Development of Simvastatin-Loaded Particles Using Spray Drying Method for Ex Tempore Preparation of Cartridges for 2D Printing Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation and Characterization of Emulsion
2.2.2. Particle Preparation by Spray Drying Emulsions
2.2.3. Experimental Design
2.2.4. Characterization of Prepared Particles
2.2.5. Drug Loading and Encapsulation Efficiency
2.2.6. Stability Study
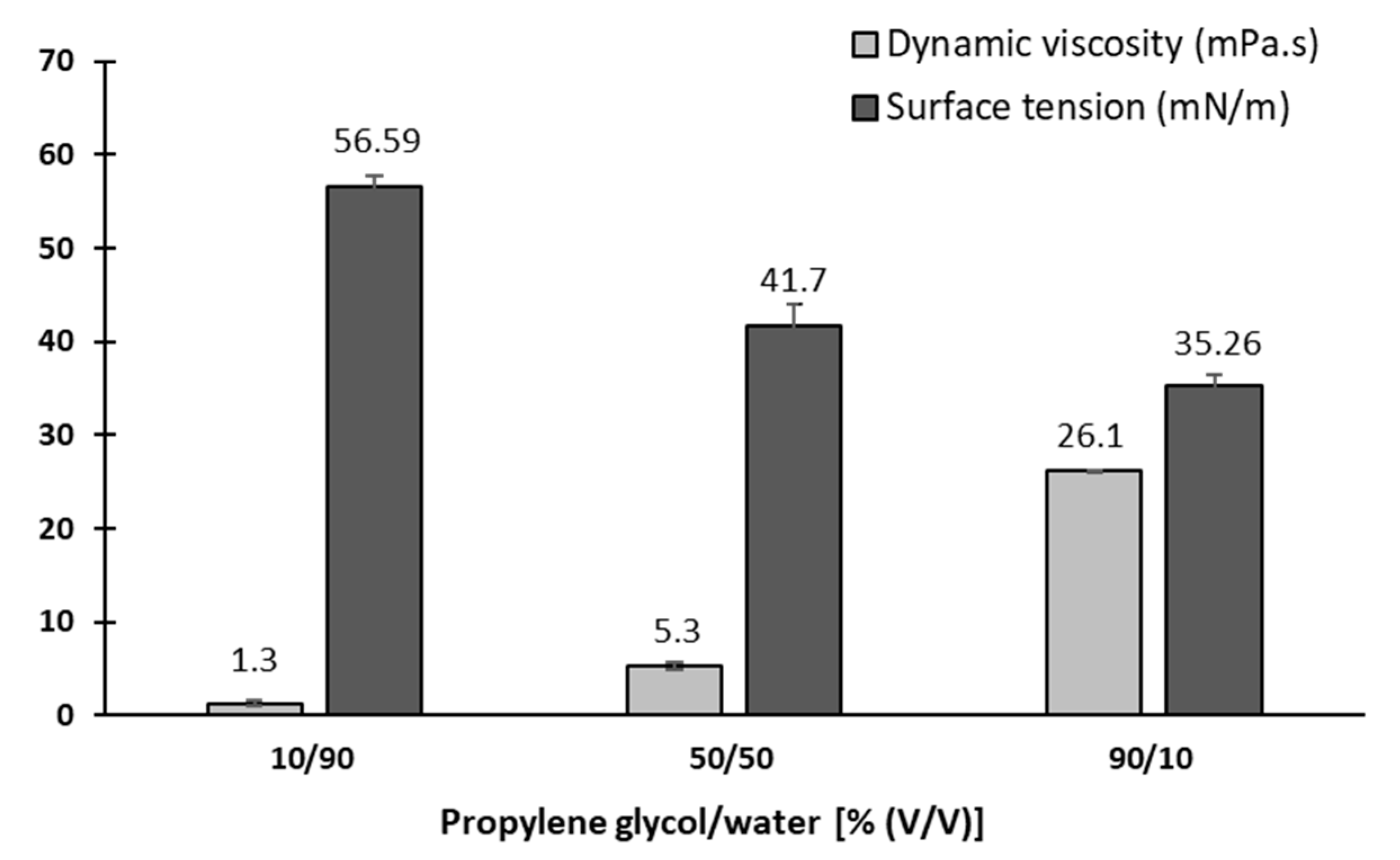
2.2.7. Preparation and Characterization of Blank Ink Media
2.2.8. Redispersion of the Dry Particles
2.2.9. UPLC Analysis
2.2.10. Statistical Analysis
3. Results and Discussion
3.1. Emulsion Optimization
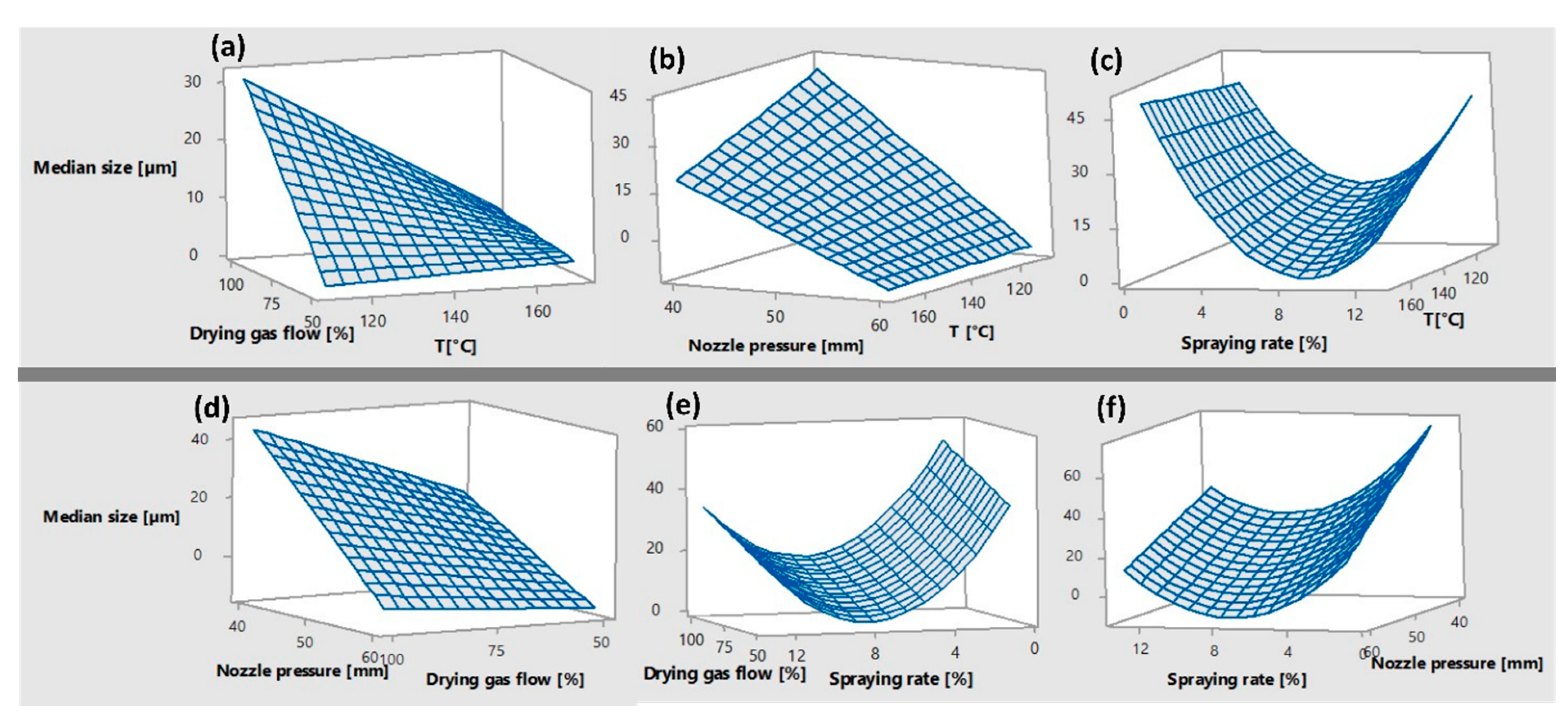
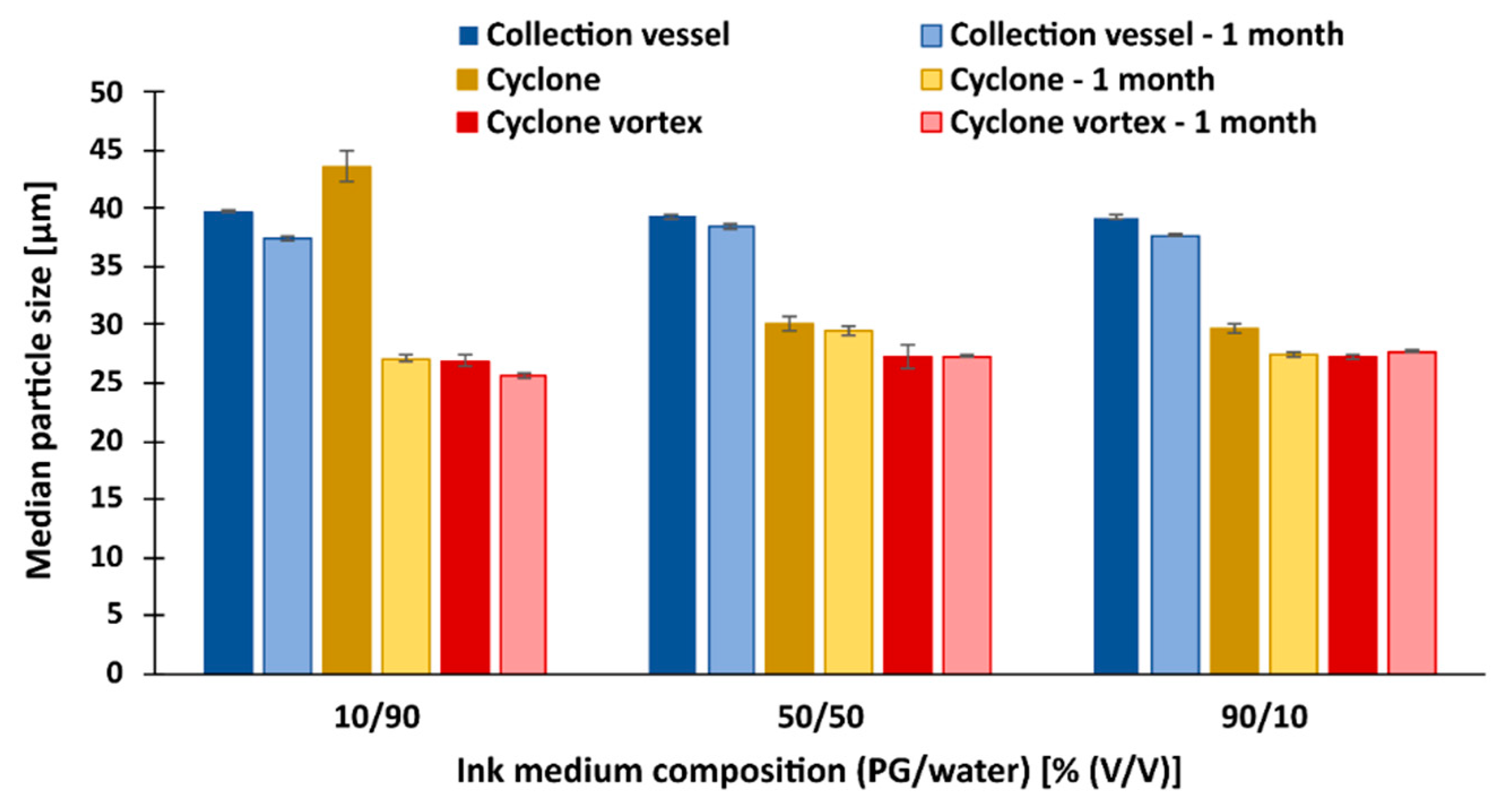
3.2. The Size of the Dry Particles after Spray Drying
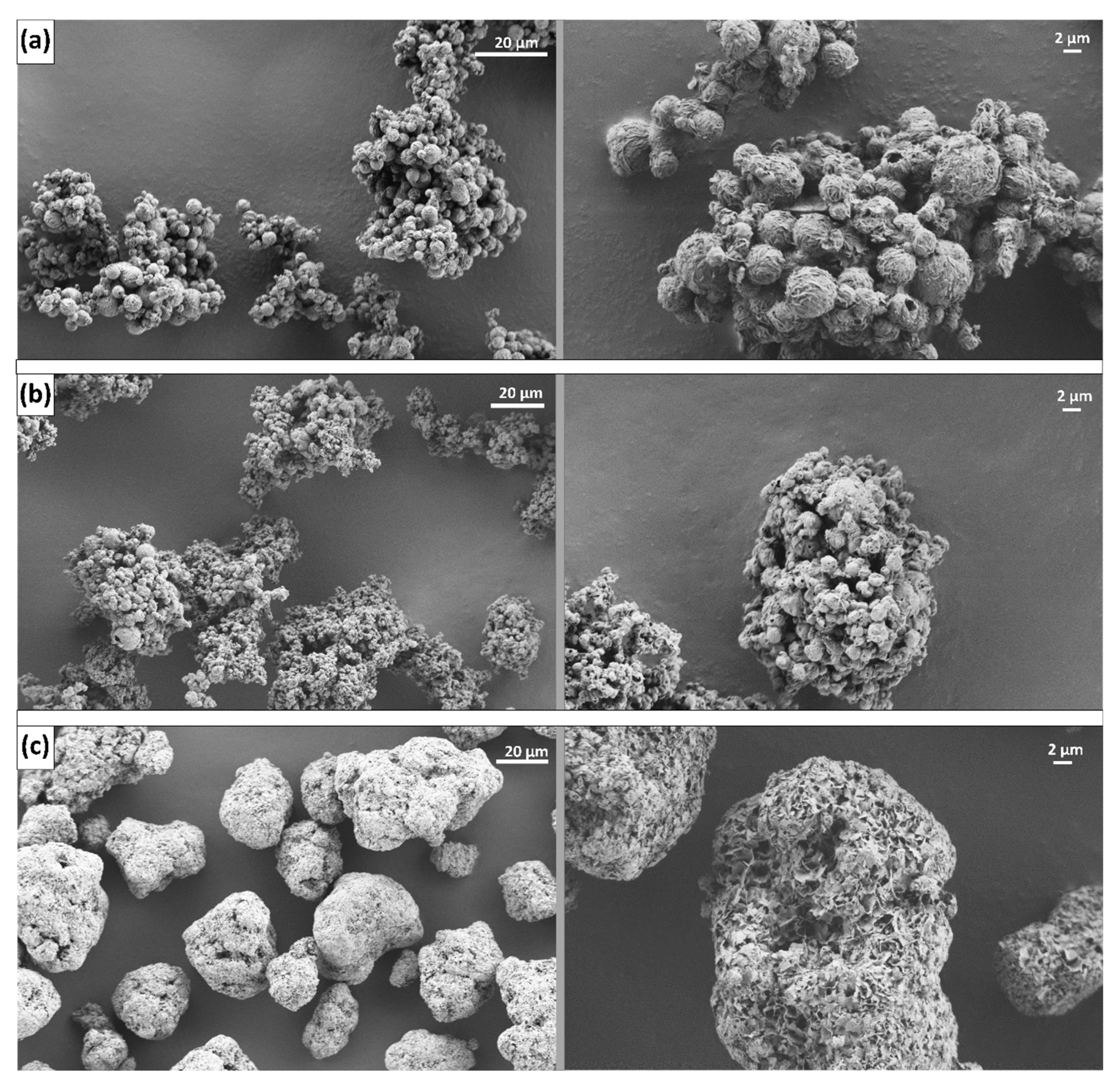
3.3. Antiadhesive Substances
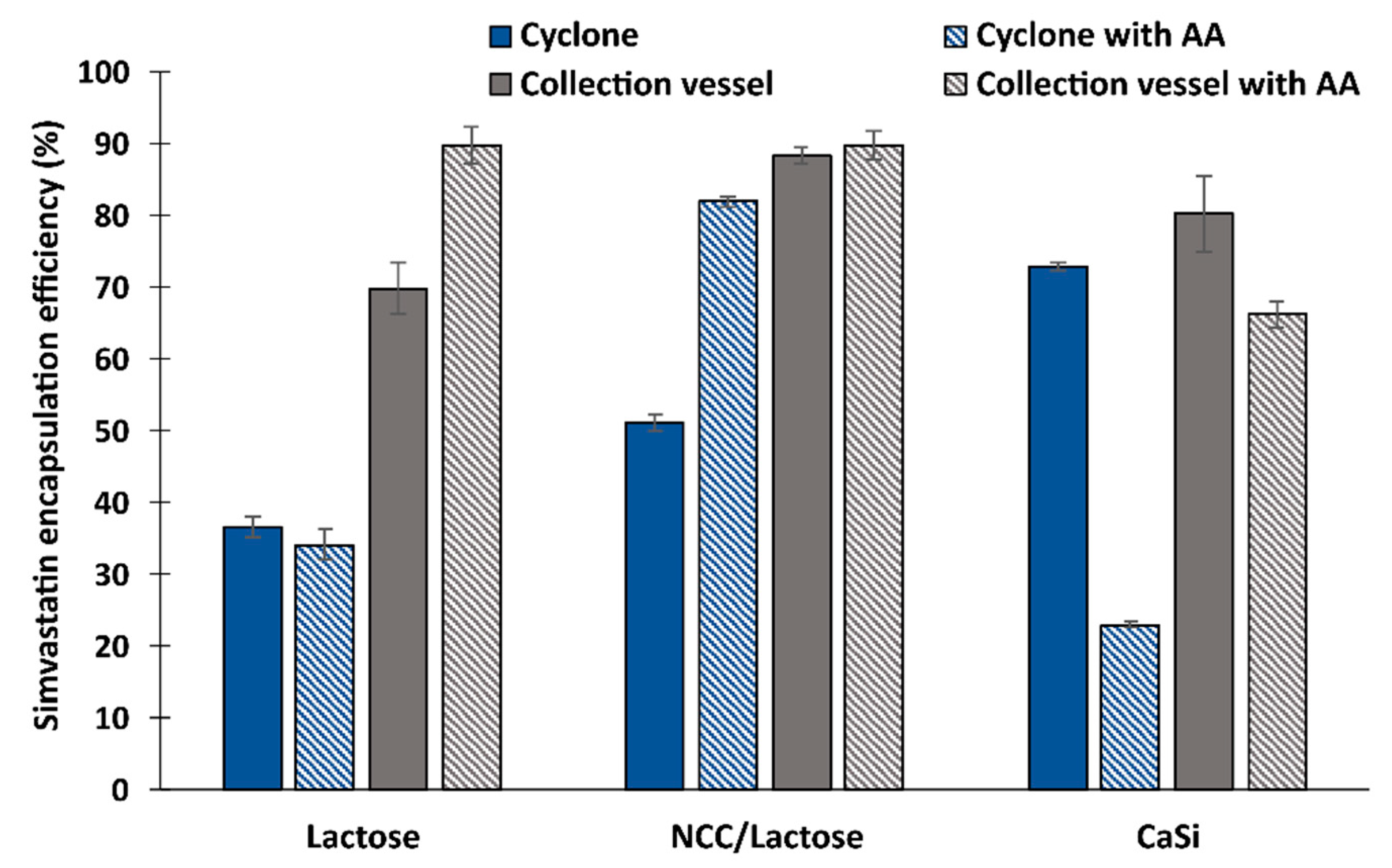
3.4. Drug Loading and Encapsulation Efficiency of Simvastatin in the Dry Particles
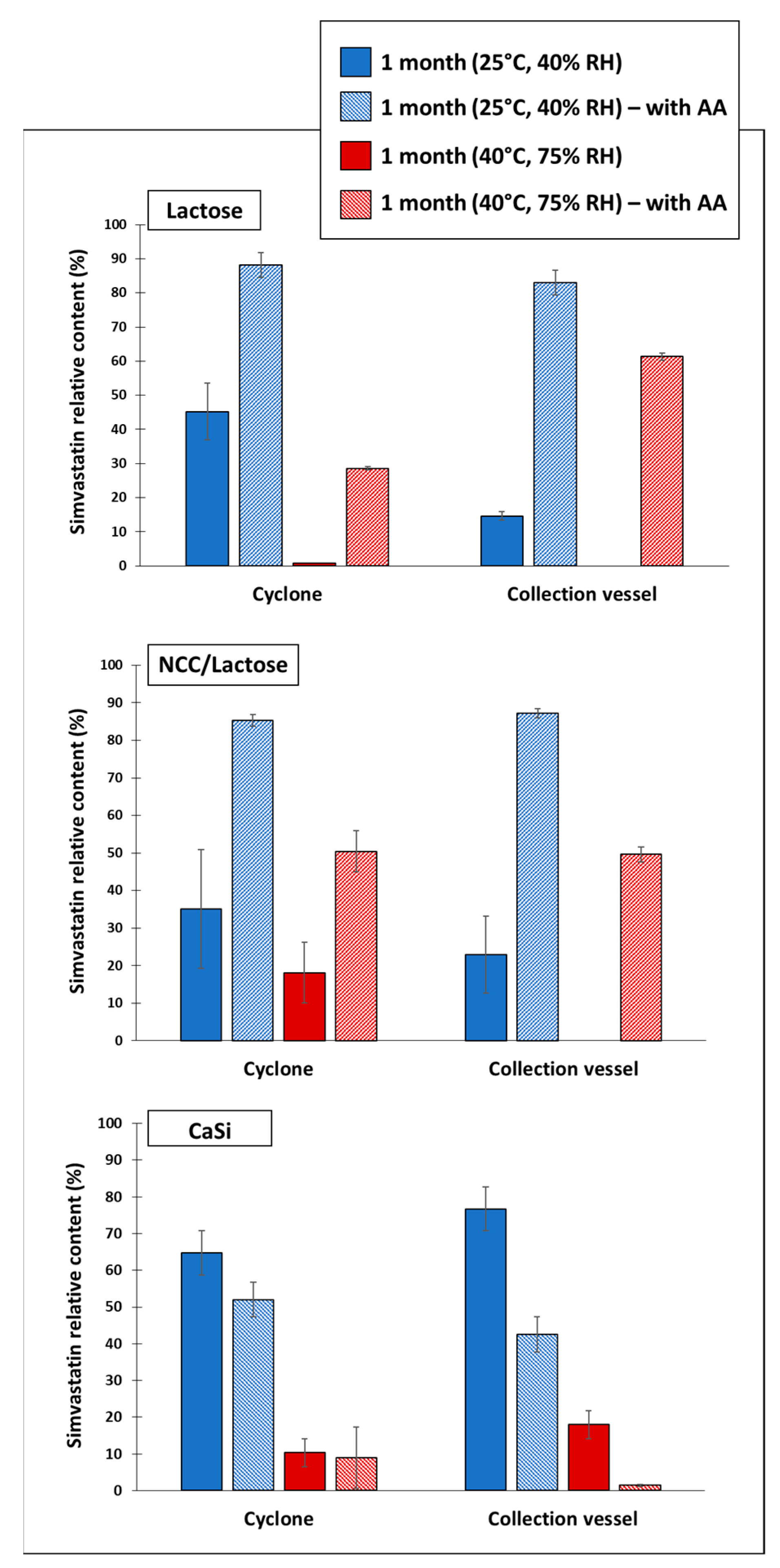
3.5. Chemical Stability of Simvastatin in Dry Particles
3.6. Moisture Content
3.7. Redispersion of Dry Particles in Blank Ink Medium
3.7.1. Redispersion of Particles with Lactose as Antiadhesive
3.7.2. Redispersion of Particles with NCC/Lactose as Antiadhesive
3.7.3. Redispersion of Particles with CaSi as Antiadhesive
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breitkreutz, J.; Boos, J. Paediatric and Geriatric Drug Delivery. Expert. Opin. Drug Deliv. 2007, 4, 37–45. [Google Scholar] [CrossRef]
- Florence, A.T.; Lee, V.H.L. Personalised Medicines: More Tailored Drugs, More Tailored Delivery. Int. J. Pharm. 2011, 415, 29–33. [Google Scholar] [CrossRef]
- Sandler, N.; Preis, M. Printed Drug-Delivery Systems for Improved Patient Treatment: (Trends in Pharmacological Sciences 37, 1070–1080). Trends Pharmacol. Sci. 2017, 38, 317. [Google Scholar] [CrossRef]
- Tarkkala, H.; Helén, I.; Snell, K. From Health to Wealth: The Future of Personalized Medicine in the Making. Futures 2019, 109, 142–152. [Google Scholar] [CrossRef]
- Ginsburg, G.S.; Willard, H.F. Genomic and Personalized Medicine: Foundations and Applications. Transl. Res. 2009, 154, 277–287. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Katsiotis, C.S.; Andreadis, D.A.; Tzetzis, D.; Ritzoulis, C.; Bouropoulos, N.; Kanellopoulou, D.; Andriotis, E.G.; Tsibouklis, J.; Fatouros, D.G. Inkjet Printing of a Thermolabile Model Drug onto FDM-Printed Substrates: Formulation and Evaluation. Drug Dev. Ind. Pharm. 2020, 46, 1253–1264. [Google Scholar] [CrossRef]
- Edinger, M.; Jacobsen, J.; Bar-Shalom, D.; Rantanen, J.; Genina, N. Analytical Aspects of Printed Oral Dosage Forms. Int. J. Pharm. 2018, 553, 97–108. [Google Scholar] [CrossRef]
- Vakili, H.; Wickström, H.; Desai, D.; Preis, M.; Sandler, N. Application of a Handheld NIR Spectrometer in Prediction of Drug Content in Inkjet Printed Orodispersible Formulations Containing Prednisolone and Levothyroxine. Int. J. Pharm. 2017, 524, 414–423. [Google Scholar] [CrossRef]
- Stranzinger, S.; Wolfgang, M.; Klotz, E.; Scheibelhofer, O.; Ghiotti, P.; Khinast, J.G.; Hsiao, W.-K.; Paudel, A. Near-Infrared Hyperspectral Imaging as a Monitoring Tool for On-Demand Manufacturing of Inkjet-Printed Formulations. AAPS PharmSciTech. 2021, 22, 211. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Januskaite, P.; Goyanes, A.; Wilsdon, D.; Rowland, M.; Gaisford, S.; Basit, A.W. Prediction of Solid-State Form of SLS 3D Printed Medicines Using NIR and Raman Spectroscopy. Pharmaceutics 2022, 14, 589. [Google Scholar] [CrossRef] [PubMed]
- Azizi Machekposhti, S.; Zhang, B.; Sachan, R.; Vanderwal, L.; Stafslien, S.J.; Narayan, R.J. Patterned Surfaces with the Controllable Drug Doses Using Inkjet Printing. J. Mater. Res. 2021, 36, 3865–3876. [Google Scholar] [CrossRef]
- Evans, S.E.; Harrington, T.; Rodriguez Rivero, M.C.; Rognin, E.; Tuladhar, T.; Daly, R. 2D and 3D Inkjet Printing of Biopharmaceuticals—A Review of Trends and Future Perspectives in Research and Manufacturing. Int. J. Pharm. 2021, 599, 120443. [Google Scholar] [CrossRef] [PubMed]
- Breithaupt, M.H.; Krohmer, E.; Taylor, L.; Koerner, E.; Hoppe-Tichy, T.; Burhenne, J.; Foerster, K.I.; Dachtler, M.; Huber, G.; Venkatesh, R.; et al. Oral Bioavailability of Microdoses and Therapeutic Doses of Midazolam as a 2-Dimensionally Printed Orodispersible Film in Healthy Volunteers. Eur. J. Clin. Pharmacol. 2022, 78, 1965–1972. [Google Scholar] [CrossRef]
- Chou, W.-H.; Gamboa, A.; Morales, J.O. Inkjet Printing of Small Molecules, Biologics, and Nanoparticles. Int. J. Pharm. 2021, 600, 120462. [Google Scholar] [CrossRef]
- Zhang, Q.; Willis-Fox, N.; Daly, R. Capturing the Value in Printed Pharmaceuticals—A Study of Inkjet Droplets Released from a Polymer Matrix. Int. J. Pharm. 2021, 599, 120436. [Google Scholar] [CrossRef]
- Alomari, M.; Vuddanda, P.R.; Trenfield, S.J.; Dodoo, C.C.; Velaga, S.; Basit, A.W.; Gaisford, S. Printing T3 and T4 Oral Drug Combinations as a Novel Strategy for Hypothyroidism. Int. J. Pharm. 2018, 549, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, O.; Fischer, B.; Breitkreutz, J. Fundamental Investigations into Metoprolol Tartrate Deposition on Orodispersible Films by Inkjet Printing for Individualised Drug Dosing. Pharmaceutics 2021, 13, 247. [Google Scholar] [CrossRef]
- Pollard, T.D.; Bonetti, M.; Day, A.; Gaisford, S.; Orlu, M.; Basit, A.W.; Murdan, S.; Goyanes, A. Printing Drugs onto Nails for Effective Treatment of Onychomycosis. Pharmaceutics 2022, 14, 448. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Seoane-Viaño, I.; Ong, J.J.; Januskaite, P.; Awwad, S.; Orlu, M.; Bande, M.F.; Basit, A.W.; Goyanes, A. Inkjet Drug Printing onto Contact Lenses: Deposition Optimisation and Non-Destructive Dose Verification. Int. J. Pharm. 2023, 5, 100150. [Google Scholar] [CrossRef]
- Pardeike, J.; Strohmeier, D.M.; Schrödl, N.; Voura, C.; Gruber, M.; Khinast, J.G.; Zimmer, A. Nanosuspensions as Advanced Printing Ink for Accurate Dosing of Poorly Soluble Drugs in Personalized Medicines. Int. J. Pharm. 2011, 420, 93–100. [Google Scholar] [CrossRef]
- Varan, C.; Wickström, H.; Sandler, N.; Aktaş, Y.; Bilensoy, E. Inkjet Printing of Antiviral PCL Nanoparticles and Anticancer Cyclodextrin Inclusion Complexes on Bioadhesive Film for Cervical Administration. Int. J. Pharm. 2017, 531, 701–713. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Baier, G.; Landfester, K.; Musin, E.; Al-Bataineh, S.A.; Cameron, D.C.; Homola, T.; Whittle, J.D.; Sillanpää, M. Attachment of Poly(l-Lactide) Nanoparticles to Plasma-Treated Non-Woven Polymer Fabrics Using Inkjet Printing. Macromol. Biosci. 2015, 15, 1274–1282. [Google Scholar] [CrossRef]
- Akagi, T.; Fujiwara, T.; Akashi, M. Inkjet Printing of Layer-by-Layer Assembled Poly(Lactide) Stereocomplex with Encapsulated Proteins. Langmuir 2014, 30, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Wickström, H.; Hilgert, E.; Nyman, J.O.; Desai, D.; Şen Karaman, D.; de Beer, T.; Sandler, N.; Rosenholm, J.M. Inkjet Printing of Drug-Loaded Mesoporous Silica Nanoparticles-A Platform for Drug Development. Molecules 2017, 22, 2020. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-H.; Galaz, A.; Jara, M.O.; Gamboa, A.; Morales, J.O. Drug-Loaded Lipid-Core Micelles in Mucoadhesive Films as a Novel Dosage Form for Buccal Administration of Poorly Water-Soluble and Biological Drugs. Pharmaceutics 2020, 12, 1168. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.M.; Liversidge, G.G. Drug Nanoparticles: Formulating Poorly Water-Soluble Compounds. Toxicol. Pathol. 2008, 36, 43–48. [Google Scholar] [CrossRef]
- Lohse, D. Fundamental Fluid Dynamics Challenges in Inkjet Printing. Annu. Rev. Fluid. Mech. 2022, 54, 349–382. [Google Scholar] [CrossRef]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray Drying of Pharmaceuticals and Biopharmaceuticals: Critical Parameters and Experimental Process Optimization Approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef]
- Pohlen, M.; Lavrič, Z.; Prestidge, C.; Dreu, R. Preparation, Physicochemical Characterisation and DoE Optimisation of a Spray-Dried Dry Emulsion Platform for Delivery of a Poorly Soluble Drug, Simvastatin. AAPS PharmSciTech. 2020, 21, 119. [Google Scholar] [CrossRef]
- Sterle Zorec, B.; Zupančič, Š.; Lavrič, Z.; Dreu, R. Particle Properties and Drug Metastable Solubility of Simvastatin Containing PVP Matrix Particles Prepared by Electrospraying Technique. Eur. J. Pharm. Sci. 2021, 158, 105649. [Google Scholar] [CrossRef] [PubMed]
- Aceves-Hernández, J.M.; Hinojosa-Torres, J.; Nicolás-Vázquez, I.; Ruvalcaba, R.M.; García, R.M.L. Solubility of Simvastatin: A Theoretical and Experimental Study. J. Mol. Struct. 2011, 995, 41–50. [Google Scholar] [CrossRef]
- Bordes, C.; Fréville, V.; Ruffin, E.; Marote, P.; Gauvrit, J.Y.; Briançon, S.; Lantéri, P. Determination of Poly(ɛ-Caprolactone) Solubility Parameters: Application to Solvent Substitution in a Microencapsulation Process. Int. J. Pharm. 2010, 383, 236–243. [Google Scholar] [CrossRef]
- Boucher, D.S. Solubility Parameters and Solvent Affinities for Polycaprolactone: A Comparison of Methods. J. Appl. Polym. Sci. 2020, 137, 48908. [Google Scholar] [CrossRef]
- Daly, R.; Harrington, T.S.; Martin, G.D.; Hutchings, I.M. Inkjet Printing for Pharmaceutics—A Review of Research and Manufacturing. Int. J. Pharm. 2015, 494, 554–567. [Google Scholar] [CrossRef]
- Lindeløv, J.S.; Wahlberg, M. Spray Drying for Processing of Nanomaterials. J. Phys. Conf. Ser. 2009, 170, 012027. [Google Scholar] [CrossRef]
- Littringer, E.M.; Zellnitz, S.; Hammernik, K.; Adamer, V.; Friedl, H.; Urbanetz, N.A. Spray Drying of Aqueous Salbutamol Sulfate Solutions Using the Nano Spray Dryer B-90—The Impact of Process Parameters on Particle Size. Dry Technol. 2013, 31, 1346–1353. [Google Scholar] [CrossRef]
- Vehring, R.; Foss, W.R.; Lechuga-Ballesteros, D. Particle Formation in Spray Drying. J. Aerosol Sci. 2007, 38, 728–746. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Okuyama, K. Progress in Developing Spray-Drying Methods for the Production of Controlled Morphology Particles: From the Nanometer to Submicrometer Size Ranges. Adv. Powder Technol. 2011, 22, 1–19. [Google Scholar] [CrossRef]
- Wong, T.W.; John, P. Advances in Spray Drying Technology for Nanoparticle Formation. In Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 329–346. [Google Scholar] [CrossRef]
- Sverdlov Arzi, R.; Sosnik, A. Electrohydrodynamic Atomization and Spray-Drying for the Production of Pure Drug Nanocrystals and Co-Crystals. Adv. Drug Deliv. Rev. 2018, 131, 79–100. [Google Scholar] [CrossRef]
- Kauppinen, A.; Broekhuis, J.; Grasmeijer, N.; Tonnis, W.; Ketolainen, J.; Frijlink, H.W.; Hinrichs, W.L.J. Efficient Production of Solid Dispersions by Spray Drying Solutions of High Solid Content Using a 3-Fluid Nozzle. Eur. J. Pharm. Biopharm. 2018, 123, 50–58. [Google Scholar] [CrossRef]
- Lallbeeharry, P.; Tian, Y.; Fu, N.; Wu, W.D.; Woo, M.W.; Selomulya, C.; Chen, X.D. Effects of Ionic and Nonionic Surfactants on Milk Shell Wettability during Co-Spray-Drying of Whole Milk Particles. J. Dairy Sci. 2014, 97, 5303–5314. [Google Scholar] [CrossRef]
- McKeen, L. Chapter11—The Effect of Heat Aging on the Properties of Sustainable Polymers. In The Effect of Long Term Thermal Exposure on Plastics and Elastomers, 2nd ed.; McKeen, L., Ed.; Plastics Design Library; William Andrew Publishing: New York, NY, USA, 2021; pp. 313–332. ISBN 978-0-323-85436-8. [Google Scholar]
- Kho, K.; Cheow, W.S.; Lie, R.H.; Hadinoto, K. Aqueous Re-Dispersibility of Spray-Dried Antibiotic-Loaded Polycaprolactone Nanoparticle Aggregates for Inhaled Anti-Biofilm Therapy. Powder Technol. 2010, 203, 432–439. [Google Scholar] [CrossRef]
- De Souza, F.J.; de Vasconcelos Salvo, R.; de Moro Martins, D.A. Large Eddy Simulation of the Gas–Particle Flow in Cyclone Separators. Sep. Purif. Technol. 2012, 94, 61–70. [Google Scholar] [CrossRef]
- Liu, F.; Ranmal, S.; Batchelor, H.K.; Orlu-Gul, M.; Ernest, T.B.; Thomas, I.W.; Flanagan, T.; Tuleu, C. Patient-Centered Pharmaceutical Design to Improve Acceptability of Medicines: Similarities and Differences in Paediatric and Geriatric Populations. Drugs 2014, 74, 1871–1889. [Google Scholar] [CrossRef]
- Nunn, T.; Williams, J. Formulation of Medicines for Children. Br. J. Clin. Pharmacol. 2005, 59, 674–676. [Google Scholar] [CrossRef]
- Kajdič, S.; Zupančič, Š.; Roškar, R.; Kocbek, P. The Potential of Nanofibers to Increase Solubility and Dissolution Rate of the Poorly Soluble and Chemically Unstable Drug Lovastatin. Int. J. Pharm. 2020, 573, 118809. [Google Scholar] [CrossRef]
- Kaufman, M.J. Applications of Oxygen Polarography to Drug Stability Testing and Formulation Development: Solution-Phase Oxidation of Hydroxymethylglutaryl Coenzyme A (HMG-CoA) Reductase Inhibitors. Pharm. Res. 1990, 7, 289–292. [Google Scholar] [CrossRef]
- Verhagen, H.; Schilderman, P.A.E.L.; Kleinjans, J.C.S. Butylated Hydroxyanisole in Perspective. Chem. Biol. Interact. 1991, 80, 109–134. [Google Scholar] [CrossRef]
- Ge, Z.; Zhang, X.; Gan, L.; Gan, Y. Redispersible, Dry Emulsion of Lovastatin Protects against Intestinal Metabolism and Improves Bioavailability. Acta Pharmacol. Sin. 2008, 29, 990–997. [Google Scholar] [CrossRef]
- Pohlen, M.; Pirker, L.; Dreu, R. The Potential of Macroporous Silica-Nanocrystalline Cellulose Combination for Formulating Dry Emulsion Systems with Improved Flow Properties: A DoE Study. Pharmaceutics 2021, 13, 1177. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, S.K.; Pandey, N.K.; Rajesh, S.Y.; Bawa, P.; Kumar, B.; Gulati, M.; Singh, S.; Verma, S.; Yadav, A.K.; et al. Impact of Solid Carriers and Spray Drying on Pre/Post-Compression Properties, Dissolution Rate and Bioavailability of Solid Self-Nanoemulsifying Drug Delivery System Loaded with Simvastatin. Powder Technol. 2018, 338, 836–846. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Guo, X.-X.; Sham, T.-K. Calcium Silicate-Based Drug Delivery Systems. Expert. Opin. Drug Deliv. 2017, 14, 215–228. [Google Scholar] [CrossRef]
- Zhang, F.; Aaltonen, J.; Tian, F.; Saville, D.J.; Rades, T. Influence of Particle Size and Preparation Methods on the Physical and Chemical Stability of Amorphous Simvastatin. Eur. J. Pharm. Biopharm. 2009, 71, 64–70. [Google Scholar] [CrossRef]
- Demirkaya, K.; Demirdöğen, B.C.; Torun, Z.Ö.; Erdem, O.; Tunca, Y.M. The Effects of Hydraulic Calcium Silicate Containing Endodontic Materials on Oxidative Stress in Erythrocytes and Liver. Turkish J. Biochem. 2018, 43, 333–341. [Google Scholar] [CrossRef]
- Lee, J.-K.; Jung, S.-H.; Lee, S.-E.; Han, J.-H.; Jo, E.; Park, H.-S.; Heo, K.-S.; Kim, D.; Park, J.-S.; Myung, C.-S. Alleviation of Ascorbic Acid-Induced Gastric High Acidity by Calcium Ascorbate In Vitro and In Vivo. Korean J. Physiol. Pharmacol. 2018, 22, 35–42. [Google Scholar] [CrossRef]
- Huang, Q.; Shen, W.; Song, W. Synthesis of Colourless Silver Precursor Ink for Printing Conductive Patterns on Silicon Nitride Substrates. Appl. Surf. Sci. 2012, 258, 7384–7388. [Google Scholar] [CrossRef]
- Kiefer, O.; Breitkreutz, J. Comparative Investigations on Key Factors and Print Head Designs for Pharmaceutical Inkjet Printing. Int. J. Pharm. 2020, 586, 119561. [Google Scholar] [CrossRef]
- Raijada, D.; Genina, N.; Fors, D.; Wisaeus, E.; Peltonen, J.; Rantanen, J.; Sandler, N. A Step toward Development of Printable Dosage Forms for Poorly Soluble Drugs. J. Pharm. Sci. 2013, 102, 3694–3704. [Google Scholar] [CrossRef]
- Sun, J.; Wei, X.; Huang, B. Influence of the Viscosity of Edible Ink to Piezoelectric Ink-Jet Printing Drop State. Appl. Mech. Mater. 2012, 200, 676–680. [Google Scholar] [CrossRef]
- Pekarovicova, A.; Husovska, V. 3—Printing Ink Formulations. In Printing on Polymers; Izdebska, J., Thomas, S., Eds.; William Andrew Publishing: New York, NY, USA, 2016; pp. 41–55. ISBN 978-0-323-37468-2. [Google Scholar]
- Song, C.; Pei, B.; Jiang, M.; Wang, B.; Xu, D.; Chen, Y. Numerical Analysis of Forces Exerted on Particles in Cyclone Separators. Powder Technol. 2016, 294, 347–448. [Google Scholar] [CrossRef]



| Emulsion | H2O to CHCl3 Ratio (w/w) | Droplet Size [nm] | PDI |
|---|---|---|---|
| EMU 1 | 4:1 | 216.2 ± 11.3 | 0.118 ± 0.024 |
| EMU 2 | 3:1 | 253.9 ± 9.4 | 0.179 ± 0.017 |
| EMU 3 | 5:2 | 289.0 ± 9.7 | 0.258 ± 0.021 |
| EMU 4 | 2:1 | 342.3 ± 10.2 | 0.268 ± 0.018 |
| Exp |
Inlet Temperature [°C] |
Drying Gas Flow [%] | Nozzle Pressure [mm] |
Spraying Rate [%] | Nozzle Geometry [mm] |
d(50) Collector
[μm] |
d(50) Cyclone [μm] |
d(50) Combined Product [μm] |
|---|---|---|---|---|---|---|---|---|
| E1 | 140 | 75 | 50 | 7 | 0.375 | 12.99 | 10.31 | 11.08 |
| E2 | 170 | 100 | 60 | 1 | 0.75 | 22.25 | 33.65 | 33.65 |
| E3 | 140 | 100 | 50 | 7 | 0.375 | 14.72 | 10.31 | 11.03 |
| E4 | 140 | 75 | 60 | 7 | 0.375 | 11.85 | 8.61 | 8.69 |
| E5 | 110 | 50 | 40 | 1 | 0 | 98.77 | 74.06 | 74.06 |
| E6 | 110 | 50 | 40 | 1 | 0.75 | 97.01 | 53.28 | 62.28 |
| E7 | 110 | 100 | 40 | 1 | 0.75 | 124.22 | 83.24 | 80.11 |
| E8 | 110 | 50 | 40 | 13 | 0 | 347.39 | 25.63 | 25.63 |
| E9 | 140 | 75 | 50 | 1 | 0.375 | 30.58 | 38.73 | 32.26 |
| E10 | 170 | 100 | 40 | 13 | 0 | 19.7 | 11.21 | 12.10 |
| E11 | 110 | 100 | 40 | 1 | 0 | 65.4 | 70.27 | 70.27 |
| E12 | 140 | 75 | 50 | 7 | 0.75 | 15.66 | 11.16 | 11.61 |
| E13 | 110 | 100 | 60 | 1 | 0.75 | 36.06 | 20.51 | 18.37 |
| E14 | 170 | 75 | 50 | 7 | 0.375 | 7.76 | 7.35 | 7.93 |
| E15 | 170 | 100 | 40 | 1 | 0 | 52.66 | 92.53 | 92.53 |
| E16 | 110 | 75 | 50 | 7 | 0.375 | 6.93 | 12.48 | 12.48 |
| E17 | 170 | 100 | 40 | 1 | 0.75 | 44.29 | 80.43 | 80.43 |
| E18 | 170 | 50 | 60 | 1 | 0 | 17.03 | 15.41 | 16.98 |
| E19 | 110 | 100 | 40 | 13 | 0.75 | 50.79 | 68.07 | 68.07 |
| E20 | 140 | 50 | 50 | 7 | 0.375 | 107.69 | 10.82 | 11.46 |
| E21 | 170 | 50 | 60 | 1 | 0.75 | 16.84 | 13.83 | 13.73 |
| E22 | 110 | 50 | 60 | 1 | 0 | 21.88 | 13.94 | 16.15 |
| E23 | 170 | 50 | 40 | 13 | 0.75 | 71.64 | 15.19 | 25.48 |
| E24 | 170 | 100 | 60 | 13 | 0.75 | 22.26 | 7.96 | 8.68 |
| E25 | 110 | 100 | 60 | 1 | 0 | 37.27 | 23.87 | 20.73 |
| E26 | 170 | 50 | 40 | 1 | 0 | 29.08 | 54.27 | 54.27 |
| E27 | 140 | 75 | 50 | 7 | 0 | 7.28 | 8.01 | 8.02 |
| E28 | 170 | 50 | 40 | 1 | 0.75 | 62.84 | 83.86 | 83.86 |
| E29 | 170 | 100 | 40 | 13 | 0.75 | 11.97 | 9.44 | 10.49 |
| E30 | 140 | 75 | 40 | 7 | 0.375 | 12.29 | 11.05 | 11.33 |
| E31 | 170 | 50 | 40 | 13 | 0 | 29.19 | 11.25 | 17.49 |
| E32 | 110 | 50 | 60 | 13 | 0.75 | 300.69 | 12.08 | 12.08 |
| E33 | 110 | 50 | 40 | 13 | 0.75 | 155.93 | 12.45 | 12.45 |
| E34 | 170 | 50 | 60 | 13 | 0 | 86.57 | 12.24 | 12.71 |
| E35 | 140 | 75 | 50 | 7 | 0.375 | 13.97 | 10.88 | 11.35 |
| E36 | 110 | 50 | 60 | 1 | 0.75 | 17.51 | 12.57 | 12.95 |
| E37 | 110 | 50 | 60 | 13 | 0 | 295.33 | 8.12 | 8.12 |
| E38 | 140 | 75 | 50 | 13 | 0.375 | 8.8 | 9.77 | 9.36 |
| E39 | 140 | 75 | 50 | 7 | 0.375 | 9.58 | 8.71 | 9.07 |
| E40 | 110 | 100 | 60 | 13 | 0 | 60.2 | 24.93 | 24.93 |
| E41 | 140 | 75 | 50 | 7 | 0.375 | 8.51 | 10.38 | 9.29 |
| E42 | 170 | 100 | 60 | 1 | 0 | 9.94 | 27.83 | 20.76 |
| E43 | 110 | 100 | 40 | 13 | 0 | 14.11 | 163.98 | 163.98 |
| E44 | 170 | 100 | 60 | 13 | 0 | 9.4 | 8.05 | 7.61 |
| E45 | 110 | 100 | 60 | 13 | 0.75 | 72.31 | 20.03 | 20.03 |
| E46 | 170 | 50 | 60 | 13 | 0.75 | 65.44 | 8.1 | 10.55 |
| E47 | 170 | 84.85 | 40 | 13 | 0.3788 | 10.37 | 8.98 | 9.15 |
| E48 | 170 | 100 | 60 | 8.39 | 0.2273 | 5.89 | 6.60 | 6.70 |
| Antiadhesives | Sampling Location | d10 [μm] | d50 [μm] | d90 [μm] | SPAN |
|---|---|---|---|---|---|
| Lactose | Collection vessel | 1.17 ± 0.28 | 5.36 ± 0.71 | 20.56 ± 2.41 | 3.62 ± 0.11 |
| Cyclone | 1.30 ± 0.45 | 6.11 ± 0.96 | 34.71 ± 2.78 | 5.47 ± 0.23 | |
| NCC/Lactose | Collection vessel | 1.15 ± 0.37 | 5.47 ± 0.88 | 15.76 ± 1.71 | 2.67 ± 0.08 |
| Cyclone | 1.38 ± 0.38 | 7.22 ± 1.01 | 18.71 ± 3.02 | 2.40 ± 0.24 | |
| CaSi | Collection vessel | 1.96 ± 0.28 | 14.81 ± 0.11 | 44.56 ± 0.56 | 2.88 ± 0.15 |
| Cyclone | 2.00 ± 0.24 | 21.51 ± 0.65 | 64.71 ± 0.78 | 2.92 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sterle Zorec, B.; Dreu, R. Development of Simvastatin-Loaded Particles Using Spray Drying Method for Ex Tempore Preparation of Cartridges for 2D Printing Technology. Pharmaceutics 2023, 15, 2221. https://doi.org/10.3390/pharmaceutics15092221
Sterle Zorec B, Dreu R. Development of Simvastatin-Loaded Particles Using Spray Drying Method for Ex Tempore Preparation of Cartridges for 2D Printing Technology. Pharmaceutics. 2023; 15(9):2221. https://doi.org/10.3390/pharmaceutics15092221
Chicago/Turabian StyleSterle Zorec, Barbara, and Rok Dreu. 2023. "Development of Simvastatin-Loaded Particles Using Spray Drying Method for Ex Tempore Preparation of Cartridges for 2D Printing Technology" Pharmaceutics 15, no. 9: 2221. https://doi.org/10.3390/pharmaceutics15092221




