Real-World Evidence of Factors Affecting Cannabidiol Exposure in Children with Drug-Resistant Developmental and Epileptic Encephalopathies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Effectiveness
2.2. Safety and Tolerability
2.3. Statistical Analysis
3. Results
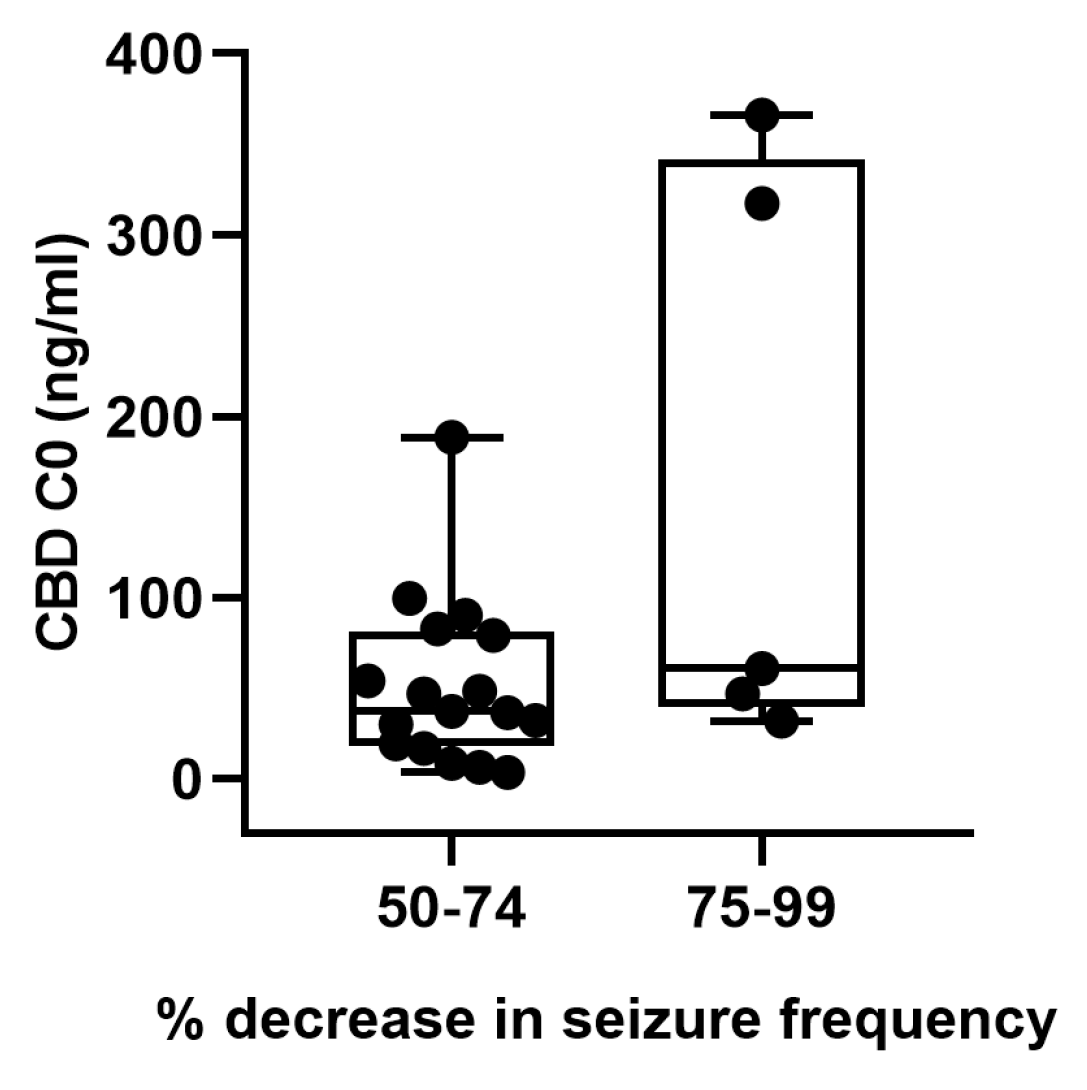
3.1. CBD Plasma Levels in Relation to Seizure Control
3.2. Safety and Tolerability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEDs | antiepileptic drugs |
| C0 | trough concentration |
| CBD | cannabidiol |
| CSWSS | continuous spikes and waves during slow sleep |
| D | dose |
| DS | Dravet syndrome |
| DEE | developmental and epileptic encephalopathies |
| LGS | Lennox Gastaut syndrome |
| Ln | natural logarithmic |
| MAE | myoclonic-atonic epilepsy |
| WS | West syndrome |
References
- Caraballo, R.; Demirdjian, G.; Reyes, G.; Huaman, M.; Gutierrez, R. Effectiveness of cannabidiol in a prospective cohort of children with drug-resistant epileptic encephalopathy in Argentina. Seizure 2020, 80, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, R.; Reyes, G.; Demirdjian, G.; Huaman, M.; Gutierrez, R. Long-term use of cannabidiol-enriched medical cannabis in a prospective cohort of children with drug-resistant developmental and epileptic encephalopathy. Seizure 2022, 95, 56–63. [Google Scholar] [CrossRef]
- Pamplona, F.A.; da Silva, L.R.; Coan, A.C. Potential Clinical Benefits of CBD-Rich Cannabis Extracts over Purified CBD in Treatment-Resistant Epilepsy: Observational Data Meta-analysis. Front. Neurol. 2018, 12, 759, Erratum in Front. Neurol. 2019, 10, 1050. [Google Scholar] [CrossRef] [Green Version]
- Perucca, E. Cannabinoids in the Treatment of Epilepsy: Hard Evidence at Last? J. Epilepsy Res. 2017, 7, 61–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Zeev, B. Medical Cannabis for Intractable Epilepsy in Childhood: A Review. Rambam Maimonides Med. J. 2020, 11, e0004. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.M.; Senn, L.; Anceschi, L.; Brighenti, V.; Pellati, F.; Biagini, G. Antiseizure Effects of Fully Characterized Non-Psychoactive Cannabis sativa, L. Extracts in the Repeated 6-Hz Corneal Stimulation Test. Pharmaceuticals 2021, 14, 1259. [Google Scholar] [CrossRef]
- Crockett, J.; Critchley, D.; Tayo, B.; Berwaerts, J.; Morrison, G. A phase 1, randomized, pharmacokinetic trial of the effect of different meal compositions, whole milk, and alcohol on cannabidiol exposure and safety in healthy subjects. Epilepsia 2020, 61, 267–277. [Google Scholar] [CrossRef]
- Taylor, L.; Gidal, B.; Blakey, G.; Tayo, B.; Morrison, G. A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects. CNS Drugs 2018, 32, 1053–1067. [Google Scholar] [CrossRef] [Green Version]
- Abbotts, K.S.S.; Ewell, T.R.; Butterklee, H.M.; Bomar, M.C.; Akagi, N.; Dooley, G.P.; Bell, C. Cannabidiol and Cannabidiol Metabolites: Pharmacokinetics, Interaction with Food, and Influence on Liver Function. Nutrients 2022, 14, 2152. [Google Scholar] [CrossRef]
- Birnbaum, A.K.; Karanam, A.; Marino, S.E.; Barkley, C.M.; Remmel, R.P.; Roslawski, M.; Gramling-Aden, M.; Leppik, I.E. Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy. Epilepsia 2019, 60, 1586–1592, Erratum in Epilepsia 2019, 60, 2009. [Google Scholar] [CrossRef]
- Perucca, E.; Bialer, M. Critical Aspects Affecting Cannabidiol Oral Bioavailability and Metabolic Elimination, and Related Clinical Implications. CNS Drugs 2020, 34, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Qin, C.; Abdelrazig, S.; Bai, Z.; Raji, M.; Darwish, R.; Chu, Y.; Ji, L.; Gray, D.A.; Stocks, M.J.; et al. Vegetable oils composition affects the intestinal lymphatic transport and systemic bioavailability of co-administered lipophilic drug cannabidiol. Int. J. Pharm. 2022, 624, 121947. [Google Scholar] [CrossRef]
- Charman, W.N.; Porter, C.J.; Mithani, S.; Dressman, J.B. Physiochemical physiological mechanisms for the effects of food on drug absorption: The role of lipids, p.H. J. Pharm Sci. 1997, 86, 269–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, V.; Gershkovich, P.; Perucca, E.; Bialer, M. The Interplay Between Liver First-Pass Effect and Lymphatic Absorption of Cannabidiol and Its Implications for Cannabidiol Oral Formulations. Clin. Pharmacokinet. 2020, 59, 1493–1500. [Google Scholar] [CrossRef]
- Jiang, R.; Yamaori, S.; Takeda, S.; Yamamoto, I.; Watanabe, K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011, 89, 165–170. [Google Scholar] [CrossRef]
- Huestis, M.A. Pharmacokinetics and metabolism of the plant cannabinoids, delta9-tetrahydrocannabinol, cannabidiol and cannabinol. Handb. Exp. Pharmacol. 2005, 168, 657–690. [Google Scholar] [CrossRef]
- Szaflarski, J.P.; Hernando, K.; Bebin, E.M.; Gaston, T.E.; Grayson, L.E.; Ampah, S.B.; Moreadith, R. Higher cannabidiol plasma levels are associated with better seizure response following treatment with a pharmaceutical grade cannabidiol. Epilepsy Behav. 2019, 95, 131–136. [Google Scholar] [CrossRef]
- Contin, M.; Mohamed, S.; Santucci, M.; Lodi, M.A.M.; Russo, E.; Mecarelli, O.; Cbd Lice Italy Study Group. Cannabidiol in Pharmacoresistant Epilepsy: Clinical Pharmacokinetic Data from an Expanded access Program. Front. Pharmacol. 2021, 12, 637801. [Google Scholar] [CrossRef]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077, Erratum in Epilepsia 2010, 51, 1922. [Google Scholar] [CrossRef]
- Pérez Montilla, C.A.; Schaiquevich, P.S.; Cáceres Guido, P.; Caraballo, R.H.; Reyes Valenzuela, G.; Cruz, C.V.; García Bournissen, F. An Ultrafast Ultrahigh-Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry Method for Cannabidiol Monitoring in Pediatric Refractory Epilepsy. Ther Drug Monit. 2021, 43, 712–717. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Clinical Investigation of Medicinal Products for the Treatment of Epileptic Disorders (Revision 2). 2018. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-epileptic-disorders-revision-2_en.pdf (accessed on 1 May 2023).
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef]
- Cáceres Guido, P.; Riva, N.; Caraballo, R.; Reyes, G.; Huaman, M.; Gutierrez, R.; Agostini, S.; Fabiana Delaven, S.; Pérez Montilla, C.A.; García Bournissen, F.; et al. Pharmacokinetics of cannabidiol in children with refractory epileptic encephalopathy. Epilepsia 2021, 62, e7–e12. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Halford, J.J.; Miller, I.; Nabbout, R.; Sanchez-Carpintero, R.; Shiloh-Malawsky, Y.; Wong, M.; Zolnowska, M.; Checketts, D.; Dunayevich, E.; et al. Add-on cannabidiol in patients with Dravet syndrome: Results of a long-term open-label extension trial. Epilepsia 2021, 62, 2505–2517. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, V.; García-Ron, A.; Smeyers, P.; Arias, E.; Soto, V.; García-Peñas, J.J.; González-Alguacil, E.; Sayas, D.; Serrano-Castro, P.; Garces, M.; et al. Outcomes from a Spanish Expanded Access Program on cannabidiol treatment in pediatric and adult patients with epilepsy. Epilepsy Behav. 2022, 137 Pt A, 108958. [Google Scholar] [CrossRef]
- Kühne, F.; Becker, L.L.; Bast, T.; Bertsche, A.; Borggraefe, I.; Boßelmann, C.M.; Fahrbach, J.; Hertzberg, C.; Herz, N.A.; Hirsch, M.; et al. Real-world data on cannabidiol treatment of various epilepsy subtypes: A retrospective, multicenter study. Epilepsia Open 2023, 8, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Wheless, J.W.; Dlugos, D.; Miller, I.; Oh, D.A.; Parikh, N.; Phillips, S.; Renfroe, J.B.; Roberts, C.M.; Saeed, I.; Sparagana, S.P.; et al. Study Investigators. Pharmacokinetics and Tolerability of Multiple Doses of Pharmaceutical-Grade Synthetic Cannabidiol in Pediatric Patients with Treatment-Resistant Epilepsy. CNS Drugs 2019, 33, 593–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devinsky, O.; Patel, A.D.; Thiele, E.A.; Wong, M.H.; Appleton, R.; Harden, C.L.; Greenwood, S.; Morrison, G.; Sommerville, K.; GWPCARE1 Part A Study Group. Randomized, dose ranging safety trial of cannabidiol in Dravet syndrome. Neurology 2018, 90, e1204–e1211. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.S.; Bourne, D.W.A.; Klawitter, J.; Sempio, C.; Chapman, K.; Knupp, K.; Wempe, M.F.; Borgelt, L.; Christians, U.; Leonard, J.; et al. Disposition of Oral Cannabidiol-Rich Cannabis Extracts in Children with Epilepsy. Clin. Pharmacokinet. 2020, 59, 1005–1012. [Google Scholar] [CrossRef]
- Kimura, T.; Higaki, K. Gastrointestinal transit and drug absorption. Biol. Pharm. Bull. 2002, 25, 149–164. [Google Scholar] [CrossRef] [Green Version]
- Prescott, L.F. Gastric emptying and drug absorption. Br. J. Clin. Pharmacol. 1974, 1, 189–190. [Google Scholar] [CrossRef] [Green Version]
- Kverneland, M.; Taubøll, E.; Selmer, K.K.; Iversen, P.O.; Nakken, K.O. Modified Atkins diet may reduce serum concentrations of antiepileptic drugs. Acta Neurol. Scand. 2015, 131, 187–190. [Google Scholar] [CrossRef]
- Heo, G.; Kim, S.H.; Chang, M.J. Effect of ketogenic diet and other dietary therapies on anti-epileptic drug concentrations in patients with epilepsy. J. Clin. Pharm. Ther. 2017, 42, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Zgair, A.; Wong, J.C.; Lee, J.B.; Mistry, J.; Sivak, O.; Wasan, K.M.; Hennig, I.M.; Barrett, D.A.; Constantinescu, C.S.; Fischer; et al. Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis-based medicines. Am. J. Transl. Res. 2016, 8, 3448–3459. [Google Scholar] [PubMed]
- Benito-Gallo, P.; Franceschetto, A.; Wong, J.C.; Marlow, M.; Zann, V.; Scholes, P.; Gershkovich, P. Chain length affects pancreatic lipase activity and the extent and pH-time profile of triglyceride lipolysis. Eur. J. Pharm. Biopharm. 2015, 93, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Hoffman, A. The effect of different lipid based formulations on the oral absorption of lipophilic drugs: The ability of in vitro lipolysis and consecutive ex vivo intestinal permeability data to predict in vivo bioavailability in rats. Eur. J. Pharm. Biopharm. 2007, 67, 96–105. [Google Scholar] [CrossRef]
- Porter, C.J.; Kaukonen, A.M.; Taillardat-Bertschinger, A.; Boyd, B.J.; O’Connor, J.M.; Edwards, G.A.; Charman, W.N. Use of in vitro lipid digestion data to explain the in vivo performance of triglyceride-based oral lipid formulations of poorly water-soluble drugs: Studies with halofantrine. J. Pharm. Sci. 2004, 93, 1110–1121. [Google Scholar] [CrossRef]
- Doohan, P.T.; Oldfield, L.D.; Arnold, J.C.; Anderson, L.L. Cannabinoid Interactions with Cytochrome P450 Drug Metabolism: A Full-Spectrum Characterization. AAPS J. 2021, 23, 91. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, P.N.; Szaflarski, J.P.; Gidal, B.; VanLandingham, K.; Critchley, D.; Morrison, G. Clinical implications of trials investigating drug-drug interactions between cannabidiol and enzyme inducers or inhibitors or common antiseizure drugs. Epilepsia 2020, 61, 1854–1868. [Google Scholar] [CrossRef]
- Yamaori, S.; Ebisawa, J.; Okushima, Y.; Yamamoto, I.; Watanabe, K. Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: Role of phenolic hydroxyl groups in the resorcinol moiety. Life Sci. 2011, 88, 730–736. [Google Scholar] [CrossRef]
- Takahashi, N.; Inui, N.; Morita, H.; Takeuchi, K.; Uchida, S.; Watanabe, H.; Nakamura, H. Effect of thyroid hormone on the activity of CYP3A enzyme in humans. J. Clin. Pharmacol. 2010, 50, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.W.; Du, Z.; Zhang, C.Z.; Zhang, W.H.; Cao, Y.F.; Sun, H.Z.; Zhu, Z.T.; Yang, K.; Liu, Y.Z.; Zhao, Z.W.; et al. The inhibition of UDP-glucuronosyltransferases (UGTs) by tetraiodothyronine (T4) and triiodothyronine (T3). Xenobiotica 2018, 48, 250–257. [Google Scholar] [CrossRef]
- Ilia, T.S.; Dragoumi, P.; Papanikolopoulou, S.; Goulis, D.G.; Pavlou, E.; Zafeiriou, D. Is the prevalence of thyroid disease higher in children receiving antiepileptic medication? A systematic review and meta-analysis. Seizure 2022, 94, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, H.H.; Barseem, N.F.; Suliman, H.A.; Talaat, E.; AlSHOKARY, A.H.; Abdelghani, W.E.; Abdulsamea, S.E.; Maksoud, Y.H.A.; Azab, S.M.; Elsadek, A.E.; et al. The Impact of Antiepileptic Drugs on Thyroid Function in Children with Epilepsy: New Versus Old. Iran J. Child. Neurol. 2020, 14, 31–41. [Google Scholar]
- Geffrey, A.L.; Pollack, S.F.; Bruno, P.L.; Thiele, E.A. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 2015, 56, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Gaston, T.E.; Bebin, E.M.; Cutter, G.R.; Liu, Y.; Szaflarski, J.P.; UAB CBD Program. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia 2017, 58, 1586–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsherbiny, M.A.; Li, C.G. Medicinal Cannabis-Potential Drug Interactions. Medicines 2018, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Morrison, G.; Crockett, J.; Blakey, G.; Sommerville, K. A Phase 1, Open-Label, Pharmacokinetic Trial to Investigate Possible Drug-Drug Interactions Between Clobazam, Stiripentol, or Valproate and Cannabidiol in Healthy Subjects. Clin. Pharmacol. Drug Dev. 2019, 8, 1009–1031. [Google Scholar] [CrossRef]
- Caraballo, R.H.; Flesler, S.; Reyes Valenzuela, G.; Fortini, S.; Chacón, S.; Ross, L.; Noli, D. Sulthiame add-on therapy in children with Lennox-Gastaut syndrome: A study of 44 patients. Seizure 2018, 62, 55–58. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Takahashi, Y.; Imai, K.; Mogami, Y.; Matsuda, K.; Nakai, M.; Kagawa, Y.; Inoue, Y. Interaction between sulthiame and clobazam: Sulthiame inhibits the metabolism of clobazam, possibly via an action on CYP2C19. Epilepsy Behav. 2014, 34, 124–126. [Google Scholar] [CrossRef]
- Miller, I.; Scheffer, I.E.; Gunning, B.; Sanchez-Carpintero, R.; Gil-Nagel, A.; Perry, M.S.; Saneto, R.P.; Checketts, D.; Dunayevich, E.; Knappertz, V.; et al. Dose-Ranging Effect of Adjunctive Oral Cannabidiol vs Placebo on Convulsive Seizure Frequency in Dravet Syndrome: A Randomized Clinical Trial. JAMA Neurol. 2020, 77, 613–621, Erratum in JAMA Neurol. 2020, 77, 655. [Google Scholar] [CrossRef] [Green Version]
- Iannone, L.F.; Arena, G.; Battaglia, D.; Bisulli, F.; Bonanni, P.; Boni, A.; Canevini, M.P.; Cantalupo, G.; Cesaroni, E.; Contin, M.; et al. Results from an Italian Expanded Access Program on Cannabidiol Treatment in Highly Refractory Dravet Syndrome and Lennox-Gastaut Syndrome. Front. Neurol. 2021, 12, 673135. [Google Scholar] [CrossRef] [PubMed]
- Knupp, K.G.; Rice, J.D.; Helmkamp, L.J.; Galinkin, J.; Sempio, C.; Jost, K.; Chapman, K.E. Prospective evaluation of oral cannabis extracts in children with epilepsy. Seizure 2019, 72, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [CrossRef]
- Calapai, F.; Cardia, L.; Sorbara, E.E.; Navarra, M.; Gangemi, S.; Calapai, G.; Mannucci, C. Cannabinoids, Blood-Brain Barrier, and Brain Disposition. Pharmaceutics 2020, 12, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brookes, A.; Jewell, A.; Feng, W.; Bradshaw, T.D.; Butler, J.; Gershkovich, P. Oral lipid-based formulations alter delivery of cannabidiol to different anatomical regions in the brain. Int. J. Pharm. 2023, 635, 122651. [Google Scholar] [CrossRef] [PubMed]

| Characteristic/Parameter | Results a |
|---|---|
| Age, years | 12.8 (4.7–17.2) |
| Sex, n | Female, 15 (68.2%); male: 7(31.8%) |
| Weight, kg | 35.0 (11.4–70.0) |
| Height, cm | 140.0 (104.0–158.0) |
| Epileptic syndrome (number of patients) | LGS (11), MAE (Doose syndrome) (5), CSWSS (2), WS (2), frontal epilepsy (1), and myoclonic epilepsy (1) |
| Concomitant drugs (number of patients) | Levetiracetam (20), Sulthiame (12), Clobazam (11), Topiramate (9), Levothyroxine (7), Zonisamide (7), Rufinamide (5), Valproic acid (5), Baclofen (4), Diazepam (4), Ethosuximide (3), Lamotrigine (2), Lorazepam (2), Lacosamide (2), Levomepromazine (2), Risperidone (2), Omeprazole (2), and Clonazepam (1) |
| CBD dose, mg/kg/day | 8.5 (2.6–22.5) |
| CBD C0, ng/mL | 48.2 (3.5–366.3) |
| CBD C0/D, (ng/mL)/(mg/kg/day) | 6.8 (0.4–24.2) |
| CBD duration treatment (days) | 749 (43–1224) |
| Variable | Estimate (β) | Standard Error | p-Value |
|---|---|---|---|
| Levothyroxine | 1.40 | 0.2485 | <0.01 |
| Sulthiame | 1.42 | 0.2598 | 0.02 |
| Clobazam | 1.42 | 0.3591 | 0.03 |
| Ketogenic diet | 1.51 | 0.2443 | <0.01 |
| Food administration | 1.34 | 0.5274 | 0.02 |
| Variable | Estimate (β) | Standard Error | p-Value |
|---|---|---|---|
| Intercept | −2.42 | 1.0779 | 0.03 |
| lnD | 1.15 | 0.2201 | <0.001 |
| Levothyroxine | 0.74 | 0.1649 | <0.001 |
| Food administration | 0.45 | 0.1550 | 0.009 |
| Ketogenic diet | 0.77 | 0.3141 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brstilo, L.; Reyes Valenzuela, G.; Caraballo, R.; Pérez Montilla, C.; García Bournissen, F.; Cáceres Guido, P.; Schaiquevich, P. Real-World Evidence of Factors Affecting Cannabidiol Exposure in Children with Drug-Resistant Developmental and Epileptic Encephalopathies. Pharmaceutics 2023, 15, 2120. https://doi.org/10.3390/pharmaceutics15082120
Brstilo L, Reyes Valenzuela G, Caraballo R, Pérez Montilla C, García Bournissen F, Cáceres Guido P, Schaiquevich P. Real-World Evidence of Factors Affecting Cannabidiol Exposure in Children with Drug-Resistant Developmental and Epileptic Encephalopathies. Pharmaceutics. 2023; 15(8):2120. https://doi.org/10.3390/pharmaceutics15082120
Chicago/Turabian StyleBrstilo, Lucas, Gabriela Reyes Valenzuela, Roberto Caraballo, Carlos Pérez Montilla, Facundo García Bournissen, Paulo Cáceres Guido, and Paula Schaiquevich. 2023. "Real-World Evidence of Factors Affecting Cannabidiol Exposure in Children with Drug-Resistant Developmental and Epileptic Encephalopathies" Pharmaceutics 15, no. 8: 2120. https://doi.org/10.3390/pharmaceutics15082120






