Influence of Manufacturing Process on the Microstructure, Stability, and Sensorial Properties of a Topical Ointment Formulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
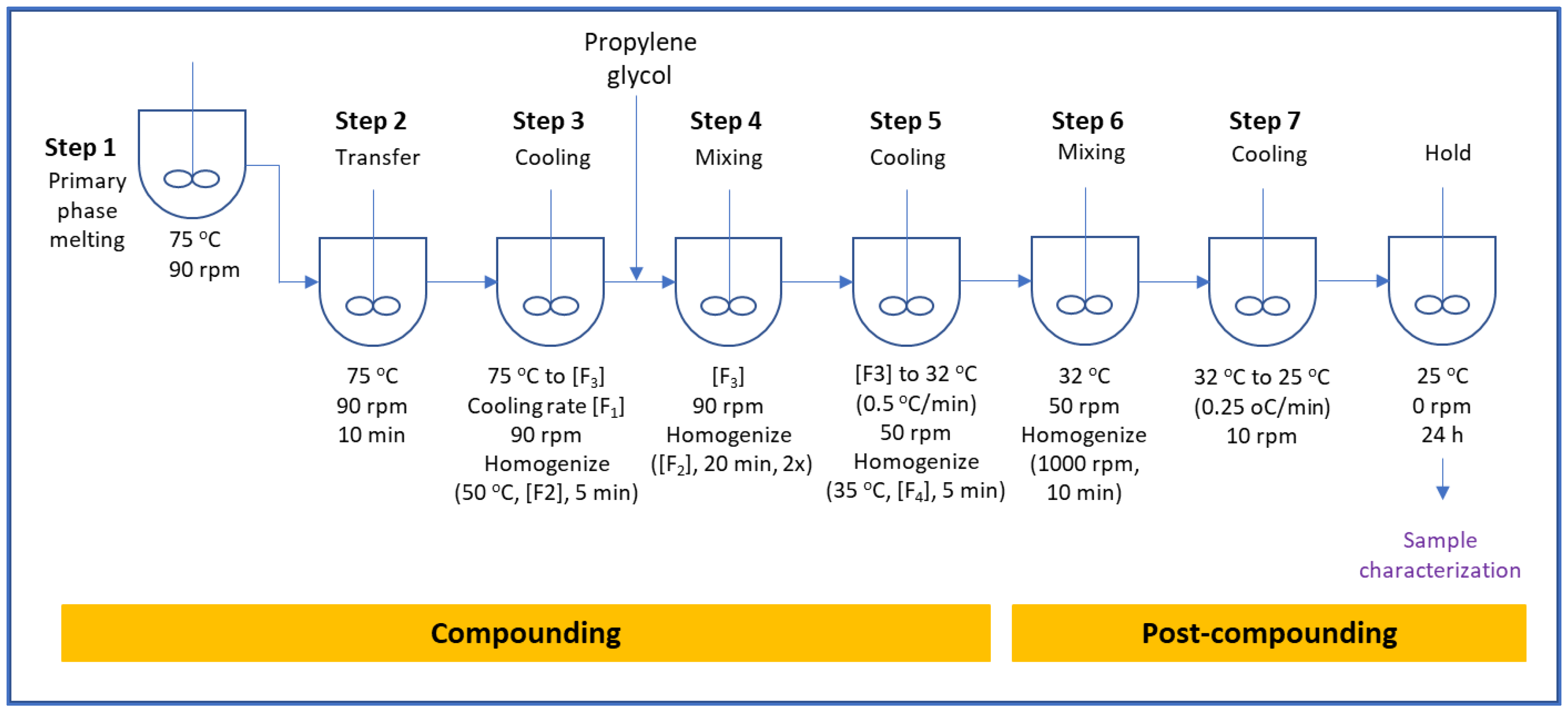
2.2. Ointment Preparation Procedure
- Step 1:
- Charge white petrolatum, paraffin wax and Mono-and-Diglycerides into the jacketed mixing vessel and heat to 75 ± 5 °C while mixing until all materials are fully melted.
- Step 2:
- Transfer melted waxes into the main vessel and mix (90 rpm) at 75 ± 5 °C for at least 10 min.
- Step 3:
- The cooling rate is set at [F1] to cool the contents of the main vessel while mixing at 90 rpm. When the temperature reaches 50 ± 3 °C, homogenize at [F2] for 5 min while continuing mixing at 90 rpm until the temperature reaches [F3]. [F1], [F2], and [F3] are critical process parameters investigated for the effect on drug product quality attributes.
- Step 4:
- Dissolve lidocaine in propylene glycol. Charge propylene glycol into main vessel while mixing (90 rpm) at [F3]. After the addition is completed, homogenize at [F2] for 20 min at every 30 min interval. Homogenization is repeated twice for a total mixing and homogenization duration of 60 min.
- Step 5:
- The cooling rate is set at 0.5 ± 0.1 °C/min to cool the contents to 32 ± 3 °C while mixing (50 rpm). Homogenization at [F4] is applied for 5 min when the temperature reaches 36 ± 3 °C.
- Step 6:
- While mixing at 50 rpm, apply shear with homogenization at 1000 rpm for 10 min at 32 ± 3 °C.
- Step 7:
- Stop shearing. The product is cooled to 25 ± 2 °C at a cooling rate of 0.25 ± 0.1 °C/min while mixing at 10 rpm. Once the temperature reaches 25 ± 2 °C, mixing is stopped, and the product is held for 24 h before characterization is conducted.
2.3. Characterization Techniques
2.3.1. Microscopy Analysis
2.3.2. Physical Stability
2.3.3. Rheological Properties
2.3.4. Sensorial Properties—Texture and Tribology Analysis
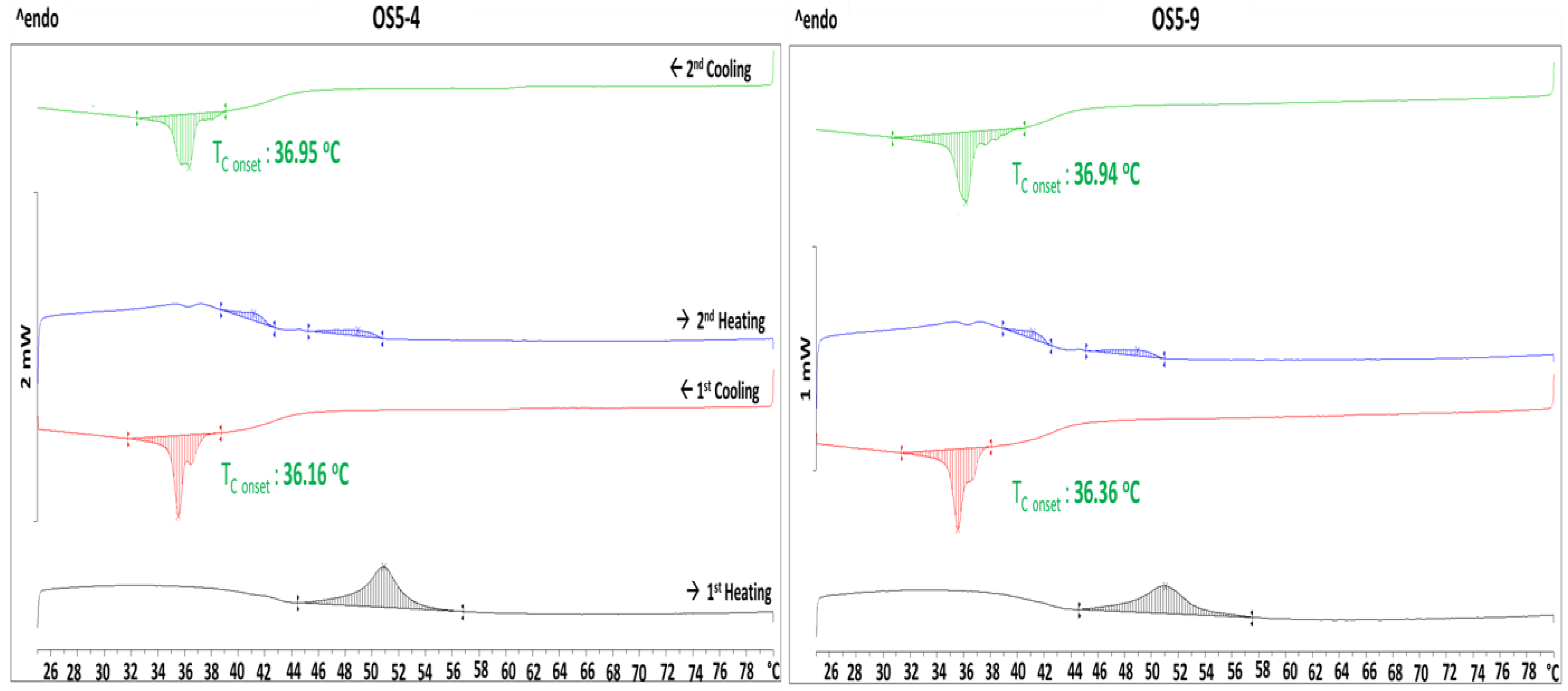
2.3.5. Thermal Analysis
2.3.6. High Performance Liquid Chromatography (HPLC) Assay of Lidocaine
2.3.7. In Vitro Release (IVRT)
2.4. Statistical Analysis—PCA
3. Results and Discussion
3.1. Preliminary Experiments
3.2. Final Design of Experiments
3.2.1. Stability of Ointment
3.2.2. Rheological Properties of Ointment
3.2.3. Sensorial Properties of Ointment
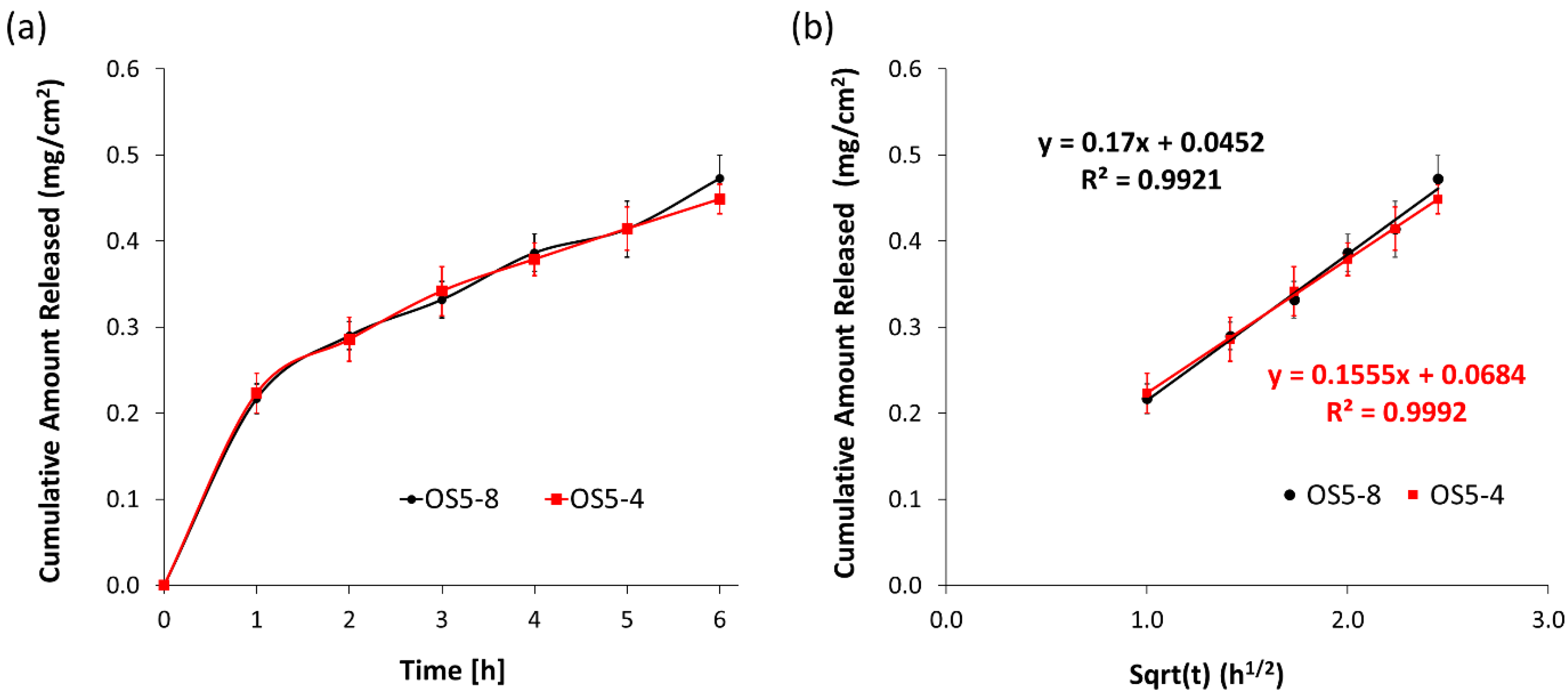
3.2.4. In Vitro Release
3.3. PCA Analysis
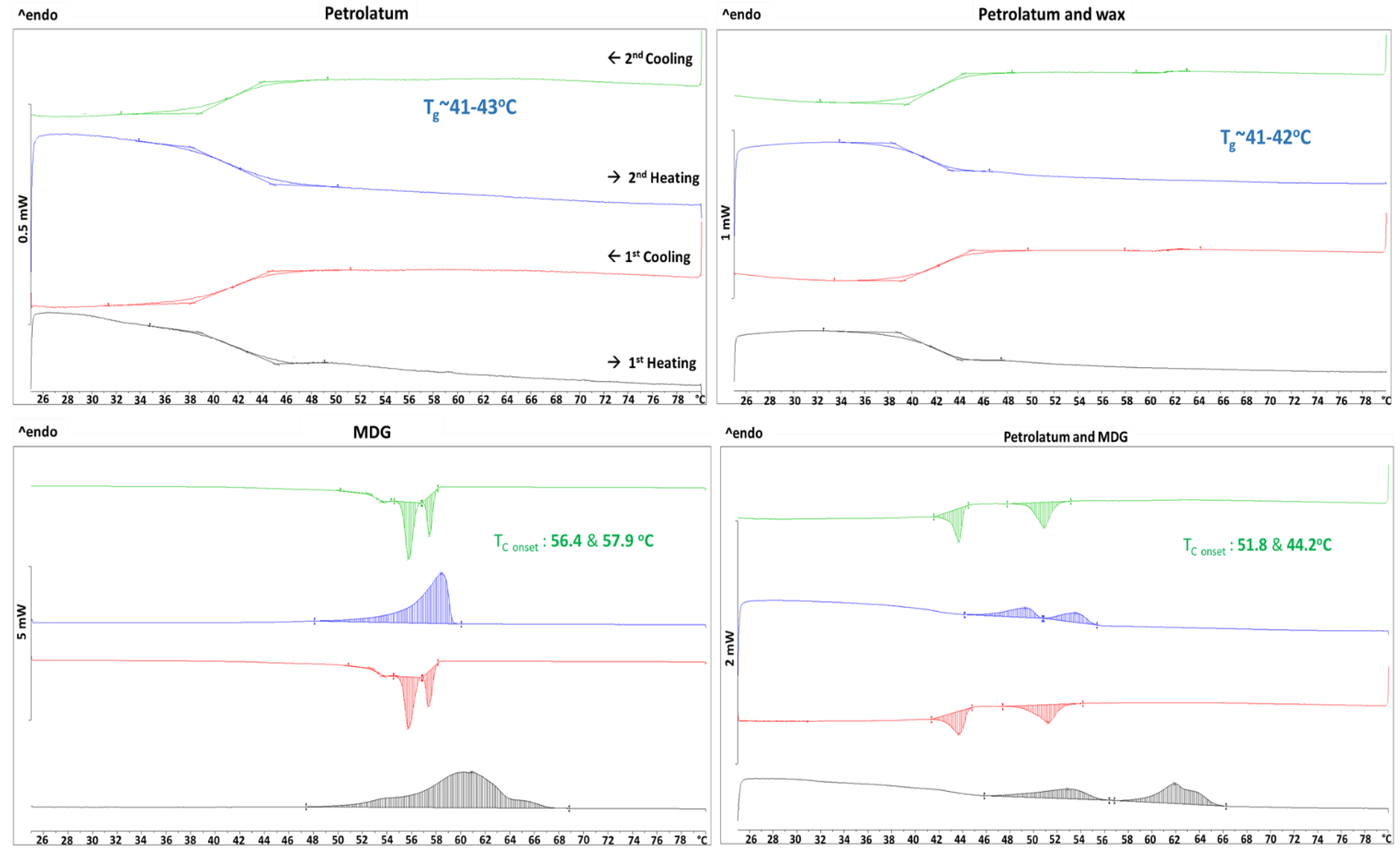
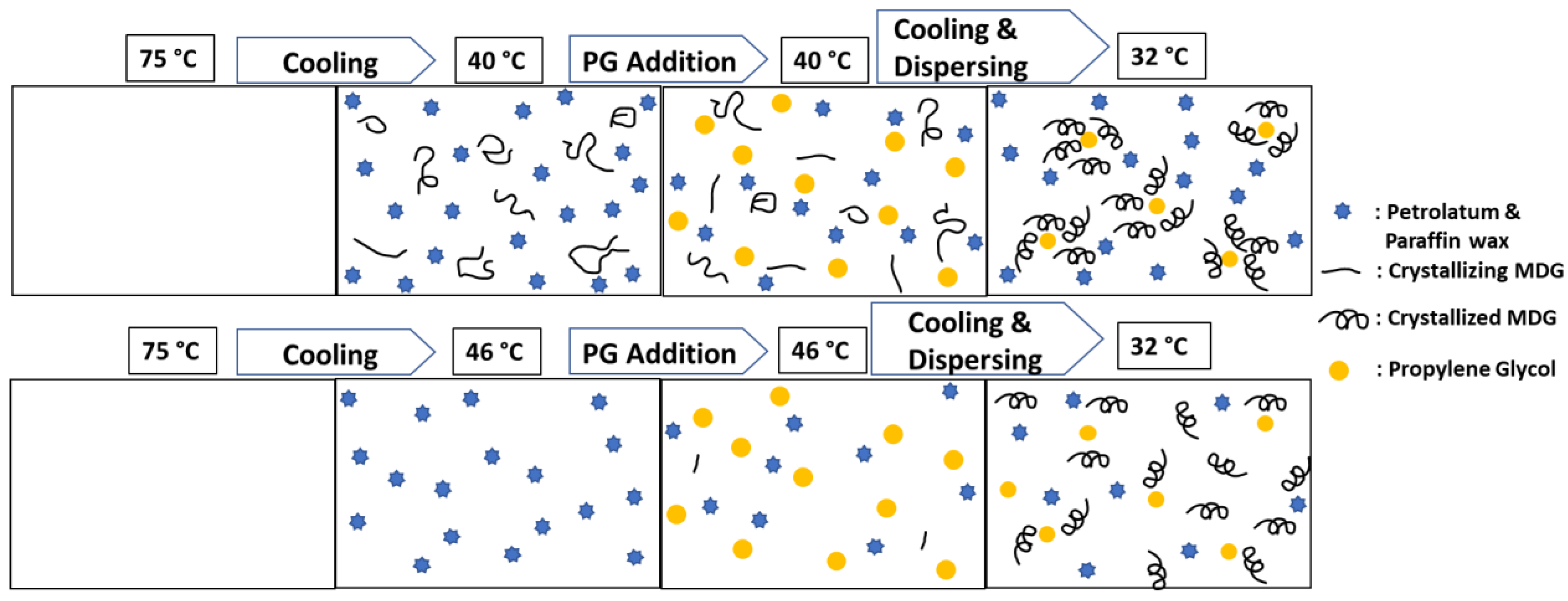
3.4. Stabilization Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawlings, A.V.; Lombard, K.J. A review on the extensive skin benefits of mineral oil. Int. J. Cosmet. Sci. 2012, 34, 511–518. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Physicochemical and Structural (Q3) Characterization of Topical Drug Products Submitted in ANDAs Guidance for Industry; US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER): Hillandale, MD, USA, 2022. [Google Scholar]
- Ali, S.; Tiwari, A.; Yeoh, T.; Doshi, P.; Kelkar, N.; Shah, J.C.; Seth, J.R. Crystallization and Rheology of Mono- and Diglycerides and Their Role in Stabilization of Emulsion Droplets in Model Topical Ointments. Langmuir 2022, 38, 8502–8512. [Google Scholar] [CrossRef]
- Baby, A.R.; Santoro, D.M.; Velasco, M.V.R.; dos Reis Serra, C.H. Emulsified systems based on glyceryl monostearate and potassium cetyl phosphate: Scale-up and characterization of physical properties. Int. J. Pharm. 2008, 361, 99–103. [Google Scholar] [CrossRef]
- Hannuksela, M.; Kousa, M.; Pirilä, V. Contact sensitivity to emulsifiers. Contact Dermat. 1976, 2, 201–204. [Google Scholar] [CrossRef]
- Kang, J.-H.; Yoo, K.-H.; Park, H.-Y.; Hyun, S.-M.; Han, S.-D.; Kim, D.-W.; Park, C.-W. Preparation and In Vivo Evaluation of a Lidocaine Self-Nanoemulsifying Ointment with Glycerol Monostearate for Local Delivery. Pharmaceutics 2021, 13, 1468. [Google Scholar] [CrossRef]
- Mahdi, E.S.N.A.; Sakeena, M.; Abdullah, G.; Abdulkarim, M.; Abdul Sattar, M.Z. Formulation and in vitro release evaluation of newly synthesized palm kernel oil esters-based nanoemulsion delivery system for 30% ethanolic dried extract derived from local Phyllanthus urinaria for skin antiaging. Int. J. Nanomed. 2011, 6, 2499–2512. [Google Scholar] [CrossRef] [PubMed]
- Rafiee-Tehrani, M.; Mehramizi, A. In Vitro Release Studies of Piroxicam from Oil-in-Water Creams and Hydroalcoholic Gel Topical Formulations. Drug Dev. Ind. Pharm. 2000, 26, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P.; Desforges, A.; Duff, D.G. Synergistic Stabilization of Emulsions by a Mixture of Surface-Active Nanoparticles and Surfactant. Langmuir 2007, 23, 1098–1106. [Google Scholar] [CrossRef]
- Kori, A.H.; Mahesar, S.A.; Sherazi, S.T.H.; Khatri, U.A.; Laghari, Z.H.; Panhwar, T. Effect of process parameters on emulsion stability and droplet size of pomegranate oil-in-water. Grasas Aceites 2021, 72, e410. [Google Scholar] [CrossRef]
- Wang, H.; Tan, P.; Barona, D.; Li, G.; Hoe, S.; Lechuga-Ballesteros, D.; Nobes, D.S.; Vehring, R. Characterization of the suspension stability of pharmaceuticals using a shadowgraphic imaging method. Int. J. Pharm. 2018, 548, 128–138. [Google Scholar] [CrossRef]
- Lerche, D.; Sobisch, T. Direct and Accelerated Characterization of Formulation Stability. J. Dispers. Sci. Technol. 2011, 32, 1799–1811. [Google Scholar] [CrossRef]
- Cyriac, F.; Xin Yi, T.; Chow, P.S.; Macbeath, C. Tactile friction and rheological studies to objectify sensory properties of topical formulations. J. Rheol. 2022, 66, 305–326. [Google Scholar] [CrossRef]
- Cyriac, F.; Xin Yi, T.; Chow, P.S. Influence of wall slip, thixotropy and lubrication regime on the instrumental sensory evaluation of topical formulations. Int. J. Cosmet. Sci. 2022, 44, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Jiang, Q.; Li, S.; Zhou, Q.; Tan, Y.; Meng, Z. Microstructure evolution and partial coalescence in the whipping process of oleofoams stabilized by monoglycerides. Food Hydrocoll. 2021, 112, 106245. [Google Scholar] [CrossRef]
- Frasch-Melnik, S.; Norton, I.T.; Spyropoulos, F. Fat-crystal stabilised w/o emulsions for controlled salt release. J. Food Eng. 2010, 98, 437–442. [Google Scholar] [CrossRef]
- Krog, N.; Larsson, K. Crystallization at Interfaces in Food Emulsions—A General Phenomenon. Lipid Fett. 1992, 94, 55–57. [Google Scholar] [CrossRef]
- Macierzanka, A.; Szeląg, H.; Szumała, P.; Pawłowicz, R.; Mackie, A.R.; Ridout, M.J. Effect of crystalline emulsifier composition on structural transformations of water-in-oil emulsions: Emulsification and quiescent conditions. Colloids Surf. Physicochem. Eng. Asp. 2009, 334, 40–52. [Google Scholar] [CrossRef]
- Ashizuka, Y.; Otoguro, S.; Horisawa, E. Effects of Manufacturing Conditions on Pharmaceutical Properties of Petrolatum Ointment–Distribution of Hydrocarbon. Chem. Pharm. Bull. 2021, 69, 352–359. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Yeoh, T.; Shah, J.C.; Walsh, T. Assessing and Predicting Physical Stability of Emulsion-Based Topical Semisolid Products: A Review. J. Pharm. Sci. 2023, 112, 1772–1793. [Google Scholar] [CrossRef]
- Echanur, V.A.; Matadh, A.V.; Pragathi, S.G.; Sarasija, S.; Thean, Y.; Badruddoza, A.Z.; Shah, J.; Kulkarni, V.; Ajjarapu, S.; Reena, N.M.; et al. Continuous Manufacturing of Oil in Water (O/W) Emulgel by Extrusion Process. AAPS PharmSciTech 2023, 24, 76. [Google Scholar] [CrossRef]
- Goswami, N.; Prajapati, P. Formulation Development and Evaluation of Matrix Type Lidocaine Topical Patch for Local Anesthetic. Int. J. Res. Dev. Pharm. Amp Life Sci. 2015, 5, 1963–1973. [Google Scholar]
- Liu, J.; Svärd, M.; Rasmuson, Å.C. Influence of Agitation on Primary Nucleation in Stirred Tank Crystallizers. Cryst. Growth Des. 2015, 15, 4177–4184. [Google Scholar] [CrossRef]
- Mitchell, N.A.; Frawley, P.J. Nucleation kinetics of paracetamol–ethanol solutions from metastable zone widths. J. Cryst. Growth 2010, 312, 2740–2746. [Google Scholar] [CrossRef]
- Mullin, J.W.; Raven, K.D. Nucleation in Agitated Solutions. Nature 1961, 190, 251. [Google Scholar] [CrossRef]
- Mullin, J.W.; Raven, K.D. Influence of Mechanical Agitation on the Nucleation of Some Aqueous Salt Solutions. Nature 1962, 195, 35–38. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Zhang, K.; Wu, S.; Liu, S.; Kangli, L.; Yu, B.; Gong, J. Nucleation behavior of eszopiclone-butyl acetate solutions from metastable zone widths. Chem. Eng. Sci. 2016, 155, 248–257. [Google Scholar] [CrossRef]









| Ingredient | Phase | Wt.% |
|---|---|---|
| Propylene glycol (PG) | Solvent for active and hence referred to as active phase | 9 |
| White petrolatum (WP) | Primary phase or continuous phase | 79 |
| Mono-and-Diglycerides (MDG) | 7 | |
| Paraffin wax (PW) | 5 | |
| Total | 100 |
| Processing Conditions | Levels | |
|---|---|---|
| [F1] | Cooling rate | 0.8 ± 0.1, 1.5 ± 0.1 °C/min |
| [F2] | Homogenization speed (compounding stage) | 2500, 4000, 8000 rpm |
| [F3] | Active phase (solvent) addition temperature | 46 ± 2, 43 ± 2, 40 ± 2 °C |
| [F4] | Compounding final cooling stage homogenization speed | 2500, 5000 or 0 rpm |
| Compounding Stages | Conditions | Sample | ||
|---|---|---|---|---|
| OT1 | OT2 | OT3 | ||
| Cool 75 °C to 43 °C (Primary phase melting) | Homogenization speed [F2] (rpm) | 2500 | 4000 | 8000 |
| Cooling rate [F1] (°C/min) | 0.8 | 0.8 | 0.8 | |
| Addition of PG at 43 °C (Active phase distribution) | Homogenization speed [F2] (rpm) | 2500 | 4000 | 8000 |
| Homogenization frequency | 4 times for 5 min | 2 times for 10 min | 2 times for 20 min | |
| Homogenization interval | Every 15 min | Every 30 min | Every 30 min | |
| Total mixing and homogenization time | 60 min | 60 min | 60 min | |
| Cool 43 to 32 °C (Final Cooling Compounding Stage, STEP 5) | Homogenization speed [F4] (rpm) | 2500 | 4000 | 5000 |
| Microscopy measurements | Crystallite size (µm) | 84.5 ± 56.5 | 71.7 ± 51.1 | 55.4 ± 34.0 |
| PG droplet (µm) | 7.2 ± 2.6 | 6.7 ± 2.6 | 6.9 ± 2.6 | |
| Instability Index (IS) | 0.149 ± 0.009 | 0.135 ± 0.004 | 0.246 ± 0.001 | |
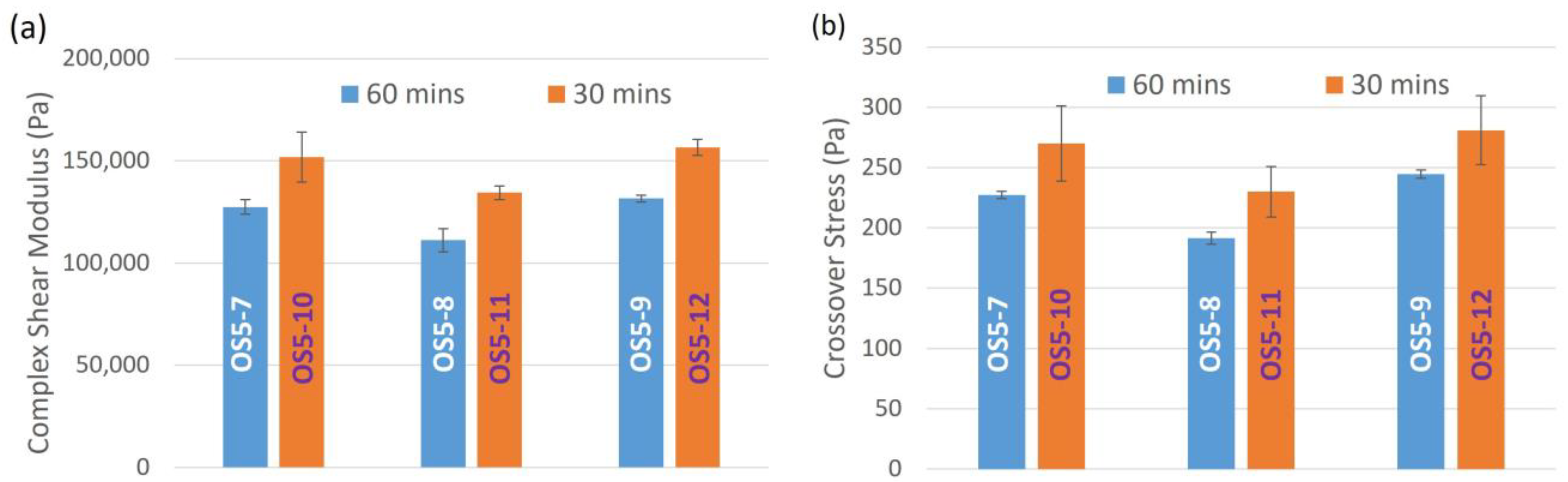
| Complex Shear Modulus (G*) (Pa) | 144,193 ± 3016 | 144,064 ± 3749 | 104,136 ± 372 | |
| Crossover Stress (σcross)(Pa) | 260 ± 5.7 | 334 ± 1.4 | 261 ± 0.0 | |
| Compounding Stages | Conditions | Sample | |||||
|---|---|---|---|---|---|---|---|
| OT4 | OT5 | OT6 | OT7 | OT8 | OT9 | ||
| Cool 75 to 40 °C (Primary Phase) | Homogenization speed [F2] (rpm) | 2500 | 4000 | 8000 | |||
| Cooling Rate [F1] (°C/min) | 0.8 | 1.5 | 0.8 | 1.5 | 0.8 | 1.5 | |
| Addition of PG at 40 °C (Active Phase) | Homogenization speed [F2] (rpm) | 2500 | 4000 | 8000 | |||
| Cooling Rate [F1] (°C/min) | 0.8 | 1.5 | 0.8 | 1.5 | 0.8 | 1.5 | |
| Cool 40 to 32 °C (Final Cooling Compounding Stage, STEP 5) | Homogenization speed [F4] (rpm) | 5000 | |||||
| Cooling rate (°C/min) | 0.5 | ||||||
| Microscopy measurements | Crystallite size (µm) | 60.8 ± 39.4 | 61.7 ± 42.2 | 49.5 ± 24.8 | 52.1 ± 22.7 | 49.0 ± 16.2 | 50.0 ± 19.0 |
| PG Droplet (µm) | 4.6 ± 2.3 | 4.4 ± 2.3 | 4.4 ± 2.2 | 4.1 ± 1.8 | 4.2 ± 1.8 | 4.8 ± 1.7 | |
| Instability Index (IS) | 0.316 ± 0.001 | 0.306 ± 0.001 | 0.312 ± 0.007 | 0.302 ± 0.006 | 0.325 ± 0.006 | 0.303 ± 0.001 | |
| Complex Shear Modulus (G*) (Pa) | 134,689 ± 1614 | 129,826 ± 4622 | 131,930 ± 8524 | 143,656 ± 208 | 103,486 ± 5604 | 106,269 ± 8579 | |
| Crossover Stress (σcross) (Pa) | 282 ± 3 | 293 ± 11 | 274 ± 7 | 271 ± 25 | 213 ± 29 | 212 ± 29 | |
| Compounding Stages | Run Conditions | Run/Sample | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Run 1/OS5-1 | Run 2/OS5-2 | Run 3/OS5-3 | Run 4/OS5-4 | Run 5/OS5-5 | Run 6/OS5-6 | Run 7/OS5-7 | Run 8/OS5-8 | Run 9/OS5-9 | Run 10/OS5-10 | Run 11/OS5-11 | Run 12/OS5-12 | ||
| Cool 75 °C to 40 or 46 °C (Primary Phase) | Homogenization speed [F2] (rpm) | 2500 | 4000 | 8000 | 2500 | 4000 | 8000 | 2500 | 8000 | 2500 | 2500 | 8000 | 2500 |
| Addition of PG at 40 or 46 °C (Active Phase) | Homogenization speed [F2] (rpm) | 2500 | 4000 | 8000 | 2500 | 4000 | 8000 | 2500 | 8000 | 2500 | 2500 | 8000 | 2500 |
| Cool at 40 or 46 °C to 32 °C (Final Cooling Compounding Stage, STEP 5) | Homogenization speed [F4] (rpm) | 2500 | 2500 | 2500 | 0 | 0 | 0 | 2500 | 2500 | 0 | 2500 | 2500 | 0 |
| Addition of PG | Temperature [F3] (°C) | 40 | 46 | 46 | |||||||||
| Mixing Duration (minutes) | 60 | 60 | 30 | ||||||||||
| Microscopy measurements | Crystallite size (µm) | 63 ± 39 | 57 ± 32 | 51 ± 26 | 69 ± 36 | 67 ± 39 | 55 ± 30 | 63 ± 41 | 57 ± 28 | 58 ± 44 | 59 ± 48 | 56 ± 31 | 57 ± 46 |
| PG droplet (µm) | 4.0 ± 2.1 | 4.0 ± 2.3 | 3.6 ± 2.1 | 3.8 ± 2.0 | 3.7 ± 1.9 | 4.1 ± 1.9 | 3.8 ± 1.9 | 4.3 ± 2.1 | 3.9 ± 2.1 | 3.6 ± 1.5 | 3.5 ± 1.5 | 3.5 ± 1.5 | |
| Instability Index (IS) | 0.274 ± 0.006 | 0.276 ± 0.007 | 0.291 ± 0.002 | 0.260 ± 0.005 | 0.266 ± 0.009 | 0.293 ± 0.008 | 0.281 ± 0.001 | 0.314 ± 0.003 | 0.282 ± 0.001 | 0.270 ± 0.003 | 0.303 ± 0.007 | 0.266 ± 0.006 | |
| Complex Shear Modulus (G*) (Pa) | 134,691 ± 2402 | 127,778 ± 3648 | 125,326 ± 5720 | 160,732 ± 8261 | 140,361 ± 2494 | 134,095 ± 6685 | 127,444 ± 3576 | 111,153 ± 5699 | 131,524 ± 1647 | 151,804 ± 12,294 | 134,379 ± 3348 | 156,473 ± 3891 | |
| Crossover Stress (σcross) (Pa) | 239 ± 6 | 226 ± 10 | 224 ± 6 | 297 ± 13 | 247 ± 3 | 234 ± 10 | 227 ± 3 | 191 ± 5 | 245 ± 4 | 243 ± 31 | 206 ± 21 | 248 ± 29 | |
| Sample | Instability Index | Firmness (g) | Adhesive Force (g) | Spreadability (N·s) | Stringiness Work Done (mJ) | Cohesion Strength (N) | Friction Coefficient (CoF) |
|---|---|---|---|---|---|---|---|
| OS5-1 | 0.274 | 54.8 ± 3.2 | 28.8 ± 2.2 | 267 ± 2 | 2.1 ± 0.3 | 1.90 ± 0.02 | 0.053 ± 0.009 |
| OS5-2 | 0.276 | 47.7 ± 3.6 | 24.9 ± 1.7 | 248 ± 11 | 1.6 ± 0.2 | 1.79 ± 0.09 | 0.042 ± 0.016 |
| OS5-3 | 0.291 | 40.7 ± 1.8 | 21.9 ± 0.8 | 231 ± 7 | 1.6 ± 0.1 | 1.66 ± 0.07 | 0.060 ± 0.022 |
| OS5-4 | 0.260 | 62.1 ± 0.9 | 31.2 ± 1.5 | 309 ± 9 | 2.2 ± 0.2 | 2.27 ± 0.06 | 0.046 ± 0.008 |
| OS5-5 | 0.266 | 51.0 ± 1.8 | 26.4 ± 1.7 | 294 ± 5 | 1.7 ± 0.1 | 2.22 ± 0.01 | 0.079 ± 0.011 |
| OS5-6 | 0.293 | 48.2 ± 2.0 | 25.4 ± 1.4 | 253 ± 0 | 1.8 ± 0.2 | 1.95 ± 0.13 | 0.095 ± 0.007 |
| OS5-7 | 0.281 | 50.2 ± 3.6 | 27.2 ± 2.3 | 213 ± 1 | 2.0 ± 0.4 | 1.41 ± 0.06 | 0.103 ± 0.016 |
| OS5-8 | 0.314 | 36.1 ± 0.8 | 20.1 ± 0.1 | 177 ± 8 | 1.4 ± 0.1 | 1.01 ± 0.10 | 0.091 ± 0.011 |
| OS5-9 | 0.282 | 43.8 ± 2.7 | 25 ± 1.3 | 210 ± 4 | 1.8 ± 0.1 | 1.34 ± 0.04 | 0.086 ± 0.003 |
| OS5-10 | 0.270 | 52.1 ± 1.1 | 29.4 ± 1.1 | 282 ± 16 | 1.7 ± 0.2 | 2.25 ± 0.09 | 0.079 ± 0.014 |
| OS5-11 | 0.303 | 41.6 ± 1.3 | 22.2 ± 2.0 | 181 ± 2 | 1.5 ± 0.2 | 1.09 ± 0.02 | 0.062 ± 0.004 |
| OS5-12 | 0.266 | 53.9 ± 4.3 | 31.8 ± 0.6 | 269 ± 20 | 2.1 ± 0.3 | 2.11 ± 0.17 | 0.081 ± 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chow, P.S.; Lim, R.T.Y.; Cyriac, F.; Shah, J.C.; Badruddoza, A.Z.M.; Yeoh, T.; Yagnik, C.K.; Tee, X.Y.; Wong, A.B.H.; Chia, V.D.; et al. Influence of Manufacturing Process on the Microstructure, Stability, and Sensorial Properties of a Topical Ointment Formulation. Pharmaceutics 2023, 15, 2219. https://doi.org/10.3390/pharmaceutics15092219
Chow PS, Lim RTY, Cyriac F, Shah JC, Badruddoza AZM, Yeoh T, Yagnik CK, Tee XY, Wong ABH, Chia VD, et al. Influence of Manufacturing Process on the Microstructure, Stability, and Sensorial Properties of a Topical Ointment Formulation. Pharmaceutics. 2023; 15(9):2219. https://doi.org/10.3390/pharmaceutics15092219
Chicago/Turabian StyleChow, Pui Shan, Ron Tau Yee Lim, Febin Cyriac, Jaymin C. Shah, Abu Zayed Md Badruddoza, Thean Yeoh, Chetan Kantilal Yagnik, Xin Yi Tee, Annie Bao Hua Wong, Vernissa Dilys Chia, and et al. 2023. "Influence of Manufacturing Process on the Microstructure, Stability, and Sensorial Properties of a Topical Ointment Formulation" Pharmaceutics 15, no. 9: 2219. https://doi.org/10.3390/pharmaceutics15092219
APA StyleChow, P. S., Lim, R. T. Y., Cyriac, F., Shah, J. C., Badruddoza, A. Z. M., Yeoh, T., Yagnik, C. K., Tee, X. Y., Wong, A. B. H., Chia, V. D., & Wang, G. (2023). Influence of Manufacturing Process on the Microstructure, Stability, and Sensorial Properties of a Topical Ointment Formulation. Pharmaceutics, 15(9), 2219. https://doi.org/10.3390/pharmaceutics15092219






