Colorectal Cancer Cells Therapy by High-Frequency Magnetic Field Treatment. Nanoscale Res. Lett. 2014, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Wydra, R.J.; Rychahou, P.G.; Evers, B.M.; Anderson, K.W.; Dziubla, T.D.; Hilt, J.Z. The Role of ROS Generation from Magnetic Nanoparticles in an Alternating Magnetic Field on Cytotoxicity. Acta Biomater. 2015, 25, 284–290. [Google Scholar] [CrossRef]
- Wang, J.T.-W.; Martino, U.; Khan, R.; Bazzar, M.; Southern, P.; Tuncel, D.; Al-Jamal, K.T. Engineering Red-Emitting Multi-Functional Nanocapsules for Magnetic Tumour Targeting and Imaging. Biomater. Sci. 2020, 8, 2590–2599. [Google Scholar] [CrossRef]
- Arriortua, O.K.; Garaio, E.; Herrero de la Parte, B.; Insausti, M.; Lezama, L.; Plazaola, F.; García, J.A.; Aizpurua, J.M.; Sagartzazu, M.; Irazola, M.; et al. Antitumor Magnetic Hyperthermia Induced by RGD-Functionalized Fe 3 O 4 Nanoparticles, in an Experimental Model of Colorectal Liver Metastases. Beilstein J. Nanotechnol. 2016, 7, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Dabaghi, M.; Quaas, R.; Hilger, I. The Treatment of Heterotopic Human Colon Xenograft Tumors in Mice with 5-Fluorouracil Attached to Magnetic Nanoparticles in Combination with Magnetic Hyperthermia Is More Efficient than Either Therapy Alone. Cancers 2020, 12, 2562. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, M.; Gneveckow, U.; Eckelt, L.; Feussner, A.; Waldöfner, N.; Scholz, R.; Deger, S.; Wust, P.; Loening, S.A.; Jordan, A. Clinical Hyperthermia of Prostate Cancer Using Magnetic Nanoparticles: Presentation of a New Interstitial Technique. Int. J. Hyperth. 2005, 21, 637–647. [Google Scholar] [CrossRef]
- Sharifi, I.; Shokrollahi, H.; Amiri, S. Ferrite-Based Magnetic Nanofluids Used in Hyperthermia Applications. J. Magn. Magn. Mater. 2012, 324, 903–915. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive Understanding of Magnetic Hyperthermia for Improving Antitumor Therapeutic Efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef]
- Giustini, A.J.; Ivkov, R.; Hoopes, P.J. Magnetic Nanoparticle Biodistribution Following Intratumoral Administration. Nanotechnology 2011, 22, 345101. [Google Scholar] [CrossRef]
- Hu, A.; Pu, Y.; Xu, N.; Cai, Z.; Sun, R.; Fu, S.; Jin, R.; Guo, Y.; Ai, H.; Nie, Y.; et al. Controlled Intracellular Aggregation of Magnetic Particles Improves Permeation and Retention for Magnetic Hyperthermia Promotion and Immune Activation. Theranostics 2023, 13, 1454–1469. [Google Scholar] [CrossRef]
- Abrahao-Machado, L.F.; Scapulatempo-Neto, C. HER2 Testing in Gastric Cancer: An Update. World J. Gastroenterol. 2016, 22, 4619. [Google Scholar] [CrossRef]
- Stephen, Z.R.; Zhang, M. Recent Progress in the Synergistic Combination of Nanoparticle-Mediated Hyperthermia and Immunotherapy for Treatment of Cancer. Adv. Healthc. Mater. 2021, 10, 2001415. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Hu, P.; Guo, Y.; Hao, J.; Ni, D.; Xu, Y.; Bao, Q.; Yao, H.; Wei, C.; Wu, Q.; et al. Combined Magnetic Hyperthermia and Immune Therapy for Primary and Metastatic Tumor Treatments. ACS Nano 2020, 14, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Chen, G.; Liang, C.; Xu, J.; Dong, Z.; Han, X.; Wang, C.; Liu, Z. Iron Nanoparticles for Low-Power Local Magnetic Hyperthermia in Combination with Immune Checkpoint Blockade for Systemic Antitumor Therapy. Nano Lett. 2019, 19, 4287–4296. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, J.; Sun, W.; Zhao, X.; Li, Y.; Gong, N.; Wang, Y.; Ma, X.; Zhang, T.; Zhao, L.-Y.; et al. Ferrimagnetic Vortex Nanoring-Mediated Mild Magnetic Hyperthermia Imparts Potent Immunological Effect for Treating Cancer Metastasis. ACS Nano 2019, 13, 8811–8825. [Google Scholar] [CrossRef] [PubMed]
- Hedayatnasab, Z.; Abnisa, F.; Daud, W.M.A.W. Review on Magnetic Nanoparticles for Magnetic Nanofluid Hyperthermia Application. Mater. Des. 2017, 123, 174–196. [Google Scholar] [CrossRef]
- Metselaar, J.M.; Lammers, T. Challenges in Nanomedicine Clinical Translation. Drug Deliv. Transl. Res. 2020, 10, 721–725. [Google Scholar] [CrossRef]



| Nanoformulation | Antitumor Agent | AMF | In Vitro Assay | In Vivo Assay | Tumor Type | Main Results | Reference |
|---|---|---|---|---|---|---|---|
| MnFe2O4-Fe3O4 core–shell NPs | - | 384.5 kHz, 27.85 kA/m | Cytotoxicity assay (HT29) | - | CRC | High cytotoxicity effect | [22] |
| Cs MNPs | 5-FU | 435 kHz, 15.4 kA/m | - | HT29 tumor-bearing mice | CRC | Decrease in tumor size | [25] |
| Exosome-FA-MNPs | DOXO | 310 kHz | Cytotoxicity assay (HT29) | HT29 tumor-bearing mice | CRC | High cytotoxicity effect and decrease in tumor size | [29] |
| MNPs loaded Cs nanofibers | - | 750–1150 kHz | Cytotoxicity assay (CT26) | - | CRC | High cytotoxicity effect | [30] |
| SPIONs loaded microrobots | 5-FU | 430 kHz, 45 kA/m | Cytotoxicity assay (HCT116) | - | CRC | High cytotoxicity effect | [31] |
| Fluorescent MNP labeled iPS | - | 63 kHz, 7 kA/m | - | MGC803 tumor-bearing mice | GC | Decrease in tumor size and good MRI results | [32] |
| SPIO-APTES anti-CD133 MNPs | IRI | 1.3–1.8 kHz | Cytotoxicity (Caco-2, HCT116, DLD1) | HCT116 tumor-bearing mice | CRC | High cytotoxicity assay, decrease in tumor size and good MRI results | [33] |
| anti-HER2 carboxydextran and amphiphilic polimer SPIONs | - | 280 kHz, 31 kA/m | Cytotoxicity assay (NUGC-4) | - | GC | High cytotoxicity effect | [34] |
| Anti-131I-labeled CC49 SPIONs | - | 252 kHz, 15.9 kA/m | - | LS174T tumor-bearing mice | CRC | Decrease in tumor size | [35] |
| MPVA-AP1 nanovehicles | DOXO | 50–100 kHz | Liberation assay | - | CRC | High drug liberation and drug release | [36] |
| TAT/CSF1R inhibitor functionalized magnetic liposomes | - | 288 kHz, 35 kA/m | - | CT26 tumor-bearing mice | CRC | Decrease in tumor size and increased magnetic targeting | [37] |
| PEG-PBA-PEG coated SPIONs | 5-FU | 13,560 kHz | Cytotoxicity assay (HT29, HCT116) | - | CRC | High cytotoxicity effect | [38] |
| Alginate coated MPNPs and QDs | DOXO | 4–6.3 kA/m | - | CT26 tumor-bearing mice | CRC | Good MRI results | [39] |
| Agar encapsulated MNPs | DOXO | 400 kHz, 0.45 kA/m | Cytotoxicity assay (HT29) | - | CRC | High cytotoxicity effect | [40] |
| APTES coated MNPs | - | 300 kHz | - | VX2 tumor-bearing rabbits | EC | Decrease in tumor size | [41] |
| (maghemite/PLGA)/Cs NPs | - | 250 kHz, 4 kA/m | Cytotoxicity assay (T84) | Healthy mice | CRC | High cytotoxicity effect and good MRI results | [42] |
| PLGA SPIONs | DOXO | 205 kHz, 2 kA/m | Cytotoxicity assay (CT26) | CT26 tumor-bearing mice | CRC | High cytotoxicity assay, drug release, decrease in tumor size and good MRI results | [43] |
| Bacteria derived MNPs | - | 187 kHz, 23 kA/m | - | HT29 tumor-bearing mice | CRC | In vivo apoptotic and necrotic areas and good MRI results | [44] |
| Solid-lipid MNPs | - | 250 kHz, 4 kA/m | Cytotoxicity assay (HT29) | - | CRC | High cytotoxicity effect | [45] |
| Bacteria-derived MNPs | 5-FU | 250 kHz, 4 kA/m | Liberation assay | - | CRC | High drug release | [46] |
| Bacteria-derived MNPs | OXA | 197 kHz, 18 kA/m | Liberation assay | - | CRC | High drug release | [47] |
| Cobalt ferrite NPs | - | 261 kHz, 8–19.8 kA/m | Cytotoxicity assay (CT26) | CT26 tumor-bearing mice | CRC | High cytotoxicity effect and decrease in tumor size | [48] |
| MNPs | CDDP | 237 kHz, 20 kA/m | Cytotoxicity assay (Caco-2) | - | CRC | High cytotoxicity effect | [49] |
| PEG-PCL-PEG/FA MNPs | 5-FU | 13,560 kHz, 0.4 kA/m | Cytotoxicity assay (HT29) | - | CRC | High cytotoxicity effect | [50] |
| MNPs | - | 100 kHz, 4 kA/m | MRI assay | - | CRC | Good MRI results | [51] |
| Iron oxide nanocubes | DOXO | 182 kHz | Patient-derived CSCs | Patient-derived CSCs tumor-bearing mice | CRC | High cytotoxicity assay, decrease in tumor size | [52] |
| Iron oxide NPs/Au NPs core/shell nanohybrid | - | 13,560 kHz | Cytotoxicity assay (CT26) | CT26 tumor-bearing mice | CRC | High cytotoxicity effect, decrease in tumor size, increased magnetic targeting and good MRI results | [53] |
| ZnCoFe2O4 and ZnMnFe2O4 NPs | - | 1.35 kA/m | Cytotoxicity assay (CT26) | CT26 tumor-bearing mice | CRC | High cytotoxicity effect, decrease in tumor size and better targeting | [54] |
| Polymers functionalized MNPs | Niclosamide | 405 kHz | Cytotoxicity assay (HCT116) | - | CRC | High Cytotoxicity effect | [55] |
| Magnetic solid lipid NPs coated with FA and Dextran | DOXO | Not specified | Cytotoxicity assay (CT26) | CT26 tumor-bearing mice | CRC | High cytotoxicity effect, decrease in tumor size and metastases | [56] |
| Acid citric and EDC/NHC functionalized MNPs | - | 87 kHz-340 kHz, 79.57 kA/m | Cytotoxicity assay (not specified) | - | CRC | High cytotoxicity effect | [57] |
| PMAO-PEG MNPs | - | 650 kHz, 16.71 kA/m | Cytotoxicity assay (HCT116) | - | CRC | High cytotoxicity effect | [58] |
| APTS/PRO functionalized SPIONs loaded with TNF-alfa | - | 110 kHz, 8.75 kA/m | Cytotoxicity assay (SW480, HepG2) | - | CRC | High cytotoxicity effect | [59] |
| Carboxydextran coated MNPs | - | 390 kHz, 28 kA/m | Cytotoxicity assay (HCT116) | Peritoneal-dissemination mice | CRC | High cytotoxicity effect and metastases decrease | [60] |
| Carboxydextran coated MNPs | Bortezomib | 233 kHz, 29.39 kA/m | Cytotoxicity assay (Caco-2) | - | CRC | High cytotoxicity effect | [61] |
| Liposome encapsulated citric acid-coated MNPs | DOXO | 300 kHz, 59.3 kA/m | Cytotoxicity assay (CT26) | - | CRC | High cytotoxicity effect and drug release | [62] |
| Monosaccharides coated MNPs | - | 292 kHz, 51.0 kA/m | Cytotoxicity assay (CT26) | - | CRC | High cytotoxicity effect | [63] |
| PLGA SPIONs | 930 kHz, 13 kA/m | - | CT26 tumor-bearing mice | CRC | Increased magnetic targeting | [64] | |
| PMAO MNPs | - | 606 kHz, 14 kA/m | - | CC-531 tumor-bearing rats | CRC | Heterogeneous cytotoxicity results | [65] |
| Cs MNPs | 5-FU | 435 kHz, 15.4 kA/m | - | HT29 tumor-bearing mice | CRC | Sensitizes cells for further therapies and DNA damage | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gago, L.; Quiñonero, F.; Perazzoli, G.; Melguizo, C.; Prados, J.; Ortiz, R.; Cabeza, L. Nanomedicine and Hyperthermia for the Treatment of Gastrointestinal Cancer: A Systematic Review. Pharmaceutics 2023, 15, 1958. https://doi.org/10.3390/pharmaceutics15071958
Gago L, Quiñonero F, Perazzoli G, Melguizo C, Prados J, Ortiz R, Cabeza L. Nanomedicine and Hyperthermia for the Treatment of Gastrointestinal Cancer: A Systematic Review. Pharmaceutics. 2023; 15(7):1958. https://doi.org/10.3390/pharmaceutics15071958
Chicago/Turabian StyleGago, Lidia, Francisco Quiñonero, Gloria Perazzoli, Consolación Melguizo, Jose Prados, Raul Ortiz, and Laura Cabeza. 2023. "Nanomedicine and Hyperthermia for the Treatment of Gastrointestinal Cancer: A Systematic Review" Pharmaceutics 15, no. 7: 1958. https://doi.org/10.3390/pharmaceutics15071958
APA StyleGago, L., Quiñonero, F., Perazzoli, G., Melguizo, C., Prados, J., Ortiz, R., & Cabeza, L. (2023). Nanomedicine and Hyperthermia for the Treatment of Gastrointestinal Cancer: A Systematic Review. Pharmaceutics, 15(7), 1958. https://doi.org/10.3390/pharmaceutics15071958