Cytotoxicity Analysis and In Silico Studies of Three Plant Extracts with Potential Application in Treatment of Endothelial Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Cytotoxicity
2.1.1. Cell Line, Cell Culture
2.1.2. Sample Solution Preparation from Plant Extracts
2.1.3. MTT Assay
2.2. In Vivo Cytotoxicity
2.3. In Silico Studies
2.3.1. Ligand Preparation
2.3.2. The Prediction of Diffusion across the Cell Membrane
2.3.3. Prediction of Skin Permeability, Subcellular Localization and Toxicity
2.3.4. Prediction of Biological Activity with PASS
2.3.5. Molecular Docking Simulations
2.4. Data Analysis
3. Results
3.1. In Vitro Cytotoxicity
3.2. In Vivo Cytotoxicity
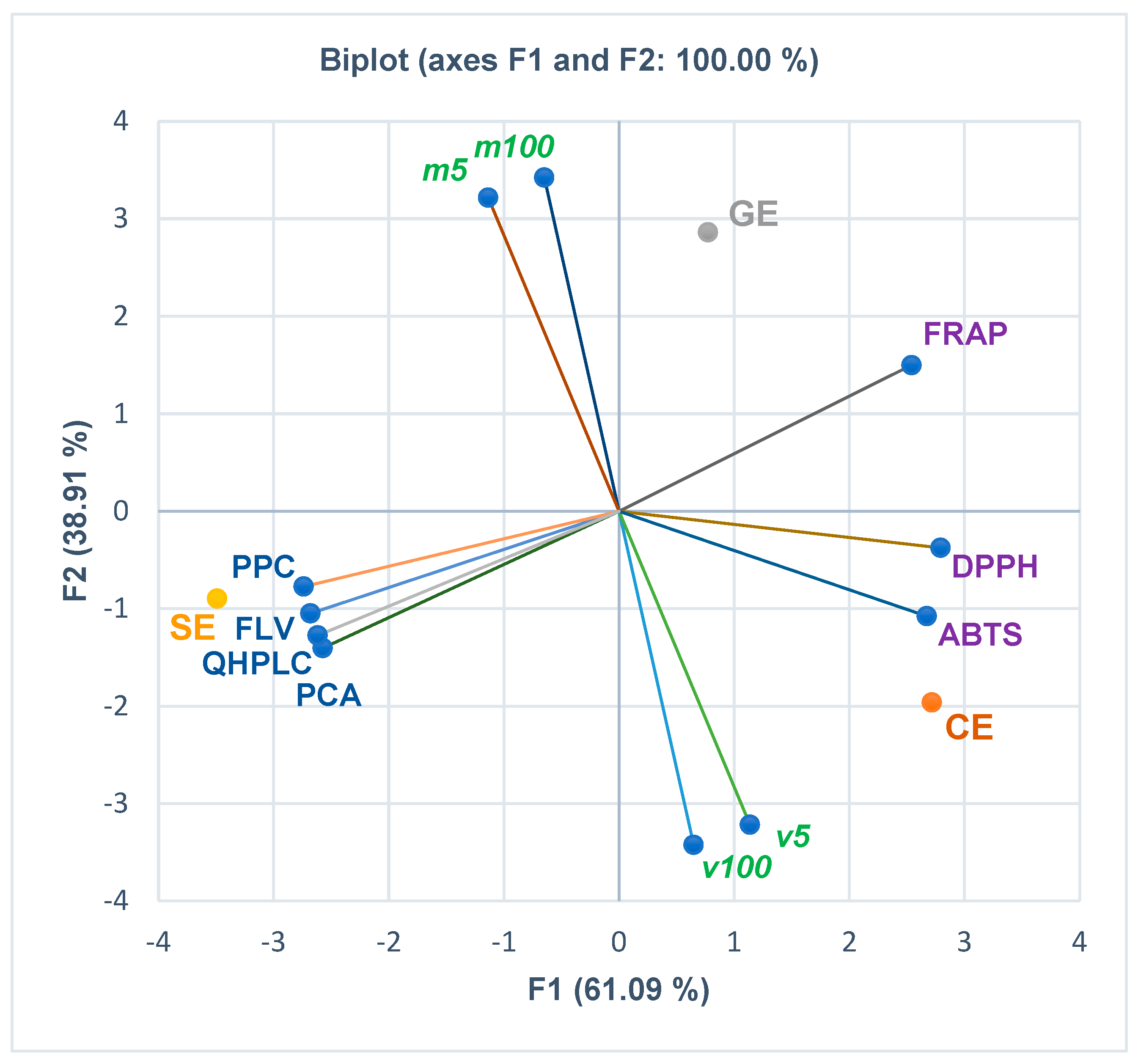
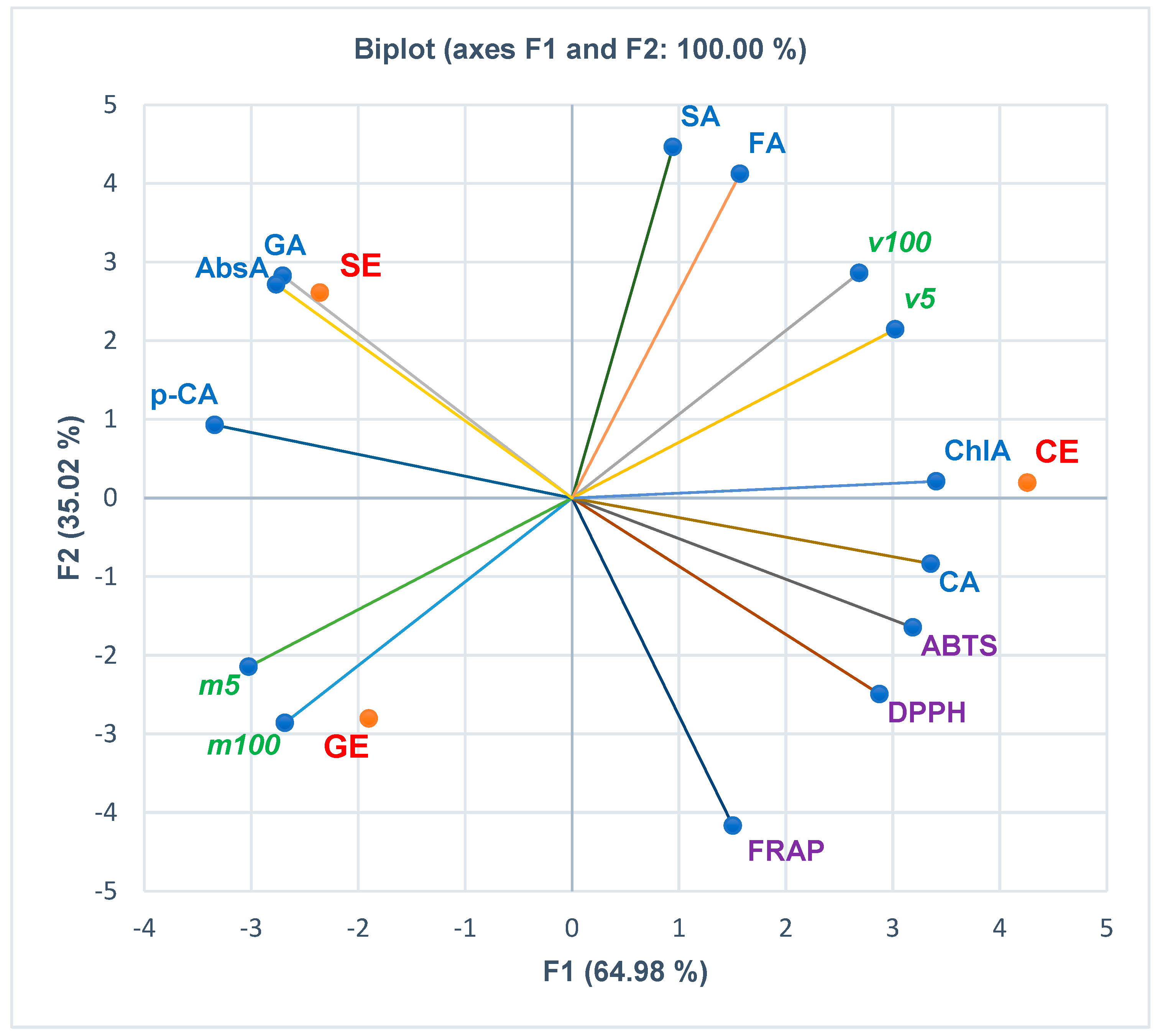
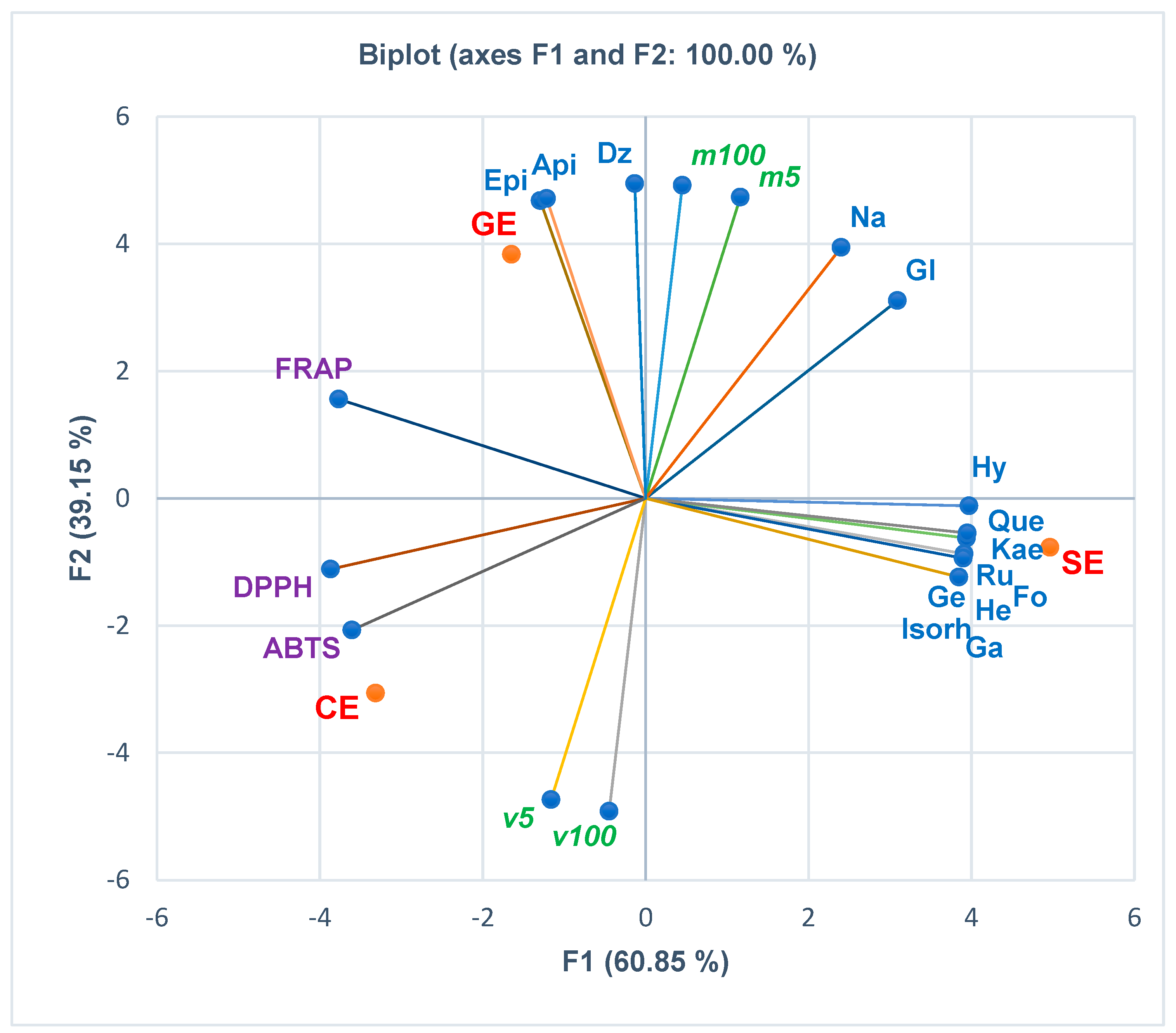
3.3. Principal Component Analysis
3.4. Computational Studies
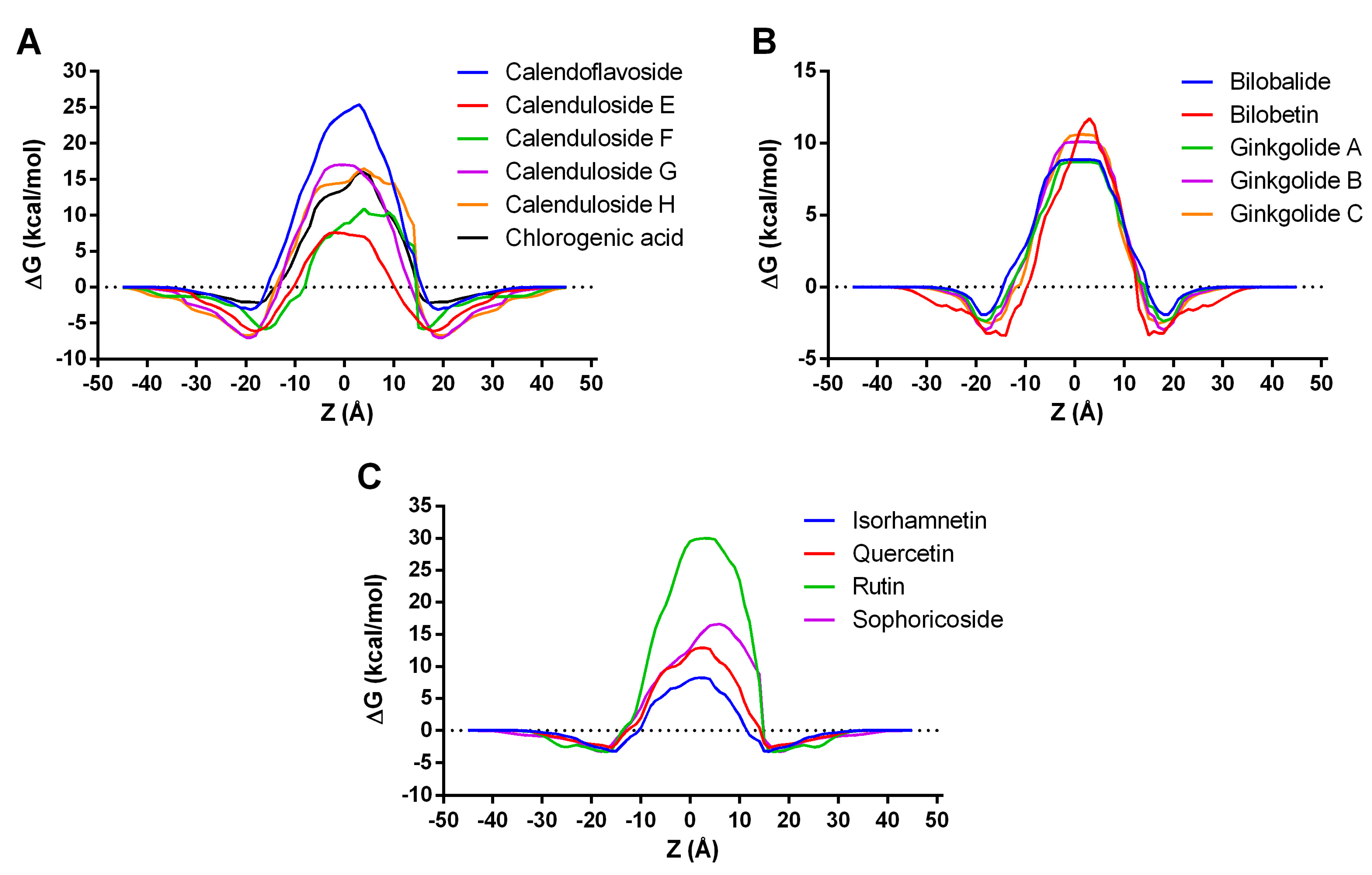
3.4.1. Permeability across Cell Membrane Prediction
3.4.2. Skin Permeability, Subcellular Localization, and Toxicity Prediction
3.4.3. PASS Activity Prediction
3.4.4. Molecular Docking Studies on Caspase-3, Caspase-8 and c-Myc
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fitridge, R.; Thompson, M. (Eds.) Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; University of Adelaide Press: Adelaide, Australia, 2011; pp. 423–471. ISBN 978-0-9871718-2-5. [Google Scholar]
- Hennigs, J.K.; Matuszcak, C.; Trepel, M.; Körbelin, J. Vascular Endothelial Cells: Heterogeneity and Targeting Approaches. Cells 2021, 10, 2712. [Google Scholar] [CrossRef] [PubMed]
- Lichota, A.; Gwozdzinski, L.; Gwozdzinski, K. Therapeutic potential of natural compounds in inflammation and chronic venous insufficiency. Eur. J. Med. Chem. 2019, 176, 68–91. [Google Scholar] [CrossRef] [PubMed]
- Castro-Ferreira, R.; Cardoso, R.; Leite-Moreira, A.; Mansilha, A. The Role of Endothelial Dysfunction and Inflammation in Chronic Venous Disease. Ann. Vasc. Surg. 2018, 46, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, A.R.; Chițescu, C.L.; Luță, E.A.; Moroșan, A.; Mihaiescu, D.E.; Mihai, D.P.; Costea, L.; Ozon, E.A.; Fița, A.C.; Balaci, T.D.; et al. Outlook on Chronic Venous Disease Treatment: Phytochemical Screening, In Vitro Antioxidant Activity and In Silico Studies for Three Vegetal Extracts. Molecules 2023, 28, 3668. [Google Scholar] [CrossRef] [PubMed]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Karampelas, O.; Musuc, A.M.; Atkinson, I.; et al. Evaluation of Usnea barbata (L.) Weber ex F.H. Wigg Extract in Canola Oil Loaded in Bioadhesive Oral Films for Potential Applications in Oral Cavity Infections and Malignancy. Antioxidants 2022, 11, 1601. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.-C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Sarbu, I.; Musuc, A.M.; Atkinson, I.; et al. Formulation and Development of Bioadhesive Oral Films Containing Usnea barbata (L.) F.H.Wigg Dry Ethanol Extract (F-UBE-HPC) with Antimicrobial and Anticancer Properties for Potential Use in Oral Cancer Complementary Therapy. Pharmaceutics 2022, 14, 1808. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Mitu, M.A.; Musuc, A.M.; Petrescu, S.; et al. Design, Characterization, and Anticancer and Antimicrobial Activities of Mucoadhesive Oral Patches Loaded with Usnea barbata (L.) F. H. Wigg Ethanol Extract F-UBE-HPMC. Antioxidants 2022, 11, 1801. [Google Scholar] [CrossRef]
- Popovici, V.; Musuc, A.M.; Matei, E.; Karampelas, O.; Ozon, E.A.; Cozaru, G.C.; Schröder, V.; Bucur, L.; Aricov, L.; Anastasescu, M.; et al. ROS-Induced DNA-Damage and Autophagy in Oral Squamous Cell Carcinoma by Usnea barbata Oil Extract—An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 14836. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; A Shoemaker, B.; A Thiessen, P.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Land, H.; Humble, M.S. YASARA: A tool to obtain structural guidance in biocatalytic investigations. In Protein Engineering; Bornscheuer, U.T., Höhne, M., Eds.; Springer: New York, NY, USA, 2018; pp. 43–67. [Google Scholar] [CrossRef]
- Lomize, A.L.; Hage, J.M.; Schnitzer, K.; Golobokov, K.; LaFaive, M.B.; Forsyth, A.C.; Pogozheva, I.D. PerMM: A Web Tool and Database for Analysis of Passive Membrane Permeability and Translocation Pathways of Bioactive Molecules. J. Chem. Inf. Model. 2019, 59, 3094–3099. [Google Scholar] [CrossRef]
- Mihai, D.P.; Boscencu, R.; Manda, G.; Burloiu, A.M.; Vasiliu, G.; Neagoe, I.V.; Socoteanu, R.P.; Lupuliasa, D. Interaction of Some Asymmetrical Porphyrins with U937 Cell Membranes–In Vitro and In Silico Studies. Molecules 2023, 28, 1640. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; O Eckert, A.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [Green Version]
- Mihai, D.P.; Trif, C.; Stancov, G.; Radulescu, D.; Nitulescu, G.M. Artificial Intelligence Algorithms for Discovering New Active Compounds Targeting TRPA1 Pain Receptors. Artif. Intell. 2020, 1, 276–285. [Google Scholar] [CrossRef]
- Filimonov, D.; Druzhilovskiy, D.; Lagunin, A.; Gloriozova, T.; Rudik, A.; Dmitriev, A.; Pogodin, P.; Poroikov, V. Computer-aided prediction of biological activity spectra for chemical compounds: Opportunities and limitation. Biomed. Chem. Res. Methods 2018, 1, e00004. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, H.; Kodandapani, L.; Mittl, P.R.; Di Marco, S.; Krebs, J.F.; Wu, J.C.; Tomaselli, K.J.; Grütter, M.G. The three-dimensional structure of caspase-8: An initiator enzyme in apoptosis. Structure 1999, 7, 1125–1133. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, H.; Donepudi, M.; Tschopp, M.; Kodandapani, L.; Wu, J.C.; Grütter, M.G. Caspase-8 specificity probed at subsite S4:crystal structure of the caspase-8-Z-DEVD-cho complex. J. Mol. Biol. 2000, 302, 9–16. [Google Scholar] [CrossRef]
- Nair, S.K.; Burley, S.K. X-Ray Structures of Myc-Max and Mad-Max Recognizing DNA: Molecular Bases of Regulation by Proto-Oncogenic Transcription Factors. Cell 2003, 112, 193–205. [Google Scholar] [CrossRef] [Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Zanfirescu, A.; Nitulescu, G.; Mihai, D.P.; Nitulescu, G.M. Identifying FAAH Inhibitors as New Therapeutic Options for the Treatment of Chronic Pain through Drug Repurposing. Pharmaceuticals 2021, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neagu, R.; Popovici, V.; Ionescu, L.E.; Ordeanu, V.; Popescu, D.M.; Ozon, E.A.; Gîrd, C.E. Antibacterial and Antibiofilm Effects of Different Samples of Five Commercially Available Essential Oils. Antibiotics 2023, 12, 1191. [Google Scholar] [CrossRef]
- Luță, E.-A.; Biță, A.; Moroșan, A.; Mihaiescu, D.E.; Mihai, D.P.; Popescu, L.; Bejenaru, L.E.; Bejenaru, C.; Popovici, V.; Olaru, O.T.; et al. Implications of the Cultivation of Rosemary and Thyme (Lamiaceae) in Plant Communities for the Development of Antioxidant Therapies. Int. J. Mol. Sci. 2023, 24, 11670. [Google Scholar] [CrossRef]
- Chavan, S.; Nicholls, I.A.; Karlsson, B.C.G.; Rosengren, A.M.; Ballabio, D.; Consonni, V.; Todeschini, R. Towards Global QSAR Model Building for Acute Toxicity: Munro Database Case Study. Int. J. Mol. Sci. 2014, 15, 18162–18174. [Google Scholar] [CrossRef] [Green Version]
- Okuyama, H.; Endo, H.; Akashika, T.; Kato, K.; Inoue, M. Downregulation of c-MYC Protein Levels Contributes to Cancer Cell Survival under Dual Deficiency of Oxygen and Glucose. Cancer Res 2010, 70, 10213–10223. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Bu, Y.; Li, B.; Zhang, H.; Guo, J.; Hu, J.; Zhang, Y. Calenduloside E alleviates cerebral ischemia/reperfusion injury by preserving mitochondrial function. Histochem. J. 2022, 53, 713–727. [Google Scholar] [CrossRef]
- Zhu, J.; Jin, Z.; Yang, L.; Zhao, C.; Hu, J.; Chen, J.; Han, Y.; Yu, P.; Luo, J.; Kong, L.; et al. Ginkgolide B targets and inhibits creatine kinase B to regulate the CCT/TRiC-SK1 axis and exerts pro-angiogenic activity in middle cerebral artery occlusion mice. Pharmacol. Res. 2022, 180, 106240. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-H.; Lee, S. Sophoricoside from Sophora japonica ameliorates allergic asthma by preventing mast cell activation and CD4+ T cell differentiation in ovalbumin-induced mice. Biomed. Pharmacother. 2021, 133, 111029. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, S.; Shang, H.; Wang, W.-Q.; Wang, B.-Q.; Zhang, X.; Xu, X.-D.; Sun, G.-B.; Sun, X.-B. The clickable activity-based probe of anti-apoptotic calenduloside E. Pharm. Biol. 2019, 57, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Di Pietro, N.; Baldassarre, M.P.A.; Cichelli, A.; Pandolfi, A.; Formoso, G.; Pipino, C. Role of Polyphenols and Carotenoids in Endothelial Dysfunction: An Overview from Classic to Innovative Biomarkers. Oxidative Med. Cell. Longev. 2020, 2020, 6381380. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, H.; He, Z. Recent advances in polyphenols improving vascular endothelial dysfunction induced by endogenous toxicity. J. Appl. Toxicol. 2021, 41, 701–712. [Google Scholar] [CrossRef]
- Chiu, Y.-L.; Tsai, W.-C.; Wu, C.-H.; Wu, C.-H.; Cheng, C.-C.; Lin, W.-S.; Tsai, T.-N.; Wu, L.-S. Ginkgo biloba Induces Thrombomodulin Expression and Tissue-Type Plasminogen Activator Secretion via the Activation of Krüppel-Like Factor 2 within Endothelial Cells. Am. J. Chin. Med. 2020, 48, 357–372. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y. Ginkgolide A Participates in LPS-Induced PMVEC Injury by Regulating miR-224 and Inhibiting p21 in a Targeted Manner. Contrast Media Mol. Imaging 2022, 2022, 6384334. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Yang, X.; Zhang, L.; Ansari, I.A.; Khan, M.S.; Han, S.; Feng, Y. Ginkgolide B ameliorates oxidized low-density lipoprotein-induced endothelial dysfunction via modulating Lectin-like ox-LDL-receptor-1 and NADPH oxidase 4 expression and inflammatory cascades. Phytother. Res. 2018, 32, 2417–2427. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, J.; Chen, B.; Zhao, Y.; Gong, H.; You, Y.; Qi, R. Ginkgolide B inhibits platelet and monocyte adhesion in TNFα-treated HUVECs under laminar shear stress. BMC Complement. Altern. Med. 2018, 18, 220. [Google Scholar] [CrossRef] [PubMed]
- Ugusman, A.; Zakaria, Z.; Chua, K.H.; Nordin, N.A.M.M.; Mahdy, Z.A. Role of Rutin on Nitric Oxide Synthesis in Human Umbilical Vein Endothelial Cells. Sci. World J. 2014, 2014, 169370. [Google Scholar] [CrossRef] [Green Version]
- Li, M.-T.; Ke, J.; Guo, S.-F.; Wu, Y.; Bian, Y.-F.; Shan, L.-L.; Liu, Q.-Y.; Huo, Y.-J.; Guo, C.; Liu, M.-Y.; et al. The Protective Effect of Quercetin on Endothelial Cells Injured by Hypoxia and Reoxygenation. Front. Pharmacol. 2021, 12, 732874. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Zhang, H.; Chen, D.; Chen, Y.; Lyu, Y.; Fang, L.; Du, G. Puerarin protects pulmonary arteries from hypoxic injury through the BMPRII and PPARγ signaling pathways in endothelial cells. Pharmacol. Rep. 2019, 71, 855–861. [Google Scholar] [CrossRef]
- Matsuo, M.; Sasaki, N.; Saga, K.; Kaneko, T. Cytotoxicity of Flavonoids toward Cultured Normal Human Cells. Biol. Pharm. Bull. 2005, 28, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Grollino, M.G.; Raschellà, G.; Cordelli, E.; Villani, P.; Pieraccioli, M.; Paximadas, I.; Malandrino, S.; Bonassi, S.; Pacchierotti, F. Cytotoxicity, genotoxicity and gene expression changes elicited by exposure of human hepatic cells to Ginkgo biloba leaf extract. Food Chem. Toxicol. 2017, 109, 486–496. [Google Scholar] [CrossRef]
- Feodorova, Y.; Tomova, T.; Minchev, D.; Turiyski, V.; Draganov, M.; Argirova, M. Cytotoxic effect of Ginkgo biloba kernel extract on HCT116 and A2058 cancer cell lines. Heliyon 2020, 6, e04941. [Google Scholar] [CrossRef] [PubMed]
- Alnuqaydan, A.M.; Lenehan, C.E.; Hughes, R.R.; Sanderson, B.J. Extracts from Calendula officinalis Offer in Vitro Protection against H2O2Induced Oxidative Stress Cell Killing of Human Skin Cells: Oxidative Stress Protection by Calendula officinalis Extract. Phytotherapy Res. 2015, 29, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Matysik, G.; Wójciak-Kosior, M.; Paduch, R. The influence of Calendulae officinalis flos extracts on cell cultures, and the chromatographic analysis of extracts. J. Pharm. Biomed. Anal. 2005, 38, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Yang, C.; Zhang, Y.; Liu, Y.; Yang, D. The Chemical Profiling and Anticancer Potential of Functional Polysaccharides from Flos Sophorae Immaturus. Molecules 2022, 27, 5978. [Google Scholar] [CrossRef]
- Li, L.; Huang, T.; Lan, C.; Ding, H.; Yan, C.; Dou, Y. Protective effect of polysaccharide from Sophora japonica L. flower buds against UVB radiation in a human keratinocyte cell line (HaCaT cells). J. Photochem. Photobiol. B Biol. 2019, 191, 135–142. [Google Scholar] [CrossRef]
- Dumitrascu, M. Artemia salina. Balneo Res. J. 2011, 2, 119–122. [Google Scholar] [CrossRef]
- Hamidi, M.R.; Jovanova, B.; Panovska, T.K. Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced. Pharm. Bull. 2014, 60, 9–18. [Google Scholar] [CrossRef]
- Campbell, D.L.; Lawton, L.A.; Beattie, K.A.; Codd, G.A. Comparative assessment of the specificity of the brine shrimp and microtox assays to hepatotoxic (microcystin-LR-containing) cyanobacteria. Environ. Toxicol. Water Qual. 1994, 9, 71–77. [Google Scholar] [CrossRef]
- Pop, A.L.; Mititelu, M. Scientific Partner: University of Medicine and Pharmacy’ Carol Davila’ Organizer: MPR Agency—A Global Health PR Partner. Available online: https://nutriterra.org/wp-content/uploads/2021/01/E520-Nutrition-2020-Bucharest.pdf (accessed on 15 July 2023).
- Popovici, V.; Bucur, L.A.; Schröder, V.; Gherghel, D.; Mihai, C.T.; Caraiane, A.; Badea, F.C.; Vochița, G.; Badea, V. Evaluation of the Cytotoxic Activity of the Usnea barbata (L.) F. H. Wigg Dry Extract. Molecules 2020, 25, 1865. [Google Scholar] [CrossRef] [Green Version]
- Lima, W.G.; Santos, L.B.; Nizer, W.S.D.C.; Castilho, R.O.; Brito, J.C.M. Brine shrimp (Artemia salina Leach) as an alternative model for assessing the in vivo antioxidant activity of rutin. Braz. J. Health Pharm. 2022, 4, 39–44. [Google Scholar] [CrossRef]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, P.J.; Folb, P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Kusaczuk, M.; Krętowski, R.; Naumowicz, M.; Stypułkowska, A.; Cechowska-Pasko, M. A Preliminary Study of the Effect of Quercetin on Cytotoxicity, Apoptosis, and Stress Responses in Glioblastoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 1345. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Karač, I.; Sirovina, D.; Kukolj, M.; Kunštić, M.; Gajski, G.; Garaj-Vrhovac, V.; Štajcar, D. Chemotherapeutic potential of quercetin on human bladder cancer cells. J. Environ. Sci. Health Part A 2016, 51, 776–781. [Google Scholar] [CrossRef]
- Sak, K.; Lust, H.; Kase, M.; Jaal, J. Cytotoxic action of methylquercetins in human lung adenocarcinoma cells. Oncol. Lett. 2017, 15, 1973–1978. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S.; Somasagara, R.R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a Natural Flavonoid Interacts with DNA, Arrests Cell Cycle and Causes Tumor Regression by Activating Mitochondrial Pathway of Apoptosis. Sci. Rep. 2016, 6, 24049. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.-Y.; Cao, L.-F.; Wan, H.-X.; Zhang, M.-Y.; Cai, J.-Y.; Shen, L.-J.; Zhong, J.-H.; Zhong, H. Quercetin enhances adriamycin cytotoxicity through induction of apoptosis and regulation of mitogen-activated protein kinase/extracellular signal-regulated kinase/c-Jun N-terminal kinase signaling in multidrug-resistant leukemia K562 cells. Mol. Med. Rep. 2015, 11, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.-H. Ginkgolide B induces apoptosis and developmental injury in mouse embryonic stem cells and blastocysts. Hum. Reprod. 2006, 21, 2985–2995. [Google Scholar] [CrossRef] [Green Version]
- Amati, B.; Land, H. Myc—Max—Mad: A transcription factor network controlling cell cycle progression, differentiation and death. Curr. Opin. Genet. Dev. 1994, 4, 102–108. [Google Scholar] [CrossRef]
- Kiessling, A.; Sperl, B.; Hollis, A.; Eick, D.; Berg, T. Selective Inhibition of c-Myc/Max Dimerization and DNA Binding by Small Molecules. Chem. Biol. 2006, 13, 745–751. [Google Scholar] [CrossRef] [Green Version]
- Florea, V.; Bhagavatula, N.; Simovic, G.; Macedo, F.Y.; Fock, R.A.; Rodrigues, C.O. c-Myc Is Essential to Prevent Endothelial Pro-Inflammatory Senescent Phenotype. PLoS ONE 2013, 8, e73146. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Sample/ Control | Concentration | ||||
|---|---|---|---|---|---|
| 5 μg/mL | 10 μg/mL | 25 μg/mL | 50 μg/mL | 100 μg/mL | |
| CE | 80.49 ± 15.03 a | 74.62 ± 13.44 | 65.11 ± 6.70 b | 53.87 ± 11.69 | 52.02 ± 9.44 a, x |
| GE | 55.56 ± 11.08 | 51.63 ± 7.73 | 42.43 ± 6.55 b | 43.07 ± 11.74 | 39.71 ± 12.33 y |
| SE | 65.74 ± 7.48 | 61.74 ± 10.09 | 55.86 ± 2.34 | 48.94 ± 10.01 | 46.70 ± 15.09 z |
| DMSO 0.1% | 99.63 ± 12.08 x, y, z | ||||
| Sample | Concentration (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | |||||||||
| Concentration | 50 | 100 | 200 | 400 | 800 | 1200 | 1500–2400 | 2500–3400 | >3500 |
| BSL% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Calendulae flos extract | |||||||||
| Concentration | 50 | 100 | 200 | 400 | 800 | 1200 | 1590 | 2500 | - |
| BSL% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Ginkgo bilobae folium extract | |||||||||
| Concentration | 50 | 100 | 200 | 400 | 800 | 1200 | 1770 | 2800 | - |
| BSL% | 0 | 0 | 0 | 0 | 0 | 0 | 29 ± 5.25 a, x | 60 ± 10.70 a, y | - |
| Sophorae flos extract | |||||||||
| Concentration | 50 | 100 | 200 | 400 | 800 | 1200 | 1610 | 3300 | 5500 |
| BSL% | 0 | 0 | 0 | 0 | 0 | 0 | 9 ± 1.38 x | 11 ± 2.83 y | 14 ± 4.1 |
| Overview | |||||||||
| Plant Extract | Minimal lethal concentration tested | Maximal lethal concentration tested | BSL% at 1200 µg/mL | Observations | |||||
| Value (µg/mL) | BSL% | Value (µg/mL) | BSL% | ||||||
| CE | NA | 0 | 2500 | 0 | 0 | No toxic | |||
| GE | 1770 | 29 | 2800 | 60 | 0 | No toxic | |||
| SE | 1610 | 9 | 5500 | 14 | 0 | No toxic | |||
| Compound | ΔG (kcal/mol) | LogPerm |
|---|---|---|
| Bilobalide | −1.92 | −7.62 |
| Bilobetin | −3.60 | −9.09 |
| Calendoflavoside | −3.14 | −17.72 |
| Calenduloside E | −6.11 | −6.89 |
| Calenduloside F | −5.83 | −8.78 |
| Calenduloside G | −7.02 | −12.89 |
| Calenduloside H | −6.72 | −12.36 |
| Chlorogenic acid | −2.19 | −11.74 |
| Ginkgolide A | −2.38 | −7.54 |
| Ginkgolide B | −2.97 | −8.42 |
| Ginkgolide C | −2.55 | −8.65 |
| Isorhamnetin | −3.40 | −7.01 |
| Quercetin | −2.67 | −9.84 |
| Rutin | −3.30 | −18.13 |
| Sophoricoside | −3.31 | −12.26 |
| Property | BBD | BBT | CDE | CDF | GKA | GKB | GKC | IRT | QCT |
|---|---|---|---|---|---|---|---|---|---|
| Skin permeability (log Kp) | −2.759 | −2.735 | −2.735 | −2.735 | −2.762 | −2.737 | −2.735 | −2.735 | −2.735 |
| Subcellular localization | mit. | mit. | mit. | mit. | mit. | mit. | mit. | mit. | mit. |
| Mitochondrial toxicity | +(0.86) | +(0.63) | +(0.63) | −(0.61) | +(0.75) | +(0.80) | +(0.66) | +(0.65) | +(0.59) |
| Cytotoxicity | −(0.66) | −(0.93) | −(0.80) | −(0.81) | −(0.62) | −(0.64) | −(0.65) | −(0.95) | −(0.99) |
| Mutagenicity | +(0.50) | −(0.81) | −(0.93) | −(0.94) | −(0.53) | −(0.56) | −(0.63) | −(0.94) | +(0.51) |
| Carcinogenicity | −(0.64) | −(0.65) | −(0.58) | −(0.72) | −(0.65) | −(0.60) | −(0.55) | −(0.68) | +(0.68) |
| Immunotoxicity | +(0.55) | +(0.84) | +(0.99) | +(0.99) | +(0.96) | +(0.94) | +(0.77) | +(0.58) | −(0.87) |
| Hepatotoxicity | −(0.87) | −(0.8) | −(0.88) | −(0.94) | −(0.83) | −(0.80) | −(0.82) | −(0.72) | −(0.69) |
| Nephrotoxicity | +(0.80) | +(0.49) | −(0.74) | −(0.91) | +(0.76) | +(0.75) | +(0.88) | −(0.79) | −(0.82) |
| Respiratory toxicity | +(0.60) | −(0.56) | −(0.50) | −(0.53) | +(0.62) | +(0.53) | +(0.61) | −(0.57) | +(0.62) |
| Reproductive toxicity | +(0.67) | +(0.72) | +(0.93) | +(0.89) | +(0.67) | +(0.64) | −(0.54) | +(0.84) | +(0.77) |
| Skin sensitization | −(0.83) | −(0.95) | −(0.80) | −(0.91) | −(0.83) | −(0.81) | −(0.81) | −(0.92) | −(0.74) |
| Honeybee toxicity | −(0.89) | −(0.72) | −(0.79) | −(0.73) | −(0.81) | −(0.81) | −(0.65) | −(0.88) | −(0.87) |
| Crustacea toxicity | +(0.56) | −(0.58) | −(0.56) | −(0.65) | −(0.50) | −(0.52) | +(0.51) | −(0.50) | −(0.53) |
| Fish toxicity | +(0.83) | +(0.88) | +(0.99) | +(0.98) | +(0.92) | −(0.39) | −(0.39) | +(0.83) | +(0.91) |
| Rat LD50 (mg/kg) | 90 | 4000 | 1750 | n.d. | 500 | 500 | 500 | 5000 | 159 |
| Apoptosis Inducer | Caspase-3 Stimulant | Caspase-8 Stimulant | c-Myc Inhibitor | |||||
|---|---|---|---|---|---|---|---|---|
| Ligand | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi |
| Bilobalide | 0.416 | 0.066 | 0.257 | 0.222 | 0.386 | 0.061 | 0.680 | 0.003 |
| Bilobetin | 0.836 | 0.006 | 0.649 | 0.014 | 0.464 | 0.026 | 0.268 | 0.128 |
| Calenduloside E | 0.876 | 0.005 | 0.987 | 0.002 | 0.964 | 0.000 | 0.689 | 0.003 |
| Calenduloside F | 0.861 | 0.005 | 0.992 | 0.001 | 0.983 | 0.000 | 0.672 | 0.003 |
| Ginkgolide A | - | - | - | - | - | - | 0.608 | 0.004 |
| Ginkgolide B | - | - | - | - | - | - | 0.608 | 0.004 |
| Ginkgolide C | - | - | - | - | - | - | 0.515 | 0.012 |
| Isorhamnetin | 0.880 | 0.005 | 0.656 | 0.013 | 0.474 | 0.023 | 0.316 | 0.082 |
| Quercetin | 0.887 | 0.005 | 0.499 | 0.028 | 0.428 | 0.039 | 0.290 | 0.104 |
| Caspase-3 | Caspase-8 | c-Myc/Max | ||||
|---|---|---|---|---|---|---|
| Ligand | ΔG (kcal/mol) | LE | ΔG (kcal/mol) | LE | ΔG (kcal/mol) | LE |
| Bilobalide | −7.399 | 0.3217 | −7.850 | 0.3413 | −6.225 | 0.2707 |
| Bilobetin | −9.921 | 0.2420 | −10.145 | 0.2474 | −7.134 | 0.1740 |
| Calenduloside E | −11.182 | 0.2485 | −9.618 | 0.2137 | −6.258 | 0.1391 |
| Calenduloside F | −9.336 | 0.1667 | −10.467 | 0.1869 | −6.489 | 0.1159 |
| Ginkgolide A | −8.517 | 0.2937 | −8.126 | 0.2802 | −6.559 | 0.2262 |
| Ginkgolide B | −8.364 | 0.2788 | −7.802 | 0.2601 | −6.368 | 0.2123 |
| Ginkgolide C | −8.128 | 0.2622 | −8.031 | 0.2591 | −6.424 | 0.2072 |
| Isorhamnetin | −7.848 | 0.3412 | −7.745 | 0.3367 | −5.907 | 0.2568 |
| Quercetin | −7.828 | 0.3558 | −7.624 | 0.3465 | −5.816 | 0.2644 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, A.R.; Popovici, V.; Oprean, C.; Danciu, C.; Schröder, V.; Olaru, O.T.; Mihai, D.P.; Popescu, L.; Luță, E.-A.; Chițescu, C.L.; et al. Cytotoxicity Analysis and In Silico Studies of Three Plant Extracts with Potential Application in Treatment of Endothelial Dysfunction. Pharmaceutics 2023, 15, 2125. https://doi.org/10.3390/pharmaceutics15082125
Ungureanu AR, Popovici V, Oprean C, Danciu C, Schröder V, Olaru OT, Mihai DP, Popescu L, Luță E-A, Chițescu CL, et al. Cytotoxicity Analysis and In Silico Studies of Three Plant Extracts with Potential Application in Treatment of Endothelial Dysfunction. Pharmaceutics. 2023; 15(8):2125. https://doi.org/10.3390/pharmaceutics15082125
Chicago/Turabian StyleUngureanu, Andreea Roxana, Violeta Popovici, Camelia Oprean, Corina Danciu, Verginica Schröder, Octavian Tudorel Olaru, Dragoș Paul Mihai, Liliana Popescu, Emanuela-Alice Luță, Carmen Lidia Chițescu, and et al. 2023. "Cytotoxicity Analysis and In Silico Studies of Three Plant Extracts with Potential Application in Treatment of Endothelial Dysfunction" Pharmaceutics 15, no. 8: 2125. https://doi.org/10.3390/pharmaceutics15082125
APA StyleUngureanu, A. R., Popovici, V., Oprean, C., Danciu, C., Schröder, V., Olaru, O. T., Mihai, D. P., Popescu, L., Luță, E.-A., Chițescu, C. L., & Gîrd, C. E. (2023). Cytotoxicity Analysis and In Silico Studies of Three Plant Extracts with Potential Application in Treatment of Endothelial Dysfunction. Pharmaceutics, 15(8), 2125. https://doi.org/10.3390/pharmaceutics15082125













