Anti-Arthritic and Immunomodulatory Potential of Methanolic, n-Hexane, and Ethyl Acetate Fractions of Bark of Acacia modesta on Complete Freund’s Adjuvant-Induced Arthritis in Rats
Abstract
1. Introduction
2. Materials and Method
2.1. Extract Formation and Fractionation
2.2. Animal Housing
2.3. Treatment Design and Induction of Arthritis
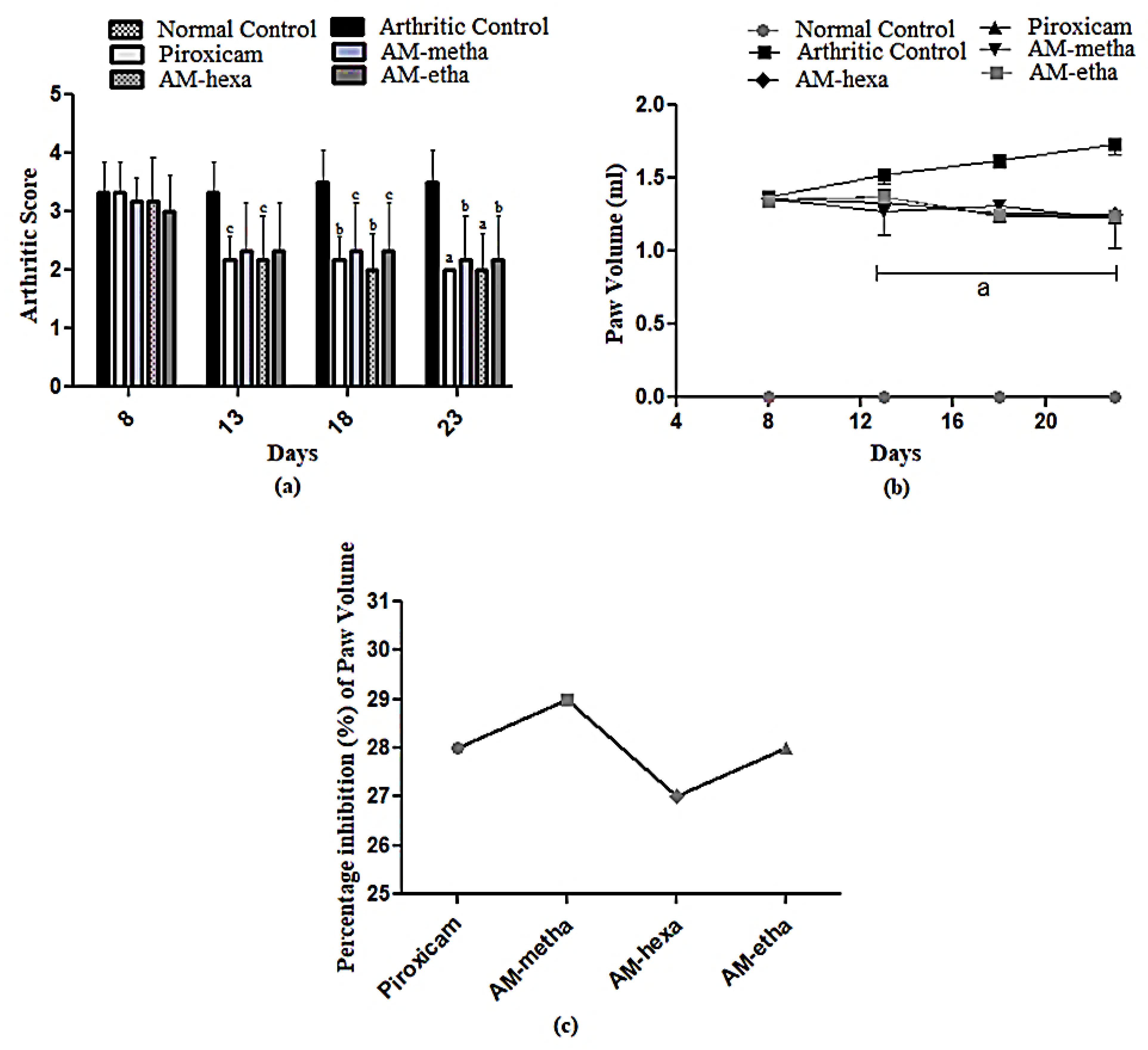
2.4. Arthritic Score and Paw Volume
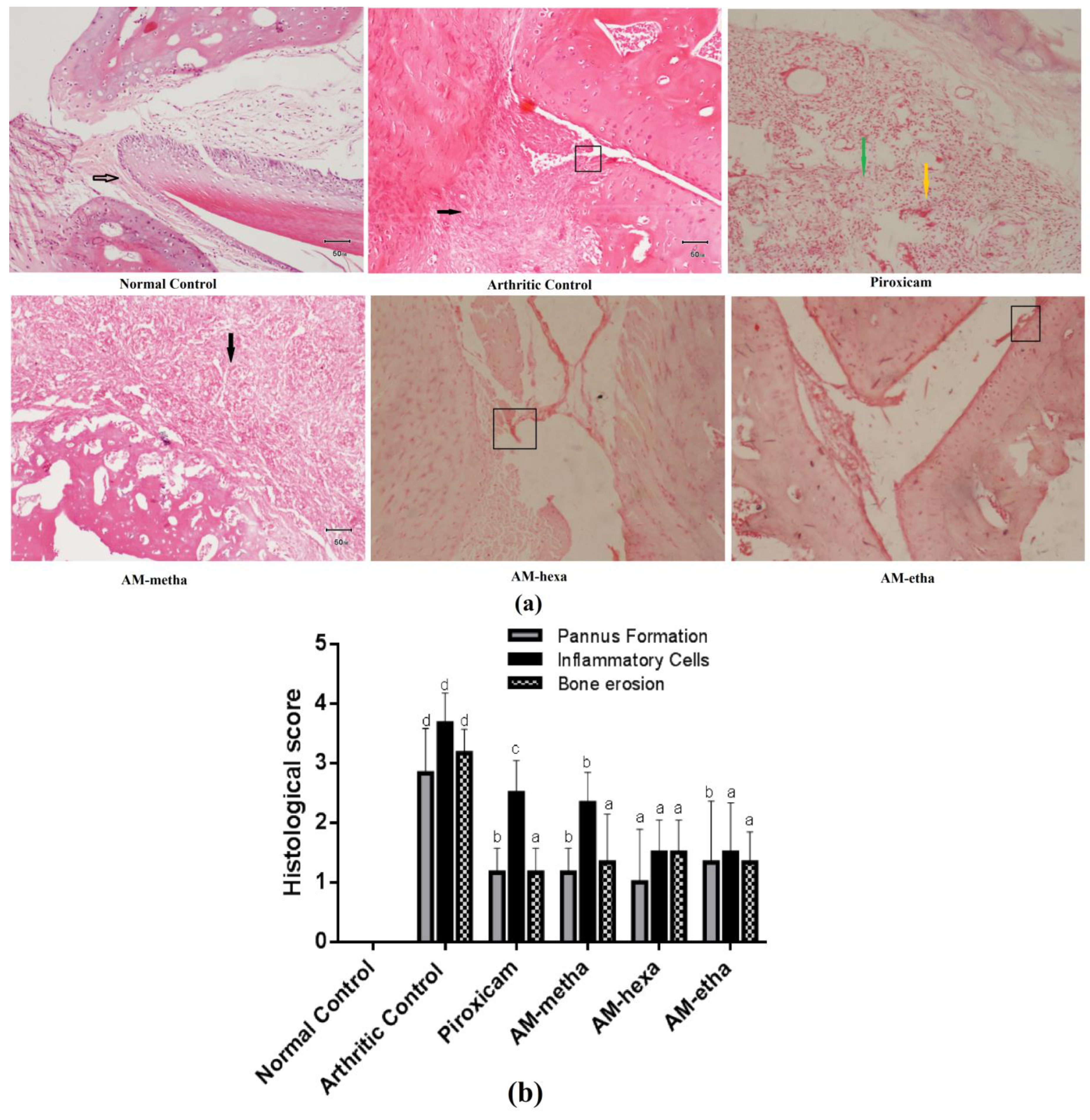
2.5. Ankle Joint Histopathology
2.6. Biochemical and Hematological Biomarkers
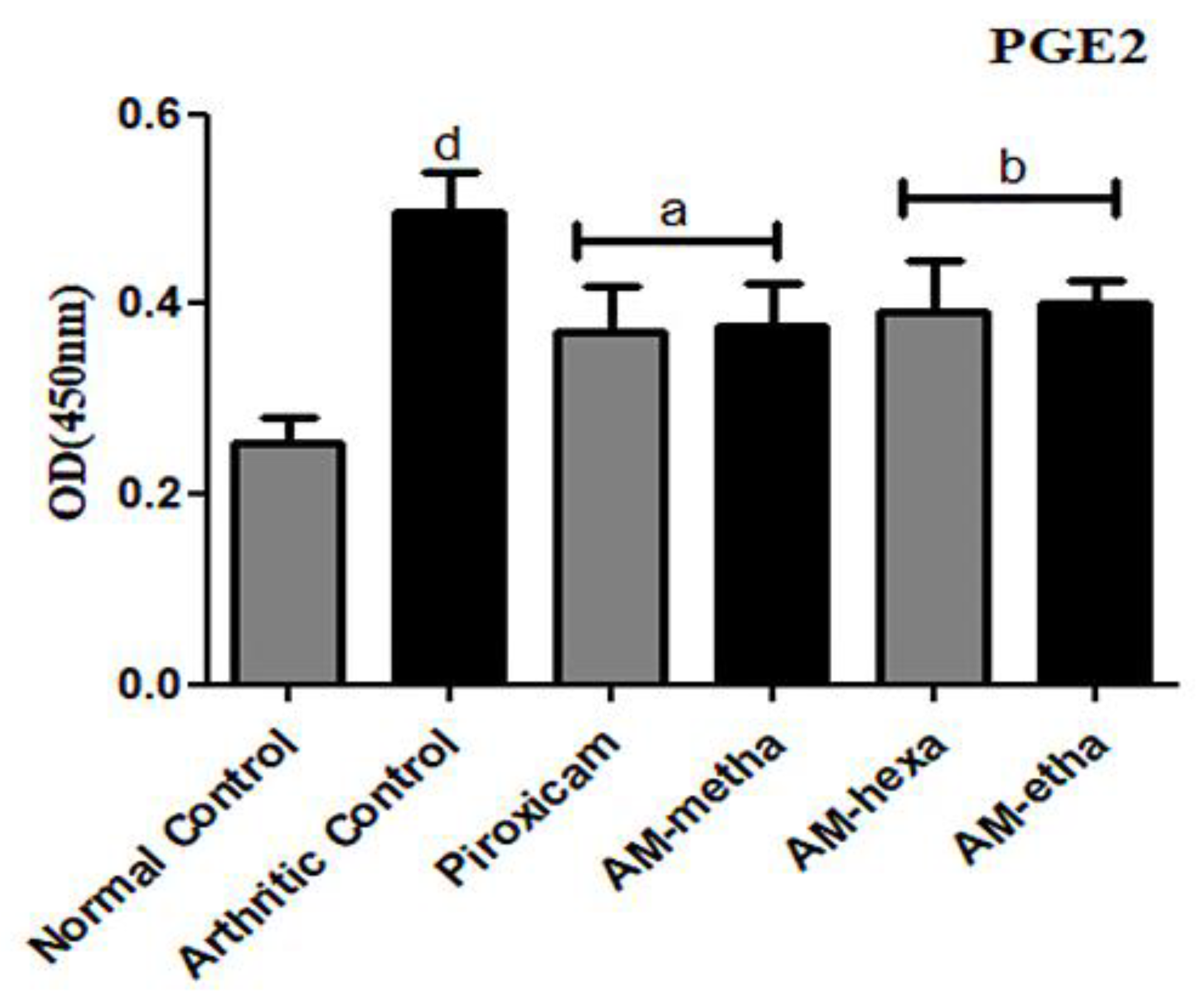
2.7. PGE2 Protein Levels
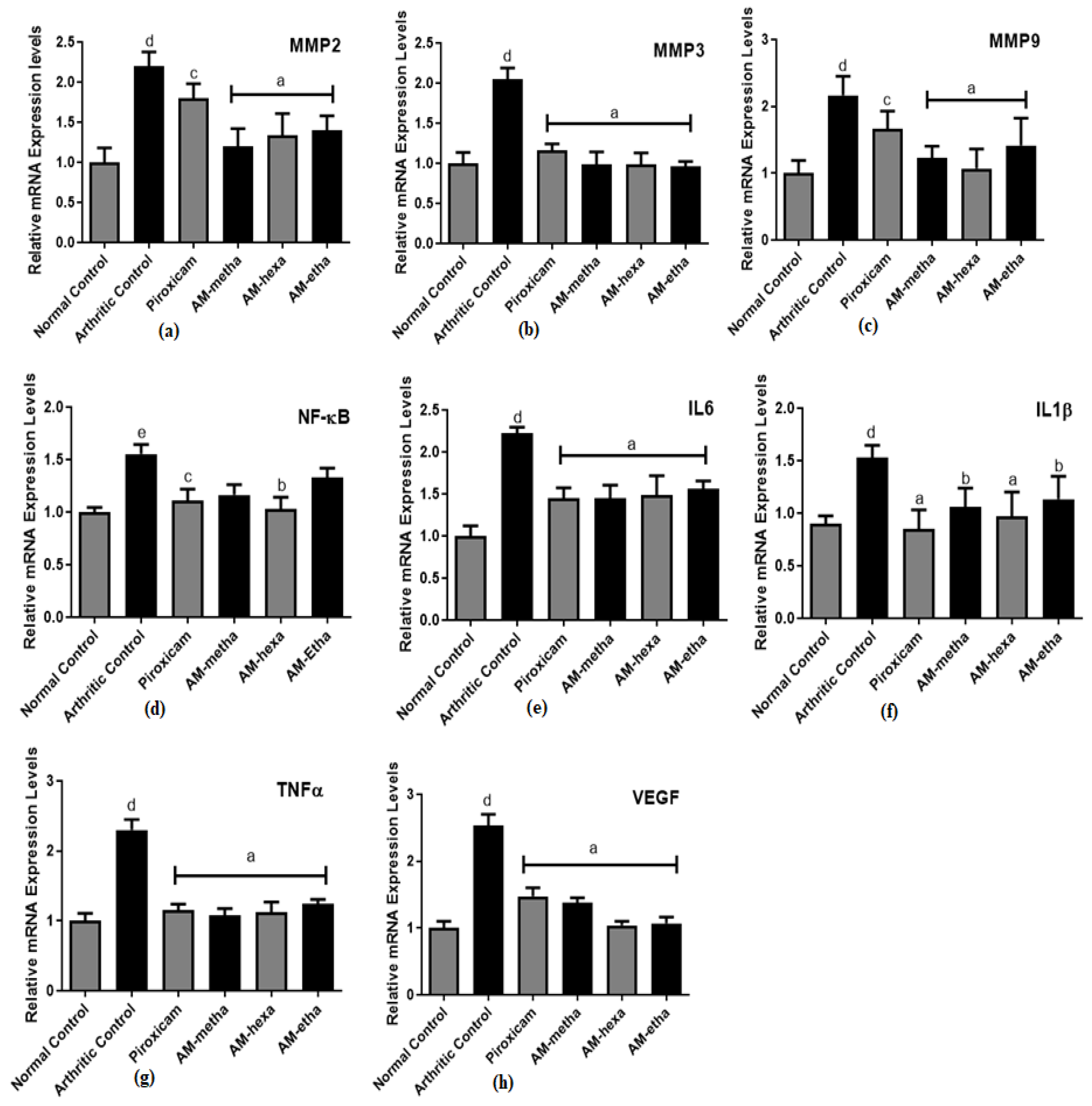
2.8. Determination of MMP2, MMP3, MMP9, NF-κB, IL6, IL1β, TNFα, and VEGF Expression Levels
2.9. GC/MS Analysis
2.10. Statistical Analysis
3. Results
3.1. A. modesta Attenuated Arthritic Score and Paw Volume
3.2. A. modesta Ameliorated Histopathological Parameters of Arthritis
3.3. A. modesta Significantly Reduced PGE2 Levels
3.4. A. modesta Significantly Reduced Matrix Metalloproteinase, Pro-Inflammatory Cytokine, Transcription Factor, and Growth Factor Expression Levels
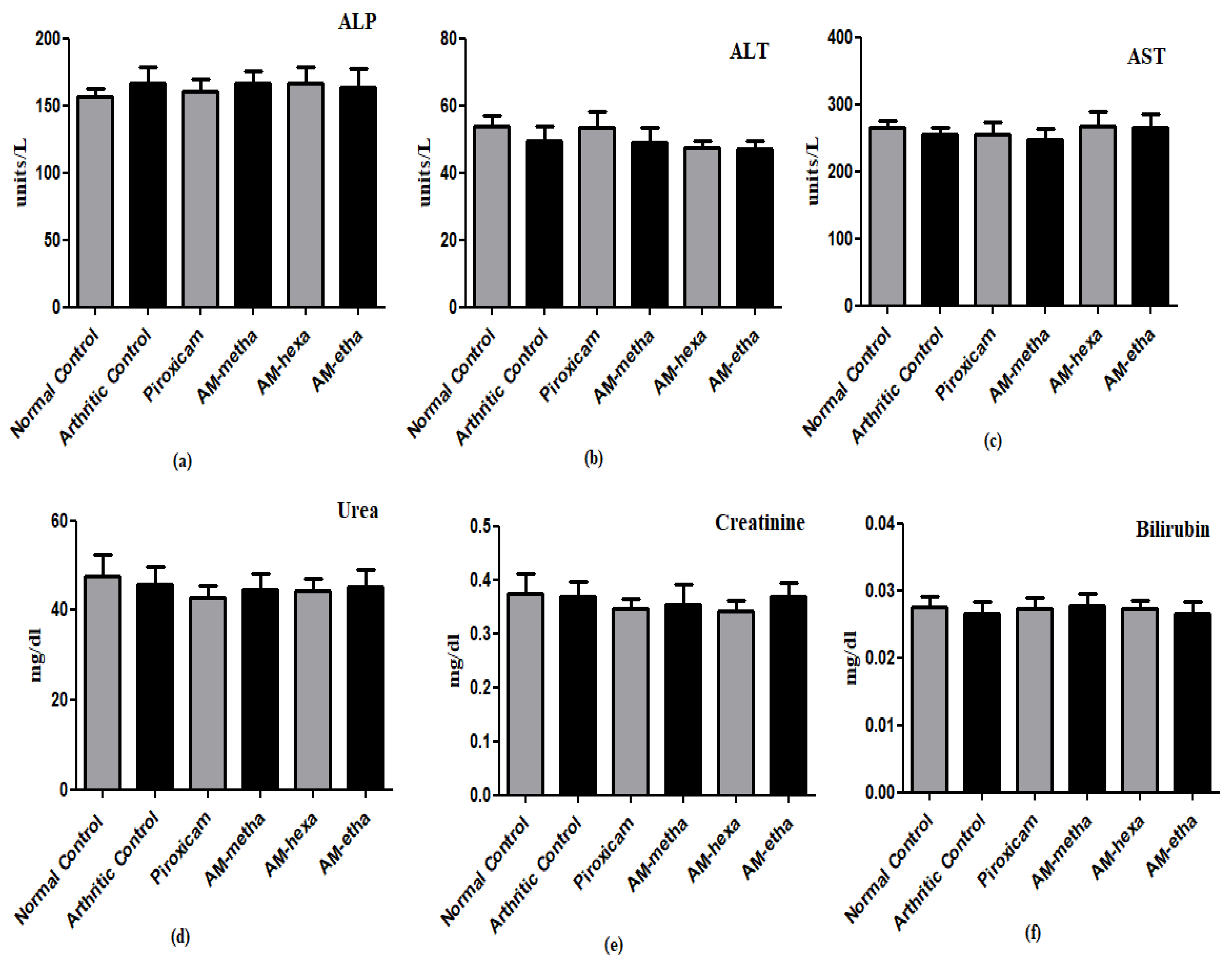
3.5. A. modesta Improved Hematological Markers and Did Not Display Harm to Liver and Kidneys
3.6. GC/MS Analysis of Extract and Fractions of A. modesta
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pezone, A.; Olivieri, F.; Napoli, M.V.; Procopio, A.; Avvedimento, E.V.; Gabrielli, A. Inflammation and DNA damage: Cause, effect or both. Nat. Rev. Rheumatol. 2023, 19, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.-S. Role of inflammasomes in inflammatory autoimmune rheumatic diseases. Korean J. Physiol. Pharmacol. 2018, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.K.; Pandey, A. Inflammation and rheumatoid arthritis. J. Physiol. Biochem. 2012, 69, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, L.; Chitti, R.; Sreeharsha, N.; Mishra, A.; Gubbiyappa, S.K.; Singh, Y. Wogonin ameliorate complete Freund’s adjuvant induced rheumatoid arthritis via targeting NF-κB/MAPK signaling pathway. Biofactors 2020, 46, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Mbiantcha, M.; Almas, J.; Shabana, S.U.; Nida, D.; Aisha, F. Anti-arthritic property of crude extracts of Piptadeniastrum africanum (Mimosaceae) in complete Freund’s adjuvant-induced arthritis in rats. BMC Complement. Altern. Med. 2017, 17, 111. [Google Scholar] [CrossRef]
- Fang, Q.; Zhou, C.; Nandakumar, K.S. Molecular and Cellular Pathways Contributing to Joint Damage in Rheumatoid Arthritis. Mediat. Inflamm. 2020, 2020, 3830212. [Google Scholar] [CrossRef]
- Sivasakthi, P.; Priya, E.S.; Selvan, P.S. Molecular insights into phytochemicals exhibiting anti-arthritic activity: Systematic review. Inflamm. Res. 2021, 70, 665–685. [Google Scholar] [CrossRef]
- Brzustewicz, E.; Bryl, E. The role of cytokines in the pathogenesis of rheumatoid arthritis–Practical and potential application of cytokines as biomarkers and targets of personalized therapy. Cytokine 2015, 76, 527–536. [Google Scholar] [CrossRef]
- Ingawale, D.K.; Patel, S.S. Hecogenin exhibits anti-arthritic activity in rats through suppression of pro-inflammatory cytokines in Complete Freund’s adjuvant-induced arthritis. Immunopharmacol. Immunotoxicol. 2017, 40, 59–71. [Google Scholar] [CrossRef]
- Ruscitti, P.; Cipriani, P.; Carubbi, F.; Liakouli, V.; Zazzeroni, F.; Di Benedetto, P.; Berardicurti, O.; Alesse, E.; Giacomelli, R. The Role of IL-1β in the Bone Loss during Rheumatic Diseases. Mediat. Inflamm. 2015, 2015, 782382. [Google Scholar] [CrossRef]
- Araki, Y.; Mimura, T. Matrix Metalloproteinase Gene Activation Resulting from Disordred Epigenetic Mechanisms in Rheumatoid Arthritis. Int. J. Mol. Sci. 2017, 18, 905. [Google Scholar] [CrossRef]
- Yan, Y.; Singh, G.K.; Zhang, F.; Wang, P.; Liu, W.; Zhong, L.; Yang, L. Comparative Study of Normal and Rheumatoid Arthritis Fibroblast-Like Synoviocytes Proliferation under Cyclic Mechanical Stretch: Role of Prostaglandin E2. Connect. Tissue Res. 2011, 53, 246–254. [Google Scholar] [CrossRef]
- Lee, Y.H.; Bae, S.-C. Correlation between circulating VEGF levels and disease activity in rheumatoid arthritis: A meta-analysis. Z. Rheumatol. 2018, 77, 240–248. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal. Transduct. Targeted. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Makarov, S.S. NF-κB in rheumatoid arthritis: A pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res. Ther. 2001, 3, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Radu, A.-F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chinnathambi, A.; Alharbi, S.A.; Bai, L. Copper oxide nanoparticles from Rabdosia rubescens attenuates the complete Freund’s adjuvant (CFA) induced rheumatoid arthritis in rats via suppressing the inflammatory proteins COX-2/PGE2. Arab. J. Chem. 2020, 13, 5639–5650. [Google Scholar] [CrossRef]
- Laurindo, L.F.; de Maio, M.C.; Minniti, G.; de Góes Corrêa, N.; Barbalho, S.M.; Quesada, K.; Guiguer, E.L.; Sloan, K.P.; Detregiachi, C.R.; Araújo, A.C.; et al. Effects of medicinal plants and phytochemicals in Nrf2 pathways during inflammatory bowel diseases and related colorectal cancer: A comprehensive review. Metabolites 2023, 13, 243. [Google Scholar] [CrossRef]
- Feng, W.; Ao, H.; Peng, C. Gut Microbiota, Short-Chain Fatty Acids, and Herbal Medicines. Front. Pharmacol. 2018, 9, 1354. [Google Scholar] [CrossRef]
- Bukhari, I.A.; Khan, R.A.; Gilani, A.H.; Ahmed, S.; Saeed, S.A. Analgesic, anti-inflammatory and anti-platelet activities of the methanolic extract of Acacia modesta leaves. Inflammopharmacology 2010, 18, 187–196. [Google Scholar] [CrossRef]
- Kamal, M.; Adnan, M.; Murad, W.; Bibi, H.; Tariq, A.; Rahman, H.; Shinwari, Z.K. Anti-rheumatic potential of Pakistani medicinal plants: A review. Pak. J. Bot. 2016, 48, 399–413. [Google Scholar]
- Murad, W.; Azizullah, A.; Adnan, M.; Tariq, A.; Khan, K.U.; Waheed, S.; Ahmad, A. Ethnobotanical assessment of plant resources of Banda Daud Shah, District Karak, Pakistan. J. Ethnobiol. Ethnomed. 2013, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Sher, H.; Aldosari, A.; Ahmad, S. Ethnoecological appraisal of Acacia modesta Wall. common tree of dry ecosystem in Pakistan. Afr. J. Agric. Res. 2012, 7, 5083–5091. [Google Scholar] [CrossRef]
- Ur, M.S.R.; Chaudhry, M.A. Evaluation of antioxidant and hepatoprotective effect of Acacia modesta wall against paracetamol induced hepatotoxicity. Br. J. Pharm. Res. 2015, 5, 336–343. [Google Scholar]
- Saleem, B.; Islam, M.; Saeed, H.; Imtiaz, F.; Asghar, M.; Saleem, Z.; Mehmood, A.; Naheed, S. Investigations of Acacia modesta Wall. leaves for in vitro anti-diabetic, proliferative and cytotoxic effects. Braz. J. Pharm. Sci. 2018, 54, e17467. [Google Scholar] [CrossRef]
- Qazi, N.G.; Khan, A.-U.; Ali, F. Anti-diarrheal, anti-secretory, anti-spasmodic and antiulcer activities of Acacia modesta (Mimosaceae) aerial parts. Trop. J. Pharm. Res. 2017, 16, 2231. [Google Scholar] [CrossRef][Green Version]
- Jawla, S.; Kumar, Y.; Khan, M.S. Antimicrobial and antihyperglycemic activities of Acacia modesta leaves. Pharmacologyonline 2011, 2, 331–343. [Google Scholar]
- Latif, S.; Ismail, H.; Khan, M.R.; Rahim, A.A.; Mehboob, R.; Dilshad, E.; Sajid, M.; Haider, S.I.; Anwaar, S.; Majeed, M.N. Pharmacological evaluation of Acacia modesta bark for antipyretic, anti-inflammatory, analgesic, antidepressant and anticoagulant activities in Sprague Dawley rats. Pak. J. Pharm. Sci. 2020, 33, 1015–1023. [Google Scholar]
- Shabbir, A.; Batool, S.A.; Basheer, M.I.; Shahzad, M.; Sultana, K.; Tareen, R.B.; Iqbal, J.; Hassan, S.U. Ziziphora clinopodioides ameliorated rheumatoid arthritis and inflammatory paw edema in different models of acute and chronic inflammation. Biomed. Pharmacother. 2018, 97, 1710–1721. [Google Scholar] [CrossRef]
- Uttra, A.M.; Hasan, U.H. Anti-arthritic activity of aqueous-methanolic extract and various fractions of Berberis orthobotrys Bien ex Aitch. BMC Complement. Altern. Med. 2017, 17, 371. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Jabbar, Z.; Alamgeer; Irfan, H.M.; Asim, M.H.; Akram, M.; Saif, A.; Alshahrani, M.A.; Alshehri, M.A.; Asiri, S.A. Venlafaxine demonstrated anti-arthritic activity possibly through down regulation of TNF-α, IL-6, IL-1β, and COX-2. Inflammopharmacology 2021, 29, 1413–1425. [Google Scholar] [CrossRef]
- Shabbir, A.; Shahzad, M.; Ali, A.; Zia-Ur-Rehman, M. Discovery of New Benzothiazine Derivative as Modulator of Pro- and Anti-inflammatory Cytokines in Rheumatoid Arthritis. Inflammation 2016, 39, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Khan, A.; Zia-Ul-Haq, M.; Dima, L. Mechanism of anti-inflammatory and anti-nociceptive actions of acacia modesta in animal models. Pak. J. Zool. 2015, 47, 1723–1730. [Google Scholar]
- Cui, X.; Wang, R.; Bian, P.; Wu, Q.; Seshadri, V.D.D.; Liu, L. Evaluation of antiarthritic activity of nimbolide against Freund’s adjuvant induced arthritis in rats. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3391–3398. [Google Scholar] [CrossRef] [PubMed]
- Uroos, M.; Abbas, Z.; Sattar, S.; Umer, N.; Shabbir, A.; Rehman, S.U.; Sharif, A. Nyctanthes arbor-tristis Ameliorated FCA-Induced Experimental Arthritis: A Comparative Study among Different Extracts. Evid.-Based Complement. Altern. Med. 2017, 2017, 4634853. [Google Scholar] [CrossRef]
- Tian, Z.; Chinnathambi, A.; Alahmadi, T.A.; Mohan, S.K.; Veeraraghavan, V.P.; Jaganathan, S.K. Anti-arthritic activity of Tin oxide-Chitosan-Polyethylene glycol carvacrol nanoparticles against Freund’s adjuvant induced arthritic rat model via the inhibition of cyclooxygenase-2 and prostaglandin E2. Arab. J. Chem. 2021, 14, 103293. [Google Scholar] [CrossRef]
- Das, C.; Bose, A.; Das, D. Ayurvedic Balarista ameliorate anti-arthritic activity in adjuvant induced arthritic rats by inhibiting pro-inflammatory cytokines and oxidative stress. J. Tradit. Complement. Med. 2020, 11, 228–237. [Google Scholar] [CrossRef]
- Mobashar, A.; Shabbir, A.; Shahzad, M.; Gobe, G. Preclinical Rodent Models of Arthritis and Acute Inflammation Indicate Immunomodulatory and Anti-Inflammatory Properties of Juglans regia Extracts. Evid.-Based Complement. Altern. Med. 2022, 2022, 1695701. [Google Scholar] [CrossRef]
- Sharif, M.; Anjum, I.; Shabbir, A.; Syed, S.K.; Mobeen, I.; Shahid, M.H.; Sarwar, K. Amelioration of Ovalbumin-Induced Allergic Asthma by Juglans regia via Downregulation of Inflammatory Cytokines and Upregulation of Aquaporin-1 and Aquaporin-5 in Mice. J. Trop. Med. 2022, 2022, 6530095. [Google Scholar] [CrossRef]
- Mobashar, A.; Shabbir, A.; Shahzad, M.; Hassan, S.U. Evaluation of Immunomodulatory and Antiarthritic Potential of Trigonella gharuensis Extracts. Evid.-Based Complement. Altern. Med. 2020, 2020, 8836080. [Google Scholar] [CrossRef]
- Akhtar, T.; Ali, G.; Sheikh, N. Immunosuppressant-Induced Oxidative Stress and Iron: A Paradigm Shift from Systemic to Intrahepatic Abnormalities. Oxid. Med. Cell. Longev. 2020, 2020, 8675275. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Su, C.; Chen, Y.; Hao, X.; Jiang, J. Electroacupuncture on ST36 and GB39 Acupoints Inhibits Synovial Angiogenesis via Downregulating HIF-1α/VEGF Expression in a Rat Model of Adjuvant Arthritis. Evid.-Based Complement. Altern. Med. 2019, 2019, 5741931. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Kuroda, T.; Kobayashi, D. Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 10922. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-L.; Chang, A.-C.; Huang, C.-C.; Tsai, C.-H.; Lin, C.-C.; Tang, C.-H. Myostatin Promotes Interleukin-1β Expression in Rheumatoid Arthritis Synovial Fibroblasts through Inhibition of miR-21-5p. Front. Immunol. 2017, 8, 1747. [Google Scholar] [CrossRef]
- Hashizume, M.; Mihara, M. The Roles of Interleukin-6 in the Pathogenesis of Rheumatoid Arthritis. Arthritis 2011, 2011, 765624. [Google Scholar] [CrossRef]
- Li, R.-L.; Duan, H.-X.; Liang, Q.; Huang, Y.-L.; Wang, L.-Y.; Zhang, Q.; Wu, C.-J.; Liu, S.-Q.; Peng, W. Targeting matrix metalloproteases: A promising strategy for herbal medicines to treat rheumatoid arthritis. Front. Immunol. 2022, 13, 1046810. [Google Scholar] [CrossRef]
- Burrage, P.S.; Mix, K.S.; Brinckerhoff, C.E. Matrix Metalloproteinases: Role in Arthritis. Front. Biosci. 2006, 11, 529–543. [Google Scholar] [CrossRef]
- Fattahi, M.J.; Mirshafiey, A. Prostaglandins and Rheumatoid Arthritis. Arthritis 2012, 2012, 239310. [Google Scholar] [CrossRef]
- Świerkot, J.; Nowak, B.; Czarny, A.; Zaczyńska, E.; Sokolik, R.; Madej, M.; Korman, L.; Sebastian, A.; Wojtala, P.; Lubiński, Ł.; et al. The Activity of JAK/STAT and NF-κB in Patients with Rheumatoid Arthritis. Adv. Clin. Exp. Med. 2016, 25, 709–717. [Google Scholar] [CrossRef]
- Hussain, A.; Aslam, B.; Muhammad, F.; Faisal, M.N.; Kousar, S.; Mushtaq, A.; Bari, M.U. Anti-arthritic activity of Ricinus communis L. and Withania somnifera L. extracts in adjuvant-induced arthritic rats via modulating inflammatory mediators and subsiding oxidative stress. Iran. J. Basic. Med. Sci. 2021, 24, 951–961. [Google Scholar] [CrossRef]
- López-Velázquez, J.A.; Chávez-Tapia, N.C.; Ponciano-Rodríguez, G.; Sánchez-Valle, V.; Caldwell, S.H.; Uribe, M.; Méndez-Sánchez, N. Bilirubin alone as a biomarker for short-term mortality in acute-on-chronic liver failure: An important prognostic indicator. Ann. Hepatol. 2014, 13, 98–104. [Google Scholar] [CrossRef]
- Harifi, G.; Sibilia, J. Pathogenic role of platelets in rheumatoid arthritis and systemic autoimmune diseases: Perspectives and therapeutic aspects. Saudi Med. J. 2016, 37, 354. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009, 285, 109–115. [Google Scholar] [CrossRef]
- Song, T.; Shi, R.; Vijayalakshmi, A.; Lei, B. Protective effect of lupeol on arthritis induced by type II collagen via the suppression of P13K / AKT signaling pathway in Sprague dawley rats. Environ. Toxicol. 2022, 37, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Wal, A.; Srivastava, R.S.; Wal, P.; Rai, A.; Sharma, S. Lupeol as a magical drug. Pharm. Biol. Eval. 2015, 2, 142–151. [Google Scholar]
- Santamarina, A.B.; Pisani, L.P.; Baker, E.J.; Marat, A.D.; Valenzuela, C.A.; Miles, E.A.; Calder, P.C. Anti-inflammatory effects of oleic acid and the anthocyanin keracyanin alone and in combination: Effects on monocyte and macrophage responses and the NF-κB pathway. Food Funct. 2021, 12, 7909–7922. [Google Scholar] [CrossRef]
- Medeiros-De-Moraes, I.M.; Gonçalves-De-Albuquerque, C.F.; Kurz, A.R.M.; Oliveira, F.M.d.J.; de Abreu, V.H.P.; Torres, R.C.; Carvalho, V.F.; Estato, V.; Bozza, P.T.; Sperandio, M.; et al. Omega-9 Oleic Acid, the Main Compound of Olive Oil, Mitigates Inflammation during Experimental Sepsis. Oxid. Med. Cell. Longev. 2018, 2018, 6053492. [Google Scholar] [CrossRef]
- Azhar, A.S.; Suhaila, H.B.; Imad, H.H. Analysis of bioactive chemical compounds of Euphorbia lathyrus using gas chromatography-mass spectrometry and Fourier-transform infrared spectroscopy. J. Pharmacogn. Phytother. 2016, 8, 109–126. [Google Scholar] [CrossRef]
- Singh, S.; Patra, A. Evaluation of adaptogenic potential of Polygonatum cirrhifolium (Wall.) Royle: In vitro, in vivo and in silico studies. S. Afr. J. Bot. 2018, 121, 159–177. [Google Scholar] [CrossRef]
- Latief, M.; Muhaimin, M.; Amanda, H.; Prahandika, G.; Tarigan, I.L. Anti-inflammatory activities of squalene compound of methanol extract of Abroma augusta L. J. Teknol. Lab. 2020, 9, 176–185. [Google Scholar] [CrossRef]
- Mujeeb, F.; Bajpai, P.; Pathak, N. Phytochemical Evaluation, Antimicrobial Activity, and Determination of Bioactive Components from Leaves of Aegle marmelos. BioMed Res. Int. 2014, 2014, 497606. [Google Scholar] [CrossRef] [PubMed]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
| Treatment Groups | Dose | Route of Administration | References |
|---|---|---|---|
| Normal Control | Normal Saline | Orally | [29] |
| Arthritic Control | i. CFA ii. Normal saline | i. Injected ii. Orally | |
| Piroxicam | 10 mg/kg b.w to arthritic rats | Intraperitonially | [32] |
| AM-metha | 500 mg/kg b.w to arthritic rats | Orally | [33] |
| AM-hexa | 500 mg/kg b.w to arthritic rats | Orally | |
| AM-etha | 500 mg/kg b.w to arthritic rats | Orally |
| Markers | Forward/Reverse Primers | Annealing Temp (°C) | Product Size | References |
|---|---|---|---|---|
| IL6 | 5′-GTCAACTCCATCTGCCCTTCAG-3′ 5′-GGCAGTGGCTGTCAACAACAT-3′ | 59 | 270 | [41] |
| MMP3 | 5′-CCTTTTGATGGGCCTGGAAT-3′ 5′-GTGACATCATCTTGTCCATCG-3′ | 54 | 107 | ENSRNOG00000032626 |
| IL1β | 5′-CCTGCTAGTGTGGATGTTC-3′ 5′-GAGGTGCTGAGTTACCAGTT-3′ | 57 | 390 | ENSRNOG00000004649 |
| MMP9 | 5′-CCACCGAGCTATCCACTCAT-3′ 5′-GTCCGGTTTCAGCATGTTTT-3′ | 56.1 | 159 | ENSRNOG00000017539 |
| NF-κB | 5′-CCGAGATAATGACAGCGTGT-3′ 5′-CCTTGGGAACGATATGATGG-3′ | 58.4 | 217 | ENSRNOG00000023258 |
| TNF-α | 5′-ACAAGGCTGCCCCGACTAT-3′ 5′-CTCCTGGTATGAAGTCCGAAATC-3′ | 60 | 67 | [41] |
| VEGF | 5′-GTTCAGAGCGGAGAAAGCATT-3′ 5′-CTTGCAACGCGAGTCTGTGT-3′ | 60 | 80 | [42] |
| MMP2 | 5′-GCAACAAGTATGAGAGCTGC-3′ 5′-CGGTCATCATCGTAGTTGGT-3′ | 57 | 85 | ENSRNOG00000016695 |
| Markers | Normal Control | Arthritic Control | Piroxicam | AM-Metha | AM-Hexa | AM-Etha |
|---|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 13.52 ± 1.357 | 9.142 ± 0.521 d | 13.50 ± 0.411 a | 13.13 ± 0.096 a | 13.99 ± 0.625 a | 13.05 ± 0.884 a |
| RBCs (106/µL) | 7.143 ± 0.662 | 5.417 ± 0.789 e | 7.188 ± 0.895 b | 7.750 ± 0.684 a | 7.503 ± 0.545 b | 7.625 ± 1.072 a |
| WBCs (103/µL) | 9.723 ± 0.416 | 17.46 ± 2.350 d | 13.77 ± 1.256 c | 14.32 ± 2.062 b | 14.04 ± 1.192 b | 13.28 ± 0.710 c |
| Platelets (103/µL) | 418.7 ± 54.64 | 711.5 ± 42.70 d | 555.7 ± 27.93 a | 550.2 ± 33.58 a | 637.0 ± 32.48 c | 547.2 ± 37.49 a |
| Identified Compounds | Molecular Formula | Retention Time | Molecular Weight (g/mol) | Total (%Age) |
|---|---|---|---|---|
| 2-Propanone, 1-cyclopentyl- | C8H14O | 8.480 | 126 | 2.294 |
| 5-Hydroxymethylfurfural | C6H6O3 | 11.502 | 126 | 1.582 |
| Phenol,2,4-bis(1,1-dimethylethyl)- | C14H22O | 15.533 | 206 | 2.230 |
| Hexadecanoic acid, methyl ester | C17H34O2 | 20.115 | 270 | 2.949 |
| Estra-1,3,5(10)-trien-17β-ol | C18H24O | 20.467 | 256 | 7.127 |
| 3-(2,6,6-Trimethyl-cyclohex-1-enyl)-propionic acid, methyl ester | C13H22O2 | 20.876 | 210 | 1.943 |
| 9,12-Octadecadienoic acid (Z, Z)-, methyl ester | C19H34O2 | 21.757 | 294 | 2.212 |
| Methyl stearate | C19H38O2 | 21.818 | 298 | 3.853 |
| 6-Octadecenoic acid, methyl ester, (Z)- | C19H36O2 | 22.029 | 296 | 1.875 |
| 9,12-Octadecadienoic acid (Z, Z)- | C18H32O2 | 22.111 | 280 | 2.663 |
| Kaur-16-ene | C20H32 | 22.857 | 272 | 2.440 |
| 17-Norkaur-15-ene, 13-methyl-, (8β, 13β)- | C20H32 | 23.340 | 272 | 3.198 |
| Podocarp-7-en-3β-ol, 13β-methyl-13-vinyl- | C20H32O | 23.490 | 288 | 1.785 |
| 1,6,10,14-Hexadecatetraen-3-ol, 3,7,11,15-tetramethyl-, (E, E)- | C20H34O | 23.603 | 290 | 1.672 |
| Naphthalene, decahydro-1,1,4a-trimethyl-6-methylene-5-(3-methyl-2,4-pentadienyl)-, [4aS-(4aα,5α | C20H32 | 23.890 | 272 | 4.854 |
| Phenol,2,2′-methylenebis [6-(1,1-dimethylethyl)-4-methyl- | C23H32O2 | 24.651 | 340 | 2.562 |
| Androst-5-en-17-ol,4,4-dimethyl- | C21H34O | 24.764 | 302 | 5.426 |
| Estra-1,3,5(10)-trien-17-one,3-hydroxy-2-methoxy- | C19H24O3 | 25.419 | 300 | 17.137 |
| Podocarp-13-ene-14-glycolic acid, 7-hydroxy-8,13-dimethyl-3-oxo-, -lactone | C21H30O4 | 25.600 | 346 | 1.911 |
| Bolasterone | C21H32O2 | 25.804 | 316 | 1.954 |
| 16-Allopregnen-3β-ol-20-one | C21H32O2 | 26.241 | 316 | 1.496 |
| Campesterol | C28H48O | 30.702 | 400 | 2.046 |
| Stigmasterol | C29H48O | 30.980 | 412 | 4.189 |
| ϒ-Sitosterol | C29H50O | 31.515 | 414 | 3.981 |
| Lupeol | C30H50O | 32.450 | 426 | 16.618 |
| Identified Compounds | Molecular Formula | Retention Time | Molecular Weight (g/mol) | Total (%Age) |
|---|---|---|---|---|
| 2-Pyrrolidinone, 1-methyl- | C5H9NO | 7.455 | 99 | 4.1584 |
| Heptadecane,2,6,10,15-tetramethyl- | C21H44 | 15.314 | 296 | 1.121 |
| Phenol,2,4-bis(1,1-dimethylethyl)- | C14H22O | 15.533 | 206 | 1.760 |
| Eicosane,2-methyl- | C21H44 | 17.876 | 296 | 1.229 |
| Hexadecanoic acid, methyl ester | C17H34O2 | 20.122 | 270 | 2.358 |
| 1,2-Benzenedicarboxylic acid, butyl 8-methylnonyl ester | C22H34O4 | 20.544 | 362 | 1.507 |
| 7,10-Octadecadienoic acid, methyl ester | C19H34O2 | 21.757 | 294 | 1.569 |
| 10-Octadecenoic acid, methyl ester | C19H36O2 | 21.810 | 296 | 1.201 |
| Ethyleneglycol dipelargonate | C20H38O4 | 23.257 | 342 | 2.256 |
| Phenol,2,2′-methylenebis [6-(1,1-dimethylethyl)-4-methyl- | C23H32O2 | 23.897 | 340 | 1.125 |
| Estra-1,3,5(10)-trien-17-one,3-hydroxy-2-methoxy- | C19H24O3 | 24.651 | 300 | 7.974 |
| Bis(2-ethylhexyl) phthalate | C24H38O4 | 25.404 | 390 | 6.118 |
| ϒ-Sitosterol | C29H50O | 25.623 | 414 | 41.430 |
| Methylenebis(2,4,6-triisopropylphenylphosphine) | C31H50P2 | 31.515 | 484 | 2.582 |
| Identified Compounds | Molecular Formula | Retention Time | Molecular Weight (g/mol) | Total (%Age) |
|---|---|---|---|---|
| Phenol,2,4, bis(1,1-dimethylethyl)- | C14H22O | 15.533 | 206 | 1.760 |
| Hexadecanoic acid, methyl ester | C17H34O2 | 20.115 | 270 | 2.004 |
| n-Hexadecanoic acid | C16H32O2 | 20.469 | 256 | 2.677 |
| 8,11-Octadecadienoic acid, methyl ester | C19H34O2 | 21.757 | 294 | 2.453 |
| 10-Octadecadienoic acid, methyl ester | C19H36O2 | 21.810 | 296 | 1.574 |
| Oleic acid | C18H34O2 | 22.149 | 282 | 1.279 |
| 9-Octadeccenoic acid,12-hydroxy-, methyl ester, [R-(Z)]- | C19H36O3 | 23.505 | 312 | 1.472 |
| 1-Naphthalenemethanol, Decahydro-5-(5-hydroxy-3-methyl-3-pentenyl)-1,4a-dimethyl-6-methyle | C20H34O2 | 23.897 | 306 | 1.333 |
| Phenol,2,2′-methylenebis [6-(1,1-dimethylethyl)-4-methyl- | C23H32O2 | 24.651 | 340 | 4.530 |
| Androst-5-en-17-ol,4,4-dimethyl- | C21H34O | 24.764 | 302 | 1.644 |
| Hexadecanoic acid,1-(hydroxymethyl)-1,2-ethanediyl ester | C35H68O5 | 25.269 | 568 | 1.008 |
| Estra-1,3,5(10)-trien-17-one,3-hydroxy-2-methoxy- | C19H24O3 | 25.404 | 300 | 4.534 |
| Bis(2-ethylhexyl) phthalate | C24H38O4 | 25.630 | 390 | 65.902 |
| Squalene | C30H5O | 27.635 | 410 | 0.911 |
| Stigmasterol | C29H48O | 30.973 | 412 | 1.033 |
| sϒ-Sitosterol | C29H50O | 31.515 | 414 | 1.918 |
| Lupeol | C30H50O | 32.435 | 426 | 3.696 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashaal, K.; Shabbir, A.; Khan, M.A.; Hameed, H.; Shahzad, M.; Irfan, A.; Shazly, G.A.; Mobashar, A.; Akhtar, T.; Shaheryar, Z.A.; et al. Anti-Arthritic and Immunomodulatory Potential of Methanolic, n-Hexane, and Ethyl Acetate Fractions of Bark of Acacia modesta on Complete Freund’s Adjuvant-Induced Arthritis in Rats. Pharmaceutics 2023, 15, 2228. https://doi.org/10.3390/pharmaceutics15092228
Mashaal K, Shabbir A, Khan MA, Hameed H, Shahzad M, Irfan A, Shazly GA, Mobashar A, Akhtar T, Shaheryar ZA, et al. Anti-Arthritic and Immunomodulatory Potential of Methanolic, n-Hexane, and Ethyl Acetate Fractions of Bark of Acacia modesta on Complete Freund’s Adjuvant-Induced Arthritis in Rats. Pharmaceutics. 2023; 15(9):2228. https://doi.org/10.3390/pharmaceutics15092228
Chicago/Turabian StyleMashaal, Kiran, Arham Shabbir, Mahtab Ahmad Khan, Huma Hameed, Muhammad Shahzad, Ali Irfan, Gamal A. Shazly, Aisha Mobashar, Tasleem Akhtar, Zaib Ali Shaheryar, and et al. 2023. "Anti-Arthritic and Immunomodulatory Potential of Methanolic, n-Hexane, and Ethyl Acetate Fractions of Bark of Acacia modesta on Complete Freund’s Adjuvant-Induced Arthritis in Rats" Pharmaceutics 15, no. 9: 2228. https://doi.org/10.3390/pharmaceutics15092228
APA StyleMashaal, K., Shabbir, A., Khan, M. A., Hameed, H., Shahzad, M., Irfan, A., Shazly, G. A., Mobashar, A., Akhtar, T., Shaheryar, Z. A., & Bin Jardan, Y. A. (2023). Anti-Arthritic and Immunomodulatory Potential of Methanolic, n-Hexane, and Ethyl Acetate Fractions of Bark of Acacia modesta on Complete Freund’s Adjuvant-Induced Arthritis in Rats. Pharmaceutics, 15(9), 2228. https://doi.org/10.3390/pharmaceutics15092228








