Targeted Doxorubicin-Loaded Dendronized Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Synthesis of PPI-N3 Dendron
2.3. Synthesis of Dendronized Gold Nanoparticles
2.4. Stimuli-Responsive DOX Release from AuNPs
2.5. In Vitro AuNP Experiments
3. Results and Discussion
3.1. Design and Synthesis of AuNP-Fab Bioconjugates
3.2. Design and Synthesis of Multifunctional AuNP-DOX/Fab
3.3. Characterization of Multifunctional AuNP-DOX/Fab
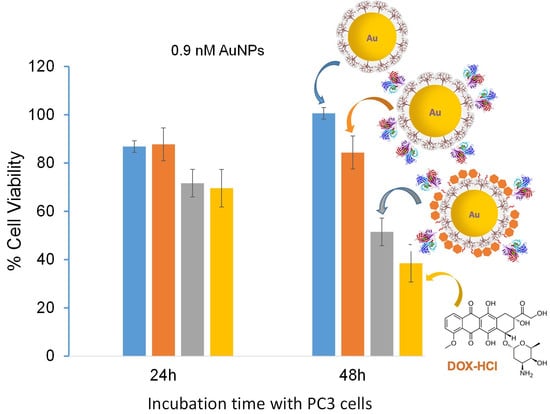
3.4. In Vitro Properties of Multifunctional AuNP-DOX/Fab
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villanueva-Flores, F.; Castro-Lugo, A.; Ramírez, O.T.; Palomares, L.A. Understanding cellular interactions with nanomaterials: Towards a rational design of medical nanodevices. Nanotechnology 2020, 31, 132002. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; FaroKhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef] [Green Version]
- Shan, X.; Gong, X.; Li, J.; Wen, J.; Li, Y.; Zhang, Z. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm. Sin. B 2022, 12, 3028–3048. [Google Scholar] [CrossRef] [PubMed]
- Rouge, J. RNA and nanocarriers: Next generation drug and delivery platform take center stage. Trends Biotechnol. 2023, 41, 281–282. [Google Scholar] [CrossRef]
- Seidi, F.; Jenjob, R.; Crespy, D. Designing Smart Polymer Conjugates for Controlled Release of Payloads. Chem. Rev. 2018, 118, 3965–4036. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Perez-Aranda, M.; Martinez, G.; Merinero, M.; Arguelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Ekladious, I.; Colson, Y.L.; Grinstaff, M.W. Polymer-drug conjugate therapeutics: Advances, insights and prospects. Nat. Rev. Drug Discov. 2019, 18, 273–294. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-based drug delivery systems: History, challenges, and latest developments. J. Biol. Eng. 2022, 16, 18. [Google Scholar] [CrossRef]
- Thakor, P.; Bhavana, V.; Sharma, R.; Srivastava, S.; Singh, S.B.; Mehra, N.K. Polymer–drug conjugates: Recent advances and future perspectives. Drug Discov. Today 2020, 25, 1718–1726. [Google Scholar] [CrossRef]
- Tomalia, D.A. Birth of a new macromolecular architecture: Dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog. Polym. Sci. 2005, 30, 294–324. [Google Scholar] [CrossRef]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Numai, S.; Yoto, R.; Kimura, M.; Simanek, E.E.; Kitano, Y. Click Chemistry of Melamine Dendrimers: Comparison of “Click-and-Grow” and “Grow-Then-Click” Strategies Using a Divergent Route to Diversity. Molecules 2023, 28, 131. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Simanek, E.E. Triazine dendrimers as drug delivery systems: From synthesis to therapy. Adv. Drug Deliv. Rev. 2012, 64, 826–835. [Google Scholar] [CrossRef]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Guan, B.; Nham, K.; Hao, G.; Sun, X.; Simanek, E.E. Tumor uptake of triazine dendrimers decorated with four, sixteen, and sixty-four PSMA-targeted ligands: Passive versus active tumor targeting. Biomolecules 2019, 9, 421. [Google Scholar] [CrossRef] [Green Version]
- Falanga, A.; Del Genio, V.; Kaufman, E.A.; Zannella, C.; Franci, G.; Weck, M.; Galdiero, S. Engineering of Janus-like dendrimers with peptides derived from glycoproteins of herpes simplex virus type 1: Toward a versatile and novel antiviral platform. Int. J. Mol. Sci. 2021, 22, 6488. [Google Scholar] [CrossRef]
- Elbert, K.C.; Jishkariani, D.; Wu, Y.; Lee, J.D.; Donnio, B.; Murray, C.B. Design, Self-Assembly, and Switchable Wettability in Hydrophobic, Hydrophilic, and Janus Dendritic Ligand–Gold Nanoparticle Hybrid Materials. Chem. Mater. 2017, 29, 8737–8746. [Google Scholar] [CrossRef]
- Nabavifard, S.; Jalili, S.; Rahmati, F.; Vasseghian, Y.; Ali, G.A.M.; Agarwal, S.; Gupta, V.K. Application of Dendrimer/Gold Nanoparticles in Cancer Therapy: A Review. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4231–4244. [Google Scholar] [CrossRef]
- Saha Ray, A.; Ghann, W.E.; Tsoi, P.S.; Szychowski, B.; Dockery, L.T.; Pak, Y.J.; Li, W.; Kane, M.A.; Swaan, P.; Daniel, M.-C. Set of Highly Stable Amine- and Carboxylate-Terminated Dendronized Au Nanoparticles with Dense Coating and Nontoxic Mixed-Dendronized Form. Langmuir 2019, 35, 3391–3403. [Google Scholar] [CrossRef]
- Dockery, L.; Daniel, M.-C. Dendronized Systems for the Delivery of Chemotherapeutics. Adv. Cancer Res. 2018, 139, 85–120. [Google Scholar] [CrossRef] [PubMed]
- Enciso, A.E.; Doni, G.; Nifosì, R.; Palazzesi, F.; Gonzalez, R.; Ellsworth, A.A.; Coffer, J.L.; Walker, A.V.; Pavan, G.M.; Mohamed, A.A.; et al. Facile synthesis of stable, water soluble, dendron-coated gold nanoparticles. Nanoscale 2017, 9, 3128–3132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, C.; Xue, C.; Chang, D.; Xu, H.; Salena, B.J.; Li, Y.; Wu, Z.-S. Ribbon of DNA Lattice on Gold Nanoparticles for Selective Drug Delivery to Cancer Cells. Angew. Chem. Int. Ed. 2020, 59, 14584–14592. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Lubin, B.C.; Bazylevich, A.; Gellerman, G.; Shpilberg, O.; Luboshits, G.; Firer, M.A. Gold nanoparticles stabilize peptide-drug-conjugates for sustained targeted drug delivery to cancer cells. J. Nanobiotechnol. 2018, 16, 34. [Google Scholar] [CrossRef]
- Khutale, G.V.; Casey, A. Synthesis and characterization of a multifunctional gold-doxorubicin nanoparticle system for pH triggered intracellular anticancer drug release. Eur. J. Pharm. Biopharm. 2017, 119, 372–380. [Google Scholar] [CrossRef] [Green Version]
- De Souza, C.D.; Nogueira, B.R.; Rostelato, M.E.C.M. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Enustun, B.V.; Turkevich, J. Coagulation of Colloidal Gold. J. Am. Chem. Soc. 1963, 85, 3317–3328. [Google Scholar] [CrossRef]
- Fu, L.; Ke, H.-T. Nanomaterials incorporated ultrasound contrast agents for cancer theranostics. Cancer Biol. Med. 2016, 13, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Zhang, J.; Gao, J.; Zhang, Z.; Zhu, H.; Wang, D. Gold Nanoparticles in Cancer Theranostics. Front. Bioeng. Biotechnol. 2021, 9, 647905. [Google Scholar] [CrossRef] [PubMed]
- Love, C.S.; Ashworth, I.; Brennan, C.; Chechik, V.; Smith, D.K. Dendron-protected Au nanoparticles—Effect of dendritic structure on chemical stability. J. Colloid Interface Sci. 2006, 302, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.J.; Zangmeister, R.A.; MacCuspie, R.I.; Patri, A.K.; Hackley, V.A. Newkome-Type Dendron-Stabilized Gold Nanoparticles: Synthesis, Reactivity, and Stability. Chem. Mater. 2011, 23, 2665–2676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, H.; Grow, M.E.; Wilson, O.; Daniel, M.-C. A new poly(propylene imine) dendron as potential convenient building-block in the construction of multifunctional systems. Tetrahedron 2013, 69, 2799–2806. [Google Scholar] [CrossRef]
- Ghann, W.E.; Aras, O.; Fleiter, T.; Daniel, M.-C. Syntheses and Characterization of Lisinopril-Coated Gold Nanoparticles as Highly Stable Targeted CT Contrast Agents in Cardiovascular Diseases. Langmuir 2012, 28, 10398–10408. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Grow, M.E.; Pan, H.; Bednarek, M.; Ghann, W.E.; Zabetakis, K.; Cornish, J. Gold nanoparticle-cored poly(propyleneimine) dendrimers as a new platform for multifunctional drug delivery systems. N. J. Chem. 2011, 35, 2366–2374. [Google Scholar] [CrossRef]
- Dockery, L.; Zalesak-Kravec, S.; Kane, M.A.; Daniel, M.-C. Modular and efficient synthesis of a poly (propylene imine) (PPI) dendron applied to acid-sensitive doxorubicin conjugation. Tetrahedron 2022, 125, 133044. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Nady, S.; Shafaa, M.W.; Khalil, M.M. Radiation and chemotherapy variable response induced by tumor cell hypoxia: Impact of radiation dose, anticancer drug, and type of cancer. Radiat. Environ. Biophys. 2022, 61, 263–277. [Google Scholar] [CrossRef]
- Gomari, H.; Moghadam, M.F.; Soleimani, M.; Ghavami, M.; Khodashenas, S. Targeted delivery of doxorubicin to HER2 positive tumor models. Int. J. Nanomed. 2019, 14, 5679–5690. [Google Scholar] [CrossRef] [Green Version]
- Korga, A.; Ostrowska, M.; Iwan, M.; Herbet, M.; Dudka, J. Inhibition of glycolysis disrupts cellular antioxidant defense and sensitizes HepG2 cells to doxorubicin treatment. FEBS Open Bio 2019, 9, 959–972. [Google Scholar] [CrossRef] [Green Version]
- Aryal, S.; Grailer, J.J.; Pilla, S.; Steeber, D.A.; Gong, S. Doxorubicin conjugated gold nanoparticles as water-soluble and pH-responsive anticancer drug nanocarriers. J. Mater. Chem. 2009, 19, 7879–7884. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, F.; Han, M.; Wang, X.; Du, J.; Zhang, H.; Li, W. Co-delivery of 5-fluorodeoxyuridine and doxorubicin via gold nanoparticle equipped with affibody-DNA hybrid strands for targeted synergistic chemotherapy of HER2 overexpressing breast cancer. Sci. Rep. 2020, 10, 22015. [Google Scholar] [CrossRef]
- Faid, A.H.; Shouman, S.A.; Badr, Y.A.; Sharaky, M. Enhanced cytotoxic effect of doxorubicin conjugated gold nanoparticles on breast cancer model. BMC Chem. 2022, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Welser, K.; Perera, M.D.A.; Aylott, J.W.; Chan, W.C. A facile method to clickable sensing polymeric nanoparticles. Chem. Commun. 2009, 6601–6603. [Google Scholar] [CrossRef]
- Florinas, S.; Liu, M.; Fleming, R.; Van Vlerken-Ysla, L.; Ayriss, J.; Gilbreth, R.; Dimasi, N.; Gao, C.; Wu, H.; Xu, Z.-Q.; et al. A Nanoparticle Platform to Evaluate Bioconjugation and Receptor-Mediated Cell Uptake Using Cross-Linked Polyion Complex Micelles Bearing Antibody Fragments. Biomacromolecules 2016, 17, 1818–1833. [Google Scholar] [CrossRef]
- Chen, S.; Florinas, S.; Teitgen, A.; Xu, Z.-Q.; Gao, C.; Wu, H.; Kataoka, K.; Cabral, H.; Christie, R.J. Controlled Fab installation onto polymeric micelle nanoparticles for tuned bioactivity. Sci. Technol. Adv. Mater. 2017, 18, 666–680. [Google Scholar] [CrossRef] [Green Version]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV−Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Chen, C.G.; Nardi, A.N.; Giustini, M.; D’Abramo, M. Absorption behavior of doxorubicin hydrochloride in visible region in different environments: A combined experimental and computational study. Phys. Chem. Chem. Phys. 2022, 24, 12027–12035. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Jäättelä, M.; Liu, B. Lysosome as a Central Hub for Rewiring PH Homeostasis in Tumors. Cancers 2020, 12, 2437. [Google Scholar] [CrossRef]
- Sherlock, S.P.; Tabakman, S.M.; Xie, L.; Dai, H. Photothermally Enhanced Drug Delivery by Ultrasmall Multifunctional FeCo/Graphitic Shell Nanocrystals. ACS Nano 2011, 5, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wang, Y.-C.; Dou, S.; Xiong, M.-H.; Sun, T.-M.; Wang, J. Doxorubicin-Tethered Responsive Gold Nanoparticles Facilitate Intracellular Drug Delivery for Overcoming Multidrug Resistance in Cancer Cells. ACS Nano 2011, 5, 3679–3692. [Google Scholar] [CrossRef] [PubMed]
- Nizamov, T.R.; Garanina, A.S.; Grebennikov, I.S.; Zhironkina, O.A.; Strelkova, O.S.; Alieva, I.B.; Kireev, I.I.; Abakumov, M.A.; Savchenko, A.G.; Majouga, A.G. Effect of Iron Oxide Nanoparticle Shape on Doxorubicin Drug Delivery Toward LNCaP and PC-3 Cell Lines. Bionanoscience 2018, 8, 394–406. [Google Scholar] [CrossRef]
- Czupiel, P.; Delplace, V.; Shoichet, M. Nanoparticle delivery of a pH-sensitive prodrug of doxorubicin and a mitochondrial targeting VES-H8R8 synergistically kill multi-drug resistant breast cancer cells. Sci. Rep. 2020, 10, 8726. [Google Scholar] [CrossRef]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trabulo, S.; Aires, A.; Aicher, A.; Heeschen, C.; Cortajarena, A.L. Multifunctionalized iron oxide nanoparticles for selective targeting of pancreatic cancer cells. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1597–1605. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dockery, L.T.; Daniel, M.-C. Targeted Doxorubicin-Loaded Dendronized Gold Nanoparticles. Pharmaceutics 2023, 15, 2103. https://doi.org/10.3390/pharmaceutics15082103
Dockery LT, Daniel M-C. Targeted Doxorubicin-Loaded Dendronized Gold Nanoparticles. Pharmaceutics. 2023; 15(8):2103. https://doi.org/10.3390/pharmaceutics15082103
Chicago/Turabian StyleDockery, Lance T., and Marie-Christine Daniel. 2023. "Targeted Doxorubicin-Loaded Dendronized Gold Nanoparticles" Pharmaceutics 15, no. 8: 2103. https://doi.org/10.3390/pharmaceutics15082103
APA StyleDockery, L. T., & Daniel, M.-C. (2023). Targeted Doxorubicin-Loaded Dendronized Gold Nanoparticles. Pharmaceutics, 15(8), 2103. https://doi.org/10.3390/pharmaceutics15082103