Ampicillin Stability in a Portable Elastomeric Infusion Pump: A Step Forward in Outpatient Parenteral Antimicrobial Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
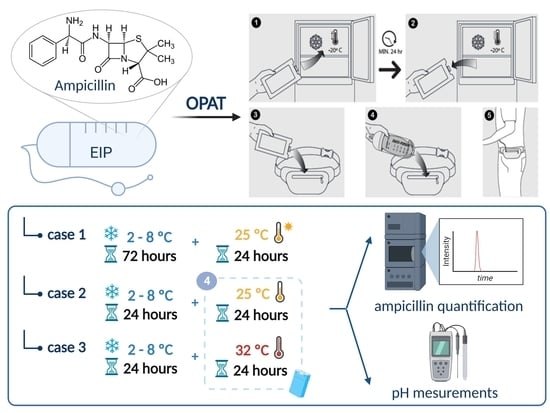
2.2. Elaboration and Packaging of Ampicillin Sterile Solutions
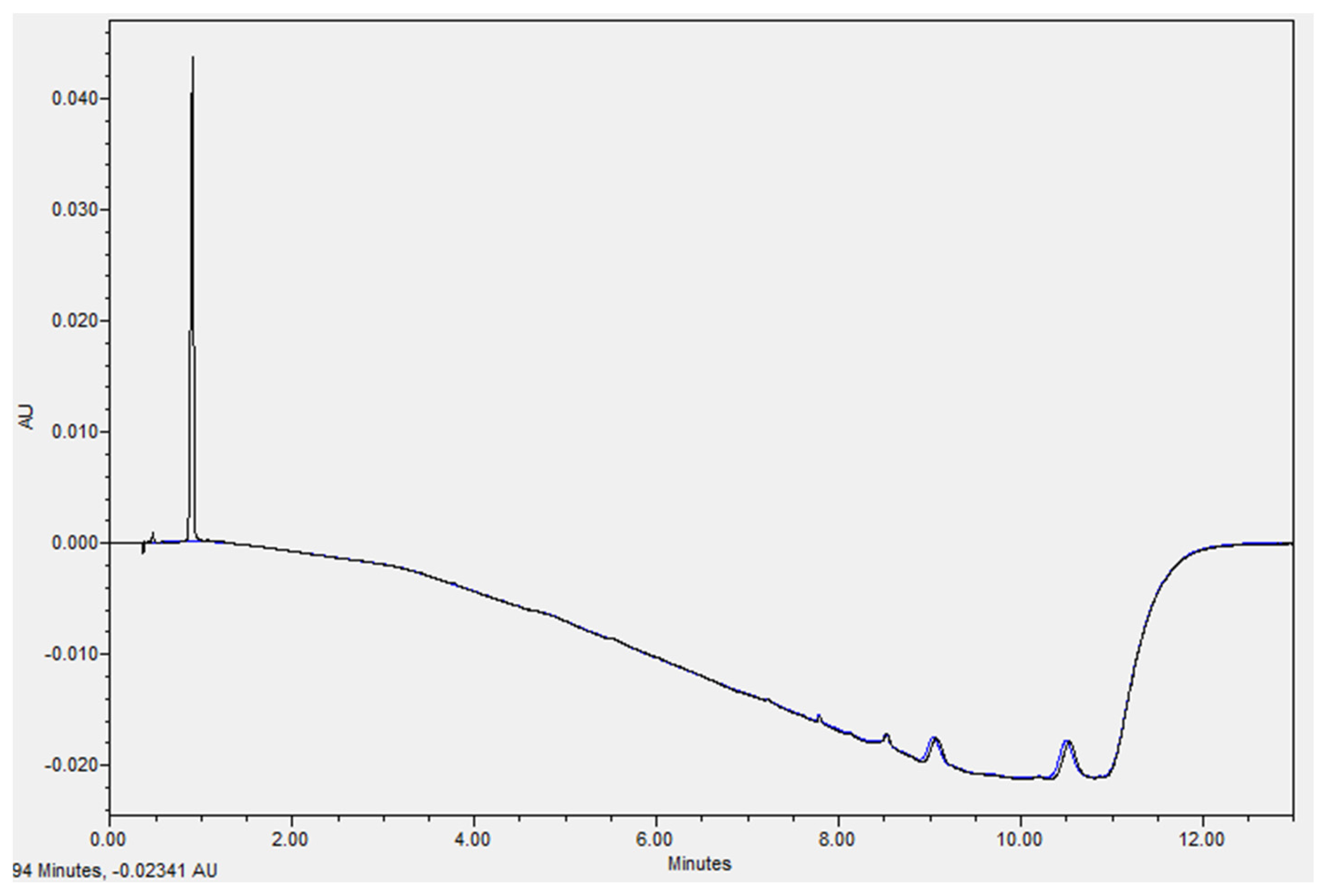
2.3. Ampicillin Quantification
2.4. Re-Validation of the Chromatographic Method
2.5. Stability Study Conditions
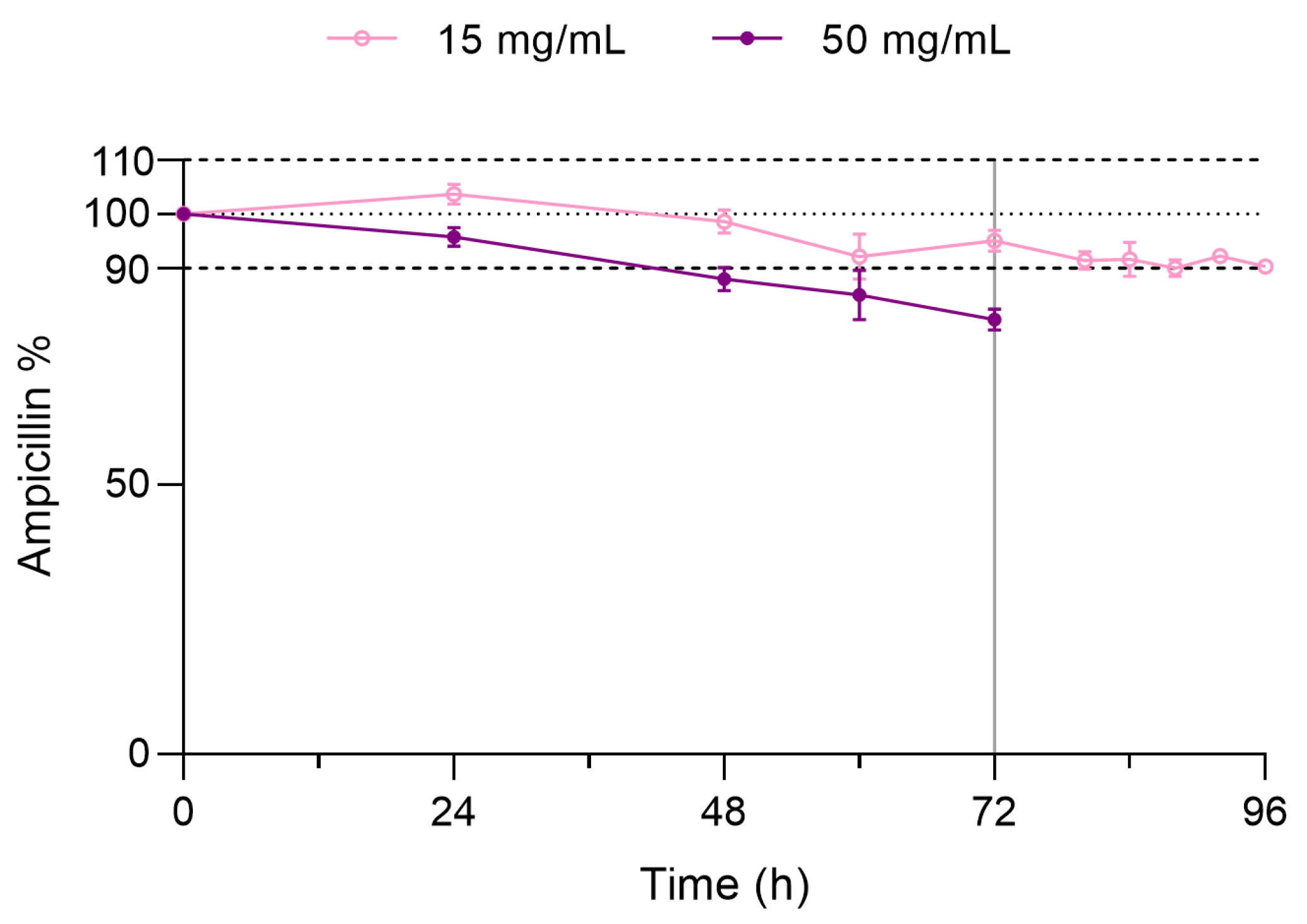
2.5.1. Extended Storage Stability Study
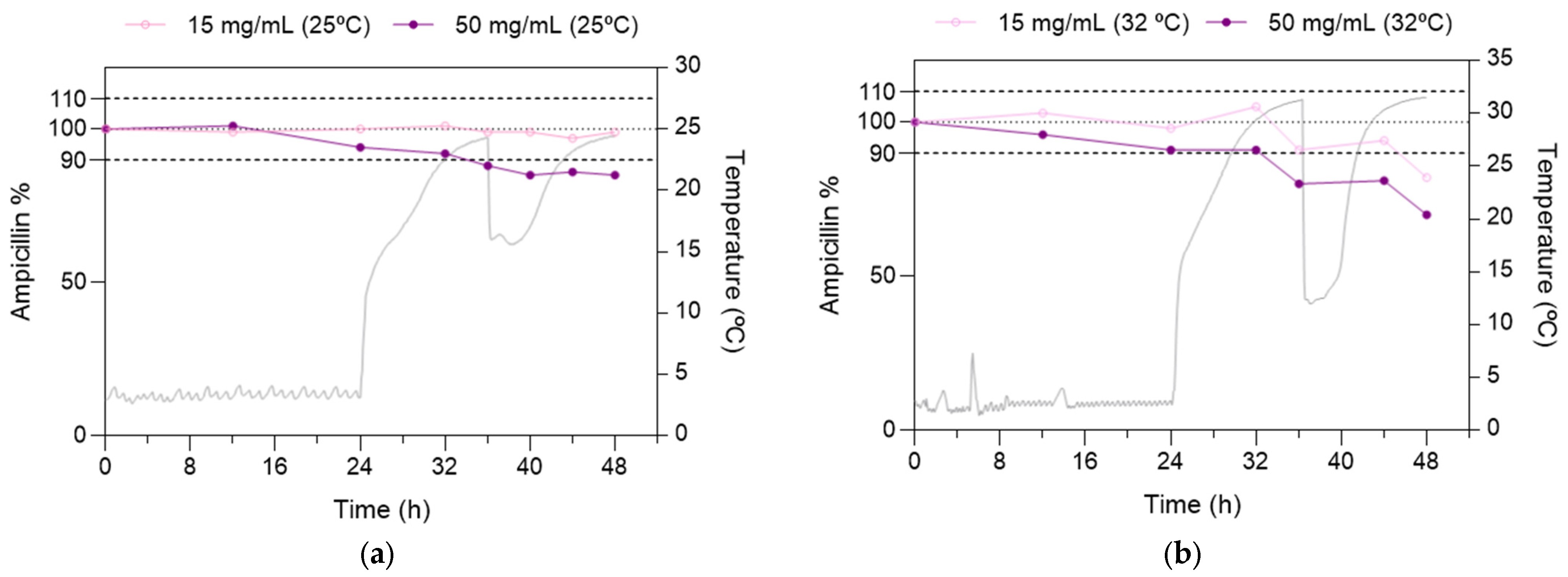
2.5.2. Real-Life Conditions Stability Study
2.6. Physical Stability
2.7. Statistical Analysis
3. Results
3.1. Re-Validation of the Chromatographic Method
3.2. Chemical Stability Study
3.2.1. Extended Storage Stability Study
3.2.2. Real-Life Conditions Stability Study
3.3. Determination of pH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esteban-Cartelle, B.; Vicente-Oliveros, N.; Pérez Menéndez-Conde, C.; Serrano, D.R.; Martín-Dávila, P.; Fortún-Abete, J.; León-Gil, L.A.; Álvarez-Díaz, A. Antibiotic Stability in Portable Elastomeric Infusion Devices: A Systematic Review. Am. J. Health Syst. Pharm. 2022, 79, 1355–1368. [Google Scholar] [CrossRef] [PubMed]
- Norris, A.H.; Shrestha, N.K.; Allison, G.M.; Keller, S.C.; Bhavan, K.P.; Zurlo, J.J.; Hersh, A.L.; Gorski, L.A.; Bosso, J.A.; Rathore, M.H.; et al. 2018 Infectious Diseases Society of America Clinical Practice Guideline for the Management of Outpatient Parenteral Antimicrobial Therapya. Clin. Infect. Dis. 2019, 68, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Chapman, A.L.N.; Patel, S.; Horner, C.; Gilchrist, M.; Seaton, R.A. Outpatient Parenteral Antimicrobial Therapy: Updated Recommendations from the UK. J. Antimicrob. Chemother. 2019, 74, 3125–3127. [Google Scholar] [CrossRef] [Green Version]
- Cortés, L.E.L.; Martinez, A.M.; de Mandojana, M.F.M.; Martín, N.; Bermejo, M.G.; Aznar, J.S.; Bruguera, E.V.; Cantero, M.J.P.; González-Ramallo, V.J.; González, M.Á.P.; et al. Resumen Ejecutivo del tratamiento antibiótico domiciliario endovenoso: Directrices de la Sociedad Española de Enfermedades Infecciosas y la Sociedad Española de Hospitalización a Domicilio. Hosp. A Domic. 2018, 2, 165–177. [Google Scholar] [CrossRef]
- Akahane, M.; Enoki, Y.; Saiki, R.; Hayashi, Y.; Hiraoka, K.; Honma, K.; Itagaki, M.; Gotoda, M.; Shinoda, K.; Hanyu, S.; et al. Stability of Antimicrobial Agents in an Elastomeric Infusion Pump Used for Outpatient Parenteral Antimicrobial Therapy. Int. J. Infect. Dis. 2021, 103, 464–468. [Google Scholar] [CrossRef]
- Vargas-Palacios, A.; Meads, D.M.; Twiddy, M.; Czoski Murray, C.; Hulme, C.; Mitchell, E.D.; Gregson, A.; Stanley, P.; Minton, J. Cost-Effectiveness of Outpatient Parenteral Antibiotic Therapy: A Simulation Modelling Approach. J. Antimicrob. Chemother. 2017, 72, 2392–2400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minton, J.; Murray, C.C.; Meads, D.; Hess, S.; Vargas-Palacios, A.; Mitchell, E.; Wright, J.; Hulme, C.; Raynor, D.K.; Gregson, A.; et al. The Community IntraVenous Antibiotic Study (CIVAS): A Mixed-Methods Evaluation of Patient Preferences for and Cost-Effectiveness of Different Service Models for Delivering Outpatient Parenteral Antimicrobial Therapy; Health Services and Delivery Research; NIHR Journals Library: Southampton, UK, 2017. [Google Scholar]
- Mitchell, E.D.; Murray, C.C.; Meads, D.; Minton, J.; Wright, J.; Twiddy, M. Clinical and Cost-Effectiveness, Safety and Acceptability of Community Intravenous Antibiotic Service Models: CIVAS Systematic Review. BMJ Open 2017, 7, e013560. [Google Scholar] [CrossRef] [Green Version]
- García-Queiruga, M.; Feal Cortizas, B.; Lamelo Alfonsín, F.; Pertega Diaz, S.; Martín-Herranz, I. Continuous Infusion of Antibiotics Using Elastomeric Pumps in the Hospital at Home Setting. Rev. Esp. Quim. 2021, 34, 200–206. [Google Scholar] [CrossRef]
- Fernández-Rubio, B.; Del Valle-Moreno, P.; Herrera-Hidalgo, L.; Gutiérrez-Valencia, A.; Luque-Márquez, R.; López-Cortés, L.E.; Gutiérrez-Urbón, J.M.; Luque-Pardos, S.; Fernández-Polo, A.; Gil-Navarro, M.V. Stability of Antimicrobials in Elastomeric Pumps: A Systematic Review. Antibiotics 2021, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.N.; Dworkin, R.J.; Raber, S.R.; Leggett, J.E. Outpatient Parenteral Antimicrobial-Drug Therapy. N. Engl. J. Med. 1997, 337, 829–838. [Google Scholar] [CrossRef]
- Brouwers, R.; Vass, H.; Dawson, A.; Squires, T.; Tavaddod, S.; Allen, R.J. Stability of β-Lactam Antibiotics in Bacterial Growth Media. PLoS ONE 2020, 15, e0236198. [Google Scholar] [CrossRef]
- Hou, J.P.; Poole, J.W. β-Lactam Antibiotics: Their Physicochemical Properties and Biological Activities in Relation to Structure. J. Pharm. Sci. 1971, 60, 503–532. [Google Scholar] [CrossRef]
- Allen, L.V.; Stiles, M.L.; Prince, S.J.; Smeeding, J. Stability of 14 Drugs in the Latex Reservoir of an Elastomeric Infusion Device. Am. J. Health Syst. Pharm. 1996, 53, 2740–2743. [Google Scholar] [CrossRef] [PubMed]
- Huskey, M.; Lewis, P.; Brown, S.D. Stability of Ampicillin in Normal Saline Following Refrigerated Storage and 24-Hour Pump Recirculation. Hosp. Pharm. 2021, 56, 507–512. [Google Scholar] [CrossRef]
- Kang, M.A.; Kang, J.-S. Stability Test of Ampicillin Sodium Solutions in the Accufuser® Elastomeric Infusion Device Using HPLC: UV Method. Pharmacol. Pharm. 2012, 3, 462–467. [Google Scholar] [CrossRef] [Green Version]
- Manning, L.; Wright, C.; Ingram, P.R.; Whitmore, T.J.; Heath, C.H.; Manson, I.; Page-Sharp, M.; Salman, S.; Dyer, J.; Davis, T.M.E. Continuous Infusions of Meropenem in Ambulatory Care: Clinical Efficacy, Safety and Stability. PLoS ONE 2014, 9, e102023. [Google Scholar] [CrossRef]
- Voumard, R.; Van Neyghem, N.; Cochet, C.; Gardiol, C.; Decosterd, L.; Buclin, T.; de Valliere, S. Antibiotic Stability Related to Temperature Variations in Elastomeric Pumps Used for Outpatient Parenteral Antimicrobial Therapy (OPAT). J. Antimicrob. Chemother. 2017, 72, 1462–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Rubio, B.; Herrera-Hidalgo, L.; Luque-Márquez, R.; de Alarcón, A.; López-Cortés, L.E.; Luque-Pardos, S.; Gutiérrez-Urbón, J.M.; Fernández-Polo, A.; Gil-Navarro, M.V.; Gutiérrez-Valencia, A. Stability of Ampicillin plus Ceftriaxone Combined in Elastomeric Infusion Devices for Outpatient Parenteral Antimicrobial Therapy. Antibiotics 2023, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Enoki, Y.; Uno, S.; Uwamino, Y.; Iketani, O.; Hasegawa, N.; Matsumoto, K. Stability of Benzylpenicillin Potassium and Ampicillin in an Elastomeric Infusion Pump. J. Infect. Chemother. 2018, 24, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Stiles, M.L.; Allen, L.V.; Prince, S.J. Stability of Various Antibiotics Kept in an Insulated Pouch during Administration via Portable Infusion Pump. Am. J. Health Syst. Pharm. 1995, 52, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.; Shanu, S.; Jamieson, C.; Santillo, M. Widening the Net: A Literature Review of Antimicrobial Agents with Potential Suitability for Outpatient Parenteral Antimicrobial Therapy Services-the Importance of Storage and Stability. Eur. J. Hosp. Pharm. 2023, 30, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Contractor, P.; Keshrala, R.; Patel, P.R.; Shridhar, B. Development and Validation of a Stability-Indicating RP-UPLC Method for Determination of Cloxacillin Sodium in Its Bulk Form and Formulation. J. Chromatogr. Sci. 2015, 53, 903–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidance for the Validation of Pharmaceutical Quality Control Analytical Methods 1st Edition (Yellow Cover). Available online: https://www.sps.nhs.uk/articles/guidance-for-the-validation-of-pharmaceutical-quality-control-analytical-methods-1st-edition/ (accessed on 23 May 2023).
- European Medicines Agency (EMA). ICH Q2(R2) Validation of Analytical Procedures—Scientific Guideline. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 21 March 2023).
- Lund, W. The Pharmaceutical Codex: Principles and Practice of Pharmaceutics; The Pharmaceutical Press: London, UK, 1994; ISBN 0-85369-290-4. [Google Scholar]
- Manrique-Rodríguez, S.; Heras-Hidalgo, I.; Pernia-López, M.S.; Herranz-Alonso, A.; del Río Pisabarro, M.C.; Suárez-Mier, M.B.; Cubero-Pérez, M.A.; Viera-Rodríguez, V.; Cortés-Rey, N.; Lafuente-Cabrero, E.; et al. Standardization and Chemical Characterization of Intravenous Therapy in Adult Patients: A Step Further in Medication Safety. Drugs R D 2021, 21, 39–64. [Google Scholar] [CrossRef] [PubMed]
- Stranz, M.; Kastango, E.S. A Review of PH and Osmolarity. Int. J. Pharm. Compd. 2002, 6, 216–220. [Google Scholar] [PubMed]
- David, P.; Nicolau, P. Optimizing Antimicrobial Therapy and Emerging Pathogens. Am. J. Man Care 1998, 4, 525–530. [Google Scholar]
- MacKenzie, M.; Rae, N.; Nathwani, D. Outcomes from Global Adult Outpatient Parenteral Antimicrobial Therapy Programmes: A Review of the Last Decade. Int. J. Antimicrob. Agents 2014, 43, 7–16. [Google Scholar] [CrossRef]
- Seaton, R.A.; Barr, D.A. Outpatient Parenteral Antibiotic Therapy: Principles and Practice. Eur. J. Intern. Med. 2013, 24, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.N.; Baker, C.A.; Kind, A.C.; Sannes, M.R. The History and Evolution of Outpatient Parenteral Antibiotic Therapy (OPAT). Int. J. Antimicrob. Agents 2015, 46, 307–312. [Google Scholar] [CrossRef]
- Durojaiye, O.C.; Bell, H.; Andrews, D.; Ntziora, F.; Cartwright, K. Clinical Efficacy, Cost Analysis and Patient Acceptability of Outpatient Parenteral Antibiotic Therapy (OPAT): A Decade of Sheffield (UK) OPAT Service. Int. J. Antimicrob. Agents 2018, 51, 26–32. [Google Scholar] [CrossRef]
- MacVane, S.H.; Kuti, J.L.; Nicolau, D.P. Prolonging β-Lactam Infusion: A Review of the Rationale and Evidence, and Guidance for Implementation. Int. J. Antimicrob. Agents 2014, 43, 105–113. [Google Scholar] [CrossRef]
- Roberts, J.A.; Croom, K.; Adomakoh, N. Continuous Infusion of Beta-Lactam Antibiotics: Narrative Review of Systematic Reviews, and Implications for Outpatient Parenteral Antibiotic Therapy. Expert Rev. Anti-Infect. Ther. 2023, 21, 375–385. [Google Scholar] [CrossRef]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef] [PubMed]
- Dahl, A.; Fowler, V.G.; Miro, J.M.; Bruun, N.E. Sign of the Times: Updating Infective Endocarditis Diagnostic Criteria to Recognize Enterococcus faecalis as a Typical Endocarditis Bacterium. Clin. Infect. Dis. 2022, 75, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Dahl, A.; Iversen, K.; Tonder, N.; Hoest, N.; Arpi, M.; Dalsgaard, M.; Chehri, M.; Soerensen, L.L.; Fanoe, S.; Junge, S.; et al. Prevalence of Infective Endocarditis in Enterococcus faecalis Bacteremia. J. Am. Coll. Cardiol. 2019, 74, 193–201. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the Management of Infective Endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef] [PubMed]
- Berbari, E.F.; Kanj, S.S.; Kowalski, T.J.; Darouiche, R.O.; Widmer, A.F.; Schmitt, S.K.; Hendershot, E.F.; Holtom, P.D.; Huddleston, P.M.; Petermann, G.W.; et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin. Infect. Dis. 2015, 61, e26–e46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatti, M.; Tedeschi, S.; Trapani, F.; Ramirez, S.; Mancini, R.; Giannella, M.; Viale, P.; Pea, F. A Proof of Concept of the Usefulness of a TDM-Guided Strategy for Optimizing Pharmacokinetic/Pharmacodynamic Target of Continuous Infusion Ampicillin-Based Regimens in a Case Series of Patients with Enterococcal Bloodstream Infections and/or Endocarditis. Antibiotics 2022, 11, 1037. [Google Scholar] [CrossRef]
- Passon, S.G.; Schmidt, A.R.; Wittmann, M.; Velten, M.; Baehner, T. Evaluation of Continuous Ampicillin/Sulbactam Infusion in Critically Ill Patients. Life Sci. 2023, 320, 121567. [Google Scholar] [CrossRef]
- Ogawa, T.; Kasahara, K.; Ikawa, K.; Shigeta, J.; Komatsu, Y.; Kuruno, N.; Uno, K.; Maeda, K.; Mikasa, K. Continuous Ampicillin Infusion as an Alternative to Intermittent Infusion for Adult Inpatients: A Case Series. J. Infect. Chemother. 2014, 20, 653–655. [Google Scholar] [CrossRef]
- Candel, F.J.; Julián-Jiménez, A.; González-Del Castillo, J. Current Status in Outpatient Parenteral Antimicrobial Therapy: A Practical View. Rev. Esp. Quim. 2016, 29, 55–68. [Google Scholar]
- Herrera-Hidalgo, L.; de Alarcón, A.; López-Cortes, L.E.; Luque-Márquez, R.; López-Cortes, L.F.; Gutiérrez-Valencia, A.; Gil-Navarro, M.V. Enterococcus faecalis Endocarditis and Outpatient Treatment: A Systematic Review of Current Alternatives. Antibiotics 2020, 9, 657. [Google Scholar] [CrossRef]
- Perks, S.J.; Robinson, N.; Pain, T.; Franklin, R. Extended Duration Infusion Temperatures in the Tropics: 2 (EDITT2). J. Pharm. Pract. Res. 2018, 48, 423–430. [Google Scholar] [CrossRef]
- Maher, M.; Jensen, K.J.; Lee, D.; Nix, D.E. Stability of Ampicillin in Normal Saline and Buffered Normal Saline. Int. J. Pharm. Compd. 2016, 20, 338–342. [Google Scholar] [PubMed]
- Jamieson, C.; Ozolina, L.; Seaton, R.A.; Gilchrist, M.; Hills, T.; Drummond, F.; Wilkinson, A.S. BSAC Drug Stability Testing Working Group Assessment of the Stability of Citrate-Buffered Piperacillin/Tazobactam for Continuous Infusion When Stored in Two Commercially Available Elastomeric Devices for Outpatient Parenteral Antimicrobial Chemotherapy: A Study Compliant with the NHS Yellow Cover Document Requirements. Eur. J. Hosp. Pharm. 2022, 29, 212–216. [Google Scholar] [CrossRef]
- Tobudic, S.; Forstner, C.; Burgmann, H.; Lagler, H.; Ramharter, M.; Steininger, C.; Vossen, M.G.; Winkler, S.; Thalhammer, F. Dalbavancin as Primary and Sequential Treatment for Gram-Positive Infective Endocarditis: 2-Year Experience at the General Hospital of Vienna. Clin. Infect. Dis. 2018, 67, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Hidalgo, L.; Fernández-Rubio, B.; Luque-Márquez, R.; López-Cortés, L.E.; Gil-Navarro, M.V.; de Alarcón, A. Treatment of Enterococcus faecalis Infective Endocarditis: A Continuing Challenge. Antibiotics 2023, 12, 704. [Google Scholar] [CrossRef]
- Hidalgo-Tenorio, C.; Vinuesa, D.; Plata, A.; Martin Dávila, P.; Iftimie, S.; Sequera, S.; Loeches, B.; Lopez-Cortés, L.E.; Fariñas, M.C.; Fernández-Roldan, C.; et al. DALBACEN Cohort: Dalbavancin as Consolidation Therapy in Patients with Endocarditis and/or Bloodstream Infection Produced by Gram-Positive Cocci. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Xu, X.; Fu, S.; Zhang, J.; Zhang, K.; Wang, S.; Zhao, M.; Ding, W.; Wang, Q. Structural Elucidation of Stress Degradation Products of Ampicillin Sodium by Liquid Chromatography/Hybrid Triple Quadrupole Linear Ion Trap Mass Spectrometry and Liquid Chromatography/Hybrid Quadrupole Time-of-Flight Mass Spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 1929–1936. [Google Scholar] [CrossRef]


| Time (min) | Mobile Phase A (%) | Mobile Phase B (%) |
|---|---|---|
| 0 | 75 | 25 |
| 2 | 70 | 30 |
| 8 | 35 | 65 |
| 10 | 35 | 65 |
| 10.01 | 75 | 25 |
| 13 | 75 | 25 |
| Parameter | Value |
|---|---|
| Range (mg/mL) | 0.05–1 |
| Correlation coefficient (r2) | 0.999 |
| Carry-over | n.d. |
| LOD (mg/mL) | 0.005 |
| LOQ (mg/mL) | 0.01 |
| Recovery (0.5 mg/mL) | 0.477 |
| C.V. (%) | 0.42 |
| Cases | 50 mg/mL %AMP | p | 15 mg/mL %AMP | p |
|---|---|---|---|---|
| 1 | 88.1 ± 2.1 | ref | 98.6 ± 2.1 | ref |
| 2 | 85.3 ± 2.2 | 0.2 | 98.7 ± 2.3 | 0.9 |
| 3 | 69.9 ± 5.5 | 0.03 | 82.3 ± 1.3 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Martínez, L.; Castro-Balado, A.; Hermelo-Vidal, G.; Bandín-Vilar, E.; Varela-Rey, I.; Toja-Camba, F.J.; Rodríguez-Jato, T.; Novo-Veleiro, I.; Varela-García, P.M.; Zarra-Ferro, I.; et al. Ampicillin Stability in a Portable Elastomeric Infusion Pump: A Step Forward in Outpatient Parenteral Antimicrobial Therapy. Pharmaceutics 2023, 15, 2099. https://doi.org/10.3390/pharmaceutics15082099
Rodríguez-Martínez L, Castro-Balado A, Hermelo-Vidal G, Bandín-Vilar E, Varela-Rey I, Toja-Camba FJ, Rodríguez-Jato T, Novo-Veleiro I, Varela-García PM, Zarra-Ferro I, et al. Ampicillin Stability in a Portable Elastomeric Infusion Pump: A Step Forward in Outpatient Parenteral Antimicrobial Therapy. Pharmaceutics. 2023; 15(8):2099. https://doi.org/10.3390/pharmaceutics15082099
Chicago/Turabian StyleRodríguez-Martínez, Lorena, Ana Castro-Balado, Gonzalo Hermelo-Vidal, Enrique Bandín-Vilar, Iria Varela-Rey, Francisco José Toja-Camba, Teresa Rodríguez-Jato, Ignacio Novo-Veleiro, Pablo Manuel Varela-García, Irene Zarra-Ferro, and et al. 2023. "Ampicillin Stability in a Portable Elastomeric Infusion Pump: A Step Forward in Outpatient Parenteral Antimicrobial Therapy" Pharmaceutics 15, no. 8: 2099. https://doi.org/10.3390/pharmaceutics15082099