Administration of mRNA-Nanomedicine-Augmented Calvarial Defect Healing via Endochondral Ossification
Abstract
:1. Introduction
2. Materials and Methods
2.1. mRNA Preparation
2.2. Cell Culture, Transfection, Luminescence Examination, and Real-Time qRT-PCR
2.3. Preparation of the PEGylated Block Copolymer and mRNA-Loaded Polyplex Nanomicelle
2.4. Critical Calvarial Bone Defect and mRNA-Loaded Polyplex Nanomicelle Administration
2.5. μCT
2.6. Alcian Blue Staining, H&E Staining, Safranin-O Staining, and Immunohistofluorescent Staining
2.7. Statistical Analysis
3. Results
3.1. Transcriptional Polyadenylation Ameliorates the Stability of mRNAs in Varied Mammalian Cells’ Transfection
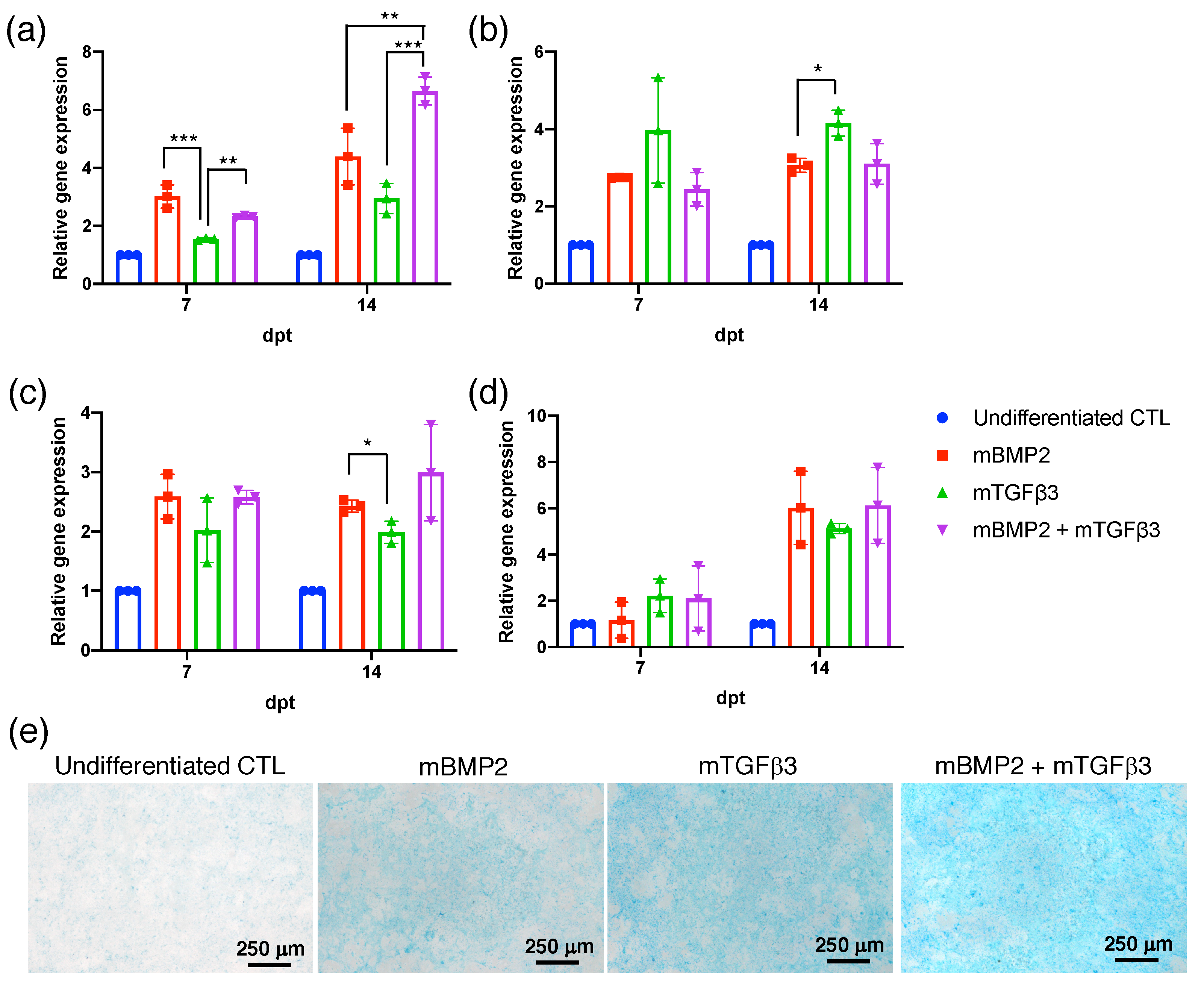
3.2. BMP2 and TGFβ3 mRNA Promote the Chondrogenesis of Bone-Marrow-Derived Stem Cells (BMSCs)
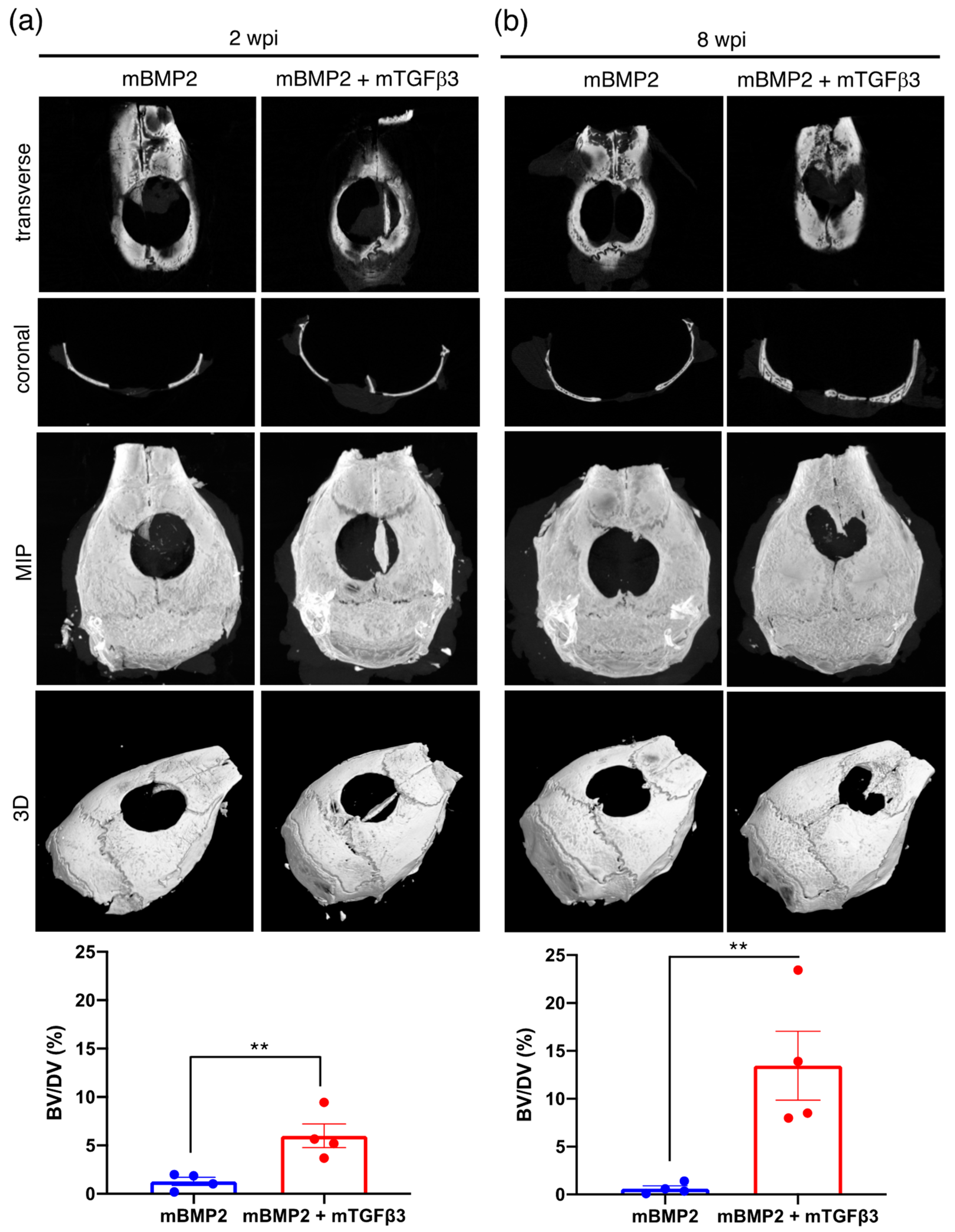
3.3. BMP2 and TGFβ3 mRNA Composite Nanomicelles Promote Significant Bone Regeneration in a Critical Calvarial Defect Model
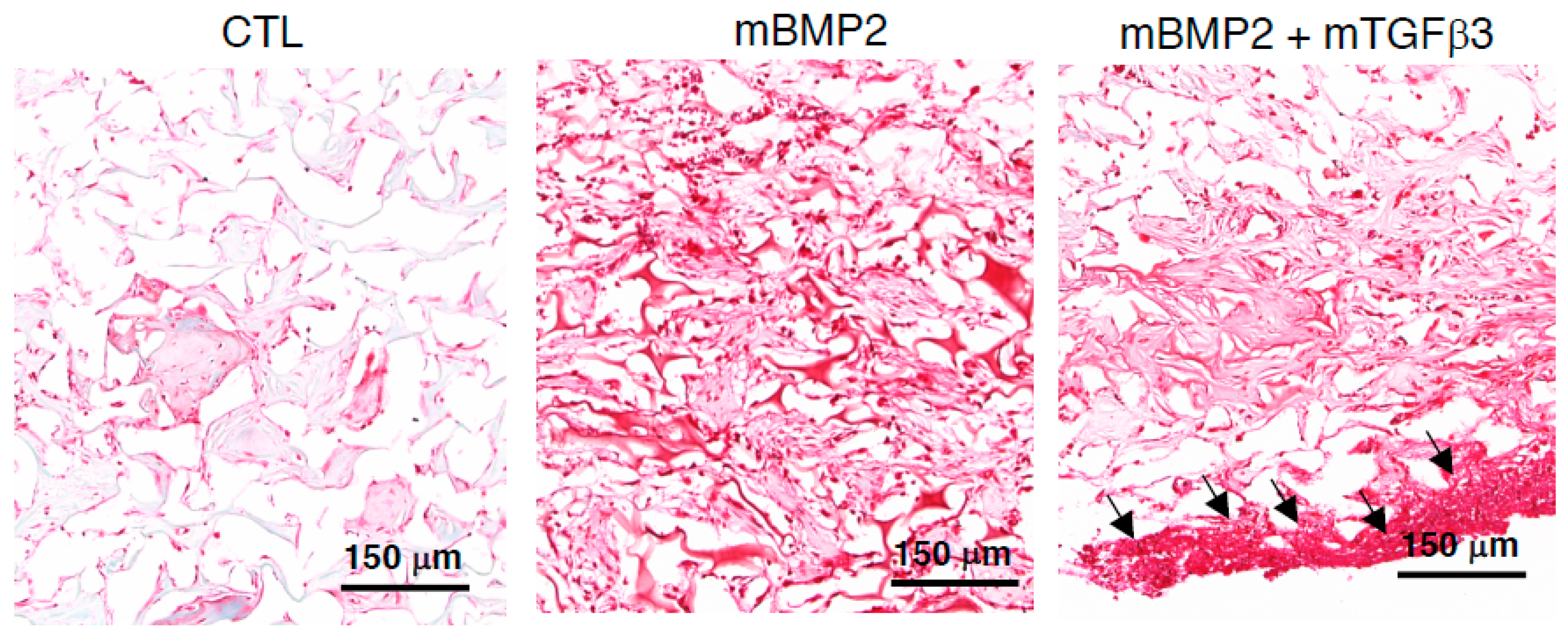
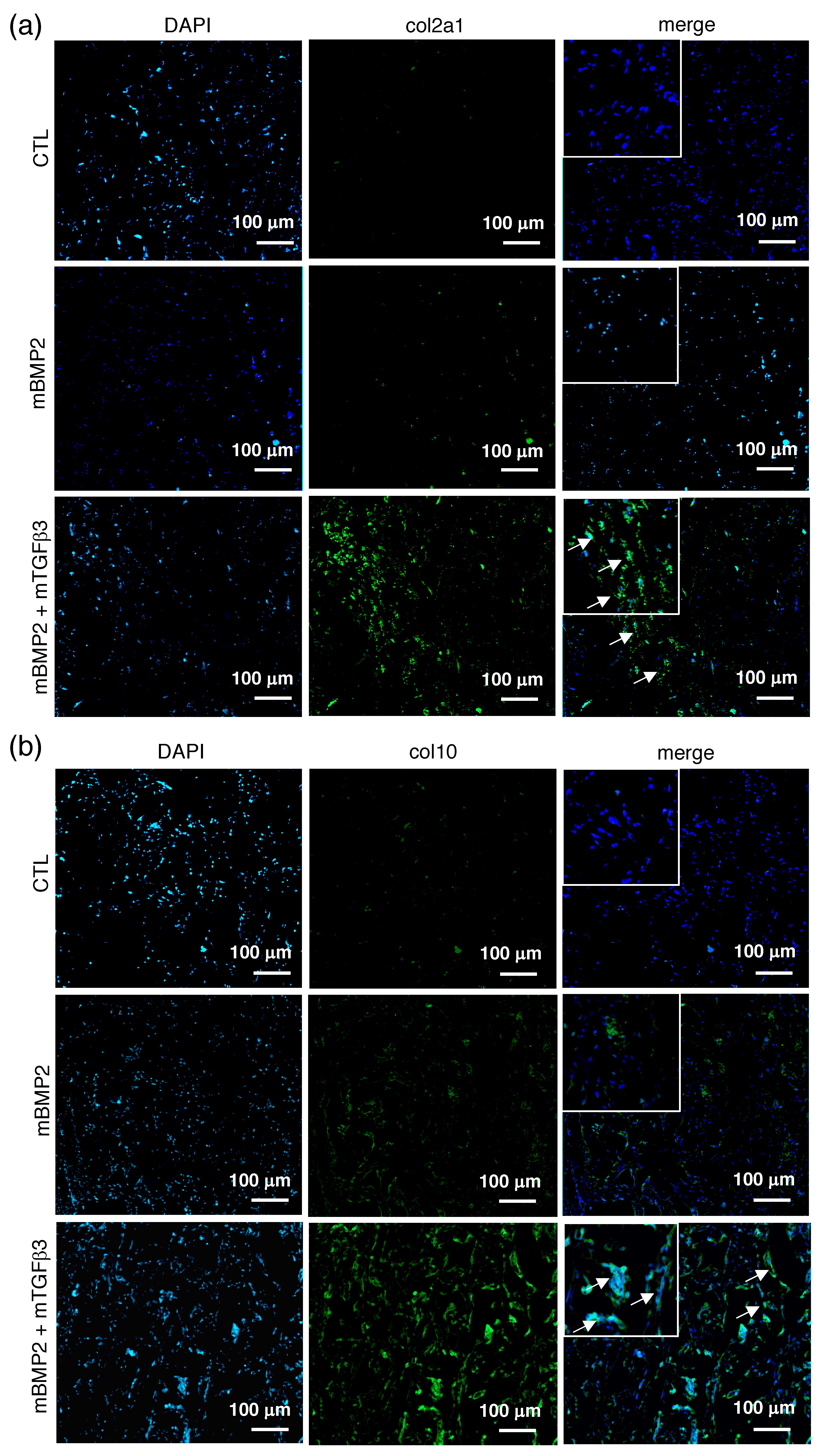
3.4. Composite mRNA Nanomicelle Induces Endochondral Ossification and Ameliorates Bone Regeneration in a Calvarial Bone Defect Healing Process
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ko, F.C.; Sumner, D.R. How faithfully does intramembranous bone regeneration recapitulate embryonic skeletal development? Dev. Dyn. 2021, 250, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Jimi, E.; Hirata, S.; Osawa, K.; Terashita, M.; Kitamura, C.; Fukushima, H. The current and future therapies of bone regeneration to repair bone defects. Int. J. Dent. 2012, 2012, 148261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, E.A.; Rosen, V.; D’Alessandro, J.S.; Bauduy, M.; Cordes, P.; Harada, T.; Israel, D.I.; Hewick, R.M.; Kerns, K.M.; LaPan, P.; et al. Recombinant human bone morphogenetic protein induces bone formation. Proc. Natl. Acad. Sci. USA 1990, 87, 2220–2224. [Google Scholar] [CrossRef] [PubMed]
- Kofron, M.D.; Laurencin, C.T. Bone tissue engineering by gene delivery. Adv. Drug Deliv. Rev. 2006, 58, 555–576. [Google Scholar] [CrossRef] [PubMed]
- Balmayor, E.R.; Geiger, J.P.; Aneja, M.K.; Berezhanskyy, T.; Utzinger, M.; Mykhaylyk, O.; Rudolph, C.; Plank, C. Chemically modified RNA induces osteogenesis of stem cells and human tissue explants as well as accelerates bone healing in rats. Biomaterials 2016, 87, 131–146. [Google Scholar] [CrossRef]
- Badieyan, Z.S.; Berezhanskyy, T.; Utzinger, M.; Aneja, M.K.; Emrich, D.; Erben, R.; Schuler, C.; Altpeter, P.; Ferizi, M.; Hasenpusch, G.; et al. Transcript-activated collagen matrix as sustained mRNA delivery system for bone regeneration. J. Control. Release 2016, 239, 137–148. [Google Scholar] [CrossRef]
- Utzinger, M.; Jarzebinska, A.; Haag, N.; Schweizer, M.; Winter, G.; Dohmen, C.; Rudolph, C.; Plank, C. cmRNA/lipoplex encapsulation in PLGA microspheres enables transfection via calcium phosphate cement (CPC)/PLGA composites. J. Control. Release 2017, 249, 143–149. [Google Scholar] [CrossRef]
- Sahin, U.; Kariko, K.; Tureci, O. mRNA-based therapeutics--developing a new class of drugs. Nat. Reviews. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chang, Y.H.; Li, K.C.; Lu, C.H.; Sung, L.Y.; Yeh, C.L.; Lin, K.J.; Huang, S.F.; Yen, T.C.; Hu, Y.C. The use of ASCs engineered to express BMP2 or TGF-beta3 within scaffold constructs to promote calvarial bone repair. Biomaterials 2013, 34, 9401–9412. [Google Scholar] [CrossRef]
- de Liyis, B.G.; Nolan, J.; Maharjana, M.A. Fibroblast growth factor receptor 1-bound extracellular vesicle as novel therapy for osteoarthritis. Biomedicine 2022, 12, 1–9. [Google Scholar] [CrossRef]
- Rosen, V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 2009, 20, 475–480. [Google Scholar] [CrossRef]
- Barry, F.; Boynton, R.E.; Liu, B.; Murphy, J.M. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: Differentiation-dependent gene expression of matrix components. Exp. Cell Res. 2001, 268, 189–200. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, C.; Li, J.; Zhu, C.; Yang, H.; Li, B. Gene expression modulation in TGF-beta3-mediated rabbit bone marrow stem cells using electrospun scaffolds of various stiffness. J. Cell. Mol. Med. 2015, 19, 1582–1592. [Google Scholar] [CrossRef]
- Bian, L.; Zhai, D.Y.; Tous, E.; Rai, R.; Mauck, R.L.; Burdick, J.A. Enhanced MSC chondrogenesis following delivery of TGF-beta3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 2011, 32, 6425–6434. [Google Scholar] [CrossRef] [Green Version]
- Hara, E.S.; Ono, M.; Pham, H.T.; Sonoyama, W.; Kubota, S.; Takigawa, M.; Matsumoto, T.; Young, M.F.; Olsen, B.R.; Kuboki, T. Fluocinolone Acetonide Is a Potent Synergistic Factor of TGF-beta3-Associated Chondrogenesis of Bone Marrow-Derived Mesenchymal Stem Cells for Articular Surface Regeneration. J. Bone Miner. Res. 2015, 30, 1585–1596. [Google Scholar] [CrossRef] [Green Version]
- Holtkamp, S.; Kreiter, S.; Selmi, A.; Simon, P.; Koslowski, M.; Huber, C.; Tureci, O.; Sahin, U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 2006, 108, 4009–4017. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.Y.; Perche, F.; Ikegami, M.; Uchida, S.; Kataoka, K.; Itaka, K. Messenger RNA-based therapeutics for brain diseases: An animal study for augmenting clearance of beta-amyloid by intracerebral administration of neprilysin mRNA loaded in polyplex nanomicelles. J. Control. Release 2016, 235, 268–275. [Google Scholar] [CrossRef]
- Chan, L.Y.; Khung, Y.L.; Lin, C.Y. Preparation of Messenger RNA Nanomicelles via Non-Cytotoxic PEG-Polyamine Nanocomplex for Intracerebroventicular Delivery: A Proof-of-Concept Study in Mouse Models. Nanomaterials 2019, 9, 67. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Tsou, H.K.; Chang, H.H.; Chan, L.Y.; Zhuo, G.Y.; Maeda, T.; Lin, C.Y. Runx1 Messenger RNA Delivered by Polyplex Nanomicelles Alleviate Spinal Disc Hydration Loss in a Rat Disc Degeneration Model. Int. J. Mol. Sci. 2022, 23, 565. [Google Scholar] [CrossRef]
- Tsou, H.-K.; Chang, C.-C.; Maeda, T.; Lin, C.-Y. Preparation of Messenger RNA-Loaded Nanomedicine Applied on Tissue Engineering and Regenerative Medicine. In Messenger RNA Therapeutics; Jurga, S., Barciszewski, J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 397–428. [Google Scholar]
- Kruijt Spanjer, E.C.; Bittermann, G.K.P.; van Hooijdonk, I.E.M.; Rosenberg, A.; Gawlitta, D. Taking the endochondral route to craniomaxillofacial bone regeneration: A logical approach? J. Craniomaxillofacial Surg. 2017, 45, 1099–1106. [Google Scholar] [CrossRef]
- Simpson, C.R.; Kelly, H.M.; Murphy, C.M. Synergistic use of biomaterials and licensed therapeutics to manipulate bone remodelling and promote non-union fracture repair. Adv. Drug Deliv. Rev. 2020, 160, 212–233. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.A.; O’Keefe, R.J.; Lee, C.H.; Mao, J.J. Bone tissue engineering: Clinical challenges and emergent advances in orthopedic and craniofacial surgery. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1733–1743. [Google Scholar]
- Thompson, E.M.; Matsiko, A.; Farrell, E.; Kelly, D.J.; O’Brien, F.J. Recapitulating endochondral ossification: A promising route to in vivo bone regeneration. J. Tissue Eng. Regen. Med. 2015, 9, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.M.; Matsiko, A.; Kelly, D.J.; Gleeson, J.P.; O’Brien, F.J. An Endochondral Ossification-Based Approach to Bone Repair: Chondrogenically Primed Mesenchymal Stem Cell-Laden Scaffolds Support Greater Repair of Critical-Sized Cranial Defects Than Osteogenically Stimulated Constructs In Vivo. Tissue Eng. Part A 2016, 22, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Freeman, F.E.; Allen, A.B.; Stevens, H.Y.; Guldberg, R.E.; McNamara, L.M. Effects of in vitro endochondral priming and pre-vascularisation of human MSC cellular aggregates in vivo. Stem Cell Res. Ther. 2015, 6, 218. [Google Scholar] [CrossRef] [Green Version]
- Dang, P.N.; Dwivedi, N.; Phillips, L.M.; Yu, X.; Herberg, S.; Bowerman, C.; Solorio, L.D.; Murphy, W.L.; Alsberg, E. Controlled Dual Growth Factor Delivery From Microparticles Incorporated Within Human Bone Marrow-Derived Mesenchymal Stem Cell Aggregates for Enhanced Bone Tissue Engineering via Endochondral Ossification. Stem Cells Transl. Med. 2015, 5, 206–217. [Google Scholar] [CrossRef]
- Fujii, T.; Ueno, T.; Kagawa, T.; Sakata, Y.; Sugahara, T. Comparison of bone formation ingrafted periosteum harvested from tibia and calvaria. Microsc. Res. Tech. 2006, 69, 580–584. [Google Scholar] [CrossRef]
- Jukes, J.M.; Both, S.K.; Leusink, A.; Sterk, L.M.T.; Van Blitterswijk, C.A.; De Boer, J. Endochondral bone tissue engineering using embryonic stem cells. Proc. Natl. Acad. Sci. USA 2008, 105, 6840–6845. [Google Scholar] [CrossRef]
- Scotti, C.; Tonnarelli, B.; Papadimitropoulos, A.; Scherberich, A.; Schaeren, S.; Schauerte, A.; Lopez-Rios, J.; Zeller, R.; Barbero, A.; Martin, I. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 7251–7256. [Google Scholar] [CrossRef]
- Freeman, F.E.; McNamara, L.M. Endochondral Priming: A Developmental Engineering Strategy for Bone Tissue Regeneration. Tissue Eng. Part B Rev. 2017, 23, 128–141. [Google Scholar] [CrossRef]
- Rowland, C.R.; Glass, K.A.; Ettyreddy, A.R.; Gloss, C.C.; Matthews, J.R.L.; Huynh, N.P.T.; Guilak, F. Regulation of decellularized tissue remodeling via scaffold-mediated lentiviral delivery in anatomically-shaped osteochondral constructs. Biomaterials 2018, 177, 161–175. [Google Scholar] [CrossRef]
- Zhdanov, V.P. mRNA function after intracellular delivery and release. Biosystems 2018, 165, 52–56. [Google Scholar] [CrossRef]
- Pascolo, S. Vaccination with messenger RNA (mRNA). In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 221–235. [Google Scholar] [CrossRef]
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef]
- Chan, L.Y.; Chang, C.C.; Lai, P.L.; Maeda, T.; Hsu, H.C.; Lin, C.Y.; Kuo, S.J. Cre/LoxP Genetic Recombination Sustains Cartilage Anabolic Factor Expression in Hyaluronan Encapsulated MSCs Alleviates Intervertebral Disc Degeneration. Biomedicines 2022, 10, 555. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsou, H.-K.; Wu, C.-H.; Chan, L.Y.; Kataoka, K.; Itokazu, N.; Tsuzuki, M.; Hu, H.; Zhuo, G.-Y.; Itaka, K.; Lin, C.-Y. Administration of mRNA-Nanomedicine-Augmented Calvarial Defect Healing via Endochondral Ossification. Pharmaceutics 2023, 15, 1965. https://doi.org/10.3390/pharmaceutics15071965
Tsou H-K, Wu C-H, Chan LY, Kataoka K, Itokazu N, Tsuzuki M, Hu H, Zhuo G-Y, Itaka K, Lin C-Y. Administration of mRNA-Nanomedicine-Augmented Calvarial Defect Healing via Endochondral Ossification. Pharmaceutics. 2023; 15(7):1965. https://doi.org/10.3390/pharmaceutics15071965
Chicago/Turabian StyleTsou, Hsi-Kai, Cheng-Hsin Wu, Long Yi Chan, Kazunori Kataoka, Nanae Itokazu, Minoru Tsuzuki, Hsuan Hu, Guan-Yu Zhuo, Keiji Itaka, and Chin-Yu Lin. 2023. "Administration of mRNA-Nanomedicine-Augmented Calvarial Defect Healing via Endochondral Ossification" Pharmaceutics 15, no. 7: 1965. https://doi.org/10.3390/pharmaceutics15071965
APA StyleTsou, H.-K., Wu, C.-H., Chan, L. Y., Kataoka, K., Itokazu, N., Tsuzuki, M., Hu, H., Zhuo, G.-Y., Itaka, K., & Lin, C.-Y. (2023). Administration of mRNA-Nanomedicine-Augmented Calvarial Defect Healing via Endochondral Ossification. Pharmaceutics, 15(7), 1965. https://doi.org/10.3390/pharmaceutics15071965







