Reversible and Non-Competitive Inhibition of Cyclooxygenase by Indobufen for Efficient Antiplatelet Action and Relief of Gastrointestinal Irritation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Reagents
2.3. Statistical Analysis and Software Setting
2.4. Study on the Antiplatelet Effects of Indobufen
2.4.1. AA-Induced Platelet Aggregation
2.4.2. ADP-Induced Platelet Aggregation
2.5. Study on the Anticoagulant Effects of Indobufen
- (1)
- The control group;
- (2)
- The high-dose indobufen group (40 mg/kg);
- (3)
- The low-dose indobufen group (20 mg/kg);
- (4)
- The positive drug dabigatran etexilate high-dose group (30 mg/kg);
- (5)
- The positive drug dabigatran etexilate low-dose group (22 mg/kg);
- (6)
- The positive drug rivaroxaban high-dose group (2 mg/kg);
- (7)
- The positive drug rivaroxaban low-dose group (1 mg/kg).
2.6. Study on the Adverse Stomach Reactions of Indobufen
- (1)
- The control group;
- (2)
- The low-dose indobufen group (20 mg/kg);
- (3)
- The medium-dose indobufen group (30 mg/kg);
- (4)
- The high-dose indobufen group (40 mg/kg);
- (5)
- The aspirin group (10.14 mg/kg).
2.7. Study on the Mechanism of Indobufen on Cyclooxygenase-1
2.7.1. Study on the Reversible Inhibition of Indobufen
2.7.2. Study on the Reversible Inhibition Type of Indobufen
2.8. Study Regarding the Site in COX-1 Interacting with Indobufen
- (1)
- An appropriate amount of 0.1 M pH 8.0 Tris-HCl buffer;
- (2)
- Phenol solution;
- (3)
- Hematin solution;
- (4)
- Arachidonic acid solution;
- (5)
- Indobufen solution;
- (6)
- Iron ion solution;
- (7)
- Magnesium ion solution;
- (8)
- EDTA solution;
- (9)
- Imidazole solution.
3. Results
3.1. Study of the Antiplatelet Effects of Indobufen
3.1.1. AA-Induced Platelet Aggregation
3.1.2. ADP-Induced Platelet Aggregation
3.2. Study on the Anticoagulant Effects of Indobufen
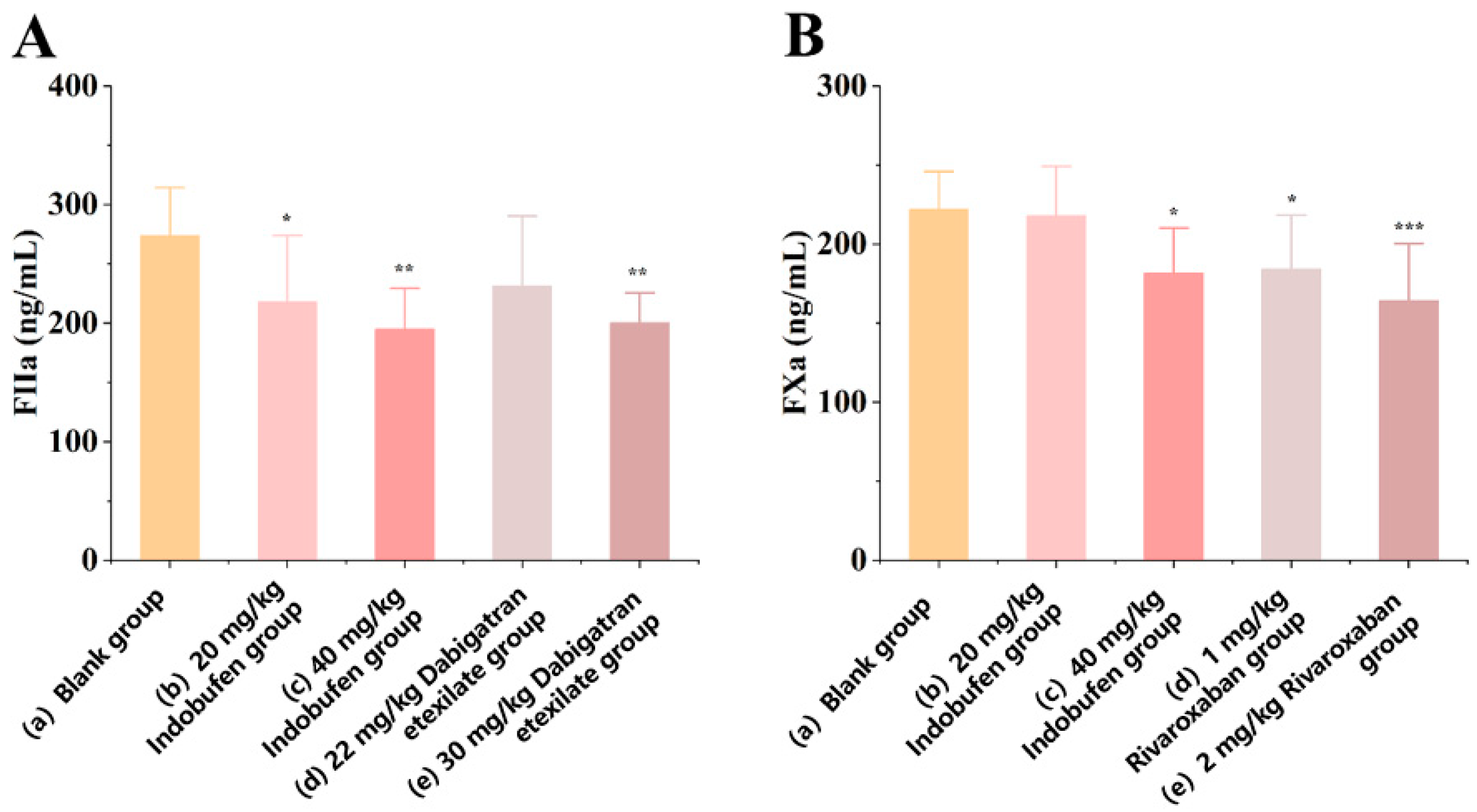
3.2.1. The Effect of Indobufen on the Level of Coagulation Factor IIa in Rats
3.2.2. The Effect of Indobufen on the Level of Coagulation Factor Xa in Rats
3.3. Study on the Adverse Stomach Reactions of Indobufen
3.3.1. The Effect of Indobufen on the Gastric Ulcer Index in Rats
3.3.2. The Effect of Indobufen on Gastric Mucosal Slices in Rats
3.3.3. The Effect of Indobufen on the Levels of TXB2 and 6-keto-PGF1α in Rat Gastric Mucosa
3.3.4. The Effect of Indobufen on the Levels of TXB2 and 6-keto-PGF1α in the Serum of Rats
3.4. Study on the Mechanism of Indobufen on Cyclooxygenase-1
3.4.1. Study on the Reversible Inhibition of Indobufen
3.4.2. Study on the Reversible Inhibition Type of Indobufen
3.5. Study on the Site in COX-1 Interacting with Indobufen
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhana, N.; McClellan, K.J. Indobufen: An updated review of its use in the management of atherothrombosis. Drugs Aging 2001, 18, 369–388. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N. Triggers, targets and treatments for thrombosis. Nature 2008, 451, 914–918. [Google Scholar] [CrossRef] [Green Version]
- Wiseman, L.R.; Fitton, A.; Buckley, M.M. Indobufen. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in cerebral, peripheral and coronary vascular disease. Drugs 1992, 44, 445–464. [Google Scholar] [CrossRef]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling During Platelet Adhesion and Activation. Arter. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, N.Z.; Gopinath, S.C. Potential blood clotting factors and anticoagulants. Biomed. Pharmacother. 2016, 84, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, D.; Xia, N.; Hou, K.; Chen, S.; Wang, Y.; Li, Y. Anticoagulant Activities of Indobufen, an Antiplatelet Drug. Molecules 2018, 23, 1452. [Google Scholar] [CrossRef] [Green Version]
- Vinazzer, H.; Fuccella, L.M.; Vinazzer, H.; Fuccella, L.M. Clinical Pharmacology Studies with Indobufen (K 3920): Inhibitor of Platelet Aggregation. J. Clin. Pharmacol. 1980, 20, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Ryu, R.; Jung, U.J.; Kim, H.-J.; Lee, W.; Bae, J.-S.; Park, Y.B.; Choi, M.-S. Anticoagulant and Antiplatelet Activities of Artemisia princeps Pampanini and Its Bioactive Components. Prev. Nutr. Food Sci. 2013, 18, 181–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuijt, T.J.; Bakhtiari, K.; Daffre, S.; DePonte, K.; Wielders, S.J.; Marquart, J.A.; Hovius, J.W.; van der Poll, T.; Fikrig, E.; Bunce, M.W.; et al. Factor Xa Activation of Factor V Is of Paramount Importance in Initiating the Coagulation System: Lessons from a tick salivary protein. Circulation 2013, 128, 254–266. [Google Scholar] [CrossRef] [Green Version]
- Eligini, S.; Violi, F.; Banfi, C.; Barbieri, S.S.; Brambilla, M.; Saliola, M.; Tremoli, E.; Colli, S. Indobufen inhibits tissue factor in human monocytes through a thromboxane-mediated mechanism. Cardiovasc. Res. 2006, 69, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ye, Z.; Mei, L.; Ullah, I.; Tan, C.; Wang, G.; Gu, Q.; Lu, Y.; Abdus, S.; Shi, L.; et al. Pharmacodynamic effects of indobufen compared with aspirin in patients with coronary atherosclerosis. Eur. J. Clin. Pharmacol. 2021, 77, 1815–1823. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Sung, K.-C.; Choi, H.-I. Comparison of aspirin and indobufen in healthy volunteers. Platelets 2016, 27, 105–109. [Google Scholar] [CrossRef]
- Rovelli, F.; Campolo, L.; Cataldo, G.; Pellegrini, A.; Mannucci, P.; Marubini, E.; Degaetano, G.; Orzan, F.; Lavezzari, M.; Petroccione, A.; et al. Indobufen versus aspirin plus dipyridamole after coronary-artery bypass-surgery. Coron. Artery Dis. 1991, 2, 897–906. [Google Scholar]
- Rajah, S.M.; Nair, U.; Rees, M.; Saunders, N.; Walker, D.; Williams, G.; Critchley, A.; Beton, D.; Campbell, C.; A Lawson, R. Effects of antiplatelet therapy with indobufen or aspirin-dipyridamole on graft patency one year after coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 1994, 107, 1146–1153. [Google Scholar] [CrossRef]
- Kulmacz, R.J.; Lands, W.E. Stoichiometry and kinetics of the interaction of prostaglandin H synthase with anti-inflammatory agents. J. Biol. Chem. 1985, 260, 12572–12578. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Bliden, K.P.; DiChiara, J.; Newcomer, J.; Weng, W.; Neerchal, N.K.; Gesheff, T.; Chaganti, S.K.; Etherington, A.; Tantry, U.S.; et al. Evaluation of Dose-Related Effects of Aspirin on Platelet Function: Results from the Aspirin-Induced Platelet Effect (ASPECT) study. Circulation 2007, 115, 3156–3164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, J.-K.; Jeon, H.-W.; Kang, M.-J. ADP-induced platelet aggregation in acute ischemic stroke patients on aspirin therapy. Eur. J. Neurol. 2008, 15, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, D.; Hou, K.; Gou, X.; Lv, N.; Fang, W.; Li, Y. Pretreatment of indobufen and aspirin and their combinations with clopidogrel or ticagrelor alleviates inflammasome mediated pyroptosis via inhibiting NF-κB/NLRP3 pathway in ischemic stroke. J. Neuroimmune Pharmacol. 2021, 16, 835–853. [Google Scholar] [CrossRef]
- Mitchell, J.A.; Warner, T.D. COX isoforms in the cardiovascular system: Understanding the activities of non-steroidal anti-inflammatory drugs. Nat. Rev. Drug Discov. 2006, 5, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K. Roles of Cyclooxygenase, Prostaglandin E2 and EP Receptors in Mucosal Protection and Ulcer Healing in the Gastrointestinal Tract. Curr. Pharm. Des. 2018, 24, 2002–2011. [Google Scholar] [CrossRef]
- Wallace, J.L. Prostaglandins, NSAIDs, and Gastric Mucosal Protection: Why Doesn’t the Stomach Digest Itself? Physiol. Rev. 2008, 88, 1547–1565. [Google Scholar] [CrossRef]
- Perez, R.A.; Fernandez-Alvarez, E.; Nieto, O.; Javier Piedrafita, F. Kinetics of the reversible tight-binding inhibition of pig liver catechol-O-methyltransferase by [2-(3,4-dihydroxy-2-nitrophenyl) vinyl] phenyl ketone. J. Enzym. Inhib. 1994, 8, 123–131. [Google Scholar] [CrossRef]
- Gierse, J.K.; Koboldt, C.M.; Walker, M.C.; Seibert, K.; Isakson, P.C. Kinetic basis for selective inhibition of cyclo-oxygenases. Biochem. J. 1999, 339, 607–614. [Google Scholar] [CrossRef]
- Tipton, K.F.; Davey, G.P.; McDonald, A.G. Kinetic behavior and reversible inhibition of monoamine oxidases—Enzymes that many want dead. Int. Rev. Neurobiol. 2011, 100, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Blat, Y. Non-Competitive Inhibition by Active Site Binders. Chem. Biol. Drug Des. 2010, 75, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.L.; Urade, Y.; Jakobsson, P.-J. Enzymes of the Cyclooxygenase Pathways of Prostanoid Biosynthesis. Chem. Rev. 2011, 111, 5821–5865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, D.; Gerrard, J. A hypothesis for the interaction of heme and arachidonic acid in the synthesis of prostaglandins. Med. Hypotheses 1979, 5, 683–693. [Google Scholar] [CrossRef]
- Peterson, D.; Gerrard, J.; Rao, G.; White, J. Inhibition of ferrous iron induced oxidation of arachidonic acid by indomethacin. Prostaglandins Med. 1979, 2, 97–108. [Google Scholar] [CrossRef]
- A Gum, P.; Kottke-Marchant, K.; A Welsh, P.; White, J.; Topol, E.J. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J. Am. Coll. Cardiol. 2003, 41, 961–965. [Google Scholar] [CrossRef] [Green Version]
- Mahmoodi, B.K.; Kate, M.K.T.; Waanders, F.; Veeger, N.J.; Brouwer, J.-L.P.; Vogt, L.; Navis, G.; van der Meer, J. High Absolute Risks and Predictors of Venous and Arterial Thromboembolic Events in Patients with Nephrotic Syndrome: Results from a large retrospective cohort study. Circulation 2008, 117, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Ji, C. Efficacy and safety of thrombolysis for acute ischemic stroke with atrial fibrillation: A meta-analysis. BMC Neurol. 2021, 21, 66. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xu, L.; Zhao, X.; Zhang, H.; Cheng, K.; Wang, X.; Chen, M.; Li, G.; Huang, J.; Lan, J.; et al. Indobufen or Aspirin on Top of Clopidogrel After Coronary Drug-Eluting Stent Implantation (OPTION): A Randomized, Open-Label, End Point–Blinded, Noninferiority Trial. Circulation 2023, 147, 212–222. [Google Scholar] [CrossRef]
- Yang, K.; Sun, J.F. Clinical observation of indobuprofen in the treatment of acute cerebral infarction. Chin. Med. 2012, 7, 144–145. [Google Scholar] [CrossRef]
- Douketis, J.D.; Spyropoulos, A.C.; Murad, M.H.; Arcelus, J.I.; Dager, W.E.; Dunn, A.S.; Fargo, R.A.; Levy, J.H.; Samama, C.M.; Shah, S.H.; et al. Perioperative Management of Antithrombotic Therapy: An American College of Chest Physicians Clinical Practice Guideline. Chest 2022, 162, e207–e243. [Google Scholar] [CrossRef] [PubMed]





| Component | Units | Concentration | ||
|---|---|---|---|---|
| (I) | (II) | (III) | ||
| Phenol | mM | 2 | 2 | 2 |
| Hematin | μM | 1 | 1 | 1 |
| Arachidonic acid | μM | 100 | 0.5, 1, 2, 4, 6, 8, 10 | 100 |
| COX-1 | U/mL | 0.625, 1.25, 2.5, 5, 7.5, 10, 12.5 | 5 | 2.5 |
| Indobufen | μM | 2, 5, 10 | 1, 2, 5 | 5 |
| Aspirin | μM | 2, 5, 10 | / | / |
| Ibuprofen | μM | 2, 5, 10 | 1, 2, 5 | / |
| pH 8.0 Tris-HCl | M | 0.1 | 0.1 | 0.1 |
| Iron ion | μM | / | / | 5, 40, 80 |
| Magnesium ion | μM | / | / | 5, 40, 80 |
| Imidazole | μM | / | / | 5, 40, 80 |
| EDTA | μM | / | / | 5, 40, 80 |
| Group | Concentration (μmol/L) | Max Aggregation (%) | Inhibition Rate (%) |
|---|---|---|---|
| Control | / | 52.23 ± 3.36 | / |
| Indobufen | 1 | 45.72 ± 1.64 | 12.48 |
| 2 | 40.17 ± 2.27 ** | 23.10 | |
| 4 | 25.08 ± 1.44 ***# | 51.98 | |
| 6 | 14.48 ± 1.01 ***+++ | 72.27 | |
| 8 | 5.43 ± 1.12 ***^^^ | 89.60 | |
| Aspirin | 10 | 44.73 ± 1.21 * | 14.36 |
| 20 | 40.28 ± 2.07 ** | 22.88 | |
| 40 | 29.48 ± 1.47 *** | 43.55 | |
| 80 | 21.22 ± 1.30 *** | 59.38 | |
| 100 | 14.90 ± 1.06 *** | 71.47 |
| Group | Concentration (mmol/L) | Max Aggregation (%) | Inhibition Rate (%) |
|---|---|---|---|
| Control | / | 32.17 ± 1.88 | / |
| Indobufen | 0.1 | 25.27 ± 1.48 ***# | 21.45 |
| 0.2 | 22.27 ± 1.28 ***# | 30.78 | |
| 0.4 | 18.18 ± 2.14 ***## | 43.47 | |
| 0.6 | 13.30 ± 2.37 *** | 59.48 | |
| 0.8 | 9.38 ± 0.91 ***## | 70.83 | |
| Ticlopidine | 0.1 | 27.98 ± 2.21 *** | 13.01 |
| 0.2 | 24.93 ± 1.98 *** | 21.30 | |
| 0.4 | 21.63 ± 1.40 *** | 32.75 | |
| 0.6 | 14.87 ± 1.78 *** | 53.78 | |
| 0.8 | 12.27 ± 1.46 *** | 61.87 |
| Number | Control Group | Indobufen Group | Aspirin Group 10.14 mg/kg | ||
|---|---|---|---|---|---|
| 20 mg/kg | 30 mg/kg | 40 mg/kg | |||
| 1 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 1 |
| 3 | 0 | 0 | 1 | 1 | 0 |
| 4 | 0 | 0 | 0 | 1 | 0 |
| 5 | 0 | 0 | 0 | 4 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 2 | 0 |
| 9 | 0 | 0 | 0 | 0 | 1 |
| 10 | 0 | 0 | 0 | 0 | 0 |
| Ulcer rate (%) | 0 | 0 | 10 | 40 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Sun, P.; Qi, X. Reversible and Non-Competitive Inhibition of Cyclooxygenase by Indobufen for Efficient Antiplatelet Action and Relief of Gastrointestinal Irritation. Pharmaceutics 2023, 15, 2135. https://doi.org/10.3390/pharmaceutics15082135
Liu J, Sun P, Qi X. Reversible and Non-Competitive Inhibition of Cyclooxygenase by Indobufen for Efficient Antiplatelet Action and Relief of Gastrointestinal Irritation. Pharmaceutics. 2023; 15(8):2135. https://doi.org/10.3390/pharmaceutics15082135
Chicago/Turabian StyleLiu, Jia, Peng Sun, and Xiaole Qi. 2023. "Reversible and Non-Competitive Inhibition of Cyclooxygenase by Indobufen for Efficient Antiplatelet Action and Relief of Gastrointestinal Irritation" Pharmaceutics 15, no. 8: 2135. https://doi.org/10.3390/pharmaceutics15082135
APA StyleLiu, J., Sun, P., & Qi, X. (2023). Reversible and Non-Competitive Inhibition of Cyclooxygenase by Indobufen for Efficient Antiplatelet Action and Relief of Gastrointestinal Irritation. Pharmaceutics, 15(8), 2135. https://doi.org/10.3390/pharmaceutics15082135







