Hyperbranched Polymers: Recent Advances in Photodynamic Therapy against Cancer
Abstract
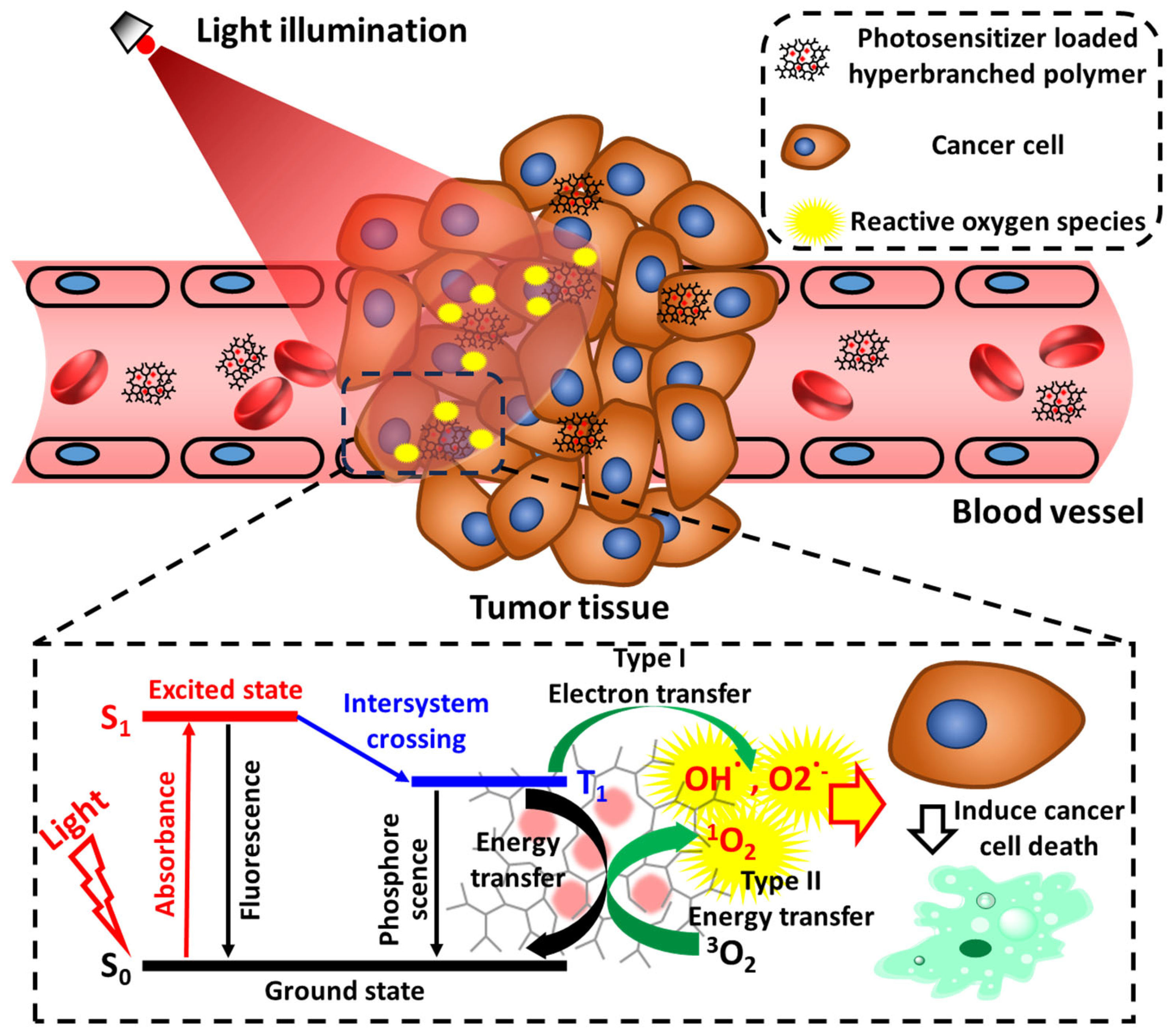
:1. Introduction
2. Hyperbranched Polymers
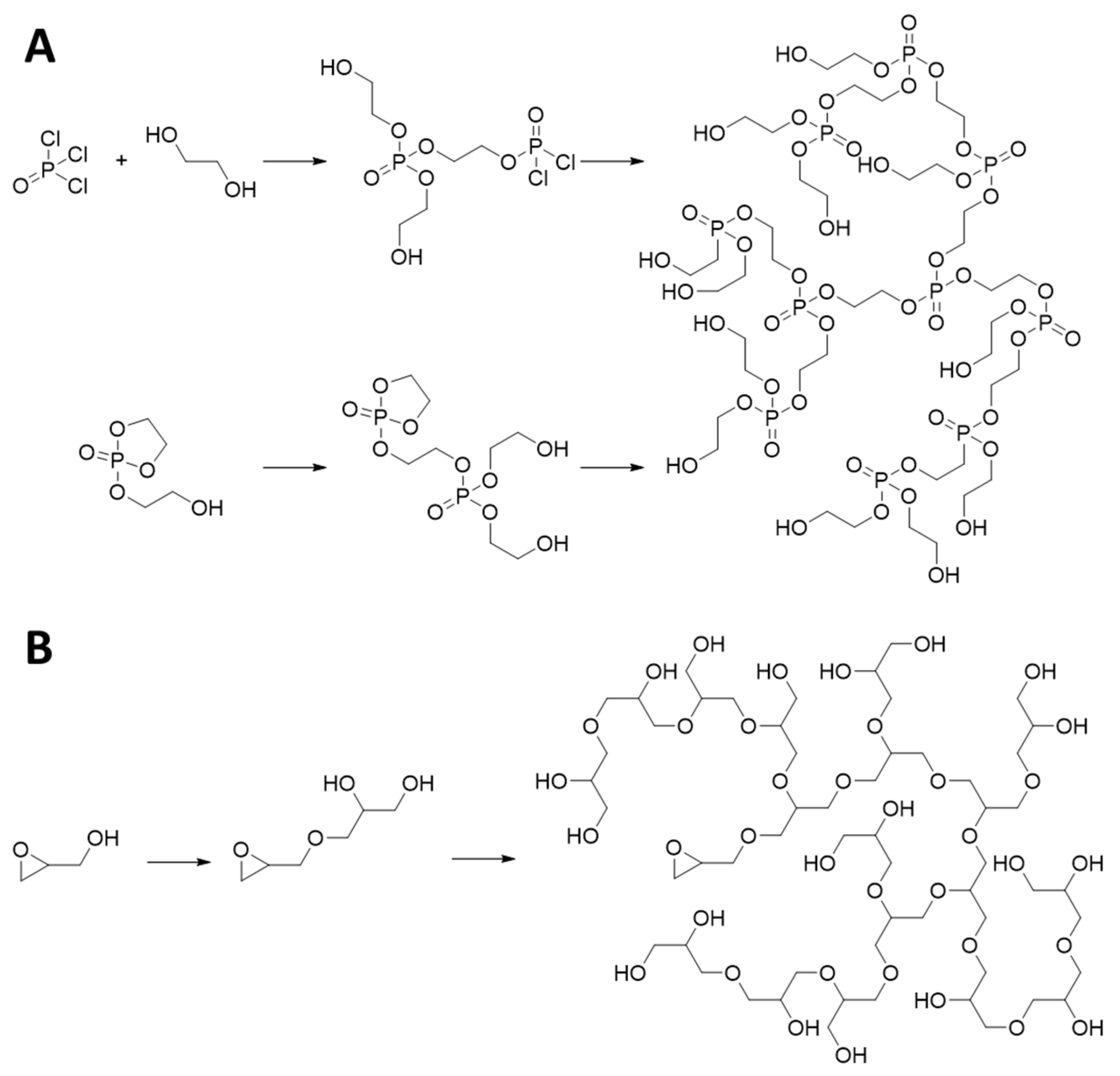
2.1. Hyperbranched-Polymer Synthesis
2.2. Stimulus-Responsive Hyperbranched Polymers
| Stimulus | Responsive Moiety | Nanocarriers | PS and/or Anticancer Drug | Cumulative Release in Physiological Environment | Cumulative Release Triggered by Internal/External Stimuli | Applications | Reference |
|---|---|---|---|---|---|---|---|
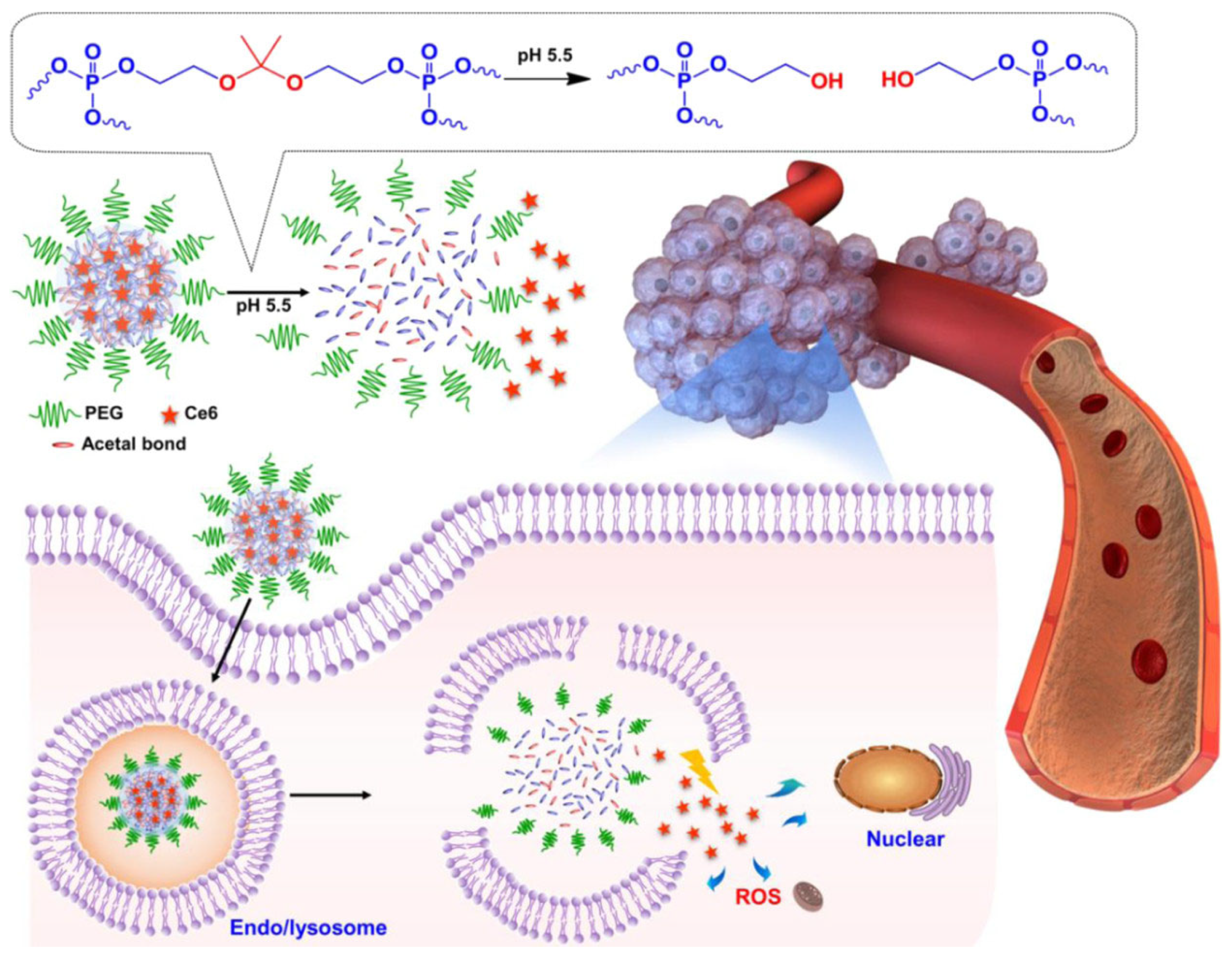
| pH | Acetal | HB polyphosphate | Ce6 | 27% release of PS over 20 h at pH 7.4 | 50% release of PS over 20 h at pH 5.5 | PDT | [52] |
| Benzacetal | HB polyglycerol | Temoporfin | - | - | PDT | [78] | |
| Amine | Cellulose nanofibril grafted with HB polyamines | Indocyanine green and doxorubicin | - | - | PDT/chemotherapy | [79] | |
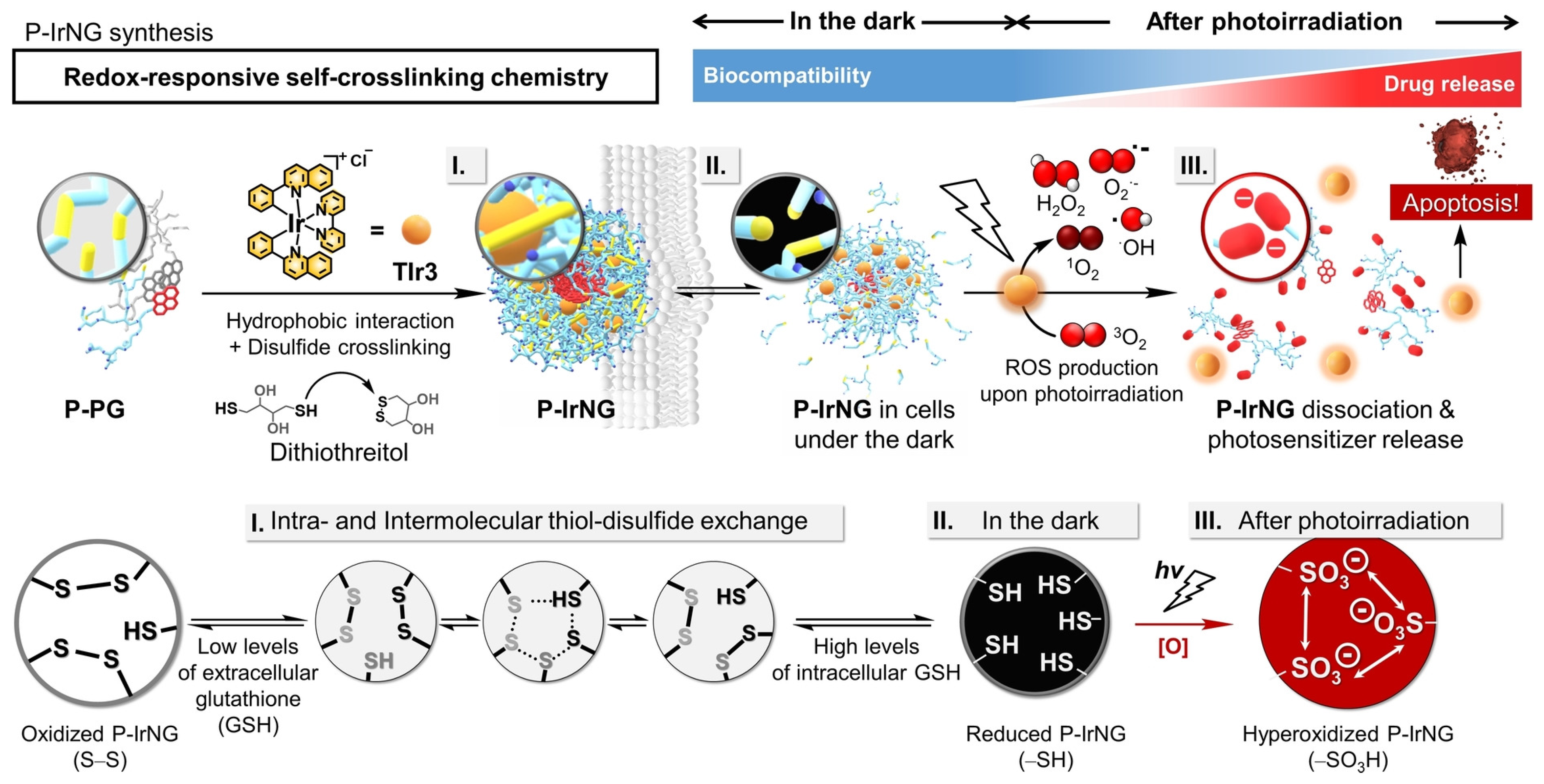
| Redox | Disulfide | HB polyglycerol | Temoporfin | - | - | PDT | [78] |
| Disulfide | HB Ce6 | Ce6 | - | - | PDT | [80] | |
| ROS | Thioether | Synthetic HB polymer constructed using MTPA and TMPTGE | Ce6 and paclitaxel | 35.4% release of paclitaxel without light illumination over 24 h | 74.8% release of paclitaxel upon 660 nm laser illumination over 24 h | PDT/chemotherapy | [41] |
| Diselenide | HB porphyrin | Porphyrin | - | - | PDT/chemotherapy | [81] | |
| Thioketal | HB polyphosphate | Ce6 and doxorubicin | 8% release of doxorubicin without light illumination over 24 h | 50% release of doxorubicin upon 660 nm laser illumination over 24 h | PDT/chemotherapy | [51] | |
| Thioketal | HB polyphosphate | Ce6 and camptothecin | 1.1% release of camptothecin without light illumination | 10.9% release of camptothecin upon 660 nm laser illumination for 5 min | PDT/chemotherapy | [53] |
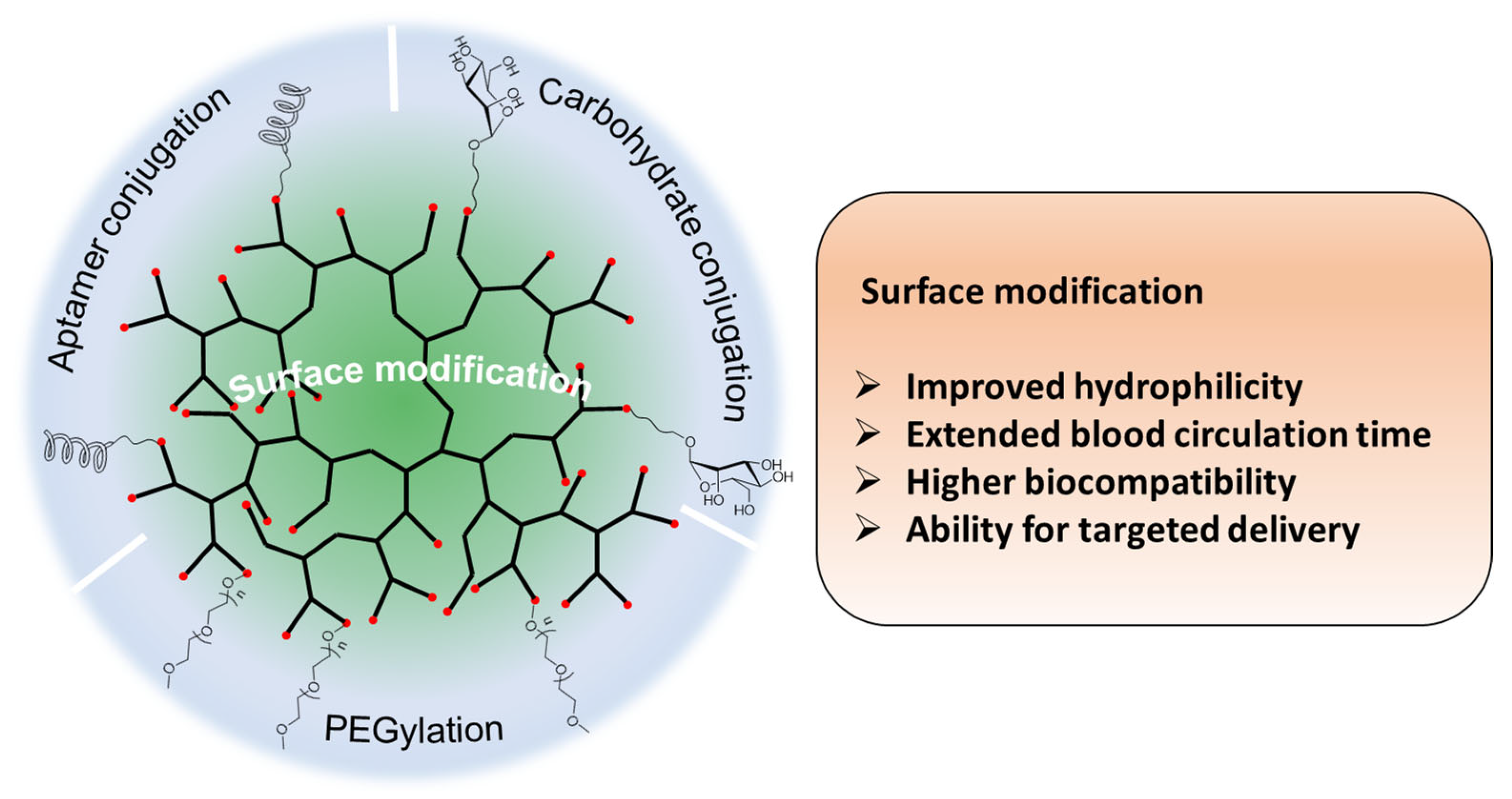
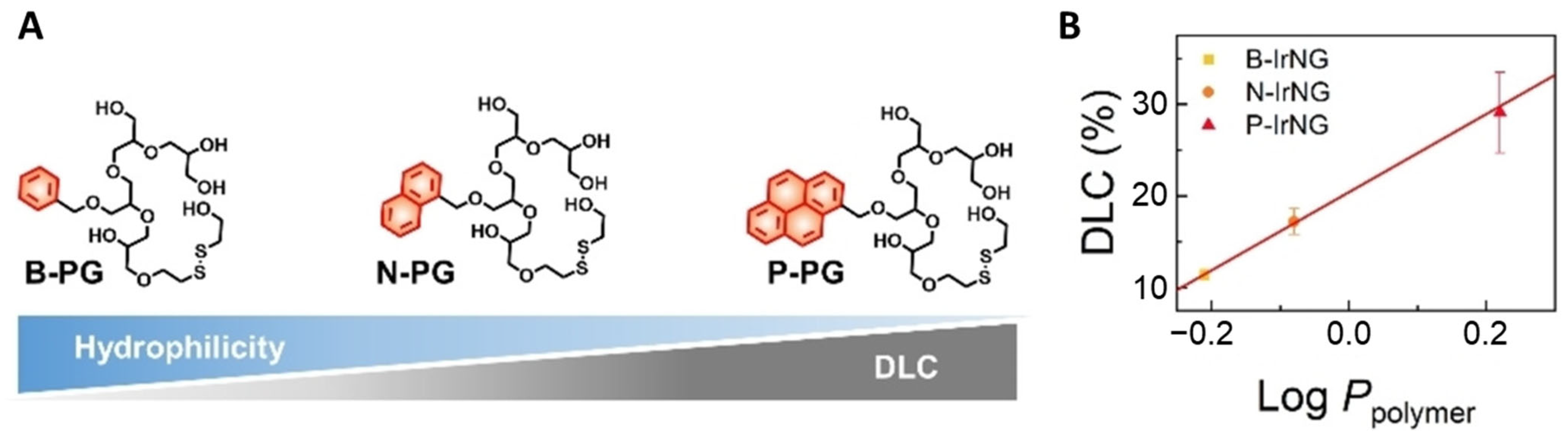
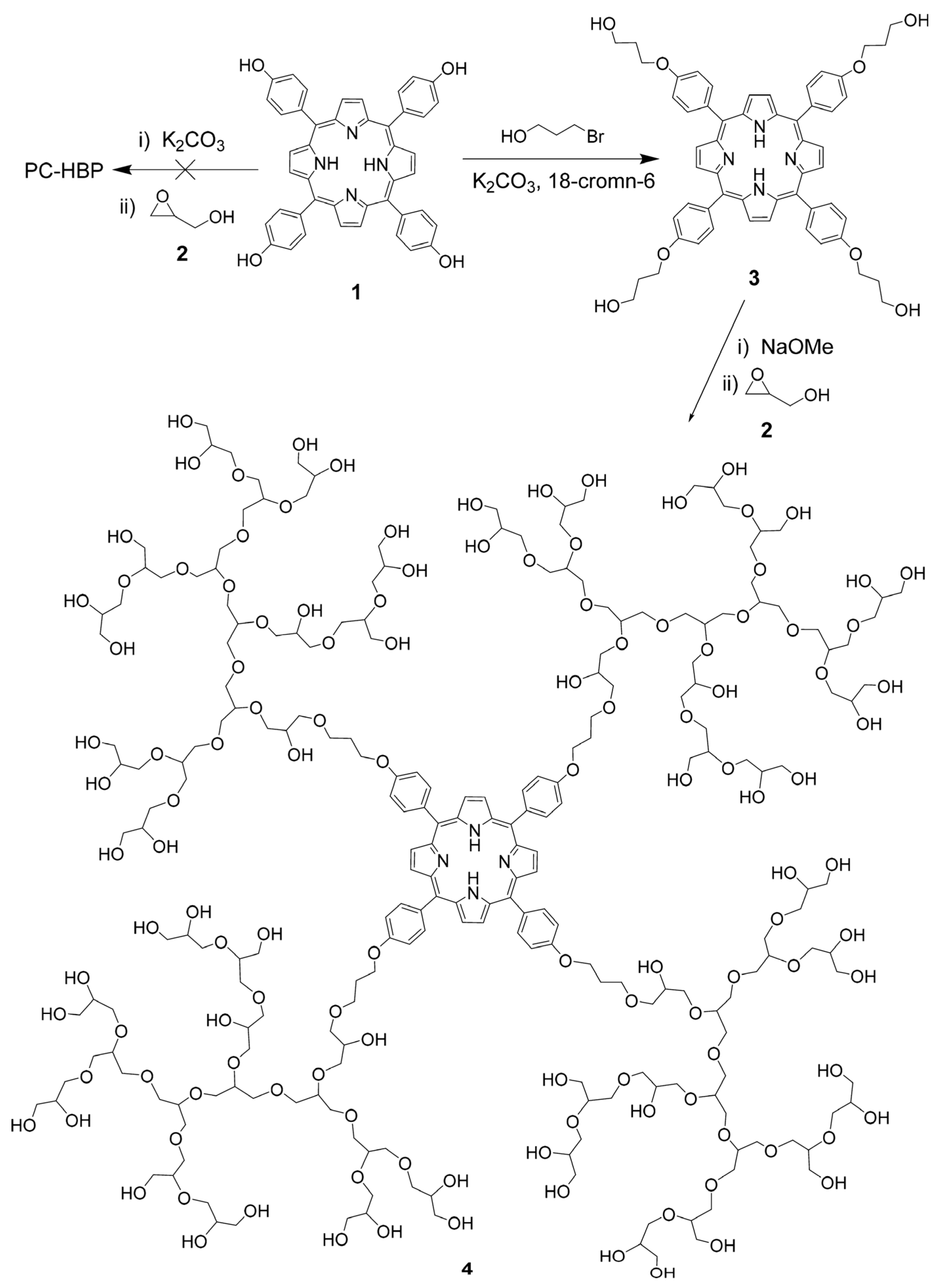
2.3. Surface Modification of Hyperbranched Polymers
3. Photosensitizers Utilized with Hyperbranched Polymers
3.1. Photosensitizers Encapsulated in Hyperbranched Polymers
3.2. Photosensitizers Covalently Conjugated to Hyperbranched Polymers
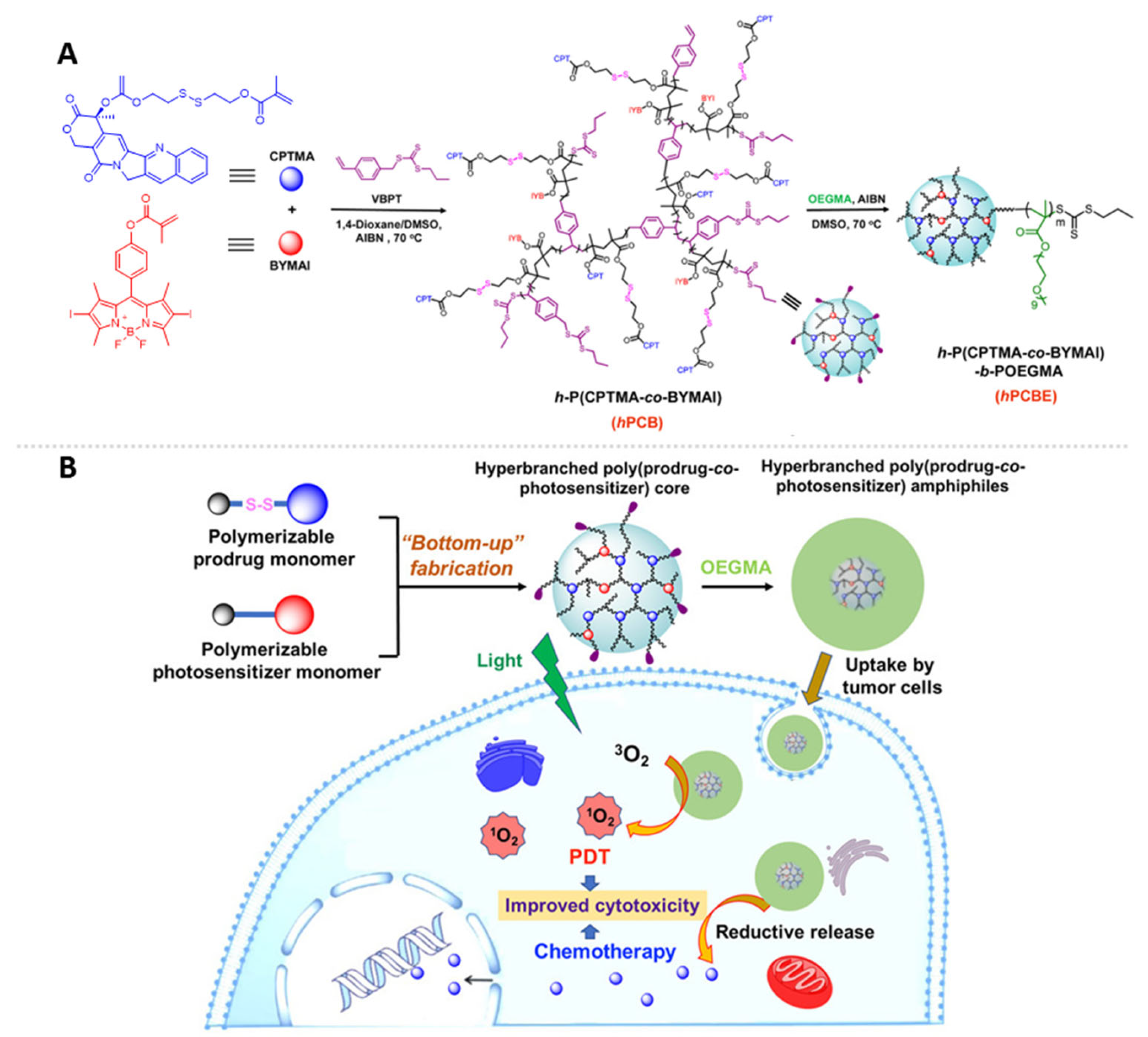
3.3. Hyperbranched Polymer Constructed with Photosensitizers
4. Chemo-/Photodynamic Combination Therapy
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Royce, T.J.; Qureshi, M.M.; Truong, M.T. Radiotherapy Utilization and Fractionation Patterns During the First Course of Cancer Treatment in the United States From 2004 to 2014. J. Am. Coll. Radiol. 2018, 15, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: From molecular design to application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar]
- Yao, Q.; Fan, J.; Long, S.; Zhao, X.; Li, H.; Du, J.; Shao, K.; Peng, X. The concept and examples of type-III photosensitizers for cancer photodynamic therapy. Chem 2022, 8, 197–209. [Google Scholar]
- Pham, T.C.; Nguyen, V.-N.; Choi, Y.; Lee, S.; Yoon, J. Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar]
- Monfrecola, G.; Megna, M.; Rovati, C.; Arisi, M.; Rossi, M.; Calzavara-Pinton, I.; Fabbrocini, G.; Calzavara-Pinton, P. A Critical Reappraisal of Off-Label Use of Photodynamic Therapy for the Treatment of Non-Neoplastic Skin Conditions. Dermatology 2021, 237, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Karrer, S.; Szeimies, R.M.; Philipp-Dormston, W.G.; Gerber, P.A.; Prager, W.; Datz, E.; Zeman, F.; Müller, K.; Koller, M. Repetitive Daylight Photodynamic Therapy versus Cryosurgery for Prevention of Actinic Keratoses in Photodamaged Facial Skin: A Prospective, Randomized Controlled Multicentre Two-armed Study. Acta Derm. Venereol. 2021, 101, adv00355. [Google Scholar] [CrossRef]
- Van Rijssen, T.J.; van Dijk, E.H.C.; Tsonaka, R.; Feenstra, H.M.A.; Dijkman, G.; Peters, P.J.H.; Diederen, R.M.H.; Hoyng, C.B.; Schlingemann, R.O.; Boon, C.J.F. Half-Dose Photodynamic Therapy Versus Eplerenone in Chronic Central Serous Chorioretinopathy (SPECTRA): A Randomized Controlled Trial. Am. J. Ophthalmol. 2022, 233, 101–110. [Google Scholar] [PubMed]
- Tichaczek-Goska, D.; Wojnicz, D.; Symonowicz, K.; Ziółkowski, P.; Hendrich, A.B. Photodynamic enhancement of the activity of antibiotics used in urinary tract infections. Lasers Med. Sci. 2019, 34, 1547–1553. [Google Scholar] [PubMed]
- Kimura, T.; Takatsuki, S.; Miyoshi, S.; Takahashi, M.; Ogawa, E.; Nakajima, K.; Kashimura, S.; Katsumata, Y.; Nishiyama, T.; Nishiyama, N.; et al. Electrical superior vena cava isolation using photodynamic therapy in a canine model. Europace 2015, 18, 294–300. [Google Scholar] [CrossRef]
- Dias, L.D.; Bagnato, V.S. An update on clinical photodynamic therapy for fighting respiratory tract infections: A promising tool against COVID-19 and its co-infections. Laser Phys. Lett. 2020, 17, 083001. [Google Scholar]
- Lashkari, S.M.; Kariminezhad, H.; Amani, H.; Mataji, P.; Rahimnejad, M. Introduction of 5-aminolevulinic acid as a theranostics agent in dentistry. Photodiagnosis Photodyn. Ther. 2019, 25, 336–343. [Google Scholar] [PubMed]
- Luby, B.M.; Walsh, C.D.; Zheng, G. Advanced Photosensitizer Activation Strategies for Smarter Photodynamic Therapy Beacons. Angew. Chem. Int. Ed. 2019, 58, 2558–2569. [Google Scholar] [CrossRef] [PubMed]
- Dysart, J.S.; Patterson, M.S. Characterization of Photofrin photobleaching for singlet oxygen dose estimation during photodynamic therapy of MLL cells in vitro. Phys. Med. Bio. 2005, 50, 2597. [Google Scholar] [CrossRef]
- Yuan, J.; Peng, R.; Su, D.; Zhang, X.; Zhao, H.; Zhuang, X.; Chen, M.; Zhang, X.; Yuan, L. Cell membranes targeted unimolecular prodrug for programmatic photodynamic-chemo therapy. Theranostics 2021, 11, 3502–3511. [Google Scholar] [CrossRef]
- Cheng, X.; Gao, J.; Ding, Y.; Lu, Y.; Wei, Q.; Cui, D.; Fan, J.; Li, X.; Zhu, E.; Lu, Y.; et al. Multi-Functional Liposome: A Powerful Theranostic Nano-Platform Enhancing Photodynamic Therapy. Adv. Sci. 2021, 8, 2100876. [Google Scholar]
- Xie, J.; Wang, Y.; Choi, W.; Jangili, P.; Ge, Y.; Xu, Y.; Kang, J.; Liu, L.; Zhang, B.; Xie, Z.; et al. Overcoming barriers in photodynamic therapy harnessing nano-formulation strategies. Chem. Soc. Rev. 2021, 50, 9152–9201. [Google Scholar]
- Dhaini, B.; Wagner, L.; Moinard, M.; Daouk, J.; Arnoux, P.; Schohn, H.; Schneller, P.; Acherar, S.; Hamieh, T.; Frochot, C. Importance of Rose Bengal Loaded with Nanoparticles for Anti-Cancer Photodynamic Therapy. Pharmaceuticals 2022, 15, 1093. [Google Scholar]
- Dhaini, B.; Daouk, J.; Schohn, H.; Jouan-Hureaux, V.; Acherar, S.; Arnoux, P.; Rocchi, P.; Lux, F.; Tillement, O.; Hamieh, T.; et al. Rose Bengal coupled to AGuIX NPs for anti-cancer photodynamic therapy. Photodiagnosis Photodyn. Ther. 2023, 41, 103424. [Google Scholar] [CrossRef]
- Youssef, Z.; Vanderesse, R.; Colombeau, L.; Baros, F.; Roques-Carmes, T.; Frochot, C.; Wahab, H.; Toufaily, J.; Hamieh, T.; Acherar, S.; et al. The application of titanium dioxide, zinc oxide, fullerene, and graphene nanoparticles in photodynamic therapy. Cancer Nanotechnol. 2017, 8, 6. [Google Scholar]
- Youssef, Z.; Arnoux, P.; Colombeau, L.; Moussaron, A.; Toufaily, J.; Hamieh, T.; Frochot, C.; Roques-Carmes, T. Two approaches for elaborating sensitized TiO2 nanoparticles of potential effect in photodynamic therapy. Photodiagnosis Photodyn. Ther. 2017, 17, A61–A62. [Google Scholar] [CrossRef]
- Lu, D.; Tao, R.; Wang, Z. Carbon-based materials for photodynamic therapy: A mini-review. Front. Chem. Sci. Eng. 2019, 13, 310–323. [Google Scholar]
- Chen, Y.; Ma, H.; Wang, W.; Zhang, M. A size-tunable nanoplatform: Enhanced MMP2-activated chemo-photodynamic immunotherapy based on biodegradable mesoporous silica nanoparticles. Biomater. Sci. 2021, 9, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Youssef, Z.; Jouan-Hureaux, V.; Colombeau, L.; Arnoux, P.; Moussaron, A.; Baros, F.; Toufaily, J.; Hamieh, T.; Roques-Carmes, T.; Frochot, C. Titania and silica nanoparticles coupled to Chlorin e6 for anti-cancer photodynamic therapy. Photodiagnosis Photodyn. Ther. 2018, 22, 115–126. [Google Scholar] [PubMed]
- Gong, B.; Shen, Y.; Li, H.; Li, X.; Huan, X.; Zhou, J.; Chen, Y.; Wu, J.; Li, W. Thermo-responsive polymer encapsulated gold nanorods for single continuous wave laser-induced photodynamic/photothermal tumour therapy. J. Nanobiotechnol. 2021, 19, 41. [Google Scholar]
- Younis, M.R.; He, G.; Qu, J.; Lin, J.; Huang, P.; Xia, X.-H. Inorganic Nanomaterials with Intrinsic Singlet Oxygen Generation for Photodynamic Therapy. Adv. Sci. 2021, 8, 2102587. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Fang, L.; Gao, C.; Xu, C.; Gou, S. Iridium(III) Complex–Derived Polymeric Micelles with Low Dark Toxicity and Strong NIR Excitation for Phototherapy and Chemotherapy. Small 2020, 16, 2000363. [Google Scholar] [CrossRef]
- Shi, L.; Hu, F.; Duan, Y.; Wu, W.; Dong, J.; Meng, X.; Zhu, X.; Liu, B. Hybrid Nanospheres to Overcome Hypoxia and Intrinsic Oxidative Resistance for Enhanced Photodynamic Therapy. ACS Nano 2020, 14, 2183–2190. [Google Scholar]
- Kawasaki, R.; Ohdake, R.; Yamana, K.; Eto, T.; Sugikawa, K.; Ikeda, A. Photodynamic therapy using self-assembled nanogels comprising chlorin e6-bearing pullulan. J. Mater. Chem. B 2021, 9, 6357–6363. [Google Scholar]
- Ouyang, Z.; Gao, Y.; Shen, M.; Shi, X. Dendrimer-based nanohybrids in cancer photomedicine. Mater. Today Bio 2021, 10, 100111. [Google Scholar] [PubMed]
- Xiang, B.; Xue, Y.; Liu, Z.; Tian, J.; Frey, H.; Gao, Y.; Zhang, W. Water-soluble hyperbranched polyglycerol photosensitizer for enhanced photodynamic therapy. Polym. Chem. 2020, 11, 3913–3921. [Google Scholar]
- Chen, L.; Huang, J.; Li, X.; Huang, M.; Zeng, S.; Zheng, J.; Peng, S.; Li, S. Progress of Nanomaterials in Photodynamic Therapy Against Tumor. Front. Bioeng. Biotechnol. 2022, 10, 920162. [Google Scholar] [PubMed]
- He, J.; Liu, S.; Zhang, Y.; Chu, X.; Lin, Z.; Zhao, Z.; Qiu, S.; Guo, Y.; Ding, H.; Pan, Y.; et al. The Application of and Strategy for Gold Nanoparticles in Cancer Immunotherapy. Front. Pharmacol. 2021, 12, 503–514. [Google Scholar]
- Lee, D.; Kwon, S.; Jang, S.-y.; Park, E.; Lee, Y.; Koo, H. Overcoming the obstacles of current photodynamic therapy in tumors using nanoparticles. Bioact. Mater. 2022, 8, 20–34. [Google Scholar]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [PubMed]
- Yakavets, I.; Millard, M.; Zorin, V.; Lassalle, H.-P.; Bezdetnaya, L. Current state of the nanoscale delivery systems for temoporfin-based photodynamic therapy: Advanced delivery strategies. J. Control Release 2019, 304, 268–287. [Google Scholar]
- Sun, W.; Zhao, X.; Fan, J.; Du, J.; Peng, X. Boron Dipyrromethene Nano-Photosensitizers for Anticancer Phototherapies. Small 2019, 15, 1804927. [Google Scholar] [CrossRef]
- Wang, G.; Huang, P.; Wang, L.; Chen, X.; Zhou, Y.; Huang, W.; Yan, D. ROS-responsive thioether-containing hyperbranched polymer micelles for light-triggered drug release. SmartMat 2022, 3, 522–531. [Google Scholar] [CrossRef]
- Pan, D.; Zheng, X.; Zhang, L.; Li, X.; Zhu, G.; Gong, M.; Kopytynski, M.; Zhou, L.; Yi, Y.; Zhu, H.; et al. Synergistic Disruption of Metabolic Homeostasis through Hyperbranched Poly(ethylene glycol) Conjugates as Nanotherapeutics to Constrain Cancer Growth. Adv. Mater. 2022, 34, 2109036. [Google Scholar] [CrossRef]
- Lee, C.G.; Lee, C.; Lee, J.; Nam, J.S.; Kim, B.-S.; Kwon, T.-H. Dual-Modulated Release of a Cytotoxic Photosensitizer Using Photogenerated Reactive Oxygen Species and Glutathione. Angew. Chem. Int. Ed. 2022, 61, e202210623. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Pan, D.; Chen, X.; Wu, L.; Chen, M.; Wang, W.; Zhang, H.; Gong, Q.; Gu, Z.; Luo, K. Self-Stabilized Supramolecular Assemblies Constructed from PEGylated Dendritic Peptide Conjugate for Augmenting Tumor Retention and Therapy. Adv. Sci. 2021, 8, 2102741. [Google Scholar]
- Zhang, Y.; Üçüncü, M.; Gambardella, A.; Baibek, A.; Geng, J.; Zhang, S.; Clavadetscher, J.; Litzen, I.; Bradley, M.; Lilienkampf, A. Bioorthogonal Swarming: In Situ Generation of Dendrimers. J. Am. Chem. Soc. 2020, 142, 21615–21621. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular Size Distribution in Three Dimensional Polymers. VI. Branched Polymers Containing A—R—Bf-1 Type Units. J. Am. Chem. Soc. 1952, 74, 2718–2723. [Google Scholar] [CrossRef]
- Liu, J.; Huang, W.; Pang, Y.; Zhu, X.; Zhou, Y.; Yan, D. Hyperbranched Polyphosphates for Drug Delivery Application: Design, Synthesis, and In Vitro Evaluation. Biomacromolecules 2010, 11, 1564–1570. [Google Scholar] [CrossRef]
- Liu, J.; Huang, W.; Pang, Y.; Yan, D. Hyperbranched polyphosphates: Synthesis, functionalization and biomedical applications. Chem. Soc. Rev. 2015, 44, 3942–3953. [Google Scholar] [PubMed]
- Tambe, P.; Sayed, N.; Paknikar, K.M.; Gajbhiye, V. Chapter 7—Poly(Phospho Ester) and Poly(Phosphazene) Nanoparticles as a Promising Tool for Anticancer Therapeutics. In Polymeric Nanoparticles as a Promising Tool for Anti-Cancer Therapeutics; Kesharwani, P., Paknikar, K.M., Gajbhiye, V., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 123–146. [Google Scholar]
- Kurniasih, I.N.; Keilitz, J.; Haag, R. Dendritic nanocarriers based on hyperbranched polymers. Chem. Soc. Rev. 2015, 44, 4145–4164. [Google Scholar]
- Sun, C.Y.; Cao, Z.Y.; Zhang, X.J.; Sun, R.; Yu, C.S.; Yang, X.Z. Cascade-amplifying synergistic effects of chemo-photodynamic therapy using ROS-responsive polymeric nanocarriers. Theranostics 2018, 8, 2939–2953. [Google Scholar] [CrossRef]
- Li, F.; Chen, C.; Yang, X.X.; He, X.Y.; Zhao, Z.Y.; Li, J.; Yu, Y.; Yang, X.Z.; Wang, J. Acetal-Linked Hyperbranched Polyphosphoester Nanocarriers Loaded with Chlorin e6 for pH-Activatable Photodynamic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 21198–21205. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, T.; Huang, X.; Sun, M.; Li, H.; Zhu, X.; Liu, M.; Xie, Y.; Huang, W.; Yan, D. ROS-responsive nanoparticles based on amphiphilic hyperbranched polyphosphoester for drug delivery: Light-triggered size-reducing and enhanced tumor penetration. Biomaterials 2019, 211, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Voit, B.I.; Lederer, A. Hyperbranched and Highly Branched Polymer Architectures—Synthetic Strategies and Major Characterization Aspects. Chem. Rev. 2009, 109, 5924–5973. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shi, W. Synthesis and thermal decomposition of a novel hyperbranched polyphosphate ester used for flame retardant systems. Polym. Degrad. Stab. 2006, 91, 1289–1294. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, W. Synthesis and properties of a novel hyperbranched polyphosphate acrylate applied to UV curable flame retardant coatings. Eur. Polym. J. 2007, 43, 1302–1312. [Google Scholar] [CrossRef]
- Yao, M.; Ma, Y.; Liu, H.; Khan, M.I.; Shen, S.; Li, S.; Zhao, Y.; Liu, Y.; Zhang, G.; Li, X.; et al. Enzyme Degradable Hyperbranched Polyphosphoester Micellar Nanomedicines for NIR Imaging-Guided Chemo-Photothermal Therapy of Drug-Resistant Cancers. Biomacromolecules 2018, 19, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Li, D.; Wang, J.; Xiong, M.; Yang, X. Direct Nucleus-Targeted Drug Delivery Using Cascade pHe/Photo Dual-Sensitive Polymeric Nanocarrier for Cancer Therapy. Small 2019, 15, 1902022. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, P.; Cao, Z.; Ma, Y.; Li, J.; Qian, H.; Tao, W.; Yang, X. PEGylated hyperbranched polyphosphoester based nanocarriers for redox-responsive delivery of doxorubicin. Biomater. Sci. 2016, 4, 412–417. [Google Scholar] [CrossRef]
- Pouyan, P.; Cherri, M.; Haag, R. Polyglycerols as Multi-Functional Platforms: Synthesis and Biomedical Applications. Polymers 2022, 14, 2684. [Google Scholar] [CrossRef]
- Rahman, M.; Alrobaian, M.; Almalki, W.H.; Mahnashi, M.H.; Alyami, B.A.; Alqarni, A.O.; Alqahtani, Y.S.; Alharbi, K.S.; Alghamdi, S.; Panda, S.K.; et al. Superbranched polyglycerol nanostructures as drug delivery and theranostics tools for cancer treatment. Drug Disco. Today 2021, 26, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Braatz, D.; Cherri, M.; Tully, M.; Dimde, M.; Ma, G.; Mohammadifar, E.; Reisbeck, F.; Ahmadi, V.; Schirner, M.; Haag, R. Chemical Approaches to Synthetic Drug Delivery Systems for Systemic Applications. Angew. Chem. Int. Ed. 2022, 61, e202203942. [Google Scholar] [CrossRef] [PubMed]
- Calderón, M.; Quadir, M.A.; Sharma, S.K.; Haag, R. Dendritic Polyglycerols for Biomedical Applications. Adv. Mater. 2010, 22, 190–218. [Google Scholar] [CrossRef]
- Roller, S.; Zhou, H.; Haag, R. High-loading polyglycerol supported reagents for Mitsunobu- and acylation-reactions and other useful polyglycerol derivatives. Mol. Divers. 2005, 9, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Bohrmann, L.; Burghardt, T.; Rodríguez-Rodríguez, C.; Herth, M.M.; Saatchi, K.; Häfeli, U.O. Quantitative Evaluation of a Multimodal Aptamer-Targeted Long-Circulating Polymer for Tumor Targeting. ACS Omega 2023, 8, 11003–11020. [Google Scholar] [CrossRef] [PubMed]
- Abbina, S.; Vappala, S.; Kumar, P.; Siren, E.M.J.; La, C.C.; Abbasi, U.; Brooks, D.E.; Kizhakkedathu, J.N. Hyperbranched polyglycerols: Recent advances in synthesis, biocompatibility and biomedical applications. J. Mater. Chem. B 2017, 5, 9249–9277. [Google Scholar] [CrossRef] [PubMed]
- Dworak, A.; Walach, W.; Trzebicka, B. Cationic polymerization of glycidol. Polymer structure and polymerization mechanism. Macromol. Chem. Phys. 1995, 196, 1963–1970. [Google Scholar] [CrossRef]
- Sunder, A.; Hanselmann, R.; Frey, H.; Mülhaupt, R. Controlled Synthesis of Hyperbranched Polyglycerols by Ring-Opening Multibranching Polymerization. Macromolecules 1999, 32, 4240–4246. [Google Scholar] [CrossRef]
- Wilms, D.; Wurm, F.; Nieberle, J.; Böhm, P.; Kemmer-Jonas, U.; Frey, H. Hyperbranched Polyglycerols with Elevated Molecular Weights: A Facile Two-Step Synthesis Protocol Based on Polyglycerol Macroinitiators. Macromolecules 2009, 42, 3230–3236. [Google Scholar] [CrossRef]
- Shenoi, R.A.; Narayanannair, J.K.; Hamilton, J.L.; Lai, B.F.L.; Horte, S.; Kainthan, R.K.; Varghese, J.P.; Rajeev, K.G.; Manoharan, M.; Kizhakkedathu, J.N. Branched Multifunctional Polyether Polyketals: Variation of Ketal Group Structure Enables Unprecedented Control over Polymer Degradation in Solution and within Cells. J. Am. Chem. Soc. 2012, 134, 14945–14957. [Google Scholar] [CrossRef]
- Son, S.; Shin, E.; Kim, B.-S. Redox-Degradable Biocompatible Hyperbranched Polyglycerols: Synthesis, Copolymerization Kinetics, Degradation, and Biocompatibility. Macromolecules 2015, 48, 600–609. [Google Scholar] [CrossRef]
- Ding, H.; Tan, P.; Fu, S.; Tian, X.; Zhang, H.; Ma, X.; Gu, Z.; Luo, K. Preparation and application of pH-responsive drug delivery systems. J. Control. Release 2022, 348, 206–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; MacEwan, S.R.; Chilkoti, A. Quantitative Mapping of the Spatial Distribution of Nanoparticles in Endo-Lysosomes by Local pH. Nano Lett. 2017, 17, 1226–1232. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Z.; Jiang, X.; Rui, L.; Gao, Y.; Zhang, W. Unimolecular micelles from POSS-based star-shaped block copolymers for photodynamic therapy. Polymer 2017, 118, 268–279. [Google Scholar] [CrossRef]
- Cheng, D.-B.; Yang, P.-P.; Cong, Y.; Liu, F.-H.; Qiao, Z.-Y.; Wang, H. One-pot synthesis of pH-responsive hyperbranched polymer–peptide conjugates with enhanced stability and loading efficiency for combined cancer therapy. Polym. Chem. 2017, 8, 2462–2471. [Google Scholar] [CrossRef]
- Wu, H.; Zhong, D.; Zhang, Z.; Li, Y.; Zhang, X.; Li, Y.; Zhang, Z.; Xu, X.; Yang, J.; Gu, Z. Bioinspired Artificial Tobacco Mosaic Virus with Combined Oncolytic Properties to Completely Destroy Multidrug-Resistant Cancer. Adv. Mater. 2020, 32, 1904958. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Wu, H.; Wu, Y.; Li, Y.; Yang, J.; Gong, Q.; Luo, K.; Gu, Z. Redox dual-responsive dendrimeric nanoparticles for mutually synergistic chemo-photodynamic therapy to overcome drug resistance. J. Control. Release 2021, 329, 1210–1221. [Google Scholar] [CrossRef]
- Staegemann, M.H.; Gräfe, S.; Gitter, B.; Achazi, K.; Quaas, E.; Haag, R.; Wiehe, A. Hyperbranched Polyglycerol Loaded with (Zinc-)Porphyrins: Photosensitizer Release Under Reductive and Acidic Conditions for Improved Photodynamic Therapy. Biomacromolecules 2018, 19, 222–238. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, R.M.; Chen, Z.P.; Lu, Q.; Zhu, H.X.; Bu, Q.; Yin, J.L.; He, H. Bioinspired Multifunctional Cellulose Nanofibril-Based In Situ Liquid Wound Dressing for Multiple Synergistic Therapy of the Postoperative Infected Wound. ACS Appl. Mater. Interfaces 2021, 13, 51578–51591. [Google Scholar] [CrossRef]
- Jung, S.; Jung, S.; Kim, D.M.; Lim, S.-H.; Shim, Y.H.; Kwon, H.; Kim, D.H.; Lee, C.-M.; Kim, B.H.; Jeong, Y.-I. Hyaluronic Acid-Conjugated with Hyperbranched Chlorin e6 Using Disulfide Linkage and Its Nanophotosensitizer for Enhanced Photodynamic Therapy of Cancer Cells. Materials 2019, 12, 3080. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.X.; Ji, S.B.; Li, F.; Xu, H.P. Diselenide-Containing Hyperbranched Polymer with Light-Induced Cytotoxicity. Acs Appl. Mater. Interfaces 2017, 9, 12924–12929. [Google Scholar] [CrossRef]
- Quinn, J.F.; Whittaker, M.R.; Davis, T.P. Glutathione responsive polymers and their application in drug delivery systems. Polym. Chem. 2017, 8, 97–126. [Google Scholar] [CrossRef]
- Pan, D.; Zheng, X.; Zhang, Q.; Li, Z.; Duan, Z.; Zheng, W.; Gong, M.; Zhu, H.; Zhang, H.; Gong, Q.; et al. Dendronized-Polymer Disturbing Cells’ Stress Protection by Targeting Metabolism Leads to Tumor Vulnerability. Adv. Mater. 2020, 32, 1907490. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, N.; Jin, X.; Zhu, X. “Bottom-Up” Construction of Hyperbranched Poly(prodrug-co-photosensitizer) Amphiphiles Unimolecular Micelles for Chemo-Photodynamic Dual Therapy. ACS Appl. Mater. Interfaces 2017, 9, 36675–36687. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, J.; Luo, H.; Li, H.; Zhou, J.; Zhou, L.; Wei, S. Photosensitizer and Autophagy Promoter Coloaded ROS-Responsive Dendrimer-Assembled Carrier for Synergistic Enhancement of Tumor Growth Suppression. Small 2018, 14, 1802337. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Li, H.-J.; Du, J.-Z.; Liu, J.; Du, X.-J.; Shen, S.; Zhu, Y.-H.; Wang, X.; Ye, X.; Nie, S.; Wang, J. Smart Superstructures with Ultrahigh pH-Sensitivity for Targeting Acidic Tumor Microenvironment: Instantaneous Size Switching and Improved Tumor Penetration. ACS Nano 2016, 10, 6753–6761. [Google Scholar] [CrossRef]
- Chen, J.; Ding, J.; Wang, Y.; Cheng, J.; Ji, S.; Zhuang, X.; Chen, X. Sequentially Responsive Shell-Stacked Nanoparticles for Deep Penetration into Solid Tumors. Adv. Mater. 2017, 29, 1701170. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Zhang, Z.; Wu, H.; Xu, X.; Gu, Z. Tumor-adapting and tumor-remodeling AuNR@dendrimer-assembly nanohybrids overcome impermeable multidrug-resistant cancer. Mater. Horiz. 2018, 5, 1047–1057. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, S.; Wu, F.; Li, D.; Zhang, X.; Chen, W.; Xing, B. Rational Design of Nanogels for Overcoming the Biological Barriers in Various Administration Routes. Angew. Chem. Int. Ed. 2021, 60, 14760–14778. [Google Scholar] [CrossRef]
- Kavand, A.; Anton, N.; Vandamme, T.; Serra, C.A.; Chan-Seng, D. Synthesis and functionalization of hyperbranched polymers for targeted drug delivery. J. Control. Release 2020, 321, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Dhaini, B.; Kenzhebayeva, B.; Ben-Mihoub, A.; Gries, M.; Acherar, S.; Baros, F.; Thomas, N.; Daouk, J.; Schohn, H.; Hamieh, T.; et al. Peptide-conjugated nanoparticles for targeted photodynamic therapy. Nanophotonics 2021, 10, 3089–3134. [Google Scholar] [CrossRef]
- Ang, M.J.Y.; Chan, S.Y.; Goh, Y.-Y.; Luo, Z.; Lau, J.W.; Liu, X. Emerging strategies in developing multifunctional nanomaterials for cancer nanotheranostics. Adv. Drug Del. Rev. 2021, 178, 113907. [Google Scholar] [CrossRef] [PubMed]
- Kolate, A.; Baradia, D.; Patil, S.; Vhora, I.; Kore, G.; Misra, A. PEG—A versatile conjugating ligand for drugs and drug delivery systems. J. Control. Release 2014, 192, 67–81. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Del. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Workie, Y.A.; Kuo, C.Y.; Riskawati, J.H.; Krathumkhet, N.; Imae, T.; Ujihara, M.; Krafft, M.P. Hierarchical Composite Nanoarchitectonics with a Graphitic Core, Dendrimer and Fluorocarbon Domains, and a Poly(ethylene glycol) Shell as O-2 Reservoirs for Reactive Oxygen Species Production. ACS Appl. Mater. Interfaces 2022, 14, 35027–35039. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; He, K.; Jiang, X.Y.; Chen, S.Y.; Zhang, R.P.; Sun, P.F.; Fan, Q.L. Polyamidoamine Dendrimer-Modified Near Infrared-II Fluorescent Conjugated Polymer Brush for Photodynamic/Gas Therapy. ACS Appl. Polym. Mater. 2022, 4, 5103–5112. [Google Scholar] [CrossRef]
- Zheng, X.; Pan, D.; Chen, M.; Dai, X.; Cai, H.; Zhang, H.; Gong, Q.; Gu, Z.; Luo, K. Tunable Hydrophile–Lipophile Balance for Manipulating Structural Stability and Tumor Retention of Amphiphilic Nanoparticles. Adv. Mater. 2019, 31, 1901586. [Google Scholar] [CrossRef]
- Li, R.-Q.; Zhang, C.; Xie, B.-R.; Yu, W.-Y.; Qiu, W.-X.; Cheng, H.; Zhang, X.-Z. A two-photon excited O2-evolving nanocomposite for efficient photodynamic therapy against hypoxic tumor. Biomaterials 2019, 194, 84–93. [Google Scholar] [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Su, L.; Feng, Y.L.; Wei, K.C.; Xu, X.Y.; Liu, R.Y.; Chen, G.S. Carbohydrate-Based Macromolecular Biomaterials. Chem. Rev. 2021, 121, 10950–11029. [Google Scholar] [CrossRef]
- Appelhans, D.; Klajnert-Maculewicz, B.; Janaszewska, A.; Lazniewska, J.; Voit, B. Dendritic glycopolymers based on dendritic polyamine scaffolds: View on their synthetic approaches, characteristics and potential for biomedical applications. Chem. Soc. Rev. 2015, 44, 3968–3996. [Google Scholar] [CrossRef]
- Machado, V.; Morais, M.; Medeiros, R. Hyaluronic Acid-Based Nanomaterials Applied to Cancer: Where Are We Now? Pharmaceutics 2022, 14, 2092. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Huang, H. Hyaluronic acid-based biopharmaceutical delivery and tumor-targeted drug delivery system. J. Control. Release 2018, 278, 122–126. [Google Scholar] [CrossRef]
- Qin, Y.; Yi, J.; Zhang, Y. Preparation and Self-Assembly of pH-Responsive Hyperbranched Polymer Peptide Hybrid Materials. Nanomaterials 2023, 13, 1725. [Google Scholar] [CrossRef]
- Gu, J.; Chen, X.; Ren, X.; Zhang, X.; Fang, X.; Sha, X. CD44-Targeted Hyaluronic Acid-Coated Redox-Responsive Hyperbranched Poly(amido amine)/Plasmid DNA Ternary Nanoassemblies for Efficient Gene Delivery. Bioconjugate Chem. 2016, 27, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Chen, X. Aptamer-based targeted therapy. Adv. Drug Del. Rev. 2018, 134, 65–78. [Google Scholar] [CrossRef]
- Xiong, H.; Liu, L.; Wang, Y.; Jiang, H.; Wang, X. Engineered Aptamer-Organic Amphiphile Self-Assemblies for Biomedical Applications: Progress and Challenges. Small 2022, 18, 2104341. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Deng, H.; Su, Y.; He, L.; Wang, R.; Tong, G.; He, D.; Zhu, X. Aptamer-Functionalized and Backbone Redox-Responsive Hyperbranched Polymer for Targeted Drug Delivery in Cancer Therapy. Biomacromolecules 2016, 17, 2050–2062. [Google Scholar] [CrossRef]
- Zhang, C.; Moonshi, S.S.; Wang, W.; Ta, H.T.; Han, Y.; Han, F.Y.; Peng, H.; Král, P.; Rolfe, B.E.; Gooding, J.J.; et al. High F-Content Perfluoropolyether-Based Nanoparticles for Targeted Detection of Breast Cancer by 19F Magnetic Resonance and Optical Imaging. ACS Nano 2018, 12, 9162–9176. [Google Scholar] [CrossRef]
- Zhao, Y.; He, M.; Xu, L.; Zhang, C.; Guo, L.; Feng, W.; Yan, H. Tuning the Emission of a Nonconventional Aggregation-Induced Emission Polymer via Silicon-Bridged Twisted Intramolecular Charge Transfer for Targeted Delivery and Visualized Drug Release. Biomacromolecules 2023, 24, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fletcher, N.L.; Liu, T.; Gemmell, A.C.; Houston, Z.H.; Blakey, I.; Thurecht, K.J. In vivo therapeutic evaluation of polymeric nanomedicines: Effect of different targeting peptides on therapeutic efficacy against breast cancer. Nanotheranostics 2018, 2, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sun, H.; Liu, Y.; Hou, W.; Yang, Y.; Cai, R.; Cui, C.; Zhang, P.; Pan, X.; Li, X.; et al. Self-Assembled Aptamer-Grafted Hyperbranched Polymer Nanocarrier for Targeted and Photoresponsive Drug Delivery. Angew. Chem. Int. Ed. 2018, 57, 17048–17052. [Google Scholar] [CrossRef]
- Fletcher, N.L.; Houston, Z.H.; Simpson, J.D.; Veedu, R.N.; Thurecht, K.J. Designed multifunctional polymeric nanomedicines: Long-term biodistribution and tumour accumulation of aptamer-targeted nanomaterials. Chem. Commun. 2018, 54, 11538–11541. [Google Scholar] [CrossRef]
- Hak, A.; Ali, M.S.; Sankaranarayanan, S.A.; Shinde, V.R.; Rengan, A.K. Chlorin e6: A Promising Photosensitizer in Photo-Based Cancer Nanomedicine. ACS Appl. Bio Mater. 2023, 6, 349–364. [Google Scholar] [CrossRef]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of third generation photosensitizers used in anticancer photodynamic therapy: Review. Photodiagnosis Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef]
- Park, J.M.; Hong, K.I.; Lee, H.; Jang, W.D. Bioinspired Applications of Porphyrin Derivatives. Acc. Chem. Res. 2021, 54, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Demartis, S.; Obinu, A.; Gavini, E.; Giunchedi, P.; Rassu, G. Nanotechnology-based rose Bengal: A broad-spectrum biomedical tool. Dyes Pigm. 2021, 188, 109236. [Google Scholar] [CrossRef]
- Tan, J.; Wang, X.; Yu, Z.; Luo, J.; Lan, B.; Li, N.; Xin, Y.; Zeng, C.; Yan, L.; Zhang, L.-M.; et al. Spectroscopic investigation of a hyperbranched cationic amylopectin derivative as a multi-guest molecular host for targeted delivery of a photosensitizer to pancreatic cancer cells. Carbohydr. Polym. 2018, 197, 38–46. [Google Scholar] [CrossRef]
- Sztandera, K.; Marcinkowska, M.; Gorzkiewicz, M.; Janaszewska, A.; Laurent, R.; Zabłocka, M.; Mignani, S.; Majoral, J.P.; Klajnert-Maculewicz, B. In Search of a Phosphorus Dendrimer-Based Carrier of Rose Bengal: Tyramine Linker Limits Fluorescent and Phototoxic Properties of a Photosensitizer. Int. J. Mol. Sci. 2020, 21, 4456. [Google Scholar] [CrossRef]
- Kadhim, A.; McKenzie, L.K.; Bryant, H.E.; Twyman, L.J. Synthesis and Aggregation of a Porphyrin-Cored Hyperbranched Polyglycidol and Its Application as a Macromolecular Photosensitizer for Photodynamic Therapy. Mol. Pharm. 2019, 16, 1132–1139. [Google Scholar] [CrossRef]
- Cheng, J.; Zhou, Y.; Xu, S.; Xie, Y.; Mao, D.; Wu, W.; Li, Z. From main-chain conjugated polymer photosensitizer to hyperbranched polymer photosensitizer: Expansion of the polymerization-enhanced photosensitization effect for photodynamic therapy. J. Mater. Chem. B 2022, 10, 5008–5015. [Google Scholar] [CrossRef]
- Wang, L.; Qian, Y. Modification of a SOCT-ISC type triphenylamine-BODIPY photosensitizer by a multipolar dendrimer design for photodynamic therapy and two-photon fluorescence imaging. Biomater. Sci. 2023, 11, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, M.; Xue, K.; Li, M.; Liu, D.; Wang, Y.; Yang, X.; Hu, Y.; Kwok, R.T.K.; Qin, A.; et al. Heteroaromatic Hyperbranched Polyelectrolytes: Multicomponent Polyannulation and Photodynamic Biopatterning. Angew. Chem. Int. Ed. 2021, 60, 19222–19231. [Google Scholar] [CrossRef]
- Hu, X.; Li, F.; Xia, F.; Wang, Q.; Lin, P.; Wei, M.; Gong, L.; Low, L.E.; Lee, J.Y.; Ling, D. Dynamic nanoassembly-based drug delivery system (DNDDS): Learning from nature. Adv. Drug Del. Rev. 2021, 175, 113830. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.F.V.; Fernandez-Megia, E.; Borges, J.; Mano, J.F. Supramolecular dendrimer-containing layer-by-layer nanoassemblies for bioapplications: Current status and future prospects. Polym. Chem. 2021, 12, 5902–5930. [Google Scholar] [CrossRef]
- Redmond, R.W.; Gamlin, J.N. A Compilation of Singlet Oxygen Yields from Biologically Relevant Molecules. Photochem. Photobiol. 1999, 70, 391–475. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Li, Z.; Wang, W.; Wu, Y.; Pan, D.; Gu, Z.; Sheng, R.; Tomás, H.; Zhang, H.; et al. Glycodendron/pyropheophorbide-a (Ppa)-functionalized hyaluronic acid as a nanosystem for tumor photodynamic therapy. Carbohydr. Polym. 2020, 247, 116749. [Google Scholar] [CrossRef] [PubMed]
- Li, W.S.; Aida, T. Dendrimer Porphyrins and Phthalocyanines. Chem. Rev. 2009, 109, 6047–6076. [Google Scholar] [CrossRef]
- Nishiyama, N.; Stapert, H.R.; Zhang, G.-D.; Takasu, D.; Jiang, D.-L.; Nagano, T.; Aida, T.; Kataoka, K. Light-Harvesting Ionic Dendrimer Porphyrins as New Photosensitizers for Photodynamic Therapy. Bioconjugate Chem. 2003, 14, 58–66. [Google Scholar] [CrossRef]
- Militello, M.P.; Hernández-Ramírez, R.E.; Lijanova, I.V.; Previtali, C.M.; Bertolotti, S.G.; Arbeloa, E.M. Novel PAMAM dendrimers with porphyrin core as potential photosensitizers for PDT applications. J. Photochem. Photobiol. A Chem. 2018, 353, 71–76. [Google Scholar] [CrossRef]
- Herlambang, S.; Kumagai, M.; Nomoto, T.; Horie, S.; Fukushima, S.; Oba, M.; Miyazaki, K.; Morimoto, Y.; Nishiyama, N.; Kataoka, K. Disulfide crosslinked polyion complex micelles encapsulating dendrimer phthalocyanine directed to improved efficiency of photodynamic therapy. J. Control. Release 2011, 155, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Nakagishi, Y.; Morimoto, Y.; Lai, P.-S.; Miyazaki, K.; Urano, K.; Horie, S.; Kumagai, M.; Fukushima, S.; Cheng, Y.; et al. Enhanced photodynamic cancer treatment by supramolecular nanocarriers charged with dendrimer phthalocyanine. J. Control. Release 2009, 133, 245–251. [Google Scholar] [CrossRef]
- Cook, A.B.; Perrier, S. Branched and Dendritic Polymer Architectures: Functional Nanomaterials for Therapeutic Delivery. Adv. Funct. Mater. 2020, 30, 1901001. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Cao, R.; Ma, Z.; Zhu, M. Development of “smart” drug delivery systems for chemo/PDT synergistic treatment. J. Mater. Chem. B 2023, 11, 1416–1433. [Google Scholar] [CrossRef]
- Zhu, L.; Lin, S.; Cui, W.; Xu, Y.; Wang, L.; Wang, Z.; Yuan, S.; Zhang, Y.; Fan, Y.; Geng, J. A nanomedicine enables synergistic chemo/photodynamic therapy for pancreatic cancer treatment. Biomater. Sci. 2022, 10, 3624–3636. [Google Scholar] [CrossRef]
- Al Dine, E.J.; Marchal, S.; Schneider, R.; Hamie, B.; Ghanbaja, J.; Roques-Carmes, T.; Hamieh, T.; Toufaily, J.; Gaffet, E.; Alem, H. A Facile Approach for Doxorubicine Delivery in Cancer Cells by Responsive and Fluorescent Core/Shell Quantum Dots. Bioconjugate Chem. 2018, 29, 2248–2256. [Google Scholar] [CrossRef]
- Recour, E.; Toufaily, J.; Schejn, A.; Roques-Carmes, T.; Kassir, M.; Obeid, H.; Hamieh, T.; Gaffet, E.; Marchal, S.; Schneider, R.; et al. Synthesis and characterization of smart nanomaterials for cancer treatment. In Proceedings of the 9th International Conference on Material Sciences (CSM9), Nancy, France, 26–28 August 2015. [Google Scholar]
- Lin, M.H.C.; Chang, L.C.; Chung, C.Y.; Huang, W.C.; Lee, M.H.; Chen, K.T.; Lai, P.S.; Yang, J.T. Photochemical Internalization of Etoposide Using Dendrimer Nanospheres Loaded with Etoposide and Protoporphyrin IX on a Glioblastoma Cell Line. Pharmaceutics 2021, 13, 1877. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, G.; Zhang, X.; Xu, F.; Xiong, X.; Zhou, S. A Step-by-Step Multiple Stimuli-Responsive Nanoplatform for Enhancing Combined Chemo-Photodynamic Therapy. Adv. Mater. 2017, 29, 1605357. [Google Scholar] [CrossRef]
- Martinez de Pinillos Bayona, A.; Moore, C.M.; Loizidou, M.; MacRobert, A.J.; Woodhams, J.H. Enhancing the efficacy of cytotoxic agents for cancer therapy using photochemical internalisation. Int. J. Cancer 2016, 138, 1049–1057. [Google Scholar] [CrossRef] [PubMed]






| Type of PS–HB Polymer Hybrid | Advantages | Disadvantages | Reference |
|---|---|---|---|
 PS encapsulated in HB polymers | High PS-loading capacity Easy fabrication | Unspecific leakage of PSs in blood circulation Burst-release behavior PS aggregation leading to low singlet-oxygen generation efficiency | [41,43,51,52,119] |
 PS surface-conjugated to HB polymers | Chemical and physiological stability Controlled PS-loading content High PS-loading efficiency Mitigated PS premature leakage in blood circulation Potential for on-demand PS release at targeted site | Complicated synthetic procedure and limited candidates of functionalized PS and HB polymers Some covalent linkers decrease photodynamic activity of PSs | [34,44,53,98,120] |
 PS-cored HB polymers | Controlled PS-loading content and distribution Negligible PS aggregation Mitigated PS premature leakage | Complicated synthetic procedure and limited candidates of functionalized PS and HB polymers Low PS-loading efficiency | [121] |
 HB polymers constructed by PSs | High ROS generation efficiency Compatible with long-wavelength light source Improved light-harvesting ability | Complicated synthetic procedure and limited candidates of functionalized PS and HB polymers Slow excretion from the body due to increased molecular weight of PSs | [122,123,124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhang, Y. Hyperbranched Polymers: Recent Advances in Photodynamic Therapy against Cancer. Pharmaceutics 2023, 15, 2222. https://doi.org/10.3390/pharmaceutics15092222
Chen J, Zhang Y. Hyperbranched Polymers: Recent Advances in Photodynamic Therapy against Cancer. Pharmaceutics. 2023; 15(9):2222. https://doi.org/10.3390/pharmaceutics15092222
Chicago/Turabian StyleChen, Jie, and Yichuan Zhang. 2023. "Hyperbranched Polymers: Recent Advances in Photodynamic Therapy against Cancer" Pharmaceutics 15, no. 9: 2222. https://doi.org/10.3390/pharmaceutics15092222





