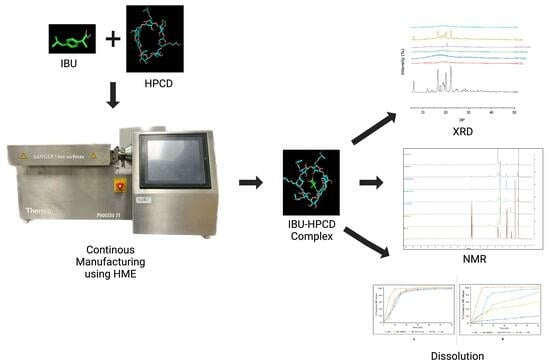
Continuous Manufacturing of Solvent-Free Cyclodextrin Inclusion Complexes for Enhanced Drug Solubility via Hot-Melt Extrusion: A Quality by Design Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. High-Performance Liquid Chromatography (HPLC) Method
2.3. Determining the Stoichiometry of the Binary Complex
2.3.1. Molecular Docking Studies
2.3.2. Phase Solubility Studies
2.4. Preparation of Samples
2.4.1. Ternary Inclusion Complex (IBU/HPβ-CD/PVP VA-64) Preparation Using QbD
Preliminary Studies
Box–Behnken Design (BBD)
2.4.2. Binary Inclusion Complex
2.4.3. IBU-PVP VA-64 Solid Dispersion
2.5. Saturation Solubility
2.6. Attenuated Total Reflection (ATR)
2.7. Differential Scanning Calorimetry (DSC)
2.8. X-ray Diffraction (XRD)
2.9. 1H Nuclear Magnetic Resonance (NMR)
2.10. Drug Content Uniformity
2.11. In Vitro Dissolution Studies
2.12. Stability Studies
3. Results and Discussion
3.1. Determining the Stoichiometry of the Binary Complex
3.1.1. Molecular Docking Studies
3.1.2. Phase Solubility Studies
3.2. QbD
3.2.1. Preliminary Studies
3.2.2. Formulation Development Using BBD
3.2.3. Effect of Independent Variables on IBU’s Solubility and Dissolution
3.2.4. Effect of Independent Factors on Saturation Solubility
3.2.5. Effect of Independent Factors on Dissolution
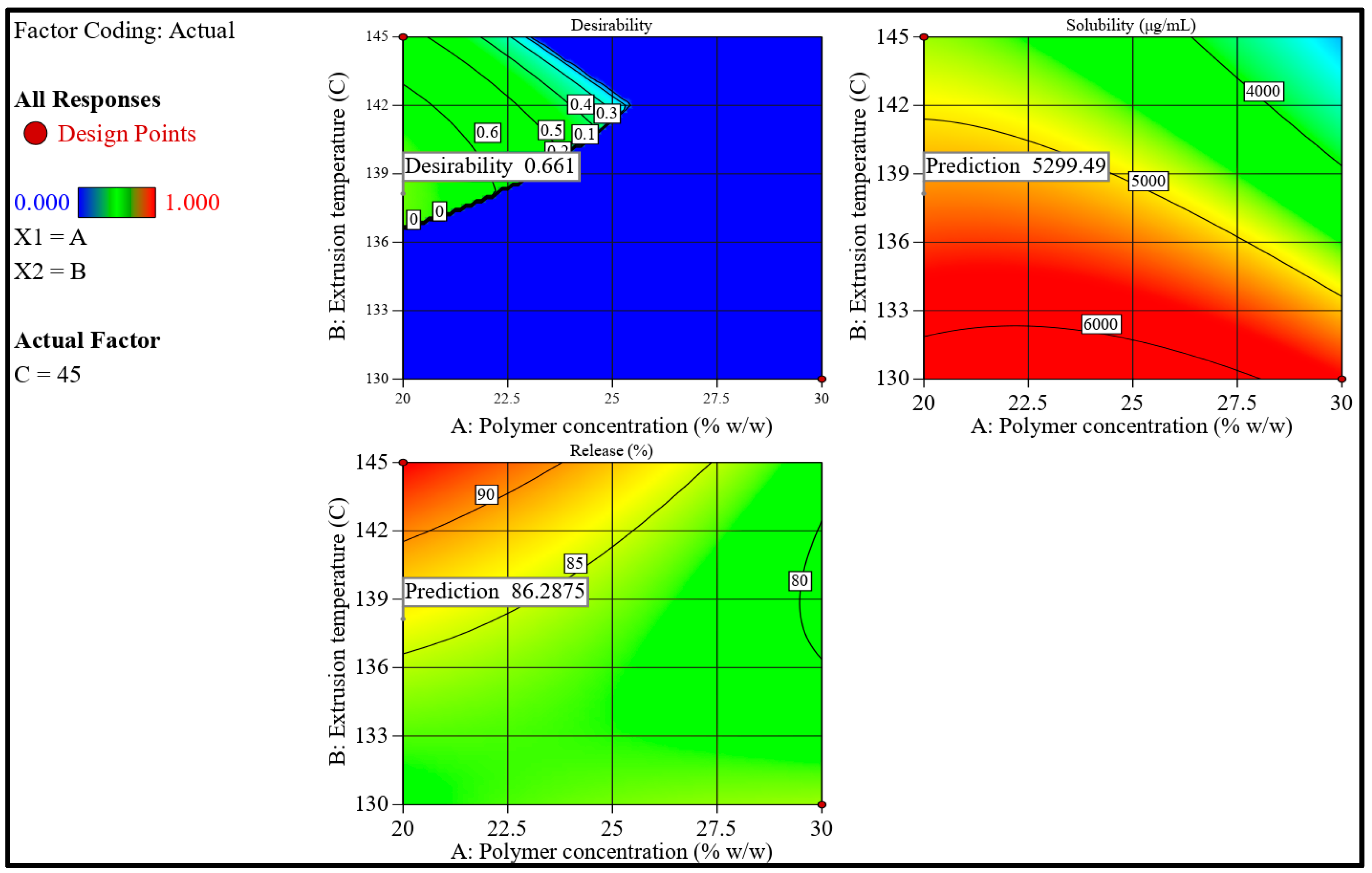
3.2.6. Optimization and Validation Trials
3.3. Saturation Solubility
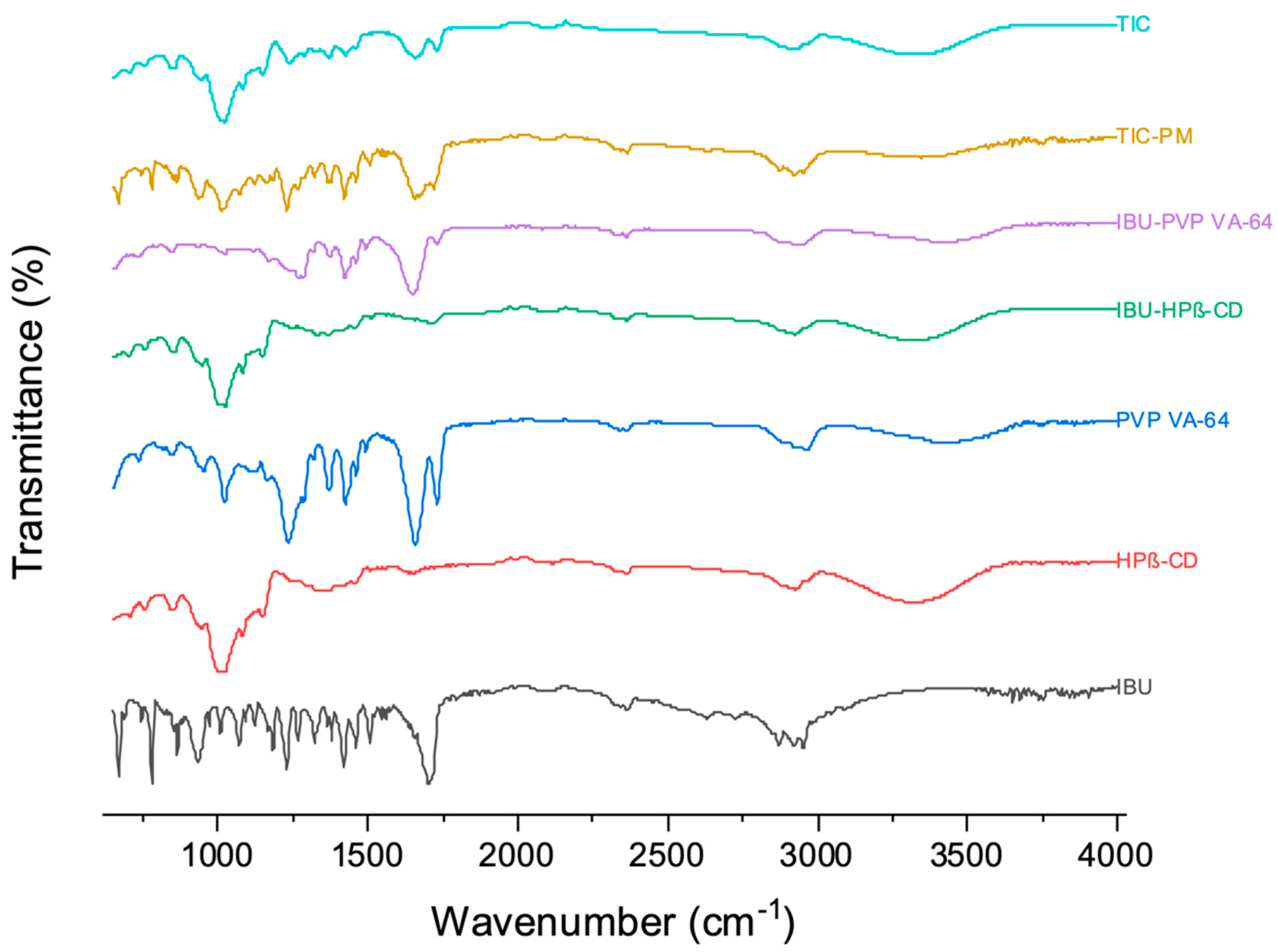
3.4. ATR
3.5. DSC
3.6. XRD
3.7. NMR
3.8. Drug Content Uniformity
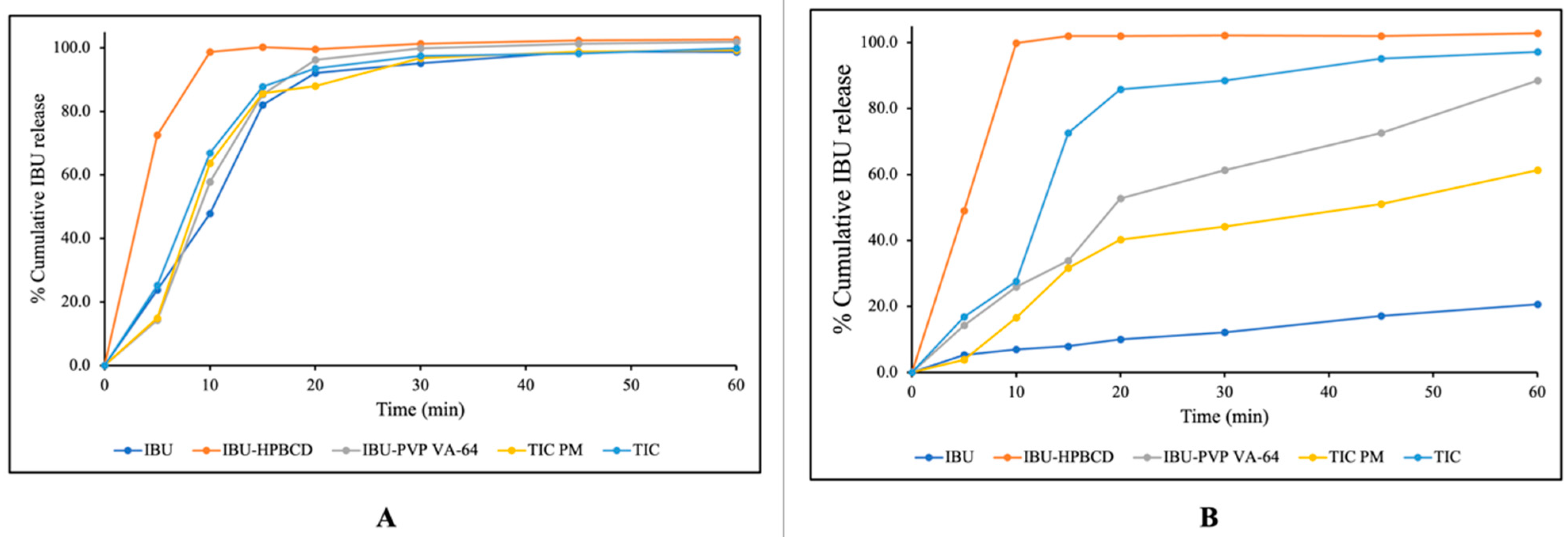
3.9. In Vitro Dissolution Studies
3.10. Stability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thiry, J.; Krier, F.; Ratwatte, S.; Thomassin, J.-M.; Jerome, C.; Evrard, B. Hot-Melt Extrusion as a Continuous Manufacturing Process to Form Ternary Cyclodextrin Inclusion Complexes. Eur. J. Pharm. Sci. 2017, 96, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Bergström, C.A.S.; Vinarov, Z.; Kuentz, M.; Brouwers, J.; Augustijns, P.; Brandl, M.; Bernkop-Schnürch, A.; Shrestha, N.; Préat, V.; et al. Successful Oral Delivery of Poorly Water-Soluble Drugs Both Depends on the Intraluminal Behavior of Drugs and of Appropriate Advanced Drug Delivery Systems. Eur. J. Pharm. Sci. 2019, 137, 104967. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.; Nyavanandi, D.; Mandati, P.; Youssef, A.A.A.; Narala, S.; Bandari, S.; Repka, M. A Systematic and Robust Assessment of Hot-Melt Extrusion-Based Amorphous Solid Dispersions: Theoretical Prediction to Practical Implementation. Int. J. Pharm. 2022, 624, 121951. [Google Scholar] [CrossRef] [PubMed]
- Munnangi, S.R.; Youssef, A.A.A.; Narala, N.; Lakkala, P.; Narala, S.; Vemula, S.K.; Repka, M. Drug Complexes: Perspective from Academic Research and Pharmaceutical Market. Pharm. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Aiassa, V.; Garnero, C.; Longhi, M.R.; Zoppi, A. Cyclodextrin Multicomponent Complexes: Pharmaceutical Applications. Pharmaceutics 2021, 13, 1099. [Google Scholar] [CrossRef]
- Almutairi, M.; Srinivasan, P.; Zhang, P.; Austin, F.; Butreddy, A.; Alharbi, M.; Bandari, S.; Ashour, E.A.; Repka, M.A. Hot-Melt Extrusion Coupled with Pressurized Carbon Dioxide for Enhanced Processability of Pharmaceutical Polymers and Drug Delivery Applications–An Integrated Review. Int. J. Pharm. 2022, 629, 122291. [Google Scholar] [CrossRef]
- Muankaew, C.; Loftsson, T. Cyclodextrin-Based Formulations: A Non-Invasive Platform for Targeted Drug Delivery. Basic Clin. Pharmacol. Toxicol. 2018, 122, 46–55. [Google Scholar] [CrossRef]
- Boczar, D.; Michalska, K. Cyclodextrin Inclusion Complexes with Antibiotics and Antibacterial Agents as Drug-Delivery Systems—A Pharmaceutical Perspective. Pharmaceutics 2022, 14, 1389. [Google Scholar] [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins Inclusion Complex: Preparation Methods, Analytical Techniques and Food Industry Applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef]
- Dodero, A.; Schlatter, G.; Hébraud, A.; Vicini, S.; Castellano, M. Polymer-Free Cyclodextrin and Natural Polymer-Cyclodextrin Electrospun Nanofibers: A Comprehensive Review on Current Applications and Future Perspectives. Carbohydr. Polym. 2021, 264, 118042. [Google Scholar] [CrossRef]
- Pereva, S.; Sarafska, T.; Bogdanova, S.; Spassov, T. Efficiency of “Cyclodextrin-Ibuprofen” Inclusion Complex Formation. J. Drug Deliv. Sci. Technol. 2016, 35, 34–39. [Google Scholar] [CrossRef]
- Granados, P.A.; Pinho, L.A.G.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. Application of Hot-Melt Extrusion in the Complexation of Naringenin with Cyclodextrin Using Hydrophilic Polymers. Adv. Powder Technol. 2022, 33, 103380. [Google Scholar] [CrossRef]
- Lima, S.G.B.; Pinho, L.A.G.; Sa-Barreto, L.L.; Gelfuso, G.M.; Gratieri, T.; Cunha-Filho, M. Granules of Finasteride and Cyclodextrin Obtained by Hot-Melt Extrusion to Target the Hair Follicles. Powder Technol. 2021, 391, 311–320. [Google Scholar] [CrossRef]
- Salave, S.; Prayag, K.; Rana, D.; Amate, P.; Pardhe, R.; Jadhav, A.; Jindal, A.B.; Benival, D. Recent Progress in Hot Melt Extrusion Technology in Pharmaceutical Dosage Form Design. Recent Adv. Drug Deliv. Formul. 2022, 16, 170–191. [Google Scholar] [CrossRef]
- Manne, A.S.N.; Hegde, A.R.; Raut, S.Y.; Rao, R.R.; Kulkarni, V.I.; Mutalik, S. Hot Liquid Extrusion Assisted Drug-Cyclodextrin Complexation: A Novel Continuous Manufacturing Method for Solubility and Bioavailability Enhancement of Drugs. Drug Deliv. Transl. Res. 2021, 11, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Folch-Cano, C.; Yazdani-Pedram, M.; Olea-Azar, C. Inclusion and Functionalization of Polymers with Cyclodextrins: Current Applications and Future Prospects. Molecules 2014, 19, 14066–14079. [Google Scholar] [CrossRef]
- Veeran, M.G.; Thomas, R.R.; Ramakrishnan, R.; Aprem, A.S. Quality-by-Design Approach for Optimization and Processing of PLGA Polymer Film by Hot Melt Extrusion. J. Pharm. Innov. 2022, 17, 1282–1294. [Google Scholar] [CrossRef]
- Pawar, J.; Suryawanshi, D.; Moravkar, K.; Aware, R.; Shetty, V.; Maniruzzaman, M.; Amin, P. Study the Influence of Formulation Process Parameters on Solubility and Dissolution Enhancement of Efavirenz Solid Solutions Prepared by Hot-Melt Extrusion: A QbD Methodology. Drug Deliv. Transl. Res. 2018, 8, 1644–1657. [Google Scholar] [CrossRef]
- Suryawanshi, D.; Wavhule, P.; Shinde, U.; Kamble, M.; Amin, P. Development, Optimization and in-Vivo Evaluation of Cyanocobalamin Loaded Orodispersible Films Using Hot-Melt Extrusion Technology: A Quality by Design (QbD) Approach. J. Drug Deliv. Sci. Technol. 2021, 63, 102559. [Google Scholar] [CrossRef]
- Das, S.K.; Kahali, N.; Bose, A.; Khanam, J. Physicochemical Characterization and in Vitro Dissolution Performance of Ibuprofen-Captisol® (Sulfobutylether Sodium Salt of β-CD) Inclusion Complexes. J. Mol. Liq. 2018, 261, 239–249. [Google Scholar] [CrossRef]
- Yani, Y.; Kanaujia, P.; Chow, P.S.; Tan, R.B.H. Effect of API-Polymer Miscibility and Interaction on the Stabilization of Amorphous Solid Dispersion: A Molecular Simulation Study. Ind. Eng. Chem. Res. 2017, 56, 12698–12707. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Nandi, U.; Scoutaris, N.; Sanfo, K.; Alexander, B.; Gong, Y.; Hui, H.-W.; Kumar, S.; Douroumis, D. Personalised Paediatric Chewable Ibuprofen Tablets Fabricated Using 3D Micro-Extrusion Printing Technology. Int. J. Pharm. 2022, 626, 122135. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Mohanty, S.; Maharana, J.; Jena, S.R.; Nayak, J.; Subuddhi, U. Microwave-Assisted β-Cyclodextrin/Chrysin Inclusion Complexation: An Economical and Green Strategy for Enhanced Hemocompatibility and Chemosensitivity in Vitro. J. Mol. Liq. 2020, 310, 113257. [Google Scholar] [CrossRef]
- Donthi, M.R.; Munnangi, S.R.; Krishna, K.V.; Marathe, S.A.; Saha, R.N.; Singhvi, G.; Dubey, S.K. Formulating Ternary Inclusion Complex of Sorafenib Tosylate Using β-Cyclodextrin and Hydrophilic Polymers: Physicochemical Characterization and In Vitro Assessment. AAPS PharmSciTech 2022, 23, 254. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken Design: An Alternative for the Optimization of Analytical Methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-W.; Sweeney, C.; Dudhipala, N.; Lakhani, P.; Chaurasiya, N.D.; Tekwani, B.L.; Majumdar, S. Primaquine Loaded Solid Lipid Nanoparticles (SLN), Nanostructured Lipid Carriers (NLC), and Nanoemulsion (NE): Effect of Lipid Matrix and Surfactant on Drug Entrapment, in Vitro Release, and Ex Vivo Hemolysis. AAPS PharmSciTech 2021, 22, 240. [Google Scholar] [CrossRef]
- Ray, R.; Maity, S.; Mandal, S.; Chatterjee, T.K.; Sa, B. Studies on the Release of Ibuprofen from Al3+ Ion Cross-Linked Homopolymeric and Interpenetrating Network Hydrogel Beads of Carboxymethyl Xanthan and Sodium Alginate. Adv. Polym. Technol. 2011, 30, 1–11. [Google Scholar] [CrossRef]
- Islam, M.S.; Renner, F.; Foster, K.; Oderinde, M.S.; Stefanski, K.; Mitra, S. Enhanced Aqueous Dissolution of Hydrophobic Apixaban via Direct Incorporation of Hydrophilic Nanographene Oxide. Colloids Surf. B Biointerfaces 2022, 216, 112512. [Google Scholar] [CrossRef]
- Patel, D.M.; Patel, S.P.; Patel, C.N. Formulation and Evaluation of Fast Dissolving Tablet Containing Domperidone Ternary Solid Dispersion. Int. J. Pharm. Investig. 2014, 4, 174–182. [Google Scholar] [CrossRef]
- Vemula, S.K.; Vangala, M. Formulation Development and Characterization of Meclizine Hydrochloride Sublimated Fast Dissolving Tablets. Int. Sch. Res. Not. 2014, 2014, 281376. [Google Scholar] [CrossRef] [PubMed]
- Karnik, I.; Youssef, A.A.A.; Joshi, P.; Munnangi, S.R.; Narala, S.; Varner, C.; Vemula, S.K.; Majumdar, S.; Repka, M. Formulation Development and Characterization of Dual Drug Loaded Hot-Melt Extruded Inserts for Better Ocular Therapeutic Outcomes: Sulfacetamide/Prednisolone. J. Drug Deliv. Sci. Technol. 2023, 84, 104558. [Google Scholar] [CrossRef]
- Dudhipala, N.; Ettireddy, S.; Youssef, A.A.A.; Puchchakayala, G. Cyclodextrin Complexed Lipid Nanoparticles of Irbesartan for Oral Applications: Design, Development, and In Vitro Characterization. Molecules 2021, 26, 7538. [Google Scholar] [CrossRef] [PubMed]
- Mady, F.M.; Aly, U.F. Experimental, Molecular Docking Investigations and Bioavailability Study on the Inclusion Complexes of Finasteride and Cyclodextrins. Drug Des. Dev. Ther. 2017, 11, 1681–1692. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, Physicochemical Properties and Pharmaceutical Applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. The Complexation Efficiency. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 545–552. [Google Scholar] [CrossRef]
- Koch, N.; Jennotte, O.; Gasparrini, Y.; Vandenbroucke, F.; Lechanteur, A.; Evrard, B. Cannabidiol Aqueous Solubility Enhancement: Comparison of Three Amorphous Formulations Strategies Using Different Type of Polymers. Int. J. Pharm. 2020, 589, 119812. [Google Scholar] [CrossRef]
- Gupta, S.S.; Meena, A.; Parikh, T.; Serajuddin, A.T. Investigation of Thermal and Viscoelastic Properties of Polymers Relevant to Hot Melt Extrusion-I: Polyvinylpyrrolidone and Related Polymers. J. Excip. Food Chem. 2016, 5, 1001. [Google Scholar]
- Krupa, A.; Majda, D.; Jachowicz, R.; Mozgawa, W. Solid-State Interaction of Ibuprofen and Neusilin US2. Thermochim. Acta 2010, 509, 12–17. [Google Scholar] [CrossRef]
- Ramukutty, S.; Ramachandran, E. Reaction Rate Models for the Thermal Decomposition of Ibuprofen Crystals. J. Cryst. Process Technol. 2014, 4, 71–78. [Google Scholar] [CrossRef]
- Thayumanasundaram, S.; Venkatesan, T.R.; Ousset, A.; Van Hollebeke, K.; Aerts, L.; Wübbenhorst, M.; Van den Mooter, G. Complementarity of MDSC, DMA, and DRS Techniques in the Study of Tg and Sub-Tg Transitions in Amorphous Solids: PVPVA, Indomethacin, and Amorphous Solid Dispersions Based on Indomethacin/PVPVA. Mol. Pharm. 2022, 19, 2299–2315. [Google Scholar] [CrossRef] [PubMed]
- Bolton, S.; Bor, S. Pharmaceutical Statistics: Practical and Clinical Applications, Revised and Expanded, 4th ed.; CRC Press: Boca Raton, FL, USA, 2003; ISBN 978-0-429-22338-9. [Google Scholar]
- Grymonpré, W.; Verstraete, G.; Vanhoorne, V.; Remon, J.P.; De Beer, T.; Vervaet, C. Downstream Processing from Melt Granulation towards Tablets: In-Depth Analysis of a Continuous Twin-Screw Melt Granulation Process Using Polymeric Binders. Eur. J. Pharm. Biopharm. 2018, 124, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Forster, S.P.; Dippold, E.; Chiang, T. Twin-Screw Melt Granulation for Oral Solid Pharmaceutical Products. Pharmaceutics 2021, 13, 665. [Google Scholar] [CrossRef]
- Munir, R.; Hadi, A.; Khan, S.-U.-D.; Asghar, S.; Irfan, M.; Khan, I.U.; Hameed, M.; Inam, S.; Islam, N.; Hassan, S.F.; et al. Solubility and Dissolution Enhancement of Dexibuprofen with Hydroxypropylbetacyclodextrin (HPβCD) and Poloxamers (188/407) Inclusion Complexes: Preparation and In Vitro Characterization. Polymers 2022, 14, 579. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, M.; Galai, H.; Amara, A.B.H.; Ben Rhaiem, H. Formation of Water Soluble and Stable Amorphous Ternary System: Ibuprofen/β-Cyclodextrin/PVP. Glass Phys. Chem. 2019, 45, 580–588. [Google Scholar] [CrossRef]
- Panda, D.S.; Alruwaili, N.K.; Pattnaik, S.; Swain, K. Ibuprofen Loaded Electrospun Polymeric Nanofibers: A Strategy to Improve Oral Absorption. Acta Chim. Slov. 2022, 69, 483–488. [Google Scholar] [CrossRef]
- Alves, T.F.R.; Barros, C.T.; Baldo, D.; Amaral, V.A.; Sever, M.; Santos, C.; Severino, P.; Chaud, M.V. Preparation, Characterization and Ex Vivo Intestinal Permeability Studies of Ibuprofen Solid Dispersion. J. Dispers. Sci. Technol. 2019, 40, 546–554. [Google Scholar] [CrossRef]
- Al-Qubaisi, M.S.; Rasedee, A.; Flaifel, M.H.; Eid, E.E.M.; Hussein-Al-Ali, S.; Alhassan, F.H.; Salih, A.M.; Hussein, M.Z.; Zainal, Z.; Sani, D.; et al. Characterization of Thymoquinone/Hydroxypropyl-β-Cyclodextrin Inclusion Complex: Application to Anti-Allergy Properties. Eur. J. Pharm. Sci. 2019, 133, 167–182. [Google Scholar] [CrossRef]
- Li, Y.; Lu, M.; Wu, C. PVP VA64 as a Novel Release-Modifier for Sustained-Release Mini-Matrices Prepared via Hot Melt Extrusion. Drug Deliv. Transl. Res. 2018, 8, 1670–1678. [Google Scholar] [CrossRef]
- Khan, I.A.; Anjum, K.; Koya, P.A.; Qadeer, A. Kabir-ud-Din Cloud Point, Fluorimetric and 1H NMR Studies of Ibuprofen-Polymer Systems. J. Mol. Struct. 2014, 1056–1057, 254–261. [Google Scholar] [CrossRef]
- El-Hinnawi, M.A.; Najib, N.M. Ibuprofen-Polyvinylpyrrolidone Dispersions. Proton Nuclear Magnetic Resonance and Infrared Studies. Int. J. Pharm. 1987, 37, 175–177. [Google Scholar] [CrossRef]
- Donthi, M.R.; Saha, R.N.; Singhvi, G.; Dubey, S.K. Dasatinib-Loaded Topical Nano-Emulgel for Rheumatoid Arthritis: Formulation Design and Optimization by QbD, In Vitro, Ex Vivo, and In Vivo Evaluation. Pharmaceutics 2023, 15, 736. [Google Scholar] [CrossRef] [PubMed]
- Macwan, J.S.; Fraczkiewicz, G.; Bertolino, M.; Krüger, P.; Peters, S.-A. Application of Physiologically Based Biopharmaceutics Modeling to Understand the Impact of Dissolution Differences on in Vivo Performance of Immediate Release Products: The Case of Bisoprolol. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Alqurshi, A.; Chan, K.L.A.; Royall, P.G. In-Situ Freeze-Drying-Forming Amorphous Solids Directly within Capsules: An Investigation of Dissolution Enhancement for a Poorly Soluble Drug. Sci. Rep. 2017, 7, 2910. [Google Scholar] [CrossRef]
- Vemula, S.K.; Daravath, B.; Repka, M. Quality by Design (QbD) Approach to Develop Fast-Dissolving Tablets Using Melt-Dispersion Paired with Surface-Adsorption Method: Formulation and Pharmacokinetics of Flurbiprofen Melt-Dispersion Granules. Drug Deliv. Transl. Res. 2023. [Google Scholar] [CrossRef]






| Independent Variables | Coded Levels | ||
|---|---|---|---|
| –1 | 0 | +1 | |
| PVP VA-64 (X1, % w/w) | 10 | 20 | 30 |
| Extrusion temperature (X2, °C) | 115 | 130 | 145 |
| Screw speed (X3, rpm) | 15 | 30 | 45 |
| Run * | Assigned Independent Variables | Actual Independent Variables | Response | |||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | PVP VA-64 (% w/w) | Extrusion Temp (°C) | Screw Speed (rpm) | Solubility (µg/mL) | Release after 30 min (%) | |
| 1 | 0 | 0 | 0 | 20 | 130 | 30 | 5131 | 78.1 |
| 2 | +1 | +1 | 0 | 30 | 145 | 30 | 2586 | 60 |
| 3 | 0 | 0 | 0 | 20 | 130 | 30 | 5132 | 78 |
| 4 | 0 | −1 | +1 | 20 | 115 | 45 | Extrusion Failure | |
| 5 | 0 | 0 | 0 | 20 | 130 | 30 | 5130 | 77.9 |
| 6 | −1 | 0 | −1 | 10 | 130 | 15 | 3238 | 87 |
| 7 | 0 | −1 | −1 | 20 | 115 | 15 | Extrusion Failure | |
| 8 | 0 | 0 | 0 | 20 | 130 | 30 | 5129 | 78.3 |
| 9 | 0 | 0 | 0 | 20 | 130 | 30 | 5133 | 77.8 |
| 10 | 0 | +1 | −1 | 20 | 145 | 15 | 4755 | 70 |
| 11 | 0 | +1 | +1 | 20 | 145 | 45 | 4723 | 95 |
| 12 | +1 | −1 | 0 | 30 | 115 | 30 | Extrusion Failure | |
| 13 | +1 | 0 | +1 | 30 | 130 | 45 | 5703 | 84 |
| 14 | −1 | −1 | 0 | 10 | 115 | 30 | 4071 | 85 |
| 15 | +1 | 0 | −1 | 30 | 130 | 15 | 3342 | 94 |
| 16 | −1 | 0 | +1 | 10 | 130 | 45 | 4297 | 78 |
| 17 | −1 | +1 | +1 | 10 | 145 | 30 | 3907 | 85 |
| Source | Solubility (X1, µg/mL) | Release after 30 min (X2, %) | ||||||
|---|---|---|---|---|---|---|---|---|
| Sum of Squares | DF | F-Value | p-Value | Sum of Squares | DF | F-Value | p-Value | |
| Model | 1.069 × 107 | 9 | 4.751 × 105 | <0.0001 | 1054.92 | 9 | 3167.91 | <0.0001 |
| A | 5.700 × 105 | 1 | 2.280 × 105 | <0.0001 | 42.25 | 1 | 1141.89 | <0.0001 |
| B | 1.673 × 106 | 1 | 6.690 × 105 | <0.0001 | 330.75 | 1 | 8939.19 | <0.0001 |
| C | 2.924 × 106 | 1 | 1.170 × 106 | <0.0001 | 90.25 | 1 | 2439.19 | <0.0001 |
| AB | 1.437 × 106 | 1 | 5.746 × 105 | <0.0001 | 330.75 | 1 | 8939.19 | <0.0001 |
| AC | 4.238 × 105 | 1 | 1.695 × 105 | <0.0001 | 0.2500 | 1 | 6.76 | 0.0601 |
| BC | 1.012 × 106 | 1 | 4.046 × 105 | <0.0001 | 396.75 | 1 | 10,722.9 | <0.0001 |
| A2 | 4.237 × 106 | 1 | 1.695 × 106 | <0.0001 | 3.55 | 1 | 96.05 | 0.0006 |
| B2 | 2.613 × 105 | 1 | 1.045 × 105 | <0.0001 | 149.75 | 1 | 4047.42 | <0.0001 |
| C2 | 1.769 × 105 | 1 | 70,770.28 | <0.0001 | 216.80 | 1 | 5859.33 | <0.0001 |
| Pure error | 10.00 | 4 | 2.50 | 0.1480 | 4 | 0.0370 | ||
| Cor total | 1.069 × 107 | 13 | 1055.06 | 13 | ||||
| Sum of squares | Type III Partial | Type III Partial | ||||||
| Model F-value | 475,081.48 | 3167.91 | ||||||
| R2 | 1.0 | 0.9999 | ||||||
| Adjusted R2 | 1.0 | 0.9995 | ||||||
| Adequate precision | 2332.5492 | 215.294 | ||||||
| Standard deviation | 1.58 | 0.1924 | ||||||
| Mean | 4448.36 | 80.58 | ||||||
| C.V. (%) | 0.0355 | 0.2387 | ||||||
| Variable | Goal | Lower Limit | Upper Limit |
|---|---|---|---|
| PVP VA-64 (X1, % w/w) | minimize | 20 | 30 |
| Extrusion temperature (X2, °C) | maximize | 130 | 145 |
| Screw speed (X3, rpm) | in range | 15 | 45 |
| Solubility (mg/mL) | maximize | 4500 | 6000 |
| Release after 30 min | in range | 85 | 100 |
| Trial No. | Kollidon VA 64 (% w/w) | Extrusion Temperature (°C) | Screw Speed (rpm) | Solubility (µg/mL) | Release after 30 min (%) | Desirability |
|---|---|---|---|---|---|---|
| 1 | 20.000 | 138.129 | 45.000 | 5299.706 | 86.286 | 0.661 |
| 2 | 20.002 | 137.500 | 45.000 | 5362.453 | 85.726 | 0.661 |
| 3 | 20.009 | 137.822 | 44.910 | 5324.795 | 85.871 | 0.660 |
| 4 | 20.000 | 138.077 | 44.862 | 5296.628 | 86.033 | 0.656 |
| 5 | 20.000 | 138.092 | 44.708 | 5286.053 | 85.821 | 0.647 |
| 6 | 20.822 | 138.373 | 45.000 | 5277.603 | 86.029 | 0.635 |
| 7 | 20.000 | 138.728 | 43.751 | 5172.099 | 85.000 | 0.632 |
| 8 | 20.987 | 137.242 | 44.970 | 5394.529 | 85.000 | 0.622 |
| 9 | 20.000 | 139.206 | 43.453 | 5113.537 | 85.000 | 0.606 |
| 10 | 20.771 | 144.523 | 45.000 | 4725.967 | 93.290 | 0.463 |
| 11 | 23.841 | 139.802 | 45.000 | 4967.657 | 85.000 | 0.271 |
| 12 | 24.210 | 142.726 | 45.000 | 4597.360 | 87.025 | 0.164 |
| 13 | 20.000 | 131.250 | 18.094 | 4579.473 | 85.000 | 0.164 |
| 14 | 20.160 | 131.232 | 18.153 | 4581.665 | 85.000 | 0.164 |
| 15 | 20.000 | 131.146 | 18.259 | 4586.201 | 85.000 | 0.163 |
| 16 | 20.216 | 131.200 | 18.214 | 4584.009 | 85.000 | 0.163 |
| 17 | 20.365 | 131.269 | 18.130 | 4579.487 | 85.000 | 0.161 |
| 18 | 20.454 | 131.175 | 18.299 | 4585.922 | 85.000 | 0.152 |
| 19 | 20.621 | 131.296 | 18.129 | 4576.392 | 85.000 | 0.150 |
| 20 | 20.234 | 130.759 | 18.933 | 4616.398 | 85.000 | 0.128 |
| 21 | 20.000 | 132.762 | 15.751 | 4509.555 | 85.000 | 0.111 |
| 22 | 20.000 | 130.023 | 20.078 | 4675.437 | 85.000 | 0.093 |
| Response | Software-Predicted Values | Standard Deviation | Standard Error | Observed Values | 95% CI Low for Mean | 95% CI High for Mean |
|---|---|---|---|---|---|---|
| Solubility (µg/mL) | 5299.71 | 1.58114 | 1.1503 | 5292.59 | 5287.59 | 5311.82 |
| Release after 30 min | 86.286 | 0.192354 | 0.13994 | 86.5 | 84.8117 | 87.7593 |
| Formulation | PD30 | IDR (%/min) | DE30 (%) | RDR30 |
|---|---|---|---|---|
| IBU | 12.10 | 0.40 | 7.88 | -- |
| TIC-PM | 44.20 | 1.47 | 26.07 | 3.65 |
| TIC | 88.50 | 2.95 | 55.68 | 7.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munnangi, S.R.; Youssef, A.A.A.; Narala, N.; Lakkala, P.; Vemula, S.K.; Alluri, R.; Zhang, F.; Repka, M.A. Continuous Manufacturing of Solvent-Free Cyclodextrin Inclusion Complexes for Enhanced Drug Solubility via Hot-Melt Extrusion: A Quality by Design Approach. Pharmaceutics 2023, 15, 2203. https://doi.org/10.3390/pharmaceutics15092203
Munnangi SR, Youssef AAA, Narala N, Lakkala P, Vemula SK, Alluri R, Zhang F, Repka MA. Continuous Manufacturing of Solvent-Free Cyclodextrin Inclusion Complexes for Enhanced Drug Solubility via Hot-Melt Extrusion: A Quality by Design Approach. Pharmaceutics. 2023; 15(9):2203. https://doi.org/10.3390/pharmaceutics15092203
Chicago/Turabian StyleMunnangi, Siva Ram, Ahmed Adel Ali Youssef, Nagarjuna Narala, Preethi Lakkala, Sateesh Kumar Vemula, Rohit Alluri, Feng Zhang, and Micheal A. Repka. 2023. "Continuous Manufacturing of Solvent-Free Cyclodextrin Inclusion Complexes for Enhanced Drug Solubility via Hot-Melt Extrusion: A Quality by Design Approach" Pharmaceutics 15, no. 9: 2203. https://doi.org/10.3390/pharmaceutics15092203