Distribution of Monocarboxylate Transporters in Brain and Choroid Plexus Epithelium
Abstract
:1. Introduction
1.1. Barrier Function in Choroid Plexus
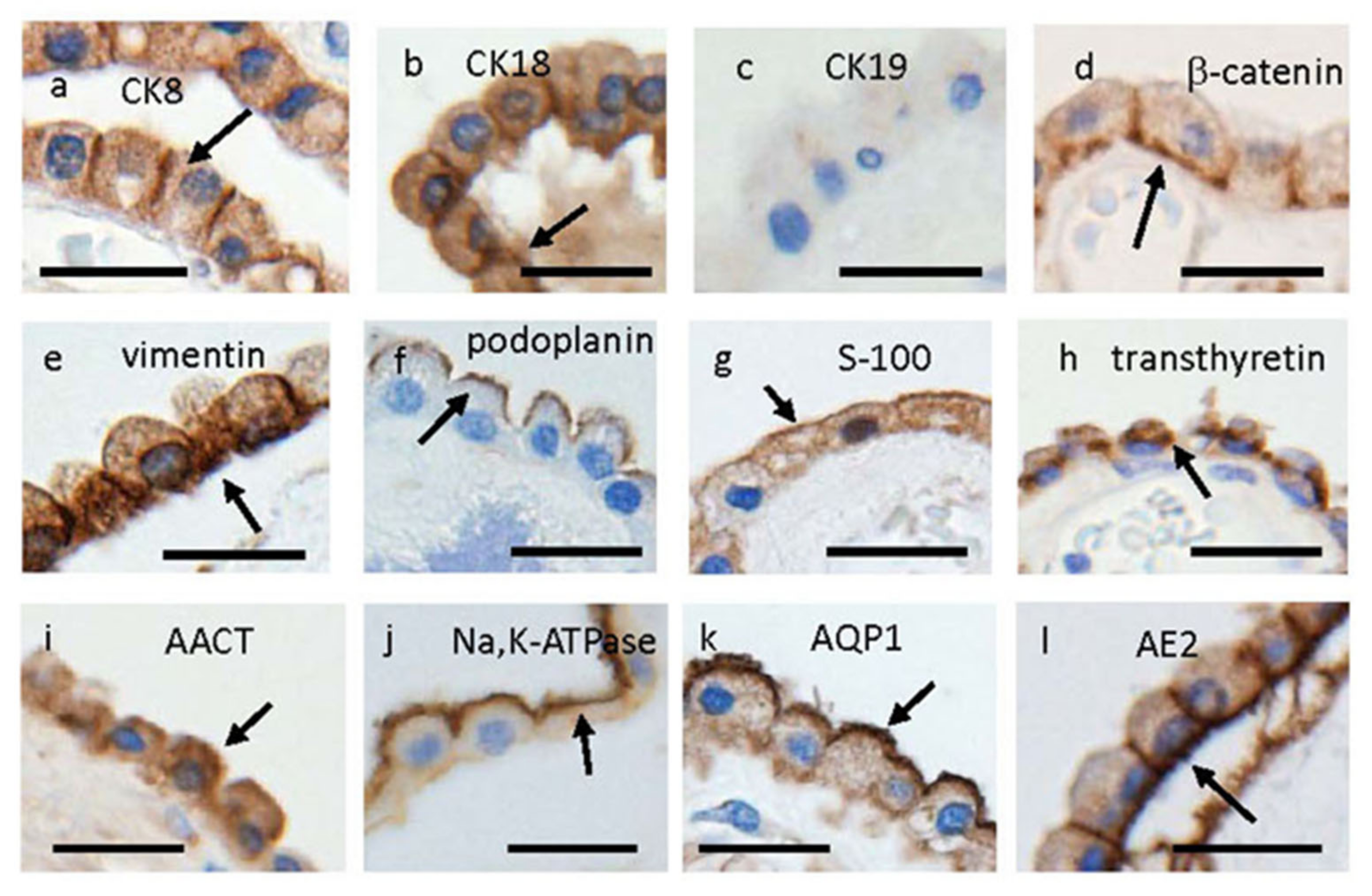
1.2. Characterization of Choroid Plexus Epithelial Cells
2. Monocarboxylate Transporters in the Brain
2.1. MCT1 (SLC16A1) Distribution in the Brain and CPEs
2.2. MCT2 (SLC16A7) Distribution in the Brain and CPEs
2.3. MCT4 (SLC16A3) Distribution in the Brain and CPEs
2.4. MCT3 (SLC16A8) Distribution in CPEs
2.5. MCT5 (SLC16A4) Distribution in the Brain
2.6. MCT8 (SLC16A2) Distribution in the Brain and CPEs
2.7. Lactate Transport in the Brain through Cerebral Microvessels and CPEs
3. Discussion
4. Conclusions and Future Direction
Author Contributions
Funding
Conflicts of Interest
References
- Redzic, Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: Similarities and differences. Fluids Barriers CNS 2011, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Goto, R.; Takeuchi, H.; Luczak, M.; Usui, T.; Tachikawa, M.; Terasaki, T. Abundant Expression of OCT2, MATE1, OAT1, OAT3, PEPT2, BCRP, MDR1, and xCT Transporters in Blood-Arachnoid Barrier of Pig and Polarized Localizations at CSF- and Blood-Facing Plasma Membranes. Drug Metab. Dispos. 2020, 48, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, K.M.; Terasaki, T.; Urtti, A.; Montaser, A.B.; Uchida, Y. Pharmacoproteomics of Brain Barrier Transporters and Substrate Design for the Brain Targeted Drug Delivery. Pharm. Res. 2022, 39, 1363–1392. [Google Scholar] [CrossRef]
- Reese, T.S.; Karnovsky, M.J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 1967, 34, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Brightman, M.W.; Reese, T.S. Junctions between intimately apposed cell membranes in the vertebrate brain. J. Cell Biol. 1969, 40, 648–677. [Google Scholar] [CrossRef]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional morphology of the blood–brain barrier in health and disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, M.; Chiba, Y.; Murakami, R.; Matsumoto, K.; Fujihara, R.; Uemura, N.; Yanase, K.; Kamada, M. Disturbance of Intracerebral Fluid Clearance and Blood–Brain Barrier in Vascular Cognitive Impairment. Int. J. Mol. Sci. 2019, 20, 2600. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.E.; Rodriguez-Cruz, V.; Felmlee, M.A. SLC and ABC Transporters: Expression, Localization, and Species Differences at the Blood-Brain and the Blood-Cerebrospinal Fluid Barriers. AAPS J. 2017, 19, 1317–1331. [Google Scholar] [CrossRef]
- Damkier, H.; Praetorius, J. Structure of the mammalian choroid plexus. In Role of the Choroid Plexus in Health and Disease; Praetorius, J., Blazer-Yost, B., Damkier, H., Eds.; Springer: New York, NY, USA, 2020; pp. 1–33. [Google Scholar]
- Praetorius, J.; Damkier, H.H. Transport across the choroid plexus epithelium. Am. J. Physiol. Physiol. 2017, 312, C673–C686. [Google Scholar] [CrossRef]
- Pardridge, W.M. CSF, blood-brain barrier, and brain drug delivery. Expert Opin. Drug Deliv. 2016, 13, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Chiba, Y.; Murakami, R.; Miyai, Y.; Matsumoto, K.; Kamada, M.; Nonaka, W.; Uemura, N.; Yanase, K.; Ueno, M. Metabolites and Biomarker Compounds of Neurodegenerative Diseases in Cerebrospinal Fluid. Metabolites 2022, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Lach, B.; Scheithauer, B.W.; Gregor, A.; Wick, M.R. Colloid cyst of the third ventricle. A comparative immunohisto-chemical study of neuraxis cysts and choroid plexus epithelium. J. Neurosurg. 1993, 78, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, J.; Kashima, T.; Kikuchi, Y.; Kunita, A.; Fukayama, M. Podoplanin is expressed in subsets of tumors of the central nervous system. Virchows Arch. 2006, 448, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Kirik, O.V.; Sufieyva, D.A.; Nazarenkova, A.; Korzhevskiy, D.E. Cell contact protein beta-catenin in ependymal and epithelial cells of the choroid plexus of the cerebral lateral ventricles. Morphology 2016, 149, 33–37. [Google Scholar] [PubMed]
- Kasper, M.; Karsten, U.; Stosiek, P. Detection of cytokeratin(s) in epithelium of human Plexus choroideus by monoclonal antibodies. Acta Histochem. 1986, 78, 101–103. [Google Scholar] [CrossRef]
- Miettinen, M.; Clark, R.; Virtanen, I. Intermediate filament proteins in choroid plexus and ependyma and their tumors. Am. J. Pathol. 1986, 123, 231–240. [Google Scholar]
- Justice, D.L.; Rhodes, R.H.; Tökés, Z.A. Immunohistochemical demonstration of proteinase inhibitor alpha-1-antichymotrypsin in normal human central nervous system. J. Cell. Biochem. 1987, 34, 227–238. [Google Scholar] [CrossRef]
- Damkier, H.H.; Brown, P.D.; Praetorius, J. Cerebrospinal Fluid Secretion by the Choroid Plexus. Physiol. Rev. 2013, 93, 1847–1892. [Google Scholar] [CrossRef] [Green Version]
- Spector, R.; Keep, R.F.; Snodgrass, S.R.; Smith, Q.R.; Johanson, C.E. A balanced view of choroid plexus structure and function: Focus on adult humans. Exp. Neurol. 2015, 267, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Johanson, C.E.; Keep, R.F. Blending Established and New Perspectives on Choroid Plexus-CSF Dynamics. In Role of the Choroid Plexus in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2020; pp. 35–81. [Google Scholar] [CrossRef]
- Chiba, Y.; Murakami, R.; Matsumoto, K.; Wakamatsu, K.; Nonaka, W.; Uemura, N.; Yanase, K.; Kamada, M.; Ueno, M. Glucose, Fructose, and Urate Transporters in the Choroid Plexus Epithelium. Int. J. Mol. Sci. 2020, 21, 7230. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Chiba, Y.; Fujihara, R.; Kubo, H.; Sakamoto, H.; Ueno, M. Immunohistochemical analysis of transporters related to clearance of amyloid-β peptides through blood–cerebrospinal fluid barrier in human brain. Histochem. Cell Biol. 2015, 144, 597–611. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Chiba, Y.; Murakami, R.; Matsumoto, K.; Miyai, Y.; Kawauchi, M.; Yanase, K.; Uemura, N.; Ueno, M. Immunohistochemical expression of osteopontin and collagens in choroid plexus of human brains. Neuropathology 2022, 42, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P. The SLC16 gene family—Structure, role and regulation in health and disease. Mol. Asp. Med. 2013, 34, 337–349. [Google Scholar] [CrossRef]
- Iwanaga, T.; Kishimoto, A. Cellular distributions of monocarboxylate transporters: A review. Biomed. Res. 2015, 36, 279–301. [Google Scholar] [CrossRef] [Green Version]
- Friesema, E.C.H.; Jansen, J.; Jachtenberg, J.-W.; Visser, W.E.; Kester, M.H.A.; Visser, T.J. Effective Cellular Uptake and Efflux of Thyroid Hormone by Human Monocarboxylate Transporter 10. Mol. Endocrinol. 2008, 22, 1357–1369. [Google Scholar] [CrossRef] [Green Version]
- Halestrap, A.P. The monocarboxylate transporter family-Structure and functional characterization. IUBMB Life 2012, 64, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pierre, K.; Pellerin, L. Monocarboxylate transporters in the central nervous system: Distribution, regulation and function. J. Neurochem. 2005, 94, 1–14. [Google Scholar] [CrossRef]
- Vijay, N.; Morris, M.E. Role of Monocarboxylate Transporters in Drug Delivery to the Brain. Curr. Pharm. Des. 2014, 20, 1487–1498. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.P.; Drewes, L.R. Modulation of Monocarboxylic Acid Transporter-1 Kinetic Function by the cAMP Signaling Pathway in Rat Brain Endothelial Cells. J. Biol. Chem. 2006, 281, 2053–2060. [Google Scholar] [CrossRef] [Green Version]
- Uhernik, A.L.; Li, L.; LaVoy, N.; Velasquez, M.J.; Smith, J.P. Regulation of Monocarboxylic Acid Transporter-1 by cAMP Dependent Vesicular Trafficking in Brain Microvascular Endothelial Cells. PLoS ONE 2014, 9, e85957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philp, N.J.; Yoon, H.; Lombardi, L.; Daniele, L.L.; Sauer, B.; Gallagher, S.M.; Pugh, E.N.; Brauchi, S.; Rauch, M.C.; Alfaro, I.E.; et al. Mouse MCT3 gene is expressed preferentially in retinal pigment and choroid plexus epithelia. Am. J. Physiol. Cell Physiol. 2001, 280, C1319–C1326. [Google Scholar] [CrossRef] [PubMed]
- Bergersen, L.H. Lactate transport and signaling in the brain: Potential therapeutic targets and roles in body-brain interaction. J. Cereb. Blood Flow. Metab. 2015, 35, 176–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, Y.; Zhang, Z.; Tachikawa, M.; Terasaki, T. Quantitative targeted absolute proteomics of rat blood-cerebrospinal fluid barrier transporters: Comparison with a human specimen. J. Neurochem. 2015, 134, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Asp. Med. 2013, 34, 121–138. [Google Scholar] [CrossRef] [Green Version]
- Wright, E.M.; Loo, D.D.F.; Hirayama, B.A. Biology of Human Sodium Glucose Transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef] [Green Version]
- Leino, R.L.; Gerhart, D.Z.; Drewes, L.R. Monocarboxylate transporter (MCT1) abundance in brains of sucking and adult rats: A quantitative electron microscopic immunogold study. Brain Res. 1999, 113, 47–54. [Google Scholar] [CrossRef]
- Smith, J.P.; Uhernik, A.L.; Li, L.; Liu, Z.; Drewes, L.R. Regulation of Mct1 by cAMP-dependent internalization in rat brain endothelial cells. Brain Res. 2012, 1480, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nijland, P.G.; Michailidou, I.; Witte, M.E.; Mizee, M.R.; van der Pol, S.M.A.; van her Hof, B.; Reijerkerk, A.; Pellerin, L.; van der Valk, P.; de Vries, H.E.; et al. Cellular distribution of glucose and monocarboxylate transporters in human brain white matter and multiple sclerosis lesions. Glia 2014, 62, 1125–1141. [Google Scholar] [CrossRef]
- Rinholm, J.E.; Hamilton, N.B.; Kessaris, N.; Richardson, W.D.; Bergersen, L.H.; Attwell, D. Regulation of Oligodendrocyte Development and Myelination by Glucose and Lactate. J. Neurosci. 2011, 31, 538–548. [Google Scholar] [CrossRef] [Green Version]
- Murakami, R.; Chiba, Y.; Nishi, N.; Matsumoto, K.; Wakamatsu, K.; Yanase, K.; Uemura, N.; Nonaka, W.; Ueno, M. Immunoreactivity of receptor and transporters for lactate located in astrocytes and epithelial cells of choroid plexus of human brain. Neurosci. Lett. 2021, 741, 135479. [Google Scholar] [CrossRef]
- Caruso, J.P.; Koch, B.J.; Benson, P.D.; Varughese, E.; Monterey, M.D.; Lee, A.E.; Dave, A.M.; Kiousis, S.; Sloan, A.E.; Mathupala, S.P. pH, Lactate, and Hypoxia: Reciprocity in Regulating High-Affinity Monocarboxylate Transporter Expression in Glioblastoma. Neoplasia 2017, 19, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Hanu, R.; McKenna, M.; O’Neill, A.; Resneck, W.G.; Bloch, R.J.; Takimoto, M.; Hamada, T.; Puchowicz, M.A.; Xu, K.; Sun, X.; et al. Monocarboxylic acid transporters, MCT1 and MCT2, in cortical astrocytes in vitro and in vivo. Am. J. Physiol. Physiol. 2000, 278, C921–C930. [Google Scholar] [CrossRef]
- Pierre, K.; Pellerin, L.; Debernardi, R.; Riederer, B.; Magistretti, P. Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience 2000, 100, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, A.J.; Llewellyn, G.N.; Kishi, S.H.; Jakowec, N.A.; Cannon, P.M.; Petzinger, G.M.; Jakowec, M.W. Knockdown of Astrocytic Monocarboxylate Transporter 4 in the Motor Cortex Leads to Loss of Dendritic Spines and a Deficit in Motor Learning. Mol. Neurobiol. 2022, 59, 1002–1017. [Google Scholar] [CrossRef] [PubMed]
- Dimmer, K.-S.; Friedrich, B.; Lang, F.; Deitmer, J.W.; Bröer, S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem. J. 2000, 350, 219–227. [Google Scholar] [CrossRef]
- Beckner, M.E.; Pollack, I.F.; Nordberg, M.L.; Hamilton, R.L. Glioblastomas with copy number gains in EGFR and RNF139 show increased expressions of carbonic anhydrase genes transformed by ENO1. BBA Clin. 2016, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Lafrenière, R.G.; Carrel, L.; Willard, H.F. A novel transmembrane transporter encoded by the XPCT gene in Xq13.2. Hum. Mol. Genet. 1994, 3, 1133–1139. [Google Scholar] [CrossRef]
- Friesema, E.C.H.; Ganguly, S.; Abdalla, A.; Manning Fox, J.E.; Halestrap, A.P.; Visser, T.J. Identification of Monocarboxylate Transporter 8 as a Specific Thyroid Hormone Transporter. J. Biol. Chem. 2003, 278, 40128–40135. [Google Scholar] [CrossRef] [Green Version]
- Visser, W.E.; Friesema, E.C.; Jansen, J.; Visser, T.J. Thyroid hormone transport in and out of cells. Trends Endocrinol. Metab. 2008, 19, 50–56. [Google Scholar] [CrossRef]
- Visser, W.E.; Friesema, E.C.H.; Visser, T.J. Minireview: Thyroid Hormone Transporters: The Knowns and the Unknowns. Mol. Endocrinol. 2011, 25, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wirth, E.K.; Schweizer, U.; Kohrle, J. Transport of Thyroid Hormone in Brain. Front. Endocrinol. 2014, 5, 98. [Google Scholar] [CrossRef] [Green Version]
- Roberts, L.M.; Woodford, K.; Zhou, M.; Black, D.S.; Haggerty, J.E.; Tate, E.H.; Grindstaff, K.K.; Mengesha, W.; Raman, C.; Zerangue, N. Expression of the Thyroid Hormone Transporters Monocarboxylate Transporter-8 (SLC16A2) and Organic Ion Transporter-14 (SLCO1C1) at the Blood-Brain Barrier. Endocrinology 2008, 149, 6251–6261. [Google Scholar] [CrossRef] [PubMed]
- Wirth, E.K.; Roth, S.; Blechschmidt, C.; Hölter, S.M.; Becker, L.; Racz, I.; Zimmer, A.; Klopstock, T.; Failus-Durner, V.; Fuchs, H.; et al. Neuronal 3’,3,5-triiodothyronine (T3) uptake and behavioral phenotype of mice deficient in Mct8, the neuronal T3 transporter mutated in Allan-Herndon-Dudley syndrome. J. Neurosci. 2009, 30, 9439–9449. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.; Kinne, A.; Bräuer, A.U.; Sapin, R.; Klein, M.O.; Köhrle, J.; Wirth, E.K.; Schweizer, U. Developmental and cell type-specific expression of thyroid hormone transporters in the mouse brain and in primary brain cells. Glia 2011, 59, 463–471. [Google Scholar] [CrossRef]
- Wilpert, N.-M.; Krueger, M.; Opitz, R.; Sebinger, D.; Paisdzior, S.; Mages, B.; Schulz, A.; Spranger, J.; Wirth, E.K.; Stachelscheid, H.; et al. Spatiotemporal Changes of Cerebral Monocarboxylate Transporter 8 Expression. Thyroid 2020, 30, 1366–1383. [Google Scholar] [CrossRef] [Green Version]
- Alkemade, A.; Friesema, E.C.; Unmehopa, U.A.; Fabriek, B.O.; Kuiper, G.G.; Leonard, J.L.; Wiersinga, W.M.; Swaab, D.F.; Visser, T.J.; Fliers, E. Neuroanatomical Pathways for Thyroid Hormone Feedback in the Human Hypothalamus. J. Clin. Endocrinol. Metab. 2005, 90, 4322–4334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuer, H.; Maier, M.K.; Iden, S.; Mittag, J.; Friesema, E.C.H.; Visser, T.J.; Bauer, K. The Monocarboxylate Transporter 8 Linked to Human Psychomotor Retardation Is Highly Expressed in Thyroid Hormone-Sensitive Neuron Populations. Endocrinology 2005, 146, 1701–1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkemade, A.; Friesema, E.C.H.; Kalsbeek, A.; Swaab, D.F.; Visser, T.J.; Fliers, E. Expression of thyroid hormone trans-porters in the human hypothalamus. J. Clin. Endocrinol. Metab. 2011, 96, E967–E971. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, C.E.; May, M.M.; Carpenter, N.J.; Rogers, R.C.; Martin, J.; Bialer, M.G.; Ward, J.; Sanabria, J.; Marsa, S.; Lewis, J.A.; et al. Allan-Herndon-Dudley syndrome and the mono-carboxylate transporter 8 (MCT8) gene. Am. J. Hum. Genet. 2005, 77, 41–53. [Google Scholar]
- Wolff, T.M.; Veil, C.; Dietrich, J.W.; Müller, M.A. Mathematical modeling and simulation of thyroid homeostasis: Implications for the Allan-Herndon-Dudley syndrome. Front. Endocrinol. 2022, 13, 882788. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S.; Rahman, B.; Pellegri, G.; Pellerin, L.; Martin, J.-L.; Verleysdonk, S.; Hamprecht, B.; Magistretti, P.J. Comparison of Lactate Transport in Astroglial Cells and Monocarboxylate Transporter 1 (MCT 1) Expressing Xenopus laevis Oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J. Biol. Chem. 1997, 272, 30096–30102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maekawa, E.; Minehira, K.; Kadomatsu, K.; Pellerin, L. Basal and stimulated lactate fluxes in primary cultures of astrocytes are differentially controlled by distinct proteins. J. Neurochem. 2008, 107, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Rostafio, K.; Pellerin, L. Oxygen tension controls the expression of the monocarboxylate transporter MCT4 in cultures mouse cortical astrocytes via a hypoxia-inducible factor-1alpha-mediated transcriptional regulation. Glia 2014, 62, 477–490. [Google Scholar] [CrossRef] [Green Version]
- Pellerin, L.; Pellegri, G.; Bittar, P.G.; Charnay, Y.; Bouras, C.; Martin, J.-L.; Stella, N.; Magistretti, P.J. Evidence Supporting the Existence of an Activity-Dependent Astrocyte-Neuron Lactate Shuttle. Dev. Neurosci. 1998, 20, 291–299. [Google Scholar] [CrossRef]
- Pellerin, L. Brain energetics (thought needs fond). Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 701–705. [Google Scholar] [CrossRef]
- Pérez-Escuredo, J.; Van Hée, V.F.; Sboarina, M.; Falces, J.; Payen, V.L.; Pellerin, L.; Sonveaux, P. Monocarboxylate transporters in the brain and in cancer. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2016, 1863, 2481–2497. [Google Scholar] [CrossRef] [Green Version]
- Bittar, P.G.; Charnay, Y.; Pellerin, L.; Bouras, C.; Magistretti, P.J. Selective Distribution of Lactate Dehydrogenase Isoenzymes in Neurons and Astrocytes of Human Brain. J. Cereb. Blood Flow Metab. 1996, 16, 1079–1089. [Google Scholar] [CrossRef] [Green Version]
- Leen, W.G.; Willemsen, M.A.; Wevers, R.A.; Verbeek, M.M. Cerebrospinal Fluid Glucose and Lactate: Age-Specific Reference Values and Implications for Clinical Practice. PLoS ONE 2012, 7, e42745. [Google Scholar] [CrossRef] [Green Version]
- Liguori, C.; Stefani, A.; Fernandes, M.; Cerroni, R.; Mercuri, N.B.; Pierantozzi, M. Biomarkers of Cerebral Glucose Metabolism and Neurodegeneration in Parkinson’s Disease: A Cerebrospinal Fluid-Based Study. J. Park. Dis. 2022, 12, 537–544. [Google Scholar] [CrossRef]
- Bonomi, C.G.; De Lucia, V.; Mascolo, A.P.; Assogna, M.; Motta, C.; Scaricamazza, E.; Sallustio, F.; Mercuri, N.B.; Koch, G.; Martorana, A. Brain energy metabolism and neurodegeneration: Hints from CSF lactate levels in dementias. Neurobiol. Aging 2021, 105, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, K.H.; Morland, C.; Puchades, M.; Holm-Hansen, S.; Hagelin, E.M.; Lauritzen, F.; Attramadal, H.; Storm-Mathisen, J.; Gjedde, A.; Bergersen, L.H. Lactate Receptor Sites Link Neurotransmission, Neurovascular Coupling, and Brain Energy Metabolism. Cereb. Cortex 2014, 24, 2784–2795. [Google Scholar] [CrossRef] [PubMed]
- Madeira, M.D.; Sousa, N.; Lima-Andrade, M.T.; Calheiros, F.; Cadete-Leite, A.; Paula-Barbosa, M.M. Selective vulnerability of the hippocampal pyramidal neurons to hypothyroidism in male and female rats. J. Comp. Neurol. 1992, 322, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Bakalov, D.; Iliev, P.; Sabit, Z.; Tafradjiiska-Hadjiolova, R.; Bocheva, G. Attenuation of Hypothyroidism-Induced Cognitive Impairment by Modulating Serotonin Mediation. Vet. Sci. 2023, 10, 122. [Google Scholar] [CrossRef]
- Stern, M.; Finch, A.; Haskard-Zolnierek, K.B.; Howard, K.; Deason, R.G. Cognitive decline in mid-life: Changes in memory and cognition related to hypothyroidism. J. Health Psychol. 2022, 28, 388–401. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Y.; Li, S.; Guo, J.; Ding, L.; Liu, M. Association of Hypothyroidism and the Risk of Cognitive Dysfunction: A Meta-Analysis. J. Clin. Med. 2022, 11, 6726. [Google Scholar] [CrossRef]
- Salehipour, A.; Dolatshahi, M.; Haghshomar, M.; Amin, J. The Role of Thyroid Dysfunction in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Prev. Alzheimer’s Dis. 2023, 10, 276–286. [Google Scholar] [CrossRef]
- Richardson, S.J.; Wijayagunaratne, R.C.; D’Souza, D.G.; Darras, V.M.; Van Herck, S.L.J. Transport of thyroid hormones via the choroid plexus into the brain: The roles of transthyretin and thyroid hormone transmembrane transporters. Front. Neurosci. 2015, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Shirbandi, K.B.; Rikhtegar, R.; Khalafi, M.; Attari, M.M.A.; Rahmani, F.; Javanmardi, P.B.; Iraji, S.M.; Aghdam, Z.B.; Rashnoudi, A.M.R. Functional Magnetic Resonance Spectroscopy of Lactate in Alzheimer Disease: A Comprehensive Review of Alzheimer Disease Pathology and the Role of Lactate. Top. Magn. Reson. Imaging 2023, 32, 15–26. [Google Scholar] [CrossRef]


| (No.) | Age/Sex | Main Diagnosis |
|---|---|---|
| 1 | 60/M | Dissecting aneurysm |
| 2 | 64/M | Multiple system atrophy, Pneumonia |
| 3 | 70/M | Myocardial infarction |
| 4 | 71/M | Cerebellar tuberculosis |
| 5 | 72/F | Pneumonia |
| 6 | 74/M | Lung cancer |
| 7 | 75/M | Gastric cancer |
| 8 | 84/M | Myocardial infarction, Cerebral infarction |
| Antibody | Cat. No. (Clone Name) | Host Species and Usage |
|---|---|---|
| CK8 | Progen, 61038 | mouse, 1:50 (¶2) |
| CK18 | ProteinTech, 66187-1-lg | mouse, 1:600 (¶2) |
| CK19 | Progen, 61010 | mouse, 1:10 (¶1) |
| β-catenin | SantaCruz, sc-7199 | rabbit, 1:50 (-) |
| vimentin | DAKO, M0725 (V9) | mouse, 1:50 (¶1) |
| D2/40(podoplanin) | DAKO, M3619 | mouse, 1:50 (¶1) |
| s-100 | Nichirei, 422091 | rabbit, diluted (-) |
| transthyretin | ProteinTech, 11891-1-AP | rabbit, 1:100 (-) |
| AACT | ProteinTech, 66078-1-lg | mouse, 1:500 (¶2) |
| Na+,-K+,-ATPase | SantaCruz, sc-48345 | rabbit, 1:100 (¶1) |
| Aquaporin-1 | ProteinTech, 20333-1-AP | rabbit, 1:250 (¶2) |
| AE2 | sc-376632 (D-3) | mouse, 1:100 (¶1) |
| MCT1 | Abcam, ab90582 | mouse, 1:100 (¶1) |
| MCT2 | Abcam, ab198272 | rabbit, 1:100 (-) |
| MCT3 | Abcam, ab60333 | rabbit, 1:200 (-) |
| MCT4 | Abcam, ab244385 | rabbit, 1:50 (¶1) |
| MCT5 | Abcam, ab191008 | rabbit, 1:500 (¶1) |
| MCT8 | Novus, NBP2-57308 | rabbit, 1:200 (¶1) |
| HCA1-R | Novus, NLS-2095 | rabbit, 1:200 (¶1) |
| Isoform (Gene) | Predominat Substrates | Regional Distribution | Cellular Localization | References |
|---|---|---|---|---|
| MCT1 (SLC16A1) | Lactate, pyruvate, | Widespread | Endothelial cells, astrocytes | [24,27,30,33,39,40,41,42,43,44,45] |
| ketone bodies | Cortex, hippocampus, | ependymocytes, microglia, | ||
| cerebellum, | oligodensrocyte, | |||
| choroid plexus | choroid plexus epithelium, | |||
| some neurons (Rt) #1 | ||||
| MCT2 (SLC16A7) | Pyruvate, lactate, | Widespread | Neurons/axon, microglia, | [26,27,41,45] |
| ketone bodies | Cortex, hippocampus, | endothelial cells, | ||
| (high affinity) | cerebellum, | choroid plexus epithelium | ||
| choroid plexus | astrocytes (Rt, MS) #2 | |||
| MCT3 (SLC16A8) | Lactate | Localized | Retinal pigment epithelium (Ms) #3, | [26,34,35] |
| choroid plexus epithelium (Ms) #3 | ||||
| MCT4 (SLC16A3) | Lactate, pyruvate, | Widespread | Astrocytes, microglia | [26,27,41,43,46] |
| ketone bodies | Cortex, hippocampus, | endothelial cells, | ||
| (low affinity) | cerebellum, | choroid plexus epithelium | ||
| (high capacity) | choroid plexus | |||
| MCT5 (SLC16A4) | Orphan | Localized | Isolated choroid plexus | [26,36] |
| MCT8 (SLC16A2) | Thyroid hormone | Widespread | Neurons, astrocytes, | [26,53,54,55,56,57] |
| (high affinity) | Cortex, hippocampus, | endothelial cells | ||
| hypothalamus, | choroid plexus epithelium | |||
| choroid plexus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueno, M.; Chiba, Y.; Murakami, R.; Miyai, Y.; Matsumoto, K.; Wakamatsu, K.; Takebayashi, G.; Uemura, N.; Yanase, K. Distribution of Monocarboxylate Transporters in Brain and Choroid Plexus Epithelium. Pharmaceutics 2023, 15, 2062. https://doi.org/10.3390/pharmaceutics15082062
Ueno M, Chiba Y, Murakami R, Miyai Y, Matsumoto K, Wakamatsu K, Takebayashi G, Uemura N, Yanase K. Distribution of Monocarboxylate Transporters in Brain and Choroid Plexus Epithelium. Pharmaceutics. 2023; 15(8):2062. https://doi.org/10.3390/pharmaceutics15082062
Chicago/Turabian StyleUeno, Masaki, Yoichi Chiba, Ryuta Murakami, Yumi Miyai, Koichi Matsumoto, Keiji Wakamatsu, Genta Takebayashi, Naoya Uemura, and Ken Yanase. 2023. "Distribution of Monocarboxylate Transporters in Brain and Choroid Plexus Epithelium" Pharmaceutics 15, no. 8: 2062. https://doi.org/10.3390/pharmaceutics15082062
APA StyleUeno, M., Chiba, Y., Murakami, R., Miyai, Y., Matsumoto, K., Wakamatsu, K., Takebayashi, G., Uemura, N., & Yanase, K. (2023). Distribution of Monocarboxylate Transporters in Brain and Choroid Plexus Epithelium. Pharmaceutics, 15(8), 2062. https://doi.org/10.3390/pharmaceutics15082062






