Induced Vascular Normalization—Can One Force Tumors to Surrender to a Better Microenvironment?
Abstract
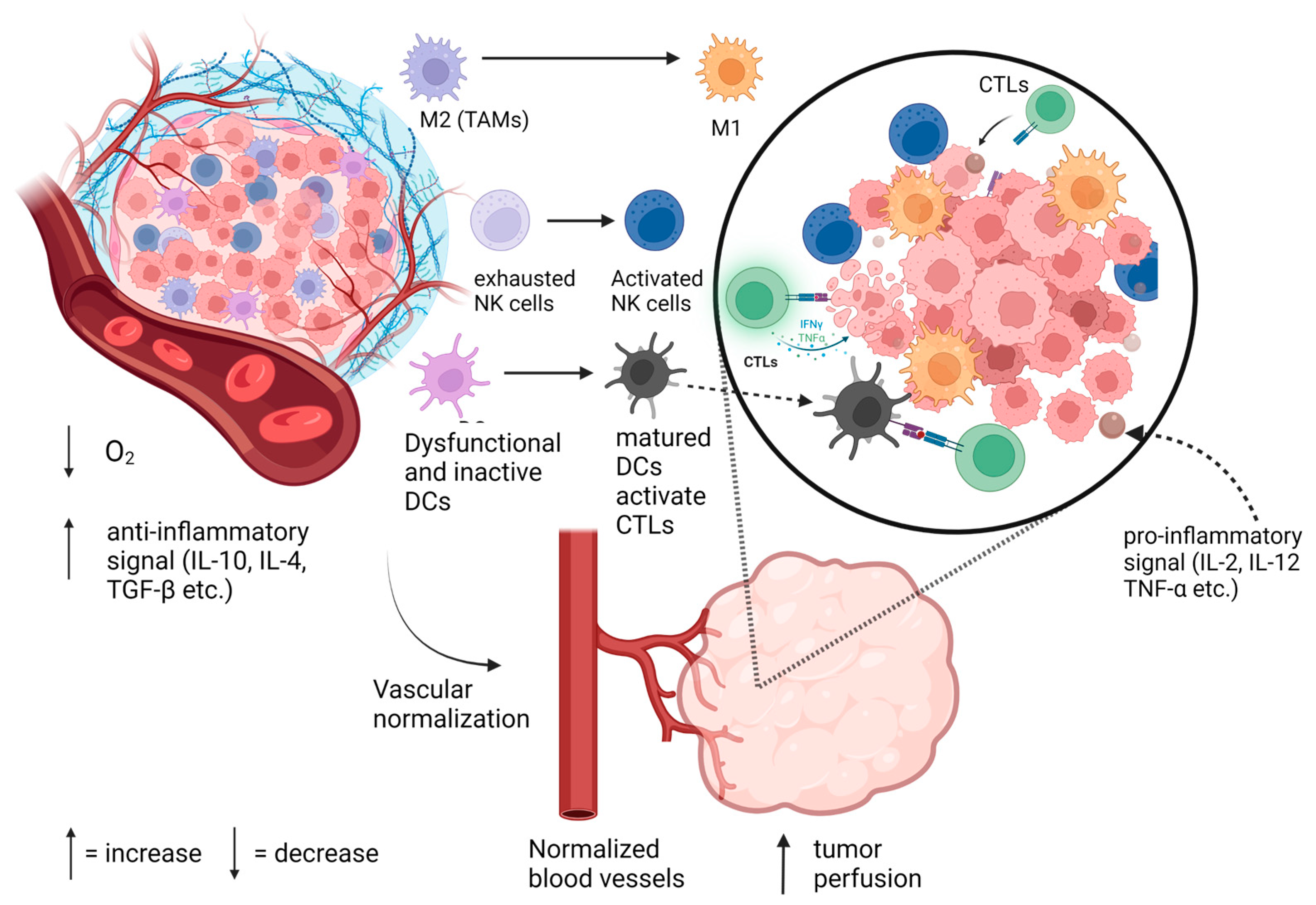
:1. Introduction
2. Cancer Angiogenesis and the TME
2.1. Role of Hypoxia in Tumor Progression
2.2. Role of Hypoxia in Tumor Immune Suppression
2.3. Major Targets in Cancer Angiogenesis That Have Immunomodulatory Effects
2.3.1. VEGF
2.3.2. bFGF
2.3.3. PDGF
3. TVN and Its Immunomodulatory Benefits
3.1. TVN and T Cells
3.2. TVN and Tumor-Associated Macrophages
3.3. TVN and Dendritic Cells and Myeloid-Derived Suppressor Cells
3.4. TVN and Natural Killer Cells
4. Anti-Cancer Treatments That Engender Tumor Vascular Normalization
4.1. Induction of Tumor Vascular Normalization by Repurposing Cardiovascular Drugs
4.1.1. Renin Angiotensin Aldosterone System Inhibitors—ARBs and ACE-Is
4.1.2. Beta-Blockers (β-Blockers)
4.1.3. Cyclooxygenase (COX) Inhibitors
4.1.4. Cardiac Glycosides (CGs)
4.2. Metronomic Dosing of Chemotherapy Drugs
4.2.1. MC and Breast Cancer
4.2.2. MC and Non-Small-Cell Lung Cancer (NSCLC)
4.2.3. MC and Ovarian Cancer
4.2.4. MC and Glioblastoma
4.3. Nanoformulations and TVN: Defining a Platform to Augment the Activity of Immunotherapeutics
A Liposomal Drug Formulation That May Be Ideal for Engendering Changes in the TME
5. Discussion and Comment on Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H., Jr. A Review of Cancer Immunotherapy: From the Past, to the Present, to the Future. Curr. Oncol. 2020, 27 (Suppl. S2), S87–S97. [Google Scholar] [CrossRef] [PubMed]
- Akkın, S.; Varan, G.; Bilensoy, E. A Review on Cancer Immunotherapy and Applications of Nanotechnology to Chemoimmunotherapy of Different Cancers. Molecules 2021, 26, 3382. [Google Scholar] [CrossRef] [PubMed]
- Naimi, A.; Mohammed, R.N.; Raji, A.; Chupradit, S.; Yumashev, A.V.; Suksatan, W.; Shalaby, M.N.; Thangavelu, L.; Kamrava, S.; Shomali, N.; et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun. Signal. 2022, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Twomey, J.D.; Zhang, B. Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics. AAPS J. 2021, 23, 39. [Google Scholar] [CrossRef]
- Liu, C.; Yang, M.; Zhang, D.; Chen, M.; Zhu, D. Clinical cancer immunotherapy: Current progress and prospects. Front. Immunol. 2022, 13, 961805. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [Green Version]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Q.; Hong, Y. Tumor Vessel Normalization: A Window to Enhancing Cancer Immunotherapy. Technol. Cancer Res. Treat. 2020, 19, 1533033820980116. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Hall, A.P. The role of angiogenesis in cancer. Comp. Clin. Pathol. 2005, 13, 95–99. [Google Scholar] [CrossRef]
- Eichhorn, M.E.; Kleespies, A.; Angele, M.K.; Jauch, K.-W.; Bruns, C.J. Angiogenesis in cancer: Molecular mechanisms, clinical impact. Langenbecks Arch. Surg. 2007, 392, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.-Q.; Du, W.-L.; Cai, M.-H.; Yao, J.-Y.; Zhao, Y.-Y.; Mou, X.-Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell. Immunol. 2020, 353, 104119. [Google Scholar] [CrossRef]
- Stockmann, C.; Schadendorf, D.; Klose, R.; Helfrich, I. The Impact of the Immune System on Tumor: Angiogenesis and Vascular Remodeling. Front. Oncol. 2014, 4, 69. [Google Scholar] [CrossRef] [Green Version]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Sun, W.; Wei, F.-Q.; Li, W.-J.; Wei, J.-W.; Zhong, H.; Wen, Y.-H.; Lei, W.-B.; Chen, L.; Li, H.; Lin, H.-Q.; et al. A positive-feedback loop between tumour infiltrating activated Treg cells and type 2-skewed macrophages is essential for progression of laryngeal squamous cell carcinoma. Br. J. Cancer 2017, 117, 1631–1643. [Google Scholar] [CrossRef] [Green Version]
- Khouzam, R.A.; Brodaczewska, K.; Filipiak, A.; Zeinelabdin, N.A.; Buart, S.; Szczylik, C.; Kieda, C.; Chouaib, S. Tumor Hypoxia Regulates Immune Escape/Invasion: Influence on Angiogenesis and Potential Impact of Hypoxic Biomarkers on Cancer Therapies. Front. Immunol. 2021, 11, 613114. [Google Scholar] [CrossRef]
- Bhandari, V.; Hoey, C.; Liu, L.Y.; Lalonde, E.; Ray, J.; Livingstone, J.; Lesurf, R.; Shiah, Y.-J.; Vujcic, T.; Huang, X.; et al. Molecular landmarks of tumor hypoxia across cancer types. Nat. Genet. 2019, 51, 308–318. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Shi, R.; Zhang, Q. Hypoxia and Oxygen-Sensing Signaling in Gene Regulation and Cancer Progression. Int. J. Mol. Sci. 2021, 21, 8162. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Semenza, G.L. Oxygen Sensing and Homeostasis. Physiology 2015, 30, 340–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korbecki, J.; Simińska, D.; Gąssowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-κB Activation: A Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 10701. [Google Scholar] [CrossRef]
- Nejad, A.E.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Javanmard, S.H.; Taherian, M.; Ahmadlou, M.; et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: A novel approach to developing treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, B.J.; Lee, C.-M.; Bennewith, K.L. Transiently hypoxic tumour cell turnover and radiation sensitivity in human tumour xenografts. Br. J. Cancer 2022, 126, 1616–1626. [Google Scholar] [CrossRef]
- Chaudary, N.; Hill, R.P. Increased expression of metastasis-related genes in hypoxic cells sorted from cervical and lymph nodal xenograft tumors. Lab. Investig. 2009, 89, 587–596. [Google Scholar] [CrossRef] [Green Version]
- Kierans, S.J.; Taylor, C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): Implications for cellular physiology. J. Physiol. 2020, 599, 23–37. [Google Scholar] [CrossRef]
- Riemann, A.; Ihling, A.; Thomas, J.; Schneider, B.; Thews, O.; Gekle, M. Acidic environment activates inflammatory programs in fibroblasts via a cAMP–MAPK pathway. Biochim. Biophys. Acta—Mol. Cell Res. 2015, 1853, 299–307. [Google Scholar] [CrossRef]
- Barnabei, L.; Laplantine, E.; Mbongo, W.; Rieux-Laucat, F.; Weil, R. NF-κB: At the Borders of Autoimmunity and Inflammation. Front. Immunol. 2021, 12, 716469. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Cao, L.; Huang, T.; Chen, X.; Li, W.; Yang, X.; Zhang, W.; Li, M.; Gao, R. Uncovering the interplay between pH receptors and immune cells: Potential drug targets (Review). Oncol. Rep. 2021, 46, 228. [Google Scholar] [CrossRef]
- Díaz, F.E.; Dantas, E.; Geffner, J. Unravelling the Interplay between Extracellular Acidosis and Immune Cells. Mediat. Inflamm. 2018, 2018, 1218297. [Google Scholar] [CrossRef] [Green Version]
- Rébé, C.; Ghiringhelli, F. Interleukin-1β and Cancer. Cancers 2020, 12, 1791. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Hoffmann, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; et al. Inhibitory effect of tumor cell–derived lactic acid on human T cells. Blood 2007, 109, 3812–3819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calcinotto, A.; Filipazzi, P.; Grioni, M.; Iero, M.; De Milito, A.; Ricupito, A.; Cova, A.; Canese, R.; Jachetti, E.; Rossetti, M.; et al. Modulation of Microenvironment Acidity Reverses Anergy in Human and Murine Tumor-Infiltrating T Lymphocytes. Cancer Res. 2012, 72, 2746–2756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, V.Y.; Ayari, A.; O’connor, R.S. Beyond the Lactate Paradox: How Lactate and Acidity Impact T Cell Therapies against Cancer. Antibodies 2021, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Bannoud, N.; Dalotto-Moreno, T.; Kindgard, L.; García, P.A.; Blidner, A.G.; Mariño, K.V.; Rabinovich, G.A.; Croci, D.O. Hypoxia Supports Differentiation of Terminally Exhausted CD8 T Cells. Front. Immunol. 2021, 12, 660944. [Google Scholar] [CrossRef]
- Dang, E.V.; Barbi, J.; Yang, H.-Y.; Jinasena, D.; Yu, H.; Zheng, Y.; Bordman, Z.; Fu, J.; Kim, Y.; Yen, H.-R.; et al. Control of TH17/Treg Balance by Hypoxia-Inducible Factor 1. Cell 2011, 146, 772–784. [Google Scholar] [CrossRef] [Green Version]
- Zahra, F.T.; Sajib, S.; Mikelis, C.M. Role of bFGF in Acquired Resistance upon Anti-VEGF Therapy in Cancer. Cancers 2021, 13, 1422. [Google Scholar] [CrossRef]
- Stavri, G.T.; Zachary, I.C.; Baskerville, P.A.; Martin, J.F.; Erusalimsky, J.D. Basic Fibroblast Growth Factor Upregulates the Expression of Vascular Endothelial Growth Factor in Vascular Smooth Muscle Cells. Circulation 1995, 92, 11–14. [Google Scholar] [CrossRef]
- Hosaka, K.; Yang, Y.; Seki, T.; Du, Q.; Jing, X.; He, X.; Wu, J.; Zhang, Y.; Morikawa, H.; Nakamura, M.; et al. Therapeutic paradigm of dual targeting VEGF and PDGF for effectively treating FGF-2 off-target tumors. Nat. Commun. 2020, 11, 3704. [Google Scholar] [CrossRef] [PubMed]
- Hellberg, C.; Östman, A.; Heldin, C.-H. PDGF and Vessel Maturation. Angiogenesis Inhib. 2010, 180, 103–114. [Google Scholar] [CrossRef]
- Mashreghi, M.; Azarpara, H.; Bazaz, M.R.; Jafari, A.; Masoudifar, A.; Mirzaei, H.; Jaafari, M.R. Angiogenesis biomarkers and their targeting ligands as potential targets for tumor angiogenesis. J. Cell. Physiol. 2018, 233, 2949–2965. [Google Scholar] [CrossRef] [PubMed]
- Akwii, R.G.; Sajib, M.S.; Zahra, F.T.; Mikelis, C.M. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 2019, 8, 471. [Google Scholar] [CrossRef] [Green Version]
- Leong, A.; Kim, M. The Angiopoietin-2 and TIE Pathway as a Therapeutic Target for Enhancing Antiangiogenic Therapy and Immunotherapy in Patients with Advanced Cancer. Int. J. Mol. Sci. 2020, 21, 8689. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef]
- DU, H.; Shi, H.; Chen, D.; Zhou, Y.; Che, G. Cross-talk between endothelial and tumor cells via basic fibroblast growth factor and vascular endothelial growth factor signaling promotes lung cancer growth and angiogenesis. Oncol. Lett. 2015, 9, 1089–1094. [Google Scholar] [CrossRef] [Green Version]
- Im, J.H.; Buzzelli, J.N.; Jones, K.; Franchini, F.; Gordon-Weeks, A.; Markelc, B.; Chen, J.; Kim, J.; Cao, Y.; Muschel, R.J. FGF2 alters macrophage polarization, tumour immunity and growth and can be targeted during radiotherapy. Nat. Commun. 2020, 11, 4064. [Google Scholar] [CrossRef]
- Cao, Y.; Cao, R.; Hedlund, E.-M. R Regulation of tumor angiogenesis and metastasis by FGF and PDGF signaling pathways. J. Mol. Med. 2008, 86, 785–789. [Google Scholar] [CrossRef]
- Chen, C.-F.; Feng, X.; Liao, H.-Y.; Jin, W.-J.; Zhang, J.; Wang, Y.; Gong, L.-L.; Liu, J.-J.; Yuan, X.-H.; Zhao, B.-B.; et al. Regulation of T cell proliferation by JMJD6 and PDGF-BB during chronic hepatitis B infection. Sci. Rep. 2014, 4, 6359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daynes, R.A.; Dowell, T.; Araneo, B.A. Platelet-derived growth factor is a potent biologic response modifier of T cells. J. Exp. Med. 1991, 174, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Van Steensel, L.; Paridaens, D.; Dingjan, G.M.; van Daele, P.L.A.; van Hagen, P.M.; Kuijpers, R.W.A.M.; Bosch, W.A.v.D.; Drexhage, H.A.; Hooijkaas, H.; Dik, W.A. Platelet-Derived Growth Factor-BB: A Stimulus for Cytokine Production by Orbital Fibroblasts in Graves’ Ophthalmopathy. Investig. Opthalmol. Vis. Sci. 2010, 51, 1002–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Wei, J.; Shang, F.; Zang, K.; Ji, T. Platelet-derived growth factor B attenuates lethal sepsis through inhibition of inflammatory responses. Int. Immunopharmacol. 2019, 75, 105792. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Coelho, F.; Martins, F.; Pereira, S.A.; Serpa, J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 3765. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, A.L.; Mills, I.G. Vascular normalisation as the stepping stone into tumour microenvironment transformation. Br. J. Cancer 2021, 125, 324–336. [Google Scholar] [CrossRef]
- Baker, J.H.; Lam, J.; Kyle, A.H.; Sy, J.; Oliver, T.; Co, S.J.; Dragowska, W.H.; Ramsay, E.; Anantha, M.; Ruth, T.J.; et al. Irinophore C, a Novel Nanoformulation of Irinotecan, Alters Tumor Vascular Function and Enhances the Distribution of 5-Fluorouracil and Doxorubicin. Clin. Cancer Res. 2008, 14, 7260–7271. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Guo, C.; Ye, Q.; Shi, Y.; Sun, Y.; Zhang, J.; Huang, J.; Huang, Y.; Zeng, C.; Zhang, X.; et al. Endothelial deletion of SHP2 suppresses tumor angiogenesis and promotes vascular normalization. Nat. Commun. 2021, 12, 6310. [Google Scholar] [CrossRef]
- Tang, D.; Zhang, S.; Shi, X.; Wu, J.; Yin, G.; Tan, X.; Liu, F.; Wu, X.; Du, X. Combination of Astragali Polysaccharide and Curcumin Improves the Morphological Structure of Tumor Vessels and Induces Tumor Vascular Normalization to Inhibit the Growth of Hepatocellular Carcinoma. Integr. Cancer Ther. 2019, 18, 1534735418824408. [Google Scholar] [CrossRef] [Green Version]
- Navarro, R.; Compte, M.; Álvarez-Vallina, L.; Sanz, L. Immune Regulation by Pericytes: Modulating Innate and Adaptive Immunity. Front. Immunol. 2016, 7, 480. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, D.K.; Bhattacharya, A.; Lozinski, B.M.; Yong, V.W. Pericytes as mediators of infiltration of macrophages in multiple sclerosis. J. Neuroinflamm. 2021, 18, 301. [Google Scholar] [CrossRef]
- Fan, Y.; Du, W.; He, B.; Fu, F.; Yuan, L.; Wu, H.; Dai, W.; Zhang, H.; Wang, X.; Wang, J.; et al. The reduction of tumor interstitial fluid pressure by liposomal imatinib and its effect on combination therapy with liposomal doxorubicin. Biomaterials 2013, 34, 2277–2288. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xiao, H.; Liu, X.; Wang, Z.; Zhang, Q.; Wei, N.; Guo, X. Vascular Normalization: A New Window Opened for Cancer Therapies. Front. Oncol. 2021, 11, 719836. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Wong, A.H.-K.; Jain, R.K. Vascular Normalization as a Therapeutic Strategy for Malignant and Nonmalignant Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006486. [Google Scholar] [CrossRef]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.-T.; Sun, Z.-J. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 2021, 11, 5365–5386. [Google Scholar] [CrossRef]
- Chelvanambi, M.; Fecek, R.J.; Taylor, J.L.; Storkus, W.J. STING agonist-based treatment promotes vascular normalization and tertiary lymphoid structure formation in the therapeutic melanoma microenvironment. J. Immunother. Cancer 2021, 9, e001906. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, R.; Zhou, P.; Liu, X.; Fan, P.; Qian, L.; Dong, L.; Zhang, C.; Zheng, X.; Deng, S.; et al. DLL1 orchestrates CD8+T cells to induce long-term vascular normalization and tumor regression. Proc. Natl. Acad. Sci. USA 2021, 118, e2020057118. [Google Scholar] [CrossRef]
- Park, S.; Oh, J.H.; Park, D.J.; Zhang, H.; Noh, M.; Kim, Y.; Kim, Y.-S.; Kim, H.; Kim, Y.-M.; Ha, S.-J.; et al. CU06-1004-Induced Vascular Normalization Improves Immunotherapy by Modulating Tumor Microenvironment via Cytotoxic T Cells. Front. Immunol. 2021, 11, 620166. [Google Scholar] [CrossRef]
- Shigeta, K.; Datta, M.; Hato, T.; Kitahara, S.; Chen, I.X.; Matsui, A.; Kikuchi, H.; Mamessier, E.; Aoki, S.; Ramjiawan, R.R.; et al. Dual Programmed Death Receptor-1 and Vascular Endothelial Growth Factor Receptor-2 Blockade Promotes Vascular Normalization and Enhances Antitumor Immune Responses in Hepatocellular Carcinoma. Hepatology 2020, 71, 1247–1261. [Google Scholar] [CrossRef]
- Principe, D.R.; Chiec, L.; Mohindra, N.A.; Munshi, H.G. Regulatory T-Cells as an Emerging Barrier to Immune Checkpoint Inhibition in Lung Cancer. Front. Oncol. 2021, 11, 684098. [Google Scholar] [CrossRef]
- Kamada, T.; Togashi, Y.; Tay, C.; Ha, D.; Sasaki, A.; Nakamura, Y.; Sato, E.; Fukuoka, S.; Tada, Y.; Tanaka, A.; et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 9999–10008. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Subudhi, S.K.; Blando, J.; Scutti, J.; Vence, L.; Wargo, J.; Allison, J.P.; Ribas, A.; Sharma, P. Anti-CTLA-4 Immunotherapy Does Not Deplete FOXP3+ Regulatory T Cells (Tregs) in Human Cancers. Clin. Cancer Res. 2019, 25, 1233–1238. [Google Scholar] [CrossRef] [Green Version]
- Facciabene, A.; Coukos, G. Abstract 308: Tumor hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Cancer Res. 2012, 72 (Suppl. S8), 308. [Google Scholar] [CrossRef]
- Riabov, V.; Gudima, A.; Wang, N.; Mickley, A.; Orekhov, A.; Kzhyshkowska, J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front. Physiol. 2014, 5, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirando, A.C.; Patil, A.; Rafie, C.I.; Christmas, B.J.; Pandey, N.B.; Stearns, V.; Jaffee, E.M.; Torres, E.T.R.; Popel, A.S. Regulation of the tumor immune microenvironment and vascular normalization in TNBC murine models by a novel peptide. Oncoimmunology 2020, 9, 1760685. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, Y.; Shi, X.; Hao, S.; Zhang, F.; Guo, Z.; Gao, Y.; Guo, H.; Liu, L. Oridonin inhibits tumor angiogenesis and induces vessel normalization in experimental colon cancer. J. Cancer 2021, 12, 3257–3264. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.; Riedemann, L.; Amoozgar, Z.; Seano, G.; Susek, K.; Yu, V.; Dalvie, N.; Amelung, R.L.; Datta, M.; Song, J.W.; et al. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc. Natl. Acad. Sci. USA 2016, 113, 4476–4481. [Google Scholar] [CrossRef]
- Lee, C.; Jeong, H.; Bae, Y.; Shin, K.; Kang, S.; Kim, H.; Oh, J.; Bae, H. Targeting of M2-like tumor-associated macrophages with a melittin-based pro-apoptotic peptide. J. Immunother. Cancer 2019, 7, 147. [Google Scholar] [CrossRef] [Green Version]
- Rolny, C.; Mazzone, M.; Tugues, S.; Laoui, D.; Johansson, I.; Coulon, C.; Squadrito, M.L.; Segura, I.; Li, X.; Knevels, E.; et al. HRG Inhibits Tumor Growth and Metastasis by Inducing Macrophage Polarization and Vessel Normalization through Downregulation of PlGF. Cancer Cell 2011, 19, 31–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastien, J.-P.; Minguy, A.; Dave, V.; Roy, D.C. Cellular therapy approaches harnessing the power of the immune system for personalized cancer treatment. Semin. Immunol. 2019, 42, 101306. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.; Ishida, T.; Oyama, T.; Ran, S.; Kravtsov, V.; Nadaf, S.; Carbone, D.P. Vascular Endothelial Growth Factor Inhibits the Development of Dendritic Cells and Dramatically Affects the Differentiation of Multiple Hematopoietic Lineages In Vivo. Blood 1998, 92, 4150–4166. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Qiu, X.; Zhang, Z.; Zhang, S.; Zhang, Y.; Liang, Y.; Guo, J.; Peng, H.; Chen, M.; Fu, Y.-X.; et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat. Commun. 2020, 11, 4835. [Google Scholar] [CrossRef]
- Shitara, K.; Nishikawa, H. Regulatory T cells: A potential target in cancer immunotherapy. Ann. N. Y. Acad. Sci. 2016, 1417, 104–115. [Google Scholar] [CrossRef]
- Boucher, Y.; Kumar, A.S.; Posada, J.M.; Gjini, E.; Pfaff, K.; Lipschitz, M.; Lako, A.; Duda, D.G.; Rodig, S.J.; Hodi, F.S.; et al. Bevacizumab improves tumor infiltration of mature dendritic cells and effector T-cells in triple-negative breast cancer patients. npj Precis. Oncol. 2021, 5, 62. [Google Scholar] [CrossRef]
- Wooster, A.L.; Girgis, L.H.; Brazeale, H.; Anderson, T.S.; Wood, L.M.; Lowe, D.B. Dendritic cell vaccine therapy for colorectal cancer. Pharmacol. Res. 2021, 164, 105374. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, A. Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment. Front. Immunol. 2018, 9, 3059. [Google Scholar] [CrossRef] [Green Version]
- Foy, K.C.; Miller, M.J.; Moldovan, N.; Carson, W.E.; Kaumaya, P.T.P. Combined vaccination with HER-2 peptide followed by therapy with VEGF peptide mimics exerts effective anti-tumor and anti-angiogenic effects in vitro and in vivo. Oncoimmunology 2012, 1, 1048–1060. [Google Scholar] [CrossRef] [Green Version]
- Manning, E.A.; Ullman, J.G.; Leatherman, J.M.; Asquith, J.M.; Hansen, T.R.; Armstrong, T.D.; Hicklin, D.J.; Jaffee, E.M.; Emens, L.A. A Vascular Endothelial Growth Factor Receptor-2 Inhibitor Enhances Antitumor Immunity through an Immune-Based Mechanism. Clin. Cancer Res. 2007, 13, 3951–3959. [Google Scholar] [CrossRef] [Green Version]
- Renner, D.N.; Malo, C.S.; Jin, F.; Parney, I.F.; Pavelko, K.D.; Johnson, A.J. Improved Treatment Efficacy of Antiangiogenic Therapy when Combined with Picornavirus Vaccination in the GL261 Glioma Model. Neurotherapeutics 2016, 13, 226–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Wang, C.; Sun, H.; Jiang, Z.; Zhang, Y.; Pan, Z. Apatinib prevents natural killer cell dysfunction to enhance the efficacy of anti-PD-1 immunotherapy in hepatocellular carcinoma. Cancer Gene Ther. 2021, 28, 89–97. [Google Scholar] [CrossRef] [PubMed]
- LaGory, E.L.; Giaccia, A.J. The ever-expanding role of HIF in tumour and stromal biology. Nature 2016, 18, 356–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, J.; Wang, X.; Stojanovic, A.; Zhang, Q.; Wincher, M.; Bühler, L.; Arnold, A.; Correia, M.P.; Winkler, M.; Koch, P.-S.; et al. Single-Cell RNA Sequencing of Tumor-Infiltrating NK Cells Reveals that Inhibition of Transcription Factor HIF-1α Unleashes NK Cell Activity. Immunity 2020, 52, 1075–1087.e8. [Google Scholar] [CrossRef] [PubMed]
- Matuszewska, K.; Pereira, M.; Petrik, D.; Lawler, J.; Petrik, J. Normalizing Tumor Vasculature to Reduce Hypoxia, Enhance Perfusion, and Optimize Therapy Uptake. Cancers 2021, 13, 4444. [Google Scholar] [CrossRef]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef] [Green Version]
- Regulska, K.; Regulski, M.; Karolak, B.; Murias, M.; Stanisz, B. Can cardiovascular drugs support cancer treatment? The rationale for drug repurposing. Drug Discov. Today 2019, 24, 1059–1065. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Chen, I.X.; Tong, R.; Ng, M.R.; Martin, J.D.; Naxerova, K.; Wu, M.W.; Huang, P.; Boucher, Y.; Kohane, D.S.; et al. Reprogramming the microenvironment with tumor-selective angiotensin blockers enhances cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2019, 116, 10674–10680. [Google Scholar] [CrossRef] [Green Version]
- Pinter, M.; Jain, R.K. Targeting the renin-angiotensin system to improve cancer treatment: Implications for immunotherapy. Sci. Transl. Med. 2017, 9, eaan5616. [Google Scholar] [CrossRef] [Green Version]
- Kosugi, M.; Miyajima, A.; Kikuchi, E.; Horiguchi, Y.; Murai, M. Angiotensin II Type 1 Receptor Antagonist Candesartan as an Angiogenic Inhibitor in a Xenograft Model of Bladder Cancer. Clin. Cancer Res. 2006, 12, 2888–2893. [Google Scholar] [CrossRef] [Green Version]
- Gelosa, P.; Castiglioni, L.; Camera, M.; Sironi, L. Repurposing of drugs approved for cardiovascular diseases: Opportunity or mirage? Biochem. Pharmacol. 2020, 177, 113895. [Google Scholar] [CrossRef]
- Kokolus, K.M.; Zhang, Y.; Sivik, J.M.; Schmeck, C.; Zhu, J.; Repasky, E.A.; Drabick, J.J.; Schell, T.D. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology 2018, 7, e1405205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrobel, L.J.; Bod, L.; Lengagne, R.; Kato, M.; Prévost-Blondel, A.; Le Gal, F.-A. Propranolol induces a favourable shift of anti-tumor immunity in a murine spontaneous model of melanoma. Oncotarget 2016, 7, 77825–77837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, M.S.; Guzner, A.; Wainwright, D.A.; Mohindra, N.A.; Chae, Y.K.; Behdad, A.; Villaflor, V.M. The Impact of Beta Blockers on Survival Outcomes in Patients with Non–small-cell Lung Cancer Treated with Immune Checkpoint Inhibitors. Clin. Lung Cancer 2021, 22, e57–e62. [Google Scholar] [CrossRef]
- Pu, D.; Yin, L.; Huang, L.; Qin, C.; Zhou, Y.; Wu, Q.; Li, Y.; Zhou, Q.; Li, L. Cyclooxygenase-2 Inhibitor: A Potential Combination Strategy with Immunotherapy in Cancer. Front. Oncol. 2021, 11, 637504. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.; Botting, R. The mechanism of action of aspirin. Thromb. Res. 2003, 110, 255–258. [Google Scholar] [CrossRef]
- Law, A.M.K.; Valdes-Mora, F.; Gallego-Ortega, D. Myeloid-Derived Suppressor Cells as a Therapeutic Target for Cancer. Cells 2020, 9, 561. [Google Scholar] [CrossRef] [Green Version]
- Winnicka, K.; Bielawski, K.; Bielawska, A. Cardiac glycosides in cancer research and cancer therapy. Acta Pol. Pharm. 2006, 63, 109–115. [Google Scholar]
- Li, X.; Zheng, J.; Chen, S.; Meng, F.-D.; Ning, J.; Sun, S.-L. Oleandrin, a cardiac glycoside, induces immunogenic cell death via the PERK/elF2α/ATF4/CHOP pathway in breast cancer. Cell Death Dis. 2021, 12, 314. [Google Scholar] [CrossRef]
- Schneider, N.F.Z.; Cerella, C.; Simões, C.M.O.; Diederich, M. Anticancer and Immunogenic Properties of Cardiac Glycosides. Molecules 2017, 22, 1932. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Yao, W.; Huang, Y.; Chen, Y.; Wang, Z.; Cai, X. Candesartan induces tumor vascular normalization to improve the efficacy of radiotherapy in the therapeutic window. Ann. Transl. Med. 2022, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Keith, S.W.; Maio, V.; Arafat, H.A.; Alcusky, M.; Karagiannis, T.; Rabinowitz, C.; Lavu, H.; Louis, D.Z. Angiotensin blockade therapy and survival in pancreatic cancer: A population study. BMC Cancer 2022, 22, 150. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhou, Z.; Xu, Z.; Zeng, S.; Chen, X.; Wang, X.; Liu, W.; Liu, M.; Gong, Z.; Yan, Y. Retrospective clinical study of renin-angiotensin system blockers in lung cancer patients with hypertension. PeerJ 2019, 7, e8188. [Google Scholar] [CrossRef] [PubMed]
- Ishida, J.; Konishi, M.; Ebner, N.; Springer, J. Repurposing of approved cardiovascular drugs. J. Transl. Med. 2016, 14, 269. [Google Scholar] [CrossRef] [Green Version]
- O’rawe, M.; Kilmister, E.J.; Mantamadiotis, T.; Kaye, A.H.; Tan, S.T.; Wickremesekera, A.C. The Renin–Angiotensin System in the Tumor Microenvironment of Glioblastoma. Cancers 2021, 13, 4004. [Google Scholar] [CrossRef] [PubMed]
- Catarata, M.J.; Ribeiro, R.; Oliveira, M.J.; Cordeiro, C.R.; Medeiros, R. Renin-Angiotensin System in Lung Tumor and Microenvironment Interactions. Cancers 2020, 12, 1457. [Google Scholar] [CrossRef]
- Wadsworth, B.J.; Cederberg, R.A.; Lee, C.-M.; Firmino, N.S.; Franks, S.E.; Pan, J.; Colpo, N.; Lin, K.-S.; Benard, F.; Bennewith, K.L. Angiotensin II type 1 receptor blocker telmisartan inhibits the development of transient hypoxia and improves tumour response to radiation. Cancer Lett. 2020, 493, 31–40. [Google Scholar] [CrossRef]
- Wadsworth, B.J.; Lee, C.-M.; Urban, R.; Hamilton, S.N.; Bennewith, K.L. Abstract PR-001: Angiotensin II receptor blockers modify the solid tumor microenvironment and improve radiation therapy response. Clin. Cancer Res. 2021, 27 (Suppl. S8), PR-001. [Google Scholar] [CrossRef]
- Stangier, J.; Su, C.; Roth, W. Pharmacokinetics of Orally and Intravenously Administered Telmisartan in Healthy Young and Elderly Volunteers and in Hypertensive Patients. J. Int. Med. Res. 2000, 28, 149–167. [Google Scholar] [CrossRef]
- Datta, M.; Coussens, L.M.; Nishikawa, H.; Hodi, F.S.; Jain, R.K. Reprogramming the Tumor Microenvironment to Improve Immunotherapy: Emerging Strategies and Combination Therapies. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 165–174. [Google Scholar] [CrossRef]
- Gandhi, S.; Pandey, M.R.; Attwood, K.; Ji, W.; Witkiewicz, A.K.; Knudsen, E.S.; Allen, C.; Tario, J.D.; Wallace, P.K.; Cedeno, C.D.; et al. Phase I Clinical Trial of Combination Propranolol and Pembrolizumab in Locally Advanced and Metastatic Melanoma: Safety, Tolerability, and Preliminary Evidence of Antitumor Activity. Clin. Cancer Res. 2021, 27, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Basu, G.D.; Tinder, T.L.; Subramani, D.B.; Bradley, J.M.; Arefayene, M.; Skaar, T.; De Petris, G. Progression of Pancreatic Adenocarcinoma Is Significantly Impeded with a Combination of Vaccine and COX-2 Inhibition. J. Immunol. 2009, 182, 216–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Fattah, E.E.A. IDO/kynurenine pathway in cancer: Possible therapeutic approaches. J. Transl. Med. 2022, 20, 347. [Google Scholar] [CrossRef]
- Müller, N. COX-2 Inhibitors, Aspirin, and Other Potential Anti-Inflammatory Treatments for Psychiatric Disorders. Front. Psychiatry 2019, 10, 375. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.C.A.; da Silva, R.J.; Franco, P.S.; de Oliveira Gomes, A.; Souza, G.; Milian, I.C.B.; Ribeiro, M.; Rosini, A.M.; Guirelli, P.M.; Ramos, E.L.P.; et al. Cyclooxygenase (COX)-2 Inhibitors Reduce Toxoplasma gondii Infection and Upregulate the Pro-inflammatory Immune Response in Calomys callosus Rodents and Human Monocyte Cell Line. Front. Microbiol. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Song, W.; Xu, Y.; Si, X.; Zhang, Y.; Tang, Z.; Chen, X. A ROS-Responsive Aspirin Polymeric Prodrug for Modulation of Tumor Microenvironment and Cancer Immunotherapy. CCS Chem. 2020, 2, 390–400. [Google Scholar] [CrossRef]
- Škubník, J.; Pavlíčková, V.; Rimpelová, S. Cardiac Glycosides as Immune System Modulators. Biomolecules 2021, 11, 659. [Google Scholar] [CrossRef]
- Reddy, D.; Kumavath, R.; Barh, D.; Azevedo, V.; Ghosh, P. Anticancer and Antiviral Properties of Cardiac Glycosides: A Review to Explore the Mechanism of Actions. Molecules 2020, 25, 3596. [Google Scholar] [CrossRef]
- Samant, R.S.; Shevde, L.A. Recent Advances in Anti-Angiogenic Therapy of Cancer. Oncotarget 2011, 2, 122–134. [Google Scholar] [CrossRef] [Green Version]
- Batchelor, T.T.; Gerstner, E.R.; Emblem, K.E.; Duda, D.G.; Kalpathy-Cramer, J.; Snuderl, M.; Ancukiewicz, M.; Polaskova, P.; Pinho, M.C.; Jennings, D.; et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc. Natl. Acad. Sci. USA 2013, 110, 19059–19064. [Google Scholar] [CrossRef]
- Wong, P.P.; Bodrug, N.; Hodivala-Dilke, K.M. Exploring Novel Methods for Modulating Tumor Blood Vessels in Cancer Treatment. Curr. Biol. 2016, 26, R1161–R1166. [Google Scholar] [CrossRef] [PubMed]
- Simsek, C.; Esin, E.; Yalcin, S. Metronomic Chemotherapy: A Systematic Review of the Literature and Clinical Experience. J. Oncol. 2019, 2019, 5483791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharovsky, O.G.; Rico, M.J.; Mainetti, L.E.; Perroud, H.A.; Rozados, V.R. Achievements and challenges in the use of metronomics for the treatment of breast cancer. Biochem. Pharmacol. 2020, 175, 113909. [Google Scholar] [CrossRef]
- Gilabert-Oriol, R.; Ryan, G.M.; Leung, A.W.; Firmino, N.S.; Bennewith, K.L.; Bally, M.B. Liposomal Formulations to Modulate the Tumour Microenvironment and Antitumour Immune Response. Int. J. Mol. Sci. 2018, 19, 2922. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Waxman, D.J. Immunogenic chemotherapy: Dose and schedule dependence and combination with immunotherapy. Cancer Lett. 2018, 419, 210–221. [Google Scholar] [CrossRef]
- Decraene, B.; Yang, Y.; De Smet, F.; Garg, A.D.; Agostinis, P.; De Vleeschouwer, S. Immunogenic cell death and its therapeutic or prognostic potential in high-grade glioma. Genes Immun. 2022, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Johnson, A.; Northcote-Smith, J.; Lu, C.; Suntharalingam, K. Immunogenic Cell Death of Breast Cancer Stem Cells Induced by an Endoplasmic Reticulum-Targeting Copper(II) Complex. Chembiochem 2020, 21, 3618–3624. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, G.; Chen, Y.; Wang, H.; Hua, Y.; Cai, Z. Immunogenic cell death in cancer therapy: Present and emerging inducers. J. Cell. Mol. Med. 2019, 23, 4854–4865. [Google Scholar] [CrossRef]
- Schaaf, M.B.; Garg, A.D.; Agostinis, P. Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis. 2018, 9, 115. [Google Scholar] [CrossRef] [Green Version]
- Winkler, F.; Kozin, S.V.; Tong, R.T.; Chae, S.-S.; Booth, M.F.; Garkavtsev, I.; Xu, L.; Hicklin, D.J.; Fukumura, D.; di Tomaso, E.; et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 2004, 6, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.; Foster, R.E.; Horgan, K.; Mounsey, K.; Nixon, H.; Smalle, N.; Hughes, T.A.; Carter, C.R. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res. 2016, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Y.; Chen, Y.-P.; Li, Y.-Q.; Na Liu, N.; Ma, J. Chemotherapeutic and targeted agents can modulate the tumor microenvironment and increase the efficacy of immune checkpoint blockades. Mol. Cancer 2021, 20, 27. [Google Scholar] [CrossRef]
- El-Arab, L.R.E.; Swellam, M.; El Mahdy, M.M. Metronomic chemotherapy in metastatic breast cancer: Impact on VEGF. J. Egypt. Natl. Cancer Inst. 2012, 24, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krajnak, S.; Battista, M.J.; Hasenburg, A.; Schmidt, M. Metronomic Chemotherapy for Metastatic Breast Cancer. Oncol. Res. Treat. 2022, 45, 12–17. [Google Scholar] [CrossRef]
- Liu, J.; He, M.; Wang, Z.; Li, Q.; Xu, B. Current Research Status of Metronomic Chemotherapy in Combination Treatment of Breast Cancer. Oncol. Res. Treat. 2022, 45, 681–692. [Google Scholar] [CrossRef]
- Mancuso, P.; Colleoni, M.; Calleri, A.; Orlando, L.; Maisonneuve, P.; Pruneri, G.; Agliano, A.; Goldhirsch, A.; Shaked, Y.; Kerbel, R.S.; et al. Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood 2006, 108, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Cazzaniga, M.E.; Cordani, N.; Capici, S.; Cogliati, V.; Riva, F.; Cerrito, M.G. Metronomic Chemotherapy. Cancers 2021, 13, 2236. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Takao, S.; Hirata, M.; Okamoto, Y.; Yamashita, S.; Kawaguchi, Y.; Takami, M.; Furusawa, H.; Morita, S.; Abe, C.; et al. Metronomic oral combination chemotherapy with capecitabine and cyclophosphamide: A phase II study in patients with HER2-negative metastatic breast cancer. Cancer Chemother. Pharmacol. 2012, 70, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Patten, S.G.; Adamcic, U.; Lacombe, K.; Minhas, K.; Skowronski, K.; Coomber, B.L. VEGFR2 heterogeneity and response to anti-angiogenic low dose metronomic cyclophosphamide treatment. BMC Cancer 2010, 10, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orecchioni, S.; Talarico, G.; Labanca, V.; Calleri, A.; Mancuso, P.; Bertolini, F. Vinorelbine, cyclophosphamide and 5-FU effects on the circulating and intratumoural landscape of immune cells improve anti-PD-L1 efficacy in preclinical models of breast cancer and lymphoma. Br. J. Cancer 2018, 118, 1329–1336. [Google Scholar] [CrossRef] [Green Version]
- Webb, E.R.; Moreno-Vicente, J.; Easton, A.; Lanati, S.; Taylor, M.; James, S.; Williams, E.L.; English, V.; Penfold, C.; Beers, S.A.; et al. Cyclophosphamide depletes tumor infiltrating T regulatory cells and combined with anti-PD-1 therapy improves survival in murine neuroblastoma. iScience 2022, 25, 104995. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Weng, S.; Zheng, S. Metronomic chemotherapy in non-small cell lung cancer (Review). Oncol. Lett. 2020, 20, 307. [Google Scholar] [CrossRef] [PubMed]
- Briasoulis, E.; Aravantinos, G.; Kouvatseas, G.; Pappas, P.; Biziota, E.; Sainis, I.; Makatsoris, T.; Varthalitis, I.; Xanthakis, I.; Vassias, A.; et al. Dose selection trial of metronomic oral vinorelbine monotherapy in patients with metastatic cancer: A hellenic cooperative oncology group clinical translational study. BMC Cancer 2013, 13, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camerini, A.; Puccetti, C.; Donati, S.; Valsuani, C.; Petrella, M.C.; Tartarelli, G.; Puccinelli, P.; Amoroso, D. Metronomic oral vinorelbine as first-line treatment in elderly patients with advanced non-small cell lung cancer: Results of a phase II trial (MOVE trial). BMC Cancer 2015, 15, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lissoni, P.; Rovelli, F.; Malugani, F.; Brivio, F.; Fumagalli, L.; Gardani, G. Changes in Circulating VEGF Levels in Relation to Clinical Response during Chemotherapy for Metastatic Cancer. Int. J. Biol. Markers 2003, 18, 152–155. [Google Scholar] [CrossRef]
- Orlandi, P.; Banchi, M.; Alì, G.; Di Desidero, T.; Fini, E.; Fontanini, G.; Bocci, G. Active metronomic vinorelbine schedules decrease plasma interleukin-2 levels in mice with Lewis lung carcinoma. J. Chemother. 2021, 33, 198–202. [Google Scholar] [CrossRef]
- Katsaounis, P.; Kotsakis, A.; Agelaki, S.; Kontopodis, E.; Agelidou, A.; Kentepozidis, N.; Vamvakas, L.; Christopoulou, A.; Karachaliou, N.; Hatzidaki, D.; et al. Cisplatin in combination with metronomic vinorelbine as front-line treatment in advanced non-small cell lung cancer: A multicenter phase II study of the Hellenic Oncology Research Group (HORG). Cancer Chemother. Pharmacol. 2015, 75, 821–827. [Google Scholar] [CrossRef]
- Correale, P.; Remondo, C.; Carbone, S.F.; Ricci, V.; Migali, C.; Martellucci, I.; Licchetta, A.; Addeo, R.; Volterrani, L.; Gotti, G.; et al. Dose/dense metronomic chemotherapy with fractioned cisplatin and oral daily etoposide enhances the anti-angiogenic effects of bevacizumab and has strong anti-tumor activity in advanced non-small-cell-lung cancer patients. Cancer Biol. Ther. 2010, 9, 685–693. [Google Scholar] [CrossRef] [Green Version]
- Skavatsou, E.; Semitekolou, M.; Morianos, I.; Karampelas, T.; Lougiakis, N.; Xanthou, G.; Tamvakopoulos, C. Immunotherapy Combined with Metronomic Dosing: An Effective Approach for the Treatment of NSCLC. Cancers 2021, 13, 1901. [Google Scholar] [CrossRef]
- Zhu, N.; Qin, R.; Zhang, Q.; Fu, S.; Liu, S.; Chen, Y.; Fan, J.; Han, Y. Efficacy of granulocyte-macrophage colony-stimulating factor combined with metronomic paclitaxel in the treatment of Lewis lung carcinoma transplanted in mice. Oncotarget 2018, 9, 4951–4960. [Google Scholar] [CrossRef] [Green Version]
- Baert, T.; Ferrero, A.; Sehouli, J.; O’Donnell, D.; González-Martín, A.; Joly, F.; van der Velden, J.; Blecharz, P.; Tan, D.; Querleu, D.; et al. The systemic treatment of recurrent ovarian cancer revisited. Ann. Oncol. 2021, 32, 710–725. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Malik, P.; Khurana, S.; Kumar, S.; Bhatla, N.; Ray, M.D.; Kumar, L. Oral metronomic chemotherapy for recurrent & refractory epithelial ovarian cancer: A retrospective analysis. Indian J. Med. Res. 2019, 150, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.A.; Hirte, H.; Fleming, G.; Yang, D.; Tsao-Wei, D.D.; Roman, L.; Groshen, S.; Swenson, S.; Markland, F.; Gandara, D.; et al. Phase II Clinical Trial of Bevacizumab and Low-Dose Metronomic Oral Cyclophosphamide in Recurrent Ovarian Cancer: A Trial of the California, Chicago, and Princess Margaret Hospital Phase II Consortia. J. Clin. Oncol. 2008, 26, 76–82. [Google Scholar] [CrossRef]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of Bevacizumab in the Primary Treatment of Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [Green Version]
- De Boo, L.W.; Vulink, A.J.E.; Bos, M.E.M.M. Metronomic cyclophosphamide-induced long-term remission after recurrent high-grade serous ovarian cancer: A case study. Mol. Clin. Oncol. 2017, 7, 1130–1134. [Google Scholar] [CrossRef] [Green Version]
- Malik, P.S.; Raina, V.; André, N. Metronomics as Maintenance Treatment in Oncology: Time for Chemo-Switch. Front. Oncol. 2014, 4, 76. [Google Scholar] [CrossRef] [Green Version]
- Zsiros, E.; Lynam, S.; Attwood, K.M.; Wang, C.; Chilakapati, S.; Gomez, E.C.; Liu, S.; Akers, S.; Lele, S.; Frederick, P.J.; et al. Efficacy and Safety of Pembrolizumab in Combination with Bevacizumab and Oral Metronomic Cyclophosphamide in the Treatment of Recurrent Ovarian Cancer. JAMA Oncol. 2021, 7, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Miyoshi, H.; Yoshida, K.; Okano, H.; Toda, M. Recent progress in the research of suicide gene therapy for malignant glioma. Neurosurg. Rev. 2021, 44, 29–49. [Google Scholar] [CrossRef]
- Ghiaseddin, A.P.; Shin, D.; Melnick, K.; Tran, D.D. Tumor Treating Fields in the Management of Patients with Malignant Gliomas. Curr. Treat. Options Oncol. 2020, 21, 76. [Google Scholar] [CrossRef]
- Sousa, M.J.; Magalhães, J.; Basto, R.; Costa, C.; Pego, A.; Sousa, G. P14.90 Survival outcomes and prognostic factors in glioblastoma patients treated with radiotherapy plus concomitant and adjuvant temozolomide—Real-world study. J. Neuro-Oncol. 2021, 23 (Suppl. S2), ii55. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Sampson, J.H. Temozolomide treatment outcomes and immunotherapy efficacy in brain tumor. J. Neuro-Oncol. 2021, 151, 55–62. [Google Scholar] [CrossRef]
- Perry, J.R.; Rizek, P.; Cashman, R.; Morrison, M.; Morrison, T. Temozolomide rechallenge in recurrent malignant glioma by using a continuous temozolomide schedule. Cancer 2008, 113, 2152–2157. [Google Scholar] [CrossRef]
- Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; Gururangan, S.; Sampson, J.H.; Sathornsumetee, S.; McLendon, R.E.; Herndon, J.E., II.; Marcello, J.E.; Norfleet, J.; et al. Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: A phase II study. Br. J. Cancer 2009, 101, 1986–1994. [Google Scholar] [CrossRef]
- Reardon, D.A.; Desjardins, A.; Peters, K.; Gururangan, S.; Sampson, J.; Rich, J.N.; McLendon, R.; Herndon, J.E.; Marcello, J.; Threatt, S.; et al. Phase II study of metronomic chemotherapy with bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. J. Neuro-Oncol. 2011, 103, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Amoozgar, Z.; Kloepper, J.; Ren, J.; Tay, R.E.; Kazer, S.W.; Kiner, E.; Krishnan, S.; Posada, J.M.; Ghosh, M.; Mamessier, E.; et al. Targeting Treg cells with GITR activation alleviates resistance to immunotherapy in murine glioblastomas. Nat. Commun. 2021, 12, 2582. [Google Scholar] [CrossRef]
- Datta, M.; Chatterjee, S.; Perez, E.M.; Gritsch, S.; Roberge, S.; Duquette, M.; Chen, I.X.; Naxerova, K.; Kumar, A.S.; Ghosh, M.; et al. Losartan controls immune checkpoint blocker-induced edema and improves survival in glioblastoma mouse models. Proc. Natl. Acad. Sci. USA 2023, 120, e2219199120. [Google Scholar] [CrossRef]
- Amoozgar, Z.; Ren, J.; Wang, N.; Andersson, P.; Ferraro, G.; Rajan, S.; Lei, P.; Subudhi, S.; Kawaguchi, K.; Tay, R.E.; et al. Combined blockade of VEGF, Angiopoietin-2, and PD1 reprograms glioblastoma endothelial cells into quasi-antigen-presenting cells. bioRxiv 2022. [Google Scholar] [CrossRef]
- Mathew, E.N.; Berry, B.C.; Yang, H.W.; Carroll, R.S.; Johnson, M.D. Delivering Therapeutics to Glioblastoma: Overcoming Biological Constraints. Int. J. Mol. Sci. 2022, 23, 1711. [Google Scholar] [CrossRef]
- Walter, I.; Schulz, U.; Vogelhuber, M.; Wiedmann, K.; Endlicher, E.; Klebl, F.; Andreesen, R.; Herr, W.; Ghibelli, L.; Hackl, C.; et al. Communicative reprogramming non-curative hepatocellular carcinoma with low-dose metronomic chemotherapy, COX-2 inhibitor and PPAR-gamma agonist: A phase II trial. Med. Oncol. 2017, 34, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wysocki, P.J.; Lubas, M.T.; Wysocka, M.L. Metronomic Chemotherapy in Prostate Cancer. J. Clin. Med. 2022, 11, 2853. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Ping, L.; Yan, H.; Yang, X.; He, Q.; Xu, Z.; Luo, P. Cardiovascular toxicity induced by anti-VEGF/VEGFR agents: A special focus on definitions, diagnoses, mechanisms and management. Expert Opin. Drug Metab. Toxicol. 2020, 16, 823–835. [Google Scholar] [CrossRef]
- Liang, Q.; Zhou, L.; Li, Y.; Liu, J.; Liu, Y. Nano drug delivery system reconstruct tumour vasculature for the tumour vascular normalisation. J. Drug Target. 2021, 30, 119–130. [Google Scholar] [CrossRef]
- Mattheolabakis, G.; Mikelis, C.M. Nanoparticle Delivery and Tumor Vascular Normalization: The Chicken or The Egg? Front. Oncol. 2019, 9, 1227. [Google Scholar] [CrossRef] [Green Version]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [Green Version]
- Gabizon, A.A. Liposome circulation time and tumor targeting: Implications for cancer chemotherapy. Adv. Drug Deliv. Rev. 1995, 16, 285–294. [Google Scholar] [CrossRef]
- Litzinger, D.C.; Buiting, A.M.; van Rooijen, N.; Huang, L. Effect of liposome size on the circulation time and intraorgan distribution of amphipathic poly(ethylene glycol)-containing liposomes. (BBA)—Biomembr. 1994, 1190, 99–107. [Google Scholar] [CrossRef]
- Li, S.-D.; Huang, L. Pharmacokinetics and Biodistribution of Nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W.; Thurston, G.; Roberge, S.; Jain, R.K.; McDonald, D.M. Openings between Defective Endothelial Cells Explain Tumor Vessel Leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef] [Green Version]
- Guyon, J.; Chapouly, C.; Andrique, L.; Bikfalvi, A.; Daubon, T. The Normal and Brain Tumor Vasculature: Morphological and Functional Characteristics and Therapeutic Targeting. Front. Physiol. 2021, 12, 622615. [Google Scholar] [CrossRef]
- Nagy, J.; Chang, S.-H.; Shih, S.-C.; Dvorak, A.; Dvorak, H. Heterogeneity of the Tumor Vasculature. Semin. Thromb. Hemost. 2010, 36, 321–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Meel, M.H.; Breur, M.; Bugiani, M.; Hulleman, E.; Phoenix, T.N. Defining tumor-associated vascular heterogeneity in pediatric high-grade and diffuse midline gliomas. Acta Neuropathol. Commun. 2021, 9, 142. [Google Scholar] [CrossRef]
- Stapleton, S.; Milosevic, M.; Allen, C.; Zheng, J.; Dunne, M.; Yeung, I.; Jaffray, D.A. A Mathematical Model of the Enhanced Permeability and Retention Effect for Liposome Transport in Solid Tumors. PLoS ONE 2013, 8, e81157. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, G.B.; Shinde, R.; Contag, C.H.; Zare, R.N. Sustained Release of Drugs Dispersed in Polymer Nanoparticles. Angew. Chem. Int. Ed. 2008, 47, 7880–7882. [Google Scholar] [CrossRef]
- Kumar, D.; Archana; Niranjan, A.K. A Comprehensive Review on Sustained Release Matrix Drug Delivery System. J. Drug Deliv. Ther. 2022, 12, 249–253. [Google Scholar] [CrossRef]
- Karumanchi, D.K.; Skrypai, Y.; Thomas, A.; Gaillard, E.R. Rational design of liposomes for sustained release drug delivery of bevacizumab to treat ocular angiogenesis. J. Drug Deliv. Sci. Technol. 2018, 47, 275–282. [Google Scholar] [CrossRef]
- Vasantha, J.; Kannan, G.; Goud, T.; Palani, T.; Vanitha, R.; Anitha, R.; Priya, J. Pharmacokinetic Evaluation of Paclitaxel in South Indian Cancer Patients: A Prospective Study. J. Young Pharm. 2011, 3, 322–328. [Google Scholar] [CrossRef]
- Emmenegger, U.; Shaked, Y.; Man, S.; Bocci, G.; Spasojevic, I.; Francia, G.; Kouri, A.; Coke, R.; Cruz-Munoz, W.; Ludeman, S.M.; et al. Pharmacodynamic and pharmacokinetic study of chronic low-dose metronomic cyclophosphamide therapy in mice. Mol. Cancer Ther. 2007, 6, 2280–2289. [Google Scholar] [CrossRef] [Green Version]
- Bocci, G.; Kerbel, R.S. Pharmacokinetics of metronomic chemotherapy: A neglected but crucial aspect. Nat. Rev. Clin. Oncol. 2016, 13, 659–673. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Ohana, P.; Amitay, Y.; Gorin, J.; Tzemach, D.; Mak, L.; Shmeeda, H. Liposome co-encapsulation of anti-cancer agents for pharmacological optimization of nanomedicine-based combination chemotherapy. Cancer Drug Resist. 2021, 4, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Daunorubicin/Cytarabine Liposome: A Review in Acute Myeloid Leukaemia. Drugs 2018, 78, 1903–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, L.D.; Harasym, T.O.; Tardi, P.G.; Harasym, N.L.; Shew, C.R.; Johnstone, S.A.; Ramsay, E.C.; Bally, M.B.; Janoff, A.S. Ratiometric dosing of anticancer drug combinations: Controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol. Cancer Ther. 2006, 5, 1854–1863. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, E.C.; Dos Santos, N.; Dragowska, W.H.; Laskin, J.J.; Bally, M. The Formulation of Lipid-Based Nanotechnologies for the Delivery of Fixed Dose Anticancer Drug Combinations. Curr. Drug Deliv. 2005, 2, 341–351. [Google Scholar] [CrossRef]
- Neijzen, R.; Wong, M.Q.; Gill, N.; Wang, H.; Karim, T.; Anantha, M.; Strutt, D.; Waterhouse, D.; Bally, M.B.; Tai, I.T.; et al. Irinophore C™, a lipid nanoparticulate formulation of irinotecan, improves vascular function, increases the delivery of sequentially administered 5-FU in HT-29 tumors, and controls tumor growth in patient derived xenografts of colon cancer. J. Control. Release 2015, 199, 72–83. [Google Scholar] [CrossRef]
- Gu, Z.; Da Silva, C.G.; Van der Maaden, K.; Ossendorp, F.; Cruz, L.J. Liposome-Based Drug Delivery Systems in Cancer Immunotherapy. Pharmaceutics 2020, 12, 1054. [Google Scholar] [CrossRef]
- Verreault, M.; Strutt, D.; Masin, D.; Anantha, M.; Yung, A.; Kozlowski, P.; Waterhouse, D.; Bally, M.B.; Yapp, D.T. Vascular normalization in orthotopic glioblastoma following intravenous treatment with lipid-based nanoparticulate formulations of irinotecan (Irinophore C™), doxorubicin (Caelyx®) or vincristine. BMC Cancer 2011, 11, 124. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, T.R.; Arnold, R.D.; Yang, J.; Turowski, S.G.; Qu, Y.; Spernyak, J.A.; Mazurchuk, R.; Mager, D.E.; Straubinger, R.M. Mechanisms of Tumor Vascular Priming by a Nanoparticulate Doxorubicin Formulation. Pharm. Res. 2012, 29, 3312–3324. [Google Scholar] [CrossRef] [Green Version]
- Rios-Doria, J.; Durham, N.; Wetzel, L.; Rothstein, R.; Chesebrough, J.; Holoweckyj, N.; Zhao, W.; Leow, C.C.; Hollingsworth, R. Doxil Synergizes with Cancer Immunotherapies to Enhance Antitumor Responses in Syngeneic Mouse Models. Neoplasia 2015, 17, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Mpekris, F.; Panagi, M.; Voutouri, C.; Martin, J.D.; Samuel, R.; Takahashi, S.; Gotohda, N.; Suzuki, T.; Papageorgis, P.; Demetriou, P.; et al. Normalizing the Microenvironment Overcomes Vessel Compression and Resistance to Nano-immunotherapy in Breast Cancer Lung Metastasis. Adv. Sci. 2020, 8, 2001917. [Google Scholar] [CrossRef]
- Ngo, W.; Ahmed, S.; Blackadar, C.; Bussin, B.; Ji, Q.; Mladjenovic, S.M.; Sepahi, Z.; Chan, W.C. Why nanoparticles prefer liver macrophage cell uptake in vivo. Adv. Drug Deliv. Rev. 2022, 185, 114238. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Tsai, T.-H.; Huang, Y.-C.; Shieh, H.-R.; Liao, H.-F.; Chen, Y.-J. Differential immunomodulating effects of pegylated liposomal doxorubicin nanoparticles on human macrophages. J. Nanosci. Nanotechnol. 2012, 12, 7739–7746. [Google Scholar] [CrossRef]
- Strieth, S.; Eichhorn, M.E.; Werner, A.; Sauer, B.; Teifel, M.; Michaelis, U.; Berghaus, A.; Dellian, M. Paclitaxel Encapsulated in Cationic Liposomes Increases Tumor Microvessel Leakiness and Improves Therapeutic Efficacy in Combination with Cisplatin. Clin. Cancer Res. 2008, 14, 4603–4611. [Google Scholar] [CrossRef] [Green Version]
- Strieth, S.; Eichhorn, M.E.; Sauer, B.; Schulze, B.; Teifel, M.; Michaelis, U.; Dellian, M. Neovascular targeting chemotherapy: Encapsulation of paclitaxel in cationic liposomes impairs functional tumor microvasculature. Int. J. Cancer 2004, 110, 117–124. [Google Scholar] [CrossRef]
- Bocci, G.; Di Paolo, A.; Danesi, R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis 2013, 16, 481–492. [Google Scholar] [CrossRef] [Green Version]
- Huo, M.; Wang, H.; Zhang, Y.; Cai, H.; Zhang, P.; Li, L.; Zhou, J.; Yin, T. Co-delivery of silybin and paclitaxel by dextran-based nanoparticles for effective anti-tumor treatment through chemotherapy sensitization and microenvironment modulation. J. Control. Release 2020, 321, 198–210. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, Y.-M. Tumor endothelial cells as a potential target of metronomic chemotherapy. Arch. Pharmacal Res. 2019, 42, 1–13. [Google Scholar] [CrossRef]
- Huang, D.; Sun, L.; Huang, L.; Chen, Y. Nanodrug Delivery Systems Modulate Tumor Vessels to Increase the Enhanced Permeability and Retention Effect. J. Pers. Med. 2021, 11, 124. [Google Scholar] [CrossRef]
- Cai, X.-J.; Fei, W.; Xu, Y.-Y.; Xu, H.; Yang, G.-Y.; Cao, J.-W.; Ni, J.-J.; Tao, K.; Wang, Z. Liposome-Encapsulated Zoledronate Favors Tumor Vascular Normalization and Enhances Anticancer Efficacy of Cisplatin. AAPS PharmSciTech 2020, 21, 57. [Google Scholar] [CrossRef]
- Luput, L.; Sesarman, A.; Porfire, A.; Achim, M.; Muntean, D.; Casian, T.; Patras, L.; Rauca, V.F.; Drotar, D.M.; Stejerean, I.; et al. Liposomal simvastatin sensitizes C26 murine colon carcinoma to the antitumor effects of liposomal 5-fluorouracil in vivo. Cancer Sci. 2020, 111, 1344–1356. [Google Scholar] [CrossRef]
- Li, Y.; Du, B.; Cheng, G. Reshaping Tumor Blood Vessels to Enhance Drug Penetration with a Multistrategy Synergistic Nanosystem. Mol. Pharm. 2020, 17, 3151–3164. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Gao, Y.; Zhu, J.; Wientjes, M.G.; Au, J.L.-S. Relationships between Liposome Properties, Cell Membrane Binding, Intracellular Processing, and Intracellular Bioavailability. AAPS J. 2011, 13, 585–597. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Jiang, J.; Liao, Y.; Tang, I.; Zheng, E.; Qiu, W.; Lin, M.; Wang, X.; Ji, Y.; Mei, K.; et al. Combination Chemo-Immunotherapy for Pancreatic Cancer Using the Immunogenic Effects of an Irinotecan Silicasome Nanocarrier Plus Anti-PD-1. Adv. Sci. 2021, 8, 2002147. [Google Scholar] [CrossRef] [PubMed]
- Piazzini, V.; Landucci, E.; Graverini, G.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Stealth and Cationic Nanoliposomes as Drug Delivery Systems to Increase Andrographolide BBB Permeability. Pharmaceutics 2018, 10, 128. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, Y.; Hada, T.; Yamamoto, S.; Kato, A.; Mizumura, W.; Harashima, H. Remodeling of the Extracellular Matrix by Endothelial Cell-Targeting siRNA Improves the EPR-Based Delivery of 100 nm Particles. Mol. Ther. 2016, 24, 2090–2099. [Google Scholar] [CrossRef] [Green Version]
- Tabernero, J.; Shapiro, G.I.; Lorusso, P.M.; Cervantes, A.; Schwartz, G.K.; Weiss, G.J.; Paz-Ares, L.; Cho, D.C.; Infante, J.R.; Alsina, M.; et al. First-in-Humans Trial of an RNA Interference Therapeutic Targeting VEGF and KSP in Cancer Patients with Liver Involvement. Cancer Discov. 2013, 3, 406–417. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Xing, L.; Wei, X.; Song, S. Effects of VEGF suppression by small hairpin RNA interference combined with radiotherapy on the growth of cervical cancer. Genet. Mol. Res. 2014, 13, 5094–5106. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Sim, M.J.W.; Sun, P.D. T Cell Recognition of Tumor Neoantigens and Insights into T Cell Immunotherapy. Front. Immunol. 2022, 13, 833017. [Google Scholar] [CrossRef]
- Hos, B.J.; Camps, M.G.; Bulk, J.V.D.; Tondini, E.; Ende, T.C.V.D.; Ruano, D.; Franken, K.; Janssen, G.M.; de Ru, A.H.; Filippov, D.V.; et al. Identification of a neo-epitope dominating endogenous CD8 T cell responses to MC-38 colorectal cancer. Oncoimmunology 2019, 9, 1673125. [Google Scholar] [CrossRef] [Green Version]
- Roviello, G.; Catalano, M.; Santi, R.; Palmieri, V.E.; Vannini, G.; Galli, I.C.; Buttitta, E.; Villari, D.; Rossi, V.; Nesi, G. Immune Checkpoint Inhibitors in Urothelial Bladder Cancer: State of the Art and Future Perspectives. Cancers 2021, 13, 4411. [Google Scholar] [CrossRef]
- Marofi, F.; Motavalli, R.; Safonov, V.A.; Thangavelu, L.; Yumashev, A.V.; Alexander, M.; Shomali, N.; Chartrand, M.S.; Pathak, Y.; Jarahian, M.; et al. CAR T cells in solid tumors: Challenges and opportunities. Stem Cell Res. Ther. 2021, 12, 81. [Google Scholar] [CrossRef]
- Newick, K.; O’Brien, S.; Moon, E.; Albelda, S.M. CAR T Cell Therapy for Solid Tumors. Annu. Rev. Med. 2017, 68, 139–152. [Google Scholar] [CrossRef]
- Ba, E.A.Y.; Shi, Y.; Jiang, W.; Feng, J.; Cheng, Y.; Xiao, L.; Zhang, Q.; Qiu, W.; Xu, B.; Xu, R.; et al. Current management of chemotherapy-induced neutropenia in adults: Key points and new challenges. Cancer Biol. Med. 2020, 17, 896–909. [Google Scholar] [CrossRef]
- Das, R.K.; O’connor, R.S.; Grupp, S.A.; Barrett, D.M. Lingering effects of chemotherapy on mature T cells impair proliferation. Blood Adv. 2020, 4, 4653–4664. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.; Al-Samkari, H. Thrombotic and bleeding risk of angiogenesis inhibitors in patients with and without malignancy. J. Thromb. Haemost. 2021, 19, 1852–1863. [Google Scholar] [CrossRef] [PubMed]
- Elice, F.; Rodeghiero, F. Side effects of anti-angiogenic drugs. Thromb. Res. 2012, 129, S50–S53. [Google Scholar] [CrossRef] [PubMed]

| Generic Name | Target * | Mechanism | Approved for ** | |
|---|---|---|---|---|
| Immune checkpoint inhibitors | Ipilimumab | CTLA-4 | Inhibit CTLA-4 and increase T cell activation | Melanoma, RCC, MCC, HCC, Metastatic NSCLC |
| Cemiplimab | PD-1 | Inhibit PD-1 and increase T cell activation | Squamous Cell Carcinoma, NSCLC | |
| Nivolumab | PD-1 | Inhibit PD-1 and increase T cell activation | Melanoma, Lung cancer, NSCLC, RCC, Hodgkin’s Lymphoma, Head and Neck Cancer, MUC, MCC, HCC NSCLC, Esophageal Carcinoma, Gastric Cancer | |
| Pembrolizumab | PD-1 | Inhibit PD-1 and increase T cell activation | Advanced Melanoma, Advanced NSCLC, Head and Neck Cancer, Hodgkin’s Lymphoma, MUC, Gastric Cancer, Cervical Cancer, HCC, Merkel Cell Carcinoma, RCC, Endometrial Cancer, Squamous Cell Carcinoma, HCC, Breast Cancer | |
| Atezolizumab | PD-L1 | Inhibit PD-L1 and increase T cell activation | NSCLC, Small Cell Lung Cancer, HCC, Melanoma | |
| Avelumab | PD-L1 | Inhibit PD-L1 and increase T cell activation | Merkel Cell Carcinoma, Urothelial Carcinoma, RCC | |
| Durvalumab | PD-L1 | Inhibit PD-L1 and increase T cell activation | NSCLC, Small Cell Lung Cancer, Biliary Tract Tumor | |
| Cytokine-based therapies | Aldesleukin | IL-2 receptor | Increase T cell activation | Metastatic Melanoma and Metastatic Renal Cell Carcinoma. |
| Interferon alpha-2b | Type I IFN receptors | Activate type 1 IFN receptors and JAK/STAT pathway | Leukemia, Follicular Lymphoma, Malignant Melanoma, AIDs-related Kaposi’s Sarcoma | |
| CAR-T cell therapies | Tisagenlecleucel | CD19 | T cell activation, expansion and elimination of target cells | ALL, NHL |
| Axicabtagene ciloleucel | CD19 | T cell activation, expansion and elimination of target cells | NHL, Follicular Lymphoma | |
| Brexucabtagene autoleucel | CD19 | T cell activation, expansion and elimination of target cells | Mantle Cell Lymphoma, ALL | |
| Lisocabtagene maraleucel | CD19 | T cell activation, expansion and elimination of target cells | NHL | |
| Idecabtagene vicleucel | BCMA | T cell activation, expansion and elimination of target cells | Multiple Myeloma | |
| Ciltacabtagene autoleucel | BCMA | T cell activation, expansion and elimination of target cells | Multiple Myeloma | |
| Vaccine | Sipuleucel-T | Prostatic acid phosphatase | Induce immune activation towards prostate cancer cells | Metastatic Prostate Cancer |
| Drug Class | Drug Names | Target | Affected Cancer Types | Beneficial Effect on Immunotherapy | References |
|---|---|---|---|---|---|
| ARBs | Telmisartan, Losartan, Candesartan | Angiotensin type 1 receptor | Breast, pancreatic ductal adenocarcinoma, bladder |
| [97,98,99,100,101] |
| Beta-blockers | Propranolol, Metoprolol | β1, β2, β3 receptor | Melanoma, breast, ovarian, colorectal |
| [102,103,104] |
| Cyclooxygenase Inhibitors | Aspirin | Cyclooxygenase enzyme | Colorectal |
| [105,106,107] |
| Cardiac Glycosides | Oleandrin, Scillaren A, Proscillaridin, Lanatoside C, Digitoxigenin | Sodium-potassium ATPase pump | Breast |
| [108,109,110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.X.; Nosrati, Z.; Ko, J.; Lee, C.-M.; Bennewith, K.L.; Bally, M.B. Induced Vascular Normalization—Can One Force Tumors to Surrender to a Better Microenvironment? Pharmaceutics 2023, 15, 2022. https://doi.org/10.3390/pharmaceutics15082022
Sun XX, Nosrati Z, Ko J, Lee C-M, Bennewith KL, Bally MB. Induced Vascular Normalization—Can One Force Tumors to Surrender to a Better Microenvironment? Pharmaceutics. 2023; 15(8):2022. https://doi.org/10.3390/pharmaceutics15082022
Chicago/Turabian StyleSun, Xu Xin, Zeynab Nosrati, Janell Ko, Che-Min Lee, Kevin L. Bennewith, and Marcel B. Bally. 2023. "Induced Vascular Normalization—Can One Force Tumors to Surrender to a Better Microenvironment?" Pharmaceutics 15, no. 8: 2022. https://doi.org/10.3390/pharmaceutics15082022
APA StyleSun, X. X., Nosrati, Z., Ko, J., Lee, C.-M., Bennewith, K. L., & Bally, M. B. (2023). Induced Vascular Normalization—Can One Force Tumors to Surrender to a Better Microenvironment? Pharmaceutics, 15(8), 2022. https://doi.org/10.3390/pharmaceutics15082022






