Cell-Penetrating and Targeted Peptides Delivery Systems as Potential Pharmaceutical Carriers for Enhanced Delivery across the Blood–Brain Barrier (BBB)
Abstract
:1. Introduction
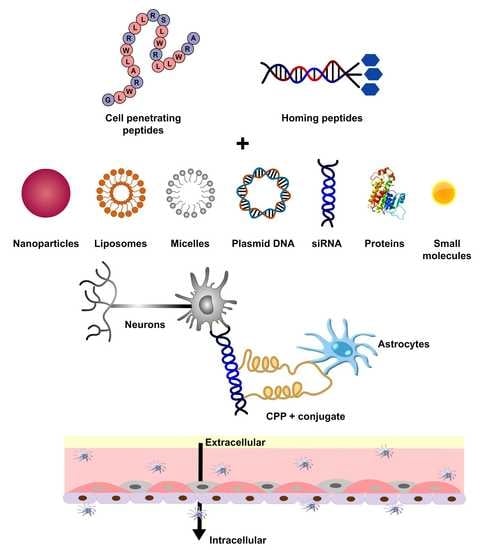
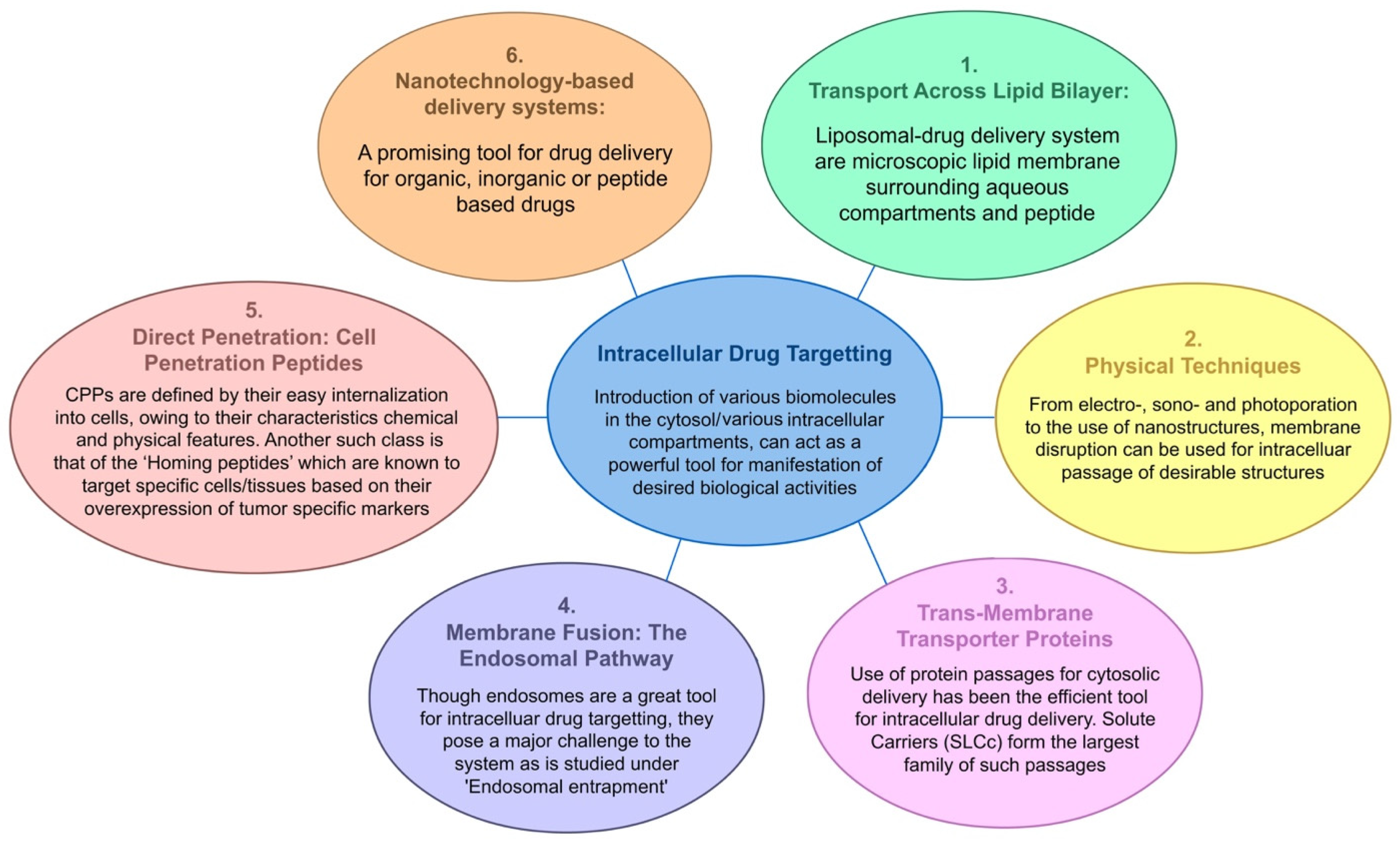
2. Cell-Penetrating Peptides (CPPs)
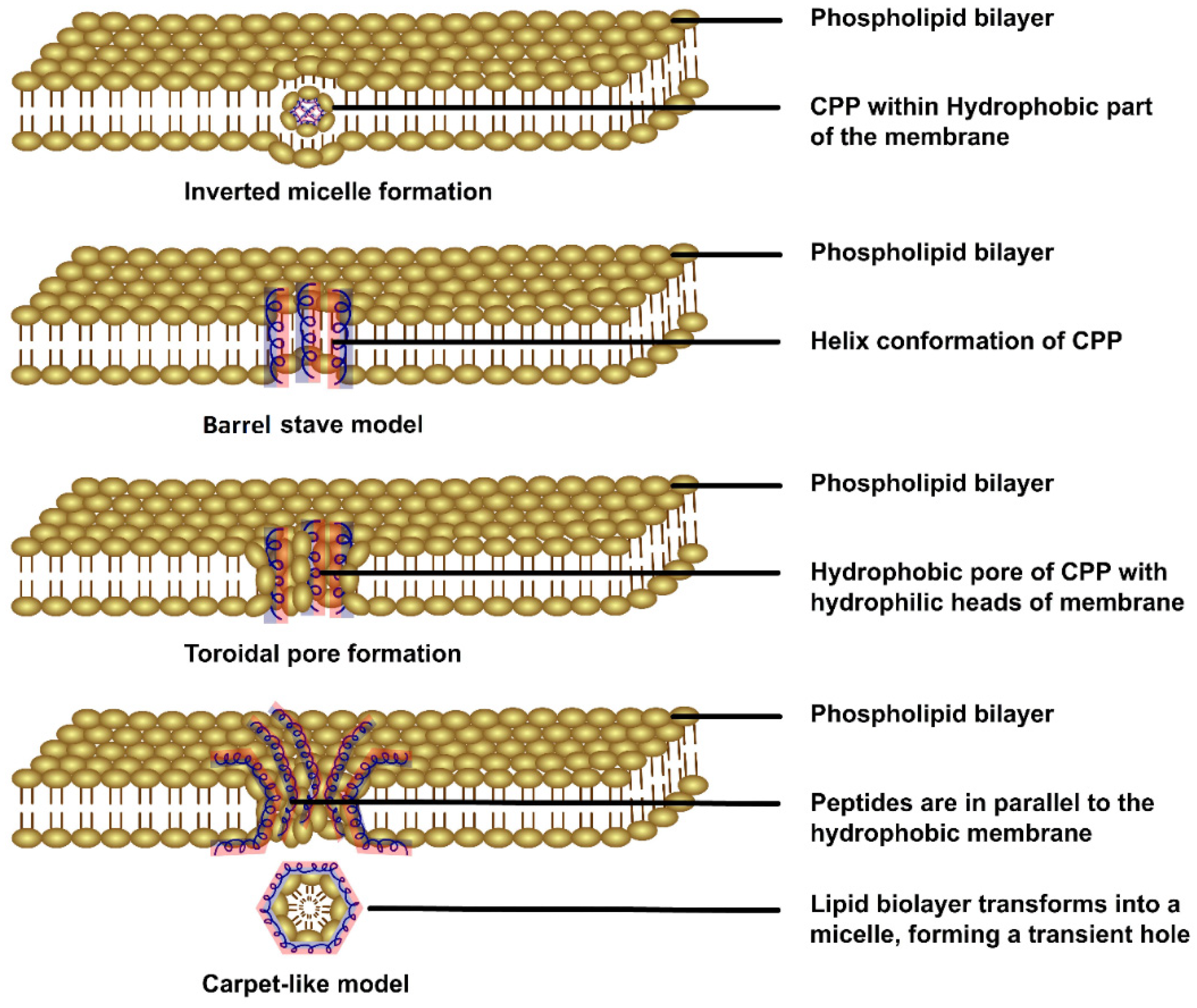
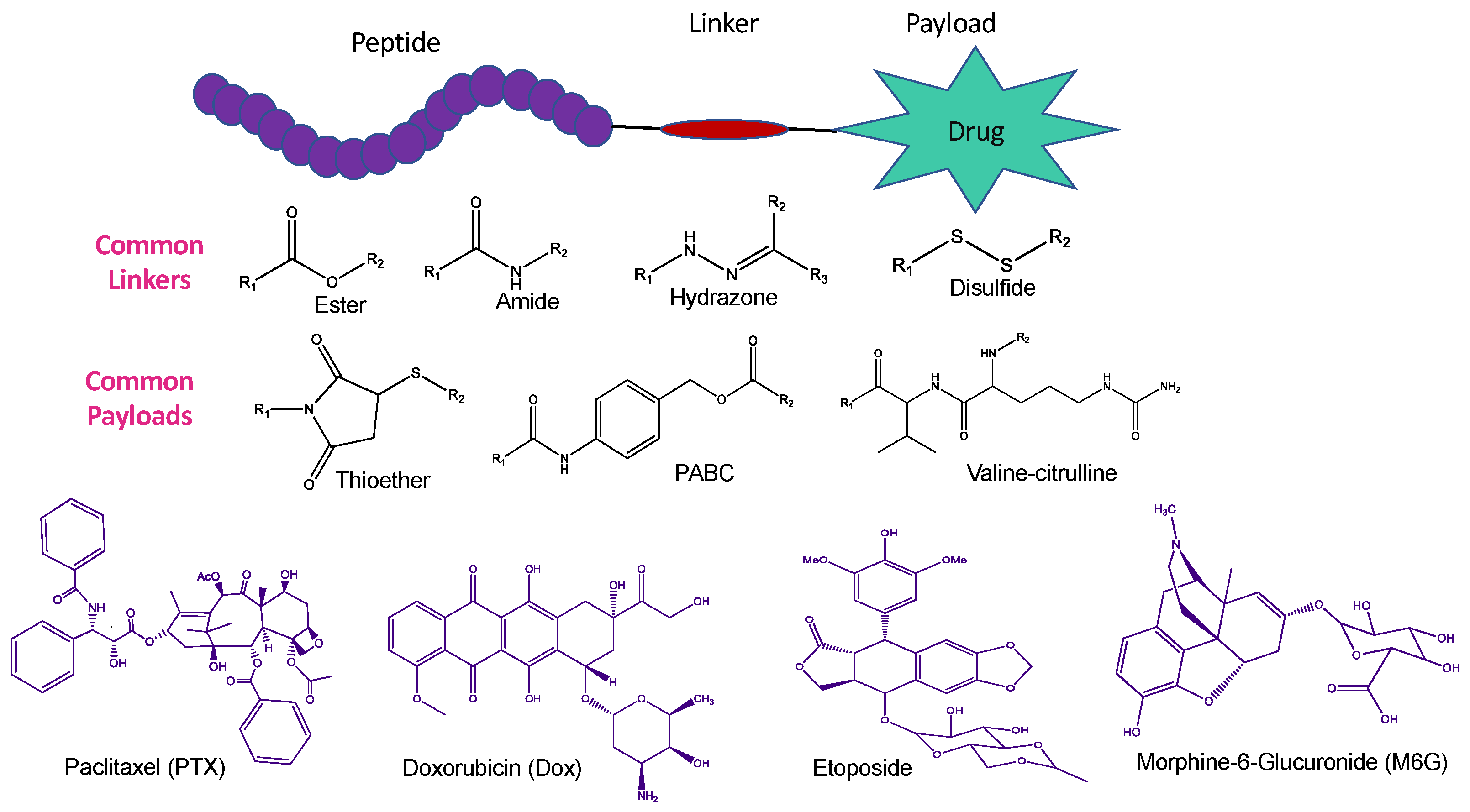
2.1. Various Strategies of CPP-Mediated Drug Delivery
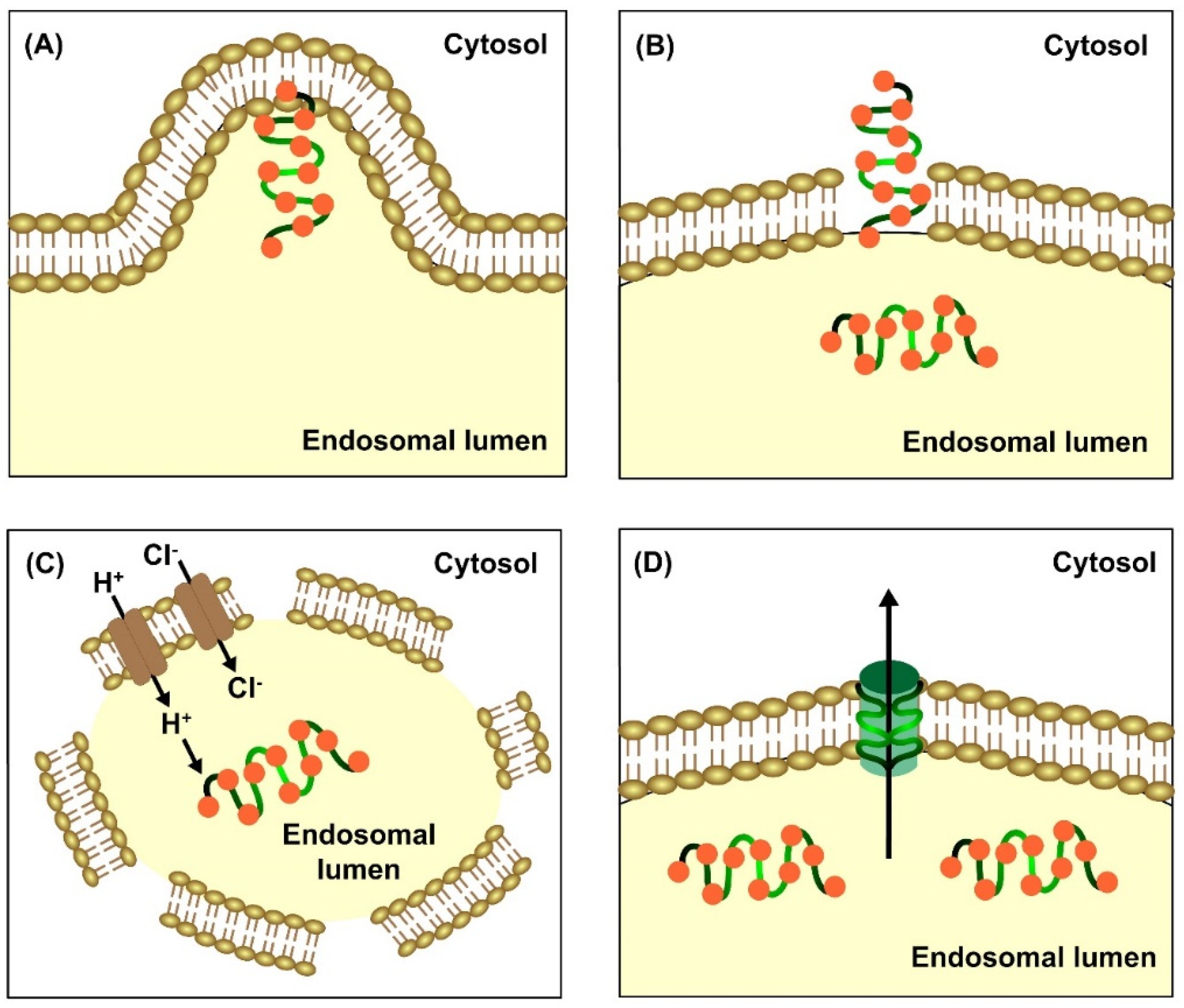
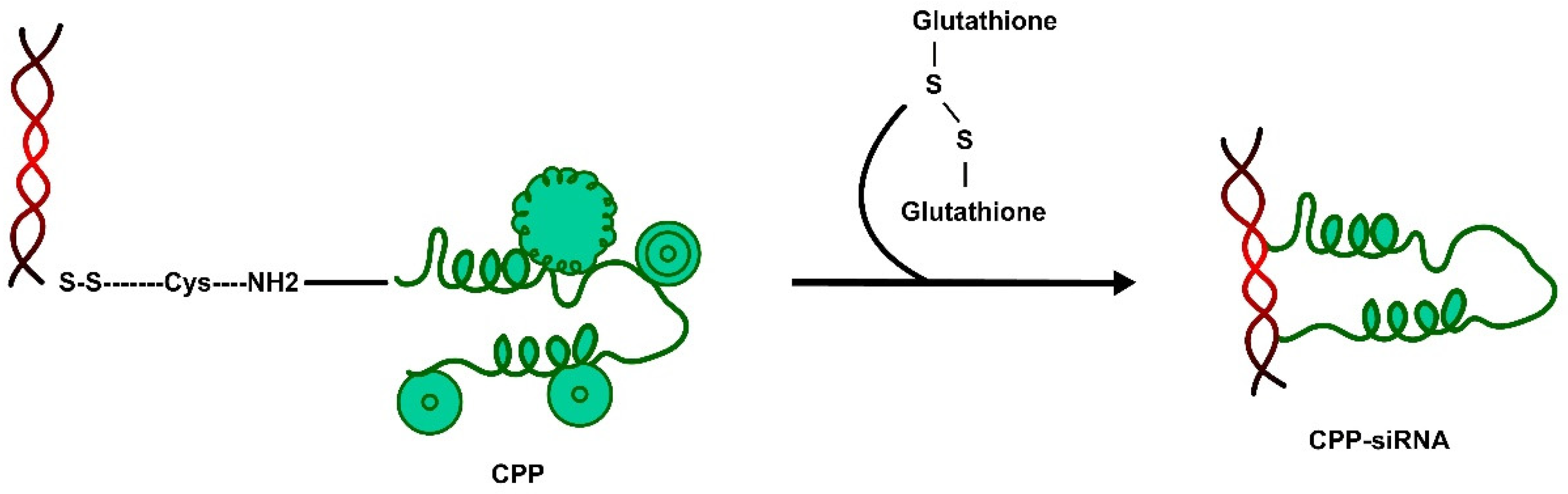
2.2. Escape of CPPs from Endosomal Entrapment and Protease Degradation
3. Homing Peptides for Targeted Drug Delivery
4. Peptide-Mediated Drug Delivery Systems across the Blood–Brain Barrier
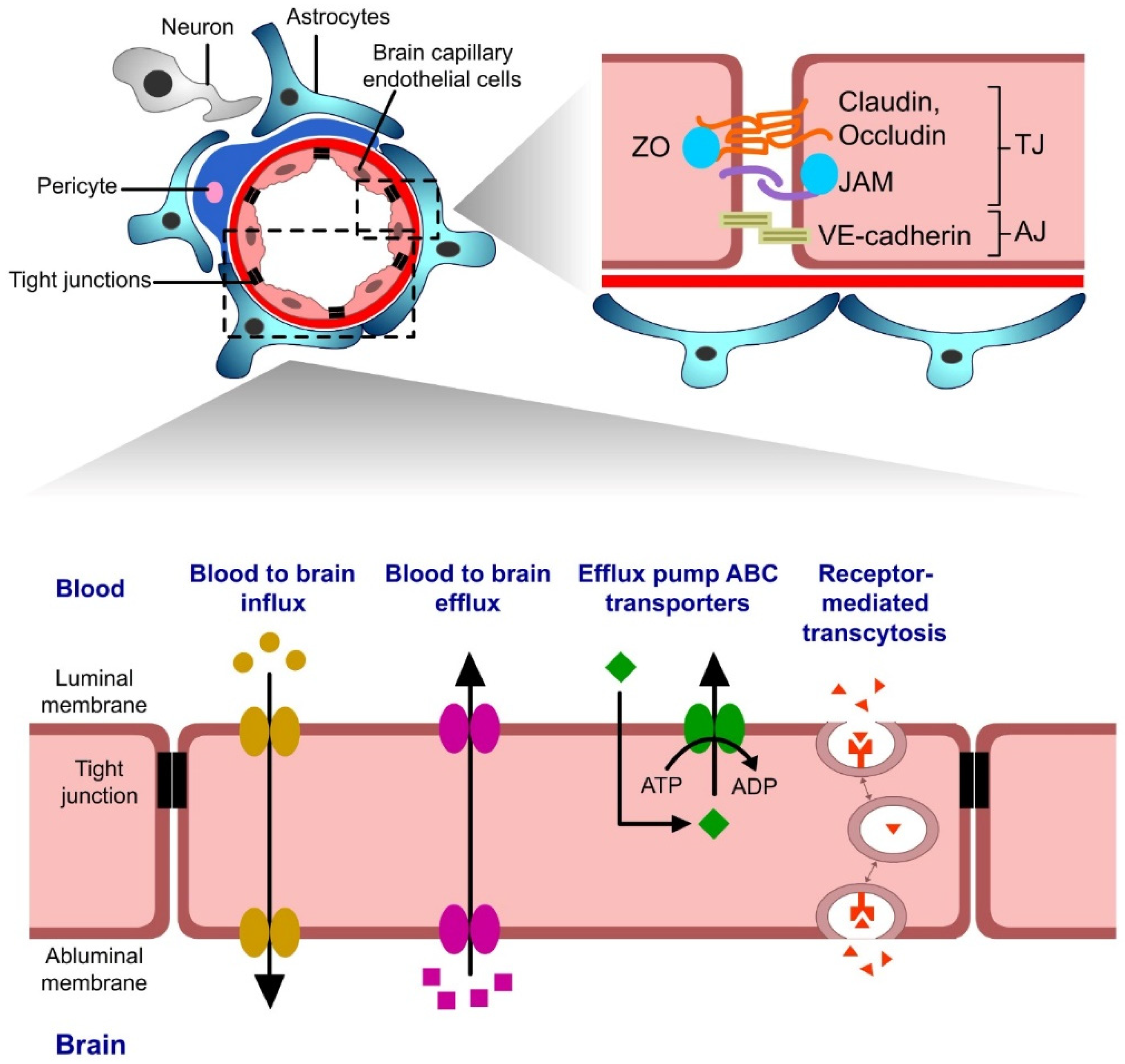
4.1. Introduction to the Blood–Brain Barrier (BBB)
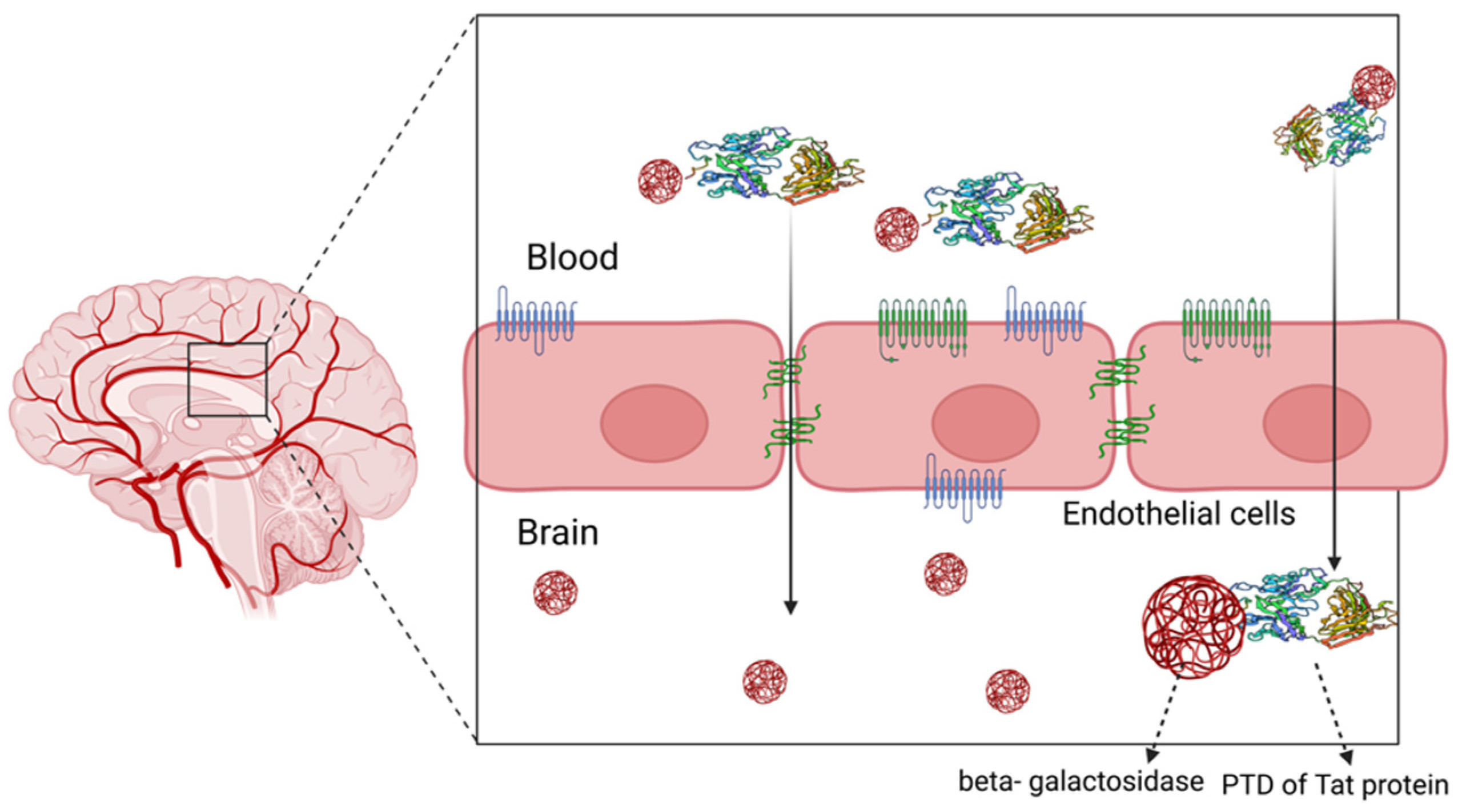
4.2. Cell-Penetrating Peptides as Delivery Systems across the Blood–Brain Barrier
4.2.1. Lipoprotein-Enabled Novel Shuttle Peptides
4.2.2. Naturally Derived CPPs
4.2.3. CPP-Mediated Nanocarriers
4.2.4. CPP-Enabled Metallic Nanopeptides (NPs)
4.2.5. CPP-Enabled Exosomes
4.2.6. CPP-Enabled Liposomes
4.2.7. Angiopep-Conjugated Polyethyleneglycol-Adapted Polyamidoamine Dendrimer (PAMAM–PEG–Angiopep)
4.3. Homing Peptides as Delivery Systems across the Blood–Brain Barrier (BBB)
5. Cell-Penetrating Peptides and siRNA Delivery to the Central Nervous System
6. siRNA-CPP Therapeutics of the Central Nervous System
6.1. siRNA Delivery by Virus
6.2. Non-Viral Route of siRNA Delivery
6.3. Liposome–siRNA–Peptide Complexes (LSPCs)
6.4. Intranasal Delivery of siRNA
6.5. siRNA-Loaded Exosomes
7. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhowmik, A.; Khan, R.; Ghosh, M.K. Blood brain barrier: A challenge for effectual therapy of brain tumours. BioMed. Res. Int. 2015, 2015, 320941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano. Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef]
- Morshedi Rad, D.; Alsadat Rad, M.; Razavi Bazaz, S.; Kashaninejad, N.; Jin, D.; Ebrahimi Warkiani, M. A comprehensive review on intracellular delivery. Adv. Mater. 2021, 33, 2005363. [Google Scholar] [CrossRef]
- Stewart, M.P.; Sharei, A.; Ding, X.; Sahay, G.; Langer, R.; Jensen, K.F. In vitro and ex vivo strategies for intracellular delivery. Nature 2016, 538, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Berillo, D.; Yeskendir, A.; Zharkinbekov, Z.; Raziyeva, K.; Saparov, A. Peptide-based drug delivery systems. Medicina 2021, 57, 1209. [Google Scholar] [CrossRef]
- Gayraud, F.; Klugman, M.; Neundorf, I. Recent Advances and Trends in Chemical CPP–Drug Conjugation Techniques. Molecules 2021, 26, 1591. [Google Scholar] [CrossRef]
- Josephson, L.; Tung, C.H.; Moore, A.; Weissleder, R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-Tat peptide conjugates. Bioconjug. Chem. 1999, 10, 186–191. [Google Scholar] [CrossRef]
- Blasi, P.; Giovagnoli, S.; Schoubben, A.; Ricci, M.; Rossi, C. Solid lipid nanoparticles for targeted brain drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 454–477. [Google Scholar] [CrossRef]
- Malhotra, M.; Prakash, S. Targeted drug delivery across blood-brain-barrier using cell penetrating peptides tagged nanoparticles. Curr. Nanosci. 2011, 7, 81–93. [Google Scholar] [CrossRef]
- Goyal, R. Peptide-Based Molecular Constructs for Cellular Targeting and Small Molecule Delivery. Ph.D. Thesis, Indian Institute of Technology, Guwahati, India, 2020. [Google Scholar]
- Heitz, F.; Morris, M.C.; Divita, G. Twenty years of cell-penetrating peptides: From molecular mechanisms to therapeutics. Br. J. Pharmacol. 2009, 157, 195–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the TAT protein from human lmmunodeficiency virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef]
- Nagahara, H.; Vocero-Akbani, A.M.; Snyder, E.L.; Ho, A.; Latham, D.G.; Lissy, N.A.; Becker-Hapak, M.; Ezhevsky, S.A.; Dowdy, S.F. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat. Med. 1998, 4, 1449–1452. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Deshayes, S.; Heitz, F.; Divita, G. Cell-penetrating peptides: From molecular mechanisms to therapeutics. Biol. Cell 2008, 100, 201–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndeboko, B.; Ramamurthy, N.; Lemamy, G.J.; Jamard, C.; Nielsen, P.E.; Cova, L. Role of cell-penetrating peptides in intracellular delivery of peptide nucleic acids targeting hepadnaviral replication. Mol. Ther.-Nucleic Acids 2017, 9, 162–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Wang, J.; Xu, D. Cell-penetrating peptides as noninvasive transmembrane vectors for the development of novel multifunctional drug-delivery systems. J. Control. Release 2016, 229, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komin, A.; Russell, L.; Hristova, K.; Searson, P. Peptide-based strategies for enhanced cell uptake, transcellular transport, and circulation: Mechanisms and challenges. Adv. Drug Deliv. Rev. 2017, 110, 52–64. [Google Scholar] [CrossRef]
- Arukuusk, P.; Pärnaste, L.; Margus, H.; Eriksson, N.J.; Vasconcelos, L.; Padari, K.R.; Pooga, M.; Langel, U.L. Differential endosomal pathways for radically modified peptide vectors. Bioconjugate Chem. 2013, 24, 1721–1732. [Google Scholar] [CrossRef]
- Ruseska, I.; Zimmer, A. Internalization mechanisms of cell-penetrating peptides. Beilstein J. Nanotechnol. 2020, 11, 101–123. [Google Scholar] [CrossRef]
- Hu, G.; Zheng, W.; Li, A.; Mu, Y.; Shi, M.; Li, T.; Zou, H.; Shao, H.; Qin, A.; Ye, J. A novel CAV derived cell-penetrating peptide efficiently delivers exogenous molecules through caveolae-mediated endocytosis. Vet. Res. 2018, 49, 16. [Google Scholar] [CrossRef] [Green Version]
- Haucke, V.; Kozlov, M.M. Membrane remodeling in clathrin-mediated endocytosis. J. Cell Sci. 2018, 131, jcs216812. [Google Scholar] [CrossRef] [Green Version]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Futaki, S.; Nakase, I. Cell-surface interactions on arginine-rich cell-penetrating peptides allow for multiplex modes of internalization. Acc. Chem. Res. 2017, 50, 2449–2456. [Google Scholar] [CrossRef]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-penetrating peptides: From basic research to clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef]
- Böhmová, E.; Machová, D.; Pechar, M.; Pola, R.; Venclíková, K.; Janoušková, O.; Etrych, T. Cell-penetrating peptides: A useful tool for the delivery of various cargoes into cells. Physiol. Res. 2018, 67, S267–S279. [Google Scholar] [CrossRef] [PubMed]
- Erazo-Oliveras, A.; Muthukrishnan, N.; Baker, R.; Wang, T.-Y.; Pellois, J.-P. Improving the endosomal escape of cell-penetrating peptides and their cargos: Strategies and challenges. Pharmaceuticals 2012, 5, 1177–1209. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Selby, L.I.; Johnston, A.P.R.; Such, G.K. The Endosomal Escape of Nanoparticles: Toward More Efficient Cellular Delivery. Bioconjugate Chem. 2019, 30, 263–272. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef]
- Martens, T.F.; Remaut, K.; Demeester, J.; De Smedt, S.C.; Braeckmans, K. Intracellular delivery of nanomaterials: How to catch endosomal escape in the act. Nano Today 2014, 9, 344–364. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.; Philipp, A.; Oskuee, R.; Schmidt, C.; Wagner, E. Breathing life into polycations: Functionalization with pH-responsive endosomolytic peptides and polyethylene glycol enables siRNA delivery. J. Am. Chem. Soc. 2008, 130, 3272–3273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, S.; Nakase, I.; Kawabata, N.; Yu, H.-H.; Pujals, S.; Imanishi, M.; Giralt, E.; Futaki, S. Cytosolic targeting of macromolecules using a pH-dependent fusogenic peptide in combination with cationic liposomes. Bioconjugate Chem. 2009, 20, 953–959. [Google Scholar] [CrossRef]
- Chen, R.; Khormaee, S.; Eccleston, M.E.; Slater, N.K. The role of hydrophobic amino acid grafts in the enhancement of membrane-disruptive activity of pH-responsive pseudo-peptides. Biomaterials 2009, 30, 1954–1961. [Google Scholar] [CrossRef] [Green Version]
- Lonn, P.; Kacsinta, A.; Cui, X.; Hamil, A.; Kaulich, M.; Gogoi, K.; Dowdy, S. Enhancing endosomal escape for intracellular delivery of macromolecular biologic therapeutics. Sci. Rep. 2016, 6, 32301. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Chen, S.; Chen, W.-H.; Lei, Q.; Liu, Y.; Zhuo, R.-X.; Zhang, X.-Z. Synergistic gene and drug tumour therapy using a chimeric peptide. Biomaterials 2013, 34, 4680–4689. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.L.; Wang, S. An endosomolytic Tat peptide produced by incorporation of histidine and cysteine residues as a nonviral vector for DNA transfection. Biomaterials 2008, 29, 2408–2414. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; LaRochelle, J.R.; Jiang, B.; Lian, W.; Hard, R.L.; Selner, N.G.; Luechapanichkul, R.; Barrios, A.M.; Pei, D. Early endosomal escape of a cyclic cell-penetrating peptide allows effective cytosolic cargo delivery. Biochemistry 2014, 53, 4034–4046. [Google Scholar] [CrossRef]
- Erazo-Oliveras, A.; Najjar, K.; Truong, D.; Wang, T.Y.; Brock, D.J.; Prater, A.R.; Pellois, J.P. The Late Endosome and Its Lipid BMP Act as Gateways for Efficient Cytosolic Access of the Delivery Agent dfTAT and Its Macromolecular Cargos. Cell Chem. Biol. 2016, 23, 598–607. [Google Scholar] [CrossRef] [Green Version]
- Erazo-Oliveras, A.; Najjar, K.; Dayani, L.; Wang, T.Y.; Johnson, G.A.; Pellois, J.P. Protein delivery into live cells by incubation with an endosomolytic agent. Nat. Methods 2014, 11, 861–867. [Google Scholar] [CrossRef] [Green Version]
- Qian, Z.; Martyna, A.; Hard, R.L.; Wang, J.; Appiah-Kubi, G.; Coss, C.; Phelps, M.A.; Rossman, J.S.; Pei, D. Discovery and Mechanism of Highly Efficient Cyclic Cell-Penetrating Peptides. Biochemistry 2016, 55, 2601–2612. [Google Scholar] [CrossRef]
- Appelbaum, J.S.; LaRochelle, J.R.; Smith, B.A.; Balkin, D.M.; Holub, J.M.; Schepartz, A. Arginine topology controls escape of minimally cationic proteins from early endosomes to the cytoplasm. Chem. Biol. 2012, 19, 819–830. [Google Scholar] [CrossRef] [Green Version]
- Bus, T.; Traeger, A.; Schubert, U.S. The great escape: How cationic polyplexes overcome the endosomal barrier. J. Mater. Chem. B 2018, 6, 6904–6918. [Google Scholar] [CrossRef]
- Pack, D.W.; Putnam, D.; Langer, R. Design of imidazole-containing endosomolytic biopolymers for gene delivery. Biotechnol. Bioeng. 2000, 67, 217–223. [Google Scholar] [CrossRef]
- Sonawane, N.D.; Szoka, F.C., Jr.; Verkman, A.S. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J. Biol. Chem. 2003, 278, 44826–44831. [Google Scholar] [CrossRef] [Green Version]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The possible “proton sponge“ effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 2013, 21, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, L.M.P.; De Smedt, S.C.; Remaut, K.; Braeckmans, K. The proton sponge hypothesis: Fable or fact? Eur. J. Pharm. Biopharm. 2018, 129, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Tweten, R.K. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect. Immun. 2005, 73, 6199–6209. [Google Scholar] [CrossRef] [Green Version]
- Gruenberg, J.; van der Goot, F.G. Mechanisms of pathogen entry through the endosomal compartments. Nat. Rev. Mol. Cell Biol. 2006, 7, 495–504. [Google Scholar] [CrossRef]
- Herce, H.D.; Garcia, A.E.; Litt, J.; Kane, R.S.; Martin, P.; Enrique, N.; Rebolledo, A.; Milesi, V. Arginine-rich peptides destabilize the plasma membrane, consistent with a pore formation translocation mechanism of cell-penetrating peptides. Biophys. J. 2009, 97, 1917–1925. [Google Scholar] [CrossRef] [Green Version]
- Nadal-Bufí, F.; Henriques, S.T. How to overcome endosomal entrapment of cell-penetrating peptides to release the therapeutic potential of peptides? Pept. Sci. 2020, 112, e24168. [Google Scholar] [CrossRef]
- Mandal, D.; Nasrolahi Shirazi, A.; Parang, K. Cell-penetrating homochiral cyclic peptides as nuclear-targeting molecular transporters. Angew. Chem. Int. Ed. Engl. 2011, 50, 9633–9637. [Google Scholar] [CrossRef]
- Lee, Y.J.; Johnson, G.; Peltier, G.C.; Pellois, J.P. A HA2-Fusion tag limits the endosomal release of its protein cargo despite causing endosomal lysis. Biochim. Biophys. Acta 2011, 1810, 752–758. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Lu, C.; Leszek, J.; Zhang, J. Design and Development of Nanomaterial-Based Drug Carriers to Overcome the Blood–Brain Barrier by Using Different Transport Mechanisms. Int. J. Mol. Sci. 2021, 22, 10118. [Google Scholar] [CrossRef] [PubMed]
- Lucana, M.C.; Arruga, Y.; Petrachi, E.; Roig, A.; Lucchi, R.; Oller-Salvia, B. Protease-Resistant Peptides for Targeting and Intracellular Delivery of Therapeutics. Pharmaceutics 2021, 13, 2065. [Google Scholar] [CrossRef] [PubMed]
- Varner, J.A.; Cheresh, D.A. Integrins and cancer. Curr. Opin. Cell Biol. 1996, 8, 724–730. [Google Scholar] [CrossRef]
- Koivunen, E.; Wang, B.; Ruoslahti, E. Phage libraries displaying cyclic peptides with different ring sizes: Ligand specificities of the RGD-directed integrins. Biotechnology 1995, 13, 265–270. [Google Scholar] [CrossRef]
- Corti, A.; Curnis, F. Tumour vasculature targeting through NGR peptide-based drug delivery systems. Curr. Pharm. Biotechnol. 2011, 12, 1128–1134. [Google Scholar] [CrossRef]
- Kondo, E.; Iioka, H.; Saito, K. Tumour-homing peptide and its utility for advanced cancer medicine. Cancer Sci. 2021, 112, 2118–2125. [Google Scholar] [CrossRef]
- Kondo, E.; Saito, K.; Tashiro, Y.; Kamide, K.; Uno, S.; Furuya, T.; Mashita, M.; Nakajima, K.; Tsumuraya, T.; Kobayashi, N.; et al. Tumour lineage-homing cell-penetrating peptides as anticancer molecular delivery systems. Nat. Commun. 2012, 3, 951. [Google Scholar] [CrossRef] [Green Version]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Treat, L.H.; McDannold, N.; Zhang, Y.; Vykhodtseva, N.; Hynynen, K. Improved anti-tumour effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound. Med. Biol. 2012, 38, 1716–1725. [Google Scholar] [CrossRef] [Green Version]
- Rip, J.; Schenk, G.J.; de Boer, A.G. Differential receptor-mediated drug targeting to the diseased brain. Expert. Opin. Drug. Deliv. 2009, 6, 227–237. [Google Scholar] [CrossRef]
- Gururangan, S.; Friedman, H.S. Innovations in design and delivery of chemotherapy for brain tumours. Neuroimaging Clin. N. Am. 2002, 12, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Romero, I.A. Transporting therapeutics across the blood-brain barrier. Mol. Med. Today 1996, 2, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Parrasia, S.; Szabò, I.; Zoratti, M.; Biasutto, L. Peptides as Pharmacological Carriers to the Brain: Promises, Shortcomings and Challenges. Mol. Pharm. 2022, 19, 3700–3729. [Google Scholar] [CrossRef]
- Reissmann, S. State of art: Cell penetration and cell-penetrating peptides and proteins. Health Educ. Public Health 2021, 4, 393–410. [Google Scholar]
- Stalmans, S.; Bracke, N.; Wynendaele, E.; Gevaert, B.; Peremans, K.; Burvenich, C.; Polis, I.; De Spiegeleer, B. Cell-Penetrating Peptides Selectively Cross the Blood-Brain Barrier In Vivo. PLoS ONE 2015, 10, e0139652. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, M.E.; Hällbrink, M.M.; Elmquist, A.M.; Langel, U. Passage of cell-penetrating peptides across a human epithelial cell layer in vitro. Biochem. J. 2004, 377, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Violini, S.; Sharma, V.; Prior, J.L.; Dyszlewski, M.; Piwnica-Worms, D. Evidence for a plasma membrane-mediated permeability barrier to Tat basic domain in well-differentiated epithelial cells: Lack of correlation with heparan sulfate. Biochemistry 2002, 41, 12652–12661. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, S.R.; Ho, A.; Vocero-Akbani, A.; Dowdy, S.F. In vivo protein transduction: Delivery of a biologically active protein into the mouse. Science 1999, 285, 1569–1572. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, P.; Ma, Z.; Lu, P.; Kebebe, D.; Liu, Z. Combination of cell-penetrating peptides with nanomaterials for the potential therapeutics of central nervous system disorders: A review. J. Nanobiotechnol. 2021, 19, 255. [Google Scholar] [CrossRef]
- Datta, G.; Chaddha, M.; Garber, D.W.; Chung, B.H.; Tytler, E.M.; Dashti, N.; Bradley, W.A.; Gianturco, S.H.; Anantharamaiah, G.M. The receptor binding domain of apolipoprotein E, linked to a model class A amphipathic helix, enhances internalization and degradation of LDL by fibroblasts. Biochemistry 2000, 39, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Spencer, B.J.; Verma, I.M. Targeted delivery of proteins across the blood-brain barrier. Proc. Natl. Acad. Sci. USA 2007, 104, 7594–7599. [Google Scholar] [CrossRef]
- Wang, D.; El-Amouri, S.S.; Dai, M.; Kuan, C.Y.; Hui, D.Y.; Brady, R.O.; Pan, D. Engineering a lysosomal enzyme with a derivative of receptor-binding domain of apoE enables delivery across the blood-brain barrier. Proc. Natl. Acad. Sci. USA 2013, 110, 2999–3004. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.X.; He, C.P.; Fan, S.; Zhu, Y.L.; Qi, C.; Huang, N.P.; Xiao, Z.D.; Lu, Z.H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
- Kumar, P.; Wu, H.; McBride, J.L.; Jung, K.E.; Kim, M.H.; Davidson, B.L.; Lee, S.K.; Shankar, P.; Manjunath, N. Transvascular delivery of small interfering RNA to the central nervous system. Nature 2007, 448, 39–43. [Google Scholar] [CrossRef]
- Javed, H.; Menon, S.A.; Al-Mansoori, K.M.; Al-Wandi, A.; Majbour, N.K.; Ardah, M.T.; Varghese, S.; Vaikath, N.N.; Haque, M.E.; Azzouz, M.; et al. Development of Nonviral Vectors Targeting the Brain as a Therapeutic Approach for Parkinson’s Disease and Other Brain Disorders. Mol. Ther. 2016, 24, 746–758. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Zhang, A.; You, S.S.; Lieber, C.M. Spontaneous Internalization of Cell Penetrating Peptide-Modified Nanowires into Primary Neurons. Nano Lett. 2016, 16, 1509–1513. [Google Scholar] [CrossRef]
- Neves, V.; Aires-da-Silva, F.; Morais, M.; Gano, L.; Ribeiro, E.; Pinto, A.; Aguiar, S.; Gaspar, D.; Fernandes, C.; Correia, J.D.G.; et al. Novel Peptides Derived from Dengue Virus Capsid Protein Translocate Reversibly the Blood-Brain Barrier through a Receptor-Free Mechanism. ACS Chem. Biol. 2017, 12, 1257–1268. [Google Scholar] [CrossRef]
- Oller-Salvia, B.; Sánchez-Navarro, M.; Ciudad, S.; Guiu, M.; Arranz-Gibert, P.; Garcia, C.; Gomis, R.R.; Cecchelli, R.; García, J.; Giralt, E.; et al. MiniAp-4: A Venom-Inspired Peptidomimetic for Brain Delivery. Angew. Chem. Int. Ed. Engl. 2016, 55, 572–575. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.H.; Liu, I.J.; Lu, R.M.; Wu, H.C. Advancement and applications of peptide phage display technology in biomedical science. J. Biomed. Sci. 2016, 23, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.; Qian, J.; Cao, S.; Yang, Z.; Pang, Z.; Pan, S.; Fan, L.; Xi, Z.; Jiang, X.; Zhang, Q. Precise glioma targeting of and penetration by aptamer and peptide dual-functioned nanoparticles. Biomaterials 2012, 33, 5115–5123. [Google Scholar] [CrossRef] [PubMed]
- Majerova, P.; Hanes, J.; Olesova, D.; Sinsky, J.; Pilipcinec, E.; Kovac, A. Novel blood–brain barrier shuttle peptides discovered through the phage display method. Molecules 2020, 25, 874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torchilin, V.P. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu. Rev. Biomed. Eng. 2006, 8, 343–375. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, K.; Lu, J.; Venkatraman, S.S.; Luo, D.; Ng, K.C.; Ling, E.A.; Moochhala, S.; Yang, Y.Y. Biologically active core/shell nanoparticles self-assembled from cholesterol-terminated PEG-TAT for drug delivery across the blood-brain barrier. Biomaterials 2008, 29, 1509–1517. [Google Scholar] [CrossRef]
- Agyare, E.K.; Curran, G.L.; Ramakrishnan, M.; Yu, C.C.; Poduslo, J.F.; Kandimalla, K.K. Development of a smart nano-vehicle to target cerebrovascular amyloid deposits and brain parenchymal plaques observed in Alzheimer’s disease and cerebral amyloid angiopathy. Pharm. Res. 2008, 25, 2674–2684. [Google Scholar] [CrossRef]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Modgil, A.; Layek, B.; Arora, K.; Sun, C.; Law, B.; Singh, J. Cell penetrating peptide tethered bi-ligand liposomes for delivery to brain in vivo: Biodistribution and transfection. J. Control. Release 2013, 167, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Urbiola, K.; Blanco-Fernández, L.; Ogris, M.; Rödl, W.; Wagner, E.; Tros de Ilarduya, C. Novel PAMAM-PEG-Peptide Conjugates for siRNA Delivery Targeted to the Transferrin and Epidermal Growth Factor Receptors. Pers. Med. 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, F.; Pang, Z.; Zhao, J.; Jin, K.; Li, H.; Pang, Q.; Zhang, L.; Pang, Z. Angiopep-2-conjugated poly(ethylene glycol)-co- poly(ε-caprolactone) polymersomes for dual-targeting drug delivery to glioma in rats. Int. J. Nanomed. 2017, 12, 2117–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlschwede, K.M.; Curran, G.L.; Rosenberg, J.T.; Grant, S.C.; Sarkar, G.; Jenkins, R.B.; Ramakrishnan, S.; Poduslo, J.F.; Kandimalla, K.K. Cationic carrier peptide enhances cerebrovascular targeting of nanoparticles in Alzheimer’s disease brain. Nanomedicine 2019, 16, 258–266. [Google Scholar] [CrossRef]
- Nosrati, H.; Tarantash, M.; Bochani, S.; Charmi, J.; Bagheri, Z.; Fridoni, M.; Abdollahifar, M.A.; Davaran, S.; Danafar, H.; Kheiri Manjili, H. Glutathione (GSH) peptide conjugated magnetic nanoparticles as blood–brain barrier shuttle for MRI-monitored brain delivery of paclitaxel. ACS Biomater. Sci. Eng. 2019, 5, 1677–1685. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, B.; Su, L.; Nie, W.; Han, D.; Han, G.; Zhang, H.; Chong, C.; Tan, J. Subcellular distributions of iron oxide nanoparticles in rat brains affected by different surface modifications. Biomed. Mater. Res. A 2019, 107, 1988–1998. [Google Scholar] [CrossRef]
- Yang, L.; Qian, W.; Scott, P.; Shao, X. Towards the development of brain-penetrating gold nanoparticle-transactivator of transcription (TAT) peptide conjugates. J. Nucl. Med. 2018, 59, 1034. [Google Scholar]
- Kang, J.; Joo, J.; Kwon, E.J.; Skalak, M.; Hussain, S.; She, Z.G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Self-Sealing Porous Silicon-Calcium Silicate Core-Shell Nanoparticles for Targeted siRNA Delivery to the Injured Brain. Adv. Mater. 2016, 28, 7962–7969. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Pusic, A.D.; Pusic, K.M.; Clayton, B.L.; Kraig, R.P. IFNγ-stimulated dendritic cell exosomes as a potential therapeutic for remyelination. J. Neuroimmunol. 2014, 266, 12–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, Y.; Leach, A.G.; Smith, J.; Pluchino, S.; Coxonl, C.R.; Sivakumaran, M.; Downing, J.; Fatokun, A.A.; Teixidò, M.; Ehtezazi, T. Peptide based drug delivery systems to the brain. Nano. Express 2020, 1, 012002. [Google Scholar] [CrossRef]
- Cooper, J.M.; Wiklander, P.B.; Nordin, J.Z.; Al-Shawi, R.; Wood, M.J.; Vithlani, M.; Schapira, A.H.; Simons, J.P.; El-Andaloussi, S.; Alvarez-Erviti, L. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov. Disord. 2014, 29, 1476–1485. [Google Scholar] [CrossRef] [Green Version]
- Pulford, B.; Reim, N.; Bell, A.; Veatch, J.; Forster, G.; Bender, H.; Meyerett, C.; Hafeman, S.; Michel, B.; Johnson, T.; et al. Liposome-siRNA-peptide complexes cross the blood-brain barrier and significantly decrease PrP on neuronal cells and PrP in infected cell cultures. PLoS ONE 2010, 5, e11085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bender, H.R.; Kane, S.; Zabel, M.D. Delivery of Therapeutic siRNA to the CNS Using Cationic and Anionic Liposomes. J. Vis. Exp. 2016, 113, e54106. [Google Scholar]
- Grinberg, S.; Linder, C.; Kolot, V.; Waner, T.; Wiesman, Z.; Shaubi, E.; Heldman, E. Novel cationic amphiphilic derivatives from vernonia oil: Synthesis and self-aggregation into bilayer vesicles, nanoparticles, and DNA complexants. Langmuir 2005, 21, 7638–7645. [Google Scholar] [CrossRef] [PubMed]
- Popov, M.; Abu Hammad, I.; Bachar, T.; Grinberg, S.; Linder, C.; Stepensky, D.; Heldman, E. Delivery of analgesic peptides to the brain by nano-sized bolaamphiphilic vesicles made of monolayer membranes. Eur. J. Pharm. Biopharm. 2013, 85, 381–389. [Google Scholar] [CrossRef]
- Conceição, M.; Mendonça, L.; Nóbrega, C.; Gomes, C.; Costa, P.; Hirai, H.; Moreira, J.N.; Lima, M.C.; Manjunath, N.; Pereira de Almeida, L. Intravenous administration of brain-targeted stable nucleic acid lipid particles alleviates Machado-Joseph disease neurological phenotype. Biomaterials 2016, 82, 124–137. [Google Scholar] [CrossRef]
- Lu, M.; Xing, H.; Xun, Z.; Yang, T.; Zhao, X.; Cai, C.; Wang, D.; Ding, P. Functionalized extracellular vesicles as advanced therapeutic nanodelivery systems. Eur. J. Pharm. Sci. 2018, 121, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Shao, K.; Huang, R.; Han, L.; Liu, Y.; Li, J.; Kuang, Y.; Ye, L.; Lou, J.; Jiang, C. Gene delivery targeted to the brain using an Angiopep-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. Biomaterials 2009, 30, 6976–6985. [Google Scholar] [CrossRef]
- Perez, A.P.; Romero, E.L.; Morilla, M.J. Ethylendiamine core PAMAM dendrimers/siRNA complexes as in vitro silencing agents. Int. J. Pharm. 2009, 380, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzawi, S.; Masheta, D.; Guildford, A.; Phillips, G.; Santin, M. Designing and Characterization of a Novel Delivery System for Improved Cellular Uptake by Brain Using Dendronised Apo-E-Derived Peptide. Front. Bioeng. Biotechnol. 2019, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Smith, Q.R.; Liu, X. Brain penetrating peptides and peptide-drug conjugates to overcome the blood-brain barrier and target CNS diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gerson, T.; Varney, M.L.; Singh, R.K.; Vinogradov, S.V. Multifunctional peptide-PEG intercalating conjugates: Programmatic of gene delivery to the blood-brain barrier. Pharm. Res. 2010, 27, 2528–2543. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.; Liu, M.; Lu, J.; Chu, B.; Yang, Y.; Song, B.; Wang, H.; He, Y. Bacteria loaded with glucose polymer and photosensitive ICG silicon-nanoparticles for glioblastoma photothermal immunotherapy. Nat. Commun. 2022, 13, 5127. [Google Scholar] [CrossRef]
- Wu, L.P.; Ahmadvand, D.; Su, J.; Hall, A.; Tan, X.; Farhangrazi, Z.S.; Moghimi, S.M. Crossing the blood-brain-barrier with nanoligand drug carriers self-assembled from a phage display peptide. Nat. Commun. 2019, 10, 4635. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.L.; Sidman, R.L.; Arap, W.; Pasqualini, R. Targeting vascular zip codes: From combinatorial selection to drug prototypes. In The Vasculome; Elsevier: Amsterdam, The Netherlands, 2022; pp. 393–401. [Google Scholar]
- Cho, C.F.; Farquhar, C.E.; Fadzen, C.M.; Scott, B.; Zhuang, P.; von Spreckelsen, N.; Loas, A.; Hartrampf, N.; Pentelute, B.L.; Lawler, S.E. A Tumour-Homing Peptide Platform Enhances Drug Solubility, Improves Blood-Brain Barrier Permeability and Targets Glioblastoma. Cancers 2022, 14, 2207. [Google Scholar] [CrossRef]
- Eriste, E.; Kurrikoff, K.; Suhorutšenko, J.; Oskolkov, N.; Copolovici, D.M.; Jones, S.; Laakkonen, P.; Howl, J.; Langel, Ü. Peptide-based glioma-targeted drug delivery vector gHoPe2. Bioconjug. Chem. 2013, 24, 305–313. [Google Scholar] [CrossRef]
- Prades, R.; Oller-Salvia, B.; Schwarzmaier, S.M.; Selva, J.; Moros, M.; Balbi, M.; Grazú, V.; de La Fuente, J.M.; Egea, G.; Plesnila, N.; et al. Applying the retro-enantio approach to obtain a peptide capable of overcoming the blood-brain barrier. Angew. Chem. Int. Ed. Engl. 2015, 54, 3967–3972. [Google Scholar] [CrossRef] [PubMed]
- Prades, R.; Guerrero, S.; Araya, E.; Molina, C.; Salas, E.; Zurita, E.; Selva, J.; Egea, G.; López-Iglesias, C.; Teixidó, M. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials 2012, 33, 7194–7205. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Shen, Q.; Pang, Y.; Li, X.; Chen, Y.; Wang, X.; Luo, X.; Wu, Z.; Bao, Z.; Zhang, J. The synthesized transporter K16APoE enabled the therapeutic HAYED peptide to cross the blood-brain barrier and remove excess iron and radicals in the brain, thus easing Alzheimer’s disease. Drug Deliv. Transl. Res. 2019, 9, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Badiali, L.; Ruozi, B.; Vergoni, A.V.; Bondioli, L.; Ferrari, A.; Rivasi, F.; Forni, F.; Vandelli, M.A. Can leptin-derived sequence-modified nanoparticles be suitable tools for brain delivery? Nanomedicine 2012, 7, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 1990, 2, 279–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatiparti, K.; Sau, S.; Kashaw, S.K.; Iyer, A.K. siRNA delivery strategies: A comprehensive review of recent developments. Nanomaterials 2017, 7, 77. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Lin, W.; Li, M.; Yang, Y.; Deng, J.; Liu, H.; Chen, Y.; Fu, X.; Liu, H.; Yang, Y. Efficient siRNA delivery using novel cell-penetrating peptide-siRNA conjugate-loaded nanobubbles and ultrasound. Ultrasound. Med. Biol. 2016, 42, 1362–1374. [Google Scholar] [CrossRef]
- Pratt, A.J.; MacRae, I.J. The RNA-induced silencing complex: A versatile gene-silencing machine. J. Biol. Chem. 2009, 284, 17897–17901. [Google Scholar] [CrossRef] [Green Version]
- Mathupala, S.P. Delivery of small-interfering RNA (siRNA) to the brain. Expert. Opin. Ther. Pat. 2009, 19, 137–140. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Ye, J.; Pei, X.; Sun, L.; Liu, E.; Wang, J.; Huang, Y.; Lee, S.J.; He, H. Improved method for synthesis of low molecular weight protamine–siRNA conjugate. Acta Pharm. Sin. B 2018, 8, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Pärnaste, L.; Arukuusk, P.; Langel, K.; Tenson, T.; Langel, Ü. The Formation of Nanoparticles between Small Interfering RNA and Amphipathic Cell-Penetrating Peptides. Mol. Ther. Nucleic Acids 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Matsushita-Ishiodori, Y.; Ohtsuki, T. Photoinduced RNA interference. Acc. Chem. Res. 2012, 45, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Zeller, S.; Choi, C.S.; Uchil, P.D.; Ban, H.-S.; Siefert, A.; Fahmy, T.M.; Mothes, W.; Lee, S.-K.; Kumar, P. Attachment of cell-binding ligands to arginine-rich cell-penetrating peptides enables cytosolic translocation of complexed siRNA. Chem. Biol. 2015, 22, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Youn, P.; Chen, Y.; Furgeson, D.Y. A myristoylated cell-penetrating peptide bearing a transferrin receptor-targeting sequence for neuro-targeted siRNA delivery. Mol. Pharm. 2014, 11, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Cao, X.W.; Fu, L.Y.; Zhang, T.Z.; Wang, F.J.; Zhao, J. Screening and characterization of a novel high-efficiency tumour-homing cell-penetrating peptide from the buffalo cathelicidin family. J. Pept. Sci. 2019, 25, e3201. [Google Scholar] [CrossRef]
- Kang, J.H.; Battogtokh, G.; Ko, Y.T. Self-assembling lipid–peptide hybrid nanoparticles of phospholipid–nonaarginine conjugates for enhanced delivery of nucleic acid therapeutics. Biomacromolecules 2017, 18, 3733–3741. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Akita, H.; Kogure, K.; Oishi, M.; Nagasaki, Y.; Kihira, Y.; Ueno, M.; Kobayashi, H.; Kikuchi, H.; Harashima, H. Development of a novel systemic gene delivery system for cancer therapy with a tumour-specific cleavable PEG-lipid. Gene Ther. 2007, 14, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Xiang, B.; Jia, X.-L.; Qi, J.-L.; Yang, L.-P.; Sun, W.-H.; Yan, X.; Yang, S.-K.; Cao, D.-Y.; Du, Q.; Qi, X.-R. Enhancing siRNA-based cancer therapy using a new pH-responsive activatable cell-penetrating peptide-modified liposomal system. Int. J. Nanomed. 2017, 12, 2385. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Xie, X.; Yang, Y.; Li, Z.; Yu, F.; Gong, W.; Li, Y.; Zhang, H.; Wang, Z.; Mei, X. Polymer nanoparticles modified with photo-and pH-dual-responsive polypeptides for enhanced and targeted cancer therapy. Mol. Pharm. 2016, 13, 1508–1519. [Google Scholar] [CrossRef]
- Richard, C.M. The basic science of gene therapy. Science 1993, 260, 926–932. [Google Scholar]
- Quinonez, R.; Sutton, R.E. Lentiviral vectors for gene delivery into cells. DNA Cell Biol. 2002, 21, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Zabel, M.D.; Mollnow, L.; Bender, H. siRNA Therapeutics for Protein Misfolding Diseases of the Central Nervous System. Des. Deliv. SiRNA Ther. 2021, 2282, 377–394. [Google Scholar]
- Chiu, Y.L.; Ali, A.; Chu, C.Y.; Cao, H.; Rana, T.M. Visualizing a correlation between siRNA localization, cellular uptake, and RNAi in living cells. Chem. Biol. 2004, 11, 1165–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meade, B.R.; Dowdy, S.F. Enhancing the cellular uptake of siRNA duplexes following noncovalent packaging with protein transduction domain peptides. Adv. Drug. Deliv. Rev. 2008, 60, 530–536. [Google Scholar] [CrossRef] [Green Version]
- Barichello, J.M.; Ishida, T.; Kiwada, H. Complexation of siRNA and pDNA with cationic liposomes: The important aspects in lipoplex preparation. Liposomes Methods Protoc. Pharm. Nanocarriers 2010, 605, 461–472. [Google Scholar]
- Malone, R.W.; Felgner, P.L.; Verma, I.M. Cationic liposome-mediated RNA transfection. Proc. Natl. Acad. Sci. USA 1989, 86, 6077–6081. [Google Scholar] [CrossRef]
- Balazs, D.A.; Godbey, W. Liposomes for use in gene delivery. J. Drug Deliv. 2011, 2011, 326497. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.D.; Burgess, D.J. DNA-based Biopharmaceuticals: Therapeutics for the 21st Century. AAPS Newsmag. 2003, 6, 27. [Google Scholar]
- Georgieva, J.V.; Hoekstra, D.; Zuhorn, I.S. Smuggling drugs into the brain: An overview of ligands targeting transcytosis for drug delivery across the blood–brain barrier. Pharmaceutics 2014, 6, 557–583. [Google Scholar] [CrossRef] [Green Version]
- Uno, Y.; Piao, W.; Miyata, K.; Nishina, K.; Mizusawa, H.; Yokota, T. High-density lipoprotein facilitates in vivo delivery of α-tocopherol–conjugated short-interfering RNA to the brain. Hum. Gene Ther. 2011, 22, 711–719. [Google Scholar] [CrossRef] [Green Version]
- Papahadjopoulos, D.; Allen, T.; Gabizon, A.; Mayhew, E.; Matthay, K.; Huang, S.; Lee, K.; Woodle, M.; Lasic, D.; Redemann, C. Sterically stabilized liposomes: Improvements in pharmacokinetics and antitumour therapeutic efficacy. Proc. Natl. Acad. Sci. USA 1991, 88, 11460–11464. [Google Scholar] [CrossRef]
- Zamboni, W.C.; Ramalingam, S.; Friedland, D.M.; Edwards, R.P.; Stoller, R.G.; Strychor, S.; Maruca, L.; Zamboni, B.A.; Belani, C.P.; Ramanathan, R.K. Phase I and pharmacokinetic study of pegylated liposomal CKD-602 in patients with advanced malignancies. Clin. Cancer Res. 2009, 15, 1466–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battaglia, L.; Panciani, P.P.; Muntoni, E.; Capucchio, M.T.; Biasibetti, E.; De Bonis, P.; Mioletti, S.; Fontanella, M.; Swaminathan, S. Lipid nanoparticles for intranasal administration: Application to nose-to-brain delivery. Expert Opin. Drug Deliv. 2018, 15, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Danielyan, L.; Schäfer, R.; von Ameln-Mayerhofer, A.; Buadze, M.; Geisler, J.; Klopfer, T.; Burkhardt, U.; Proksch, B.; Verleysdonk, S.; Ayturan, M.; et al. Intranasal delivery of cells to the brain. Eur. J. Cell Biol. 2009, 88, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Danielyan, L.; Schäfer, R.; von Ameln-Mayerhofer, A.; Bernhard, F.; Verleysdonk, S.; Buadze, M.; Lourhmati, A.; Klopfer, T.; Schaumann, F.; Schmid, B. Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson disease. Rejuvenation Res. 2011, 14, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Danielyan, L.; Beer-Hammer, S.; Stolzing, A.; Schäfer, R.; Siegel, G.; Fabian, C.; Kahle, P.; Biedermann, T.; Lourhmati, A.; Buadze, M. Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer’s and Parkinson’s disease. Cell Transplant. 2014, 23, 123–139. [Google Scholar] [CrossRef] [Green Version]
- Donega, V.; Nijboer, C.H.; van Tilborg, G.; Dijkhuizen, R.M.; Kavelaars, A.; Heijnen, C.J. Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury. Exp. Neurol. 2014, 261, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Oppliger, B.; Joerger-Messerli, M.; Mueller, M.; Reinhart, U.; Schneider, P.; Surbek, D.V.; Schoeberlein, A. Intranasal delivery of umbilical cord-derived mesenchymal stem cells preserves myelination in perinatal brain damage. Stem Cells Dev. 2016, 25, 1234–1242. [Google Scholar] [CrossRef]
- Balyasnikova, I.V.; Prasol, M.S.; Ferguson, S.D.; Han, Y.; Ahmed, A.U.; Gutova, M.; Tobias, A.L.; Mustafi, D.; Rincón, E.; Zhang, L. Intranasal delivery of mesenchymal stem cells significantly extends survival of irradiated mice with experimental brain tumours. Mol. Ther. 2014, 22, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izco, M.; Carlos, E.; Alvarez-Erviti, L. The two faces of Exosomes in Parkinson’s disease: From pathology to therapy. Neuroscientist 2022, 28, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Al Zaki, A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional nanoparticles: Cost versus benefit of adding targeting and imaging capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef] [Green Version]
- Castanotto, D.; Rossi, J.J. The promises and pitfalls of RNA-interference-based therapeutics. Nature 2009, 457, 426–433. [Google Scholar] [CrossRef] [Green Version]
| Name of the Peptide | Sequence of the Peptide | Peptide Source | Formulations/Carriers | Ref. No. |
|---|---|---|---|---|
| ApoE | LRKLRKRLL | Apolipoprotein E | Shuttle synthetic peptides | [77] |
| ApoB | SSVIDALQYKLEGTTRLTRKRGLKLATALSLSNKFVEGS | Apolipoprotein B | Shuttle synthetic peptides | [78] |
| hApoE | LRKLRKRLLR | Human apolipoprotein E (hApoE) | Shuttle synthetic peptides | [79] |
| RVG-29 | YTIWMPENPRPGTPCDIFTNSRGKRASNG | Rabies virus glycoprotein | Shuttle natural peptide | [81] |
| TAT | GGGGYGRKKRRQRRR | Human immunodeficiency virus 1 | Shuttle natural peptide | [83] |
| PepH3 | AGILKRW | Dengue virus type 2 capsid protein (DEN2C) | Shuttle natural peptide | [84] |
| Apamin | H-CNCKAPETALCARRCQQH-NH2 | Venom neurotoxin | Shuttle natural peptide | [85] |
| MiniAp-4 | H-DapKAPETALD-NH2 | Venom neurotoxin | Shuttle natural peptide | [85] |
| THRre | PWVPSWMPPRHT | Phage display | Shuttle natural peptide | [86] |
| TGN | TGNYKALHPHNG | Phage display | Shuttle natural peptide | [87] |
| THR | THRPPMWSPVWP | Phage display | Shuttle natural peptide | [123] |
| THRre_2f | (PWVPSWMPPRHT)2KKGK(CF)G | Phage display | Shuttle natural peptide | [124] |
| K16APoE | HAYED | Apolipoprotein E (LDLR) | Shuttle natural peptide | [125] |
| TAT peptide | Tat-PEG-b-chol | Nanoparticles | NPs (PMNPs) | [90] |
| Polyamine (putrescine) | F(ab’) anti-amyloid antibody | Nanoparticles | Polymeric NPs (PMNPs) | [91] |
| TfR-peptide | TfR poly-L-arginine | Poly-ethylene glycol liposomes | Polymeric NPs (PMNPs) | [93] |
| GE11 peptide | TfR-endothelial factor receptor (EGFR) | siRNA/TMC–PEG-RV | Polymeric NPs (PMNPs) | [94] |
| Angiopep-2 | TFFYGGSRGKRNNFKTEEY | Neurotropic endogenous protein | Polymeric NPs (PMNPs) | [95] |
| K16APoE | HAYED | PLGA-NPs | Polymeric NPs (PMNPs) | [96] |
| g7 | GFtGPLS (O-β-d-glucose) CONH2 | Enkephalin analogues/opioid | Polymeric NPs (PMNPs) | [119,126] |
| Mal-SPIONs | [C2H2(CO)2O]Fe₂O₃ | Superparamagnetic iron oxide nanoparticles | Metallic NPs | [88] |
| GSH-peptide | IONPs@Asp-PTX-PEG-GSH | Glutathione nanoparticles (GSHIONPs) | Metallic NPs | [97] |
| Silicon NPs | pSiNPs | Rabies virus-mimetic silica-coated gold nanorods | Metallic NPs | [100] |
| cyclo-peptide | c(RGDy)K | Macrophages/monocytes | Exosomes | [80] |
| neuron-specific RVG peptide | siRNA-RVG-9R | Dendritic cells | Exosomes | [103] |
| miR-219 | Dendritic cells | Exosomes | [104] | |
| siRNA3 RVG | Bone marrow | Exosomes | [106] | |
| siRNA-peptide | octadecenolyoxy[ethyl-2-heptadecenyl-3 hydroxyethyl] imidazolinium chloride | Bone marrow | Exosomes | [107] |
| neuroleptin-1-targeted peptide | RGERPRR | Macrophages/monocytes | Exosomes | [127] |
| siRNA-RVG peptide | 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) | Cationic liposomes | Liposomes | [108] |
| siRNA-RVG peptide | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE) | Cationic liposomes | Liposomes | [108] |
| siRNA-peptide (RVG-9r) | RVG-29-PEG-PLGA/DTX | Cationic liposomes | Liposomes | [109] |
| kyotorphin or leu-enkephalin | methyl ester-methyl vernolate | Self-assembled liposomes | Liposomes | [110] |
| siRNA-RVG peptide | Stable nucleic acid lipid particles [SNALPs] | Self-assembled liposomes | Liposomes | [111] |
| LRP1 | ANG-PEG– poly(ε-caprolactone) | Self-assembled liposomes | Liposomes | [102,112] |
| Angiopep peptide | TFFYGGSRGKRNNFKTEEYC | PAMAM–PEG–Angiopep/DNA | Dendrimer nanoparticles | [113] |
| ApoE derived peptide | LRKLRKRLLR | Lysine dendrons | Dendrimer nanoparticles | [115] |
| pPAC | CNAFTPD | Peptide-PEG-tris-acridine conjugates (pPAC) | Brain-homing peptide (BH) | [117] |
| phage-derived peptide | NLC-β-secretase 1 (BACE1) siRNA | Photosensitive ICG silicon-nanoparticles | Brain-homing peptide (BH) | [119] |
| phage-derived peptide | CNSRLHLRC, CENWWGDVC, WRCVLREGPAGGCAWFNRHRL | Nanoparticles | Brain-homing peptide (BH) | [120] |
| BTP-7 | BTP-7-Camptothecin (CPT) | Patient-derived GBM stem cells | Brain-homing peptide (BH) | [121] |
| gHoPe2 | NHQQQNPHQPPM | Phage-derived | Glioma-homing peptide (gHo) | [122] |
| S. No. | siRNA-CPP Therapeutics | Route of Delivery | Formulations | Consequences/Concerns | Refs. |
|---|---|---|---|---|---|
| 1. | Virus-delivered siRNAs | ||||
| Intracranial injections of hRNA to CNS | Vesicular stomatitis virus glycoprotein envelope (VSV-G) | Can turn brain cells cancerous | [144] | |
| Intravascular injection targeted to neuronal PrPC | siRNA encapsulated in either cationic or anionic liposomes | Decreased levels of cellular prion protein (PrPC) | [146] | |
| 2. | Non-viral delivery of siRNAs | Intravenous administration of cholesterol-conjugated siRNA lipoplexes | Cationic, anionic, or neutral, or a mixture, liposomes |
| [148] |
| 3. | Liposome-siRNA-peptide complexes (LSPCs) | In vitro RNA transfection with DOTMA-containing liposomes (lipofectin) | Cationic liposome |
| [149] |
| In vitro transfection with anionic lipoplexes (DOPG:DOPE) | Anionic liposome |
| [152] | ||
| Intravenous injection | PEGylated liposomes |
| [154] | ||
| Intravenous injection | PEGylated liposomes plus monoclonal antibody |
| [155] | ||
| 4. | Intranasal delivery of siRNA | Direct administration of drugs or stem cells into the nasal cavity | Human bone marrow-derived mesenchymal stem cells (MSC) |
| [159,160,161] |
| 5. | siRNA-loaded exosomes | Intravenous injection | Exosomes |
| [164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghorai, S.M.; Deep, A.; Magoo, D.; Gupta, C.; Gupta, N. Cell-Penetrating and Targeted Peptides Delivery Systems as Potential Pharmaceutical Carriers for Enhanced Delivery across the Blood–Brain Barrier (BBB). Pharmaceutics 2023, 15, 1999. https://doi.org/10.3390/pharmaceutics15071999
Ghorai SM, Deep A, Magoo D, Gupta C, Gupta N. Cell-Penetrating and Targeted Peptides Delivery Systems as Potential Pharmaceutical Carriers for Enhanced Delivery across the Blood–Brain Barrier (BBB). Pharmaceutics. 2023; 15(7):1999. https://doi.org/10.3390/pharmaceutics15071999
Chicago/Turabian StyleGhorai, Soma Mondal, Auroni Deep, Devanshi Magoo, Chetna Gupta, and Nikesh Gupta. 2023. "Cell-Penetrating and Targeted Peptides Delivery Systems as Potential Pharmaceutical Carriers for Enhanced Delivery across the Blood–Brain Barrier (BBB)" Pharmaceutics 15, no. 7: 1999. https://doi.org/10.3390/pharmaceutics15071999
APA StyleGhorai, S. M., Deep, A., Magoo, D., Gupta, C., & Gupta, N. (2023). Cell-Penetrating and Targeted Peptides Delivery Systems as Potential Pharmaceutical Carriers for Enhanced Delivery across the Blood–Brain Barrier (BBB). Pharmaceutics, 15(7), 1999. https://doi.org/10.3390/pharmaceutics15071999