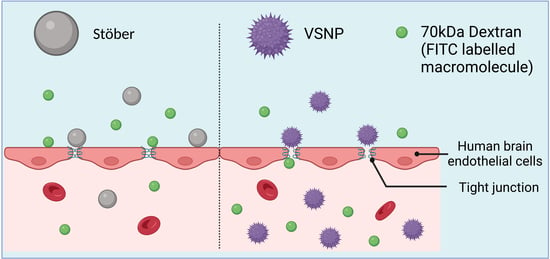
Virus-like Silica Nanoparticles Improve Permeability of Macromolecules across the Blood–Brain Barrier In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Silica Nanoparticles
2.2.1. Virus-like Silica Nanoparticles (VSNP)
2.2.2. Stöber Silica Nanoparticles
2.3. Fluorescent Dye Cy5 Labelling on Silica Nanoparticles
2.4. Physiochemical Characterisation of Nanoparticles
2.5. Cell Culture
2.6. Cell Viability
2.7. Cellular Uptake
2.8. In Vitro Transwell BBB Model
2.9. Modulation of In Vitro BBB Model for Transport of Macromolecules
2.10. Statistical Analysis
3. Results and Discussion
3.1. Synthesis, Functionalization, and Characterization of Nanoparticles
3.2. Cell Viability and Cellular Uptake
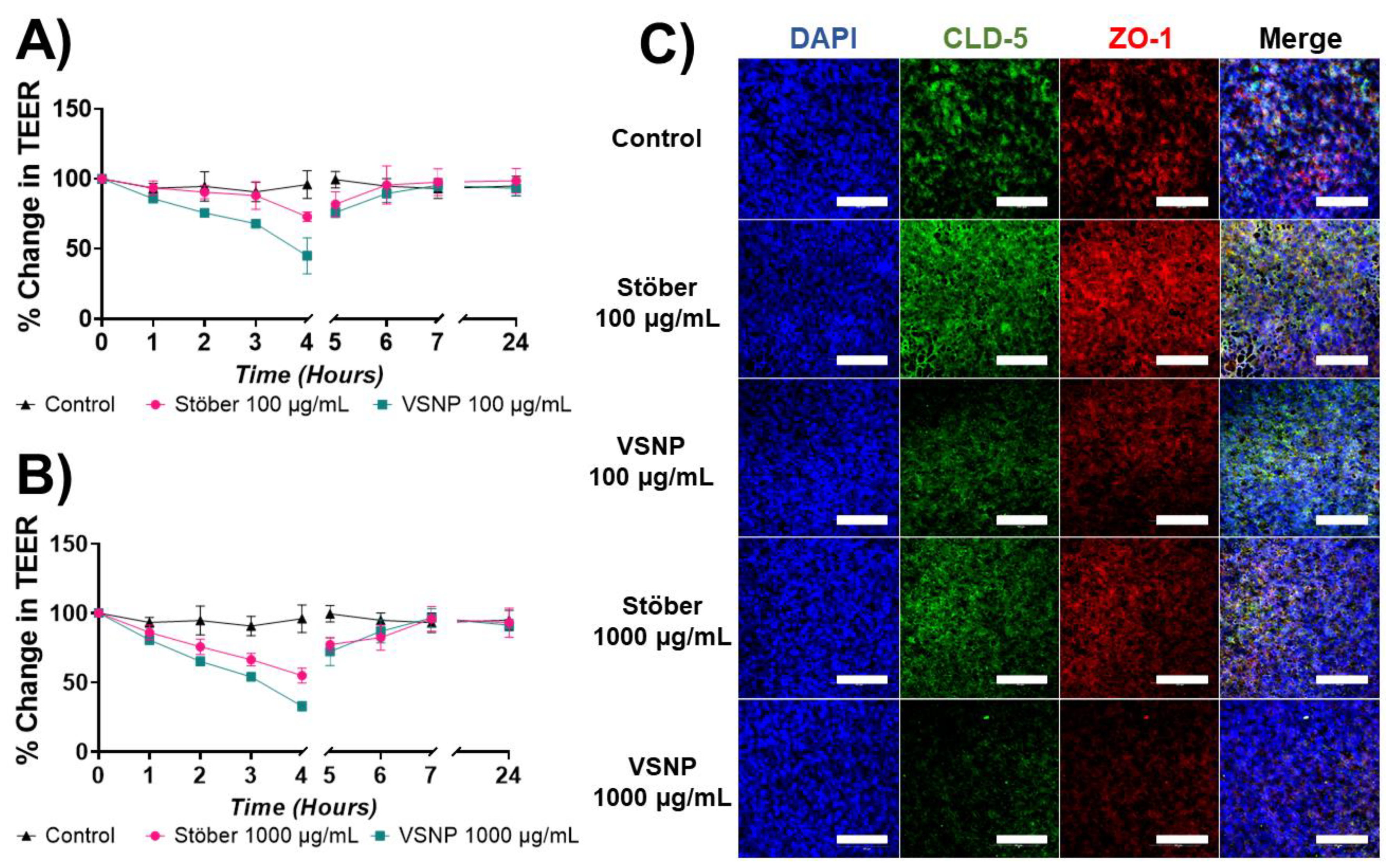
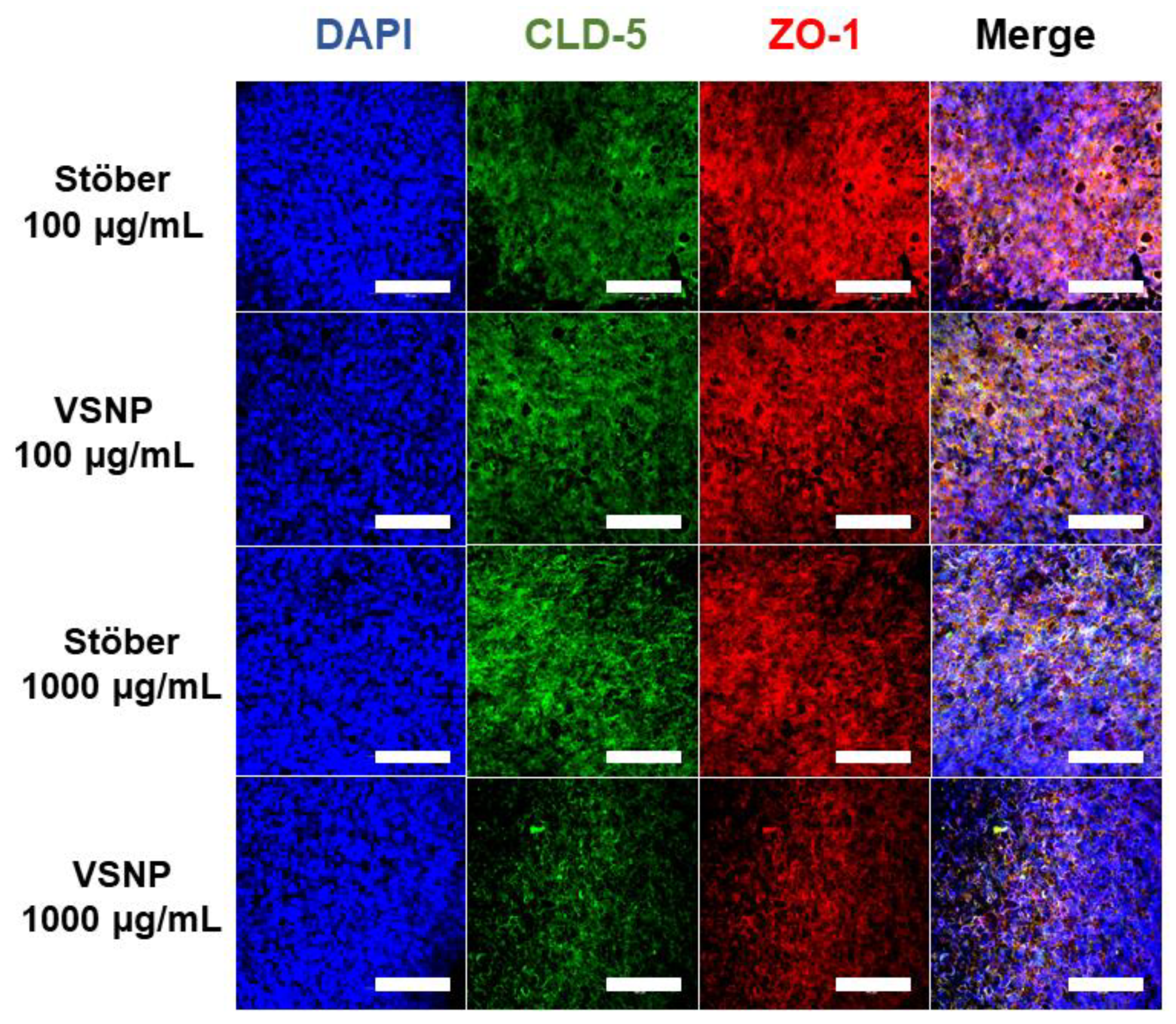
3.3. Effect of Silica Nanoparticles on the Integrity of the Blood–Brain Barrier In Vitro
3.4. Transient Relaxation of Tight Junctions in the Blood–Brain Barrier for Macromolecule Delivery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Janjua, T.I.; Rewatkar, P.; Ahmed-Cox, A.; Saeed, I.; Mansfeld, F.M.; Kulshreshtha, R.; Kumeria, T.; Ziegler, D.S.; Kavallaris, M.; Mazzieri, R. Frontiers in the treatment of glioblastoma: Past, present and emerging. Adv. Drug Deliv. Rev. 2021, 171, 108–138. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood–brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood–brain barrier delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef]
- Ung, C.; Upton, D.H.; Venkat, P.; Pandher, R.; Curnis, F.; Corti, A.; Nicolazzo, J.; Mayoh, C.; Tsoli, M.; Ziegler, D.S. DIPG-21. DIPG cells alter the permeability of the blood-brain barrier in the brainstem leading to treatment failure. Neuro-Oncology 2022, 24, i22. [Google Scholar] [CrossRef]
- Thakur, A.; Faujdar, C.; Sharma, R.; Sharma, S.; Malik, B.; Nepali, K.; Liou, J.P. Glioblastoma: Current Status, Emerging Targets, and Recent Advances. J. Med. Chem. 2022, 65, 8596–8685. [Google Scholar] [CrossRef]
- Marenco-Hillembrand, L.; Wijesekera, O.; Suarez-Meade, P.; Mampre, D.; Jackson, C.; Peterson, J.; Trifiletti, D.; Hammack, J.; Ortiz, K.; Lesser, E. Trends in glioblastoma: Outcomes over time and type of intervention: A systematic evidence based analysis. J. Neuro-Oncol. 2020, 147, 297–307. [Google Scholar] [CrossRef]
- Janjua, T.I.; Cao, Y.; Yu, C.; Popat, A. Clinical translation of silica nanoparticles. Nat. Rev. Mater. 2021, 6, 1072–1074. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update post COVID-19 vaccines. Bioeng. Transl. Med. 2021, 6, e10246. [Google Scholar] [CrossRef]
- Huang, H.; Feng, W.; Chen, Y.; Shi, J. Inorganic nanoparticles in clinical trials and translations. Nano Today 2020, 35, 100972. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles for drug delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Schüth, F.; Lozano, D.; Colilla, M.; Manzano, M. Engineering mesoporous silica nanoparticles for drug delivery: Where are we after two decades? Chem. Soc. Rev. 2022, 51, 5365–5451. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.; Neffgen, N.; Lindén, M. Straightforward adsorption-based formulation of mesoporous silica nanoparticles for drug delivery applications. J. Colloid Interface Sci. 2023, 640, 961–974. [Google Scholar] [CrossRef]
- Theivendran, S.; Lazarev, S.; Yu, C. Mesoporous silica/organosilica nanoparticles for cancer immunotherapy. Exploration 2023, 3, 20220086. [Google Scholar] [CrossRef]
- Lamson, N.G.; Berger, A.; Fein, K.C.; Whitehead, K.A. Anionic nanoparticles enable the oral delivery of proteins by enhancing intestinal permeability. Nat. Biomed. Eng. 2020, 4, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Janjua, T.I.; Qu, Z.; Draphoen, B.; Bai, Y.; Linden, M.; Moniruzzaman, M.; Hasnain, S.; Kumeria, T.; Popat, A. Virus-like silica nanoparticles enhance macromolecule permeation in vivo. Biomater. Sci. 2023, 11, 4508–4521. [Google Scholar] [CrossRef]
- Niu, Y.; Yu, M.; Meka, A.; Liu, Y.; Zhang, J.; Yang, Y.; Yu, C. Understanding the contribution of surface roughness and hydrophobic modification of silica nanoparticles to enhanced therapeutic protein delivery. J. Mater. Chem. B 2016, 4, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, C.; Lu, L.; Li, X.; Wang, W.; Zhao, M.; Hu, L.; El-Toni, A.M.; Li, Q.; Zhang, F. Kinetics-mediate fabrication of multi-model bioimaging lanthanide nanoplates with controllable surface roughness for blood brain barrier transportation. Biomaterials 2017, 141, 223–232. [Google Scholar] [CrossRef]
- Wang, X.; Hou, Y.; Ai, X.; Sun, J.; Xu, B.; Meng, X.; Zhang, Y.; Zhang, S. Potential applications of microfluidics based blood brain barrier (BBB)-on-chips for in vitro drug development. Biomed. Pharmacother. 2020, 132, 110822. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Liu, D.; Subramanyam, K.; Wang, B.; Yang, J.; Huang, J.; Auguste, D.T.; Moses, M.A. Nanoparticle elasticity directs tumor uptake. Nat. Commun. 2018, 9, 130. [Google Scholar] [CrossRef]
- Wang, W.; Wang, P.; Tang, X.; Elzatahry, A.A.; Wang, S.; Al-Dahyan, D.; Zhao, M.; Yao, C.; Hung, C.-T.; Zhu, X. Facile synthesis of uniform virus-like mesoporous silica nanoparticles for enhanced cellular internalization. ACS Cent. Sci. 2017, 3, 839–846. [Google Scholar] [CrossRef]
- Meka, A.K.; Gopalakrishna, A.; Iriarte-Mesa, C.; Rewatkar, P.; Qu, Z.; Wu, X.; Cao, Y.; Prasadam, I.; Janjua, T.I.; Kleitz, F. Influence of Pore Size and Surface Functionalization of Mesoporous Silica Nanoparticles on the Solubility and Antioxidant Activity of Confined Coenzyme Q10. Mol. Pharm. 2023, 20, 2966–2977. [Google Scholar] [CrossRef]
- Janjua, T.I.; Ahmed-Cox, A.; Meka, A.K.; Mansfeld, F.M.; Forgham, H.; Ignacio, R.M.C.; Cao, Y.; McCarroll, J.A.; Mazzieri, R.; Kavallaris, M. Facile synthesis of lactoferrin conjugated ultra small large pore silica nanoparticles for the treatment of glioblastoma. Nanoscale 2021, 13, 16909–16922. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, Z.; Subramaniam, S.; Khan, G.M.; Abeer, M.M.; Qu, Z.; Janjua, T.; Kumeria, T.; Batra, J.; Popat, A. Encapsulation and controlled release of resveratrol within functionalized mesoporous silica nanoparticles for prostate cancer therapy. Front. Bioeng. Biotechnol. 2019, 7, 225. [Google Scholar] [CrossRef]
- Ang, C.W.; Tan, L.; Qu, Z.; West, N.P.; Cooper, M.A.; Popat, A.; Blaskovich, M.A. Mesoporous silica nanoparticles improve oral delivery of antitubercular bicyclic nitroimidazoles. ACS Biomater. Sci. Eng. 2021, 8, 4196–4206. [Google Scholar] [CrossRef]
- Janjua, T.I.; Cao, Y.; Ahmed-Cox, A.; Raza, A.; Moniruzzaman, M.; Akhter, D.T.; Fletcher, N.L.; Kavallaris, M.; Thurecht, K.J.; Popat, A. Efficient delivery of Temozolomide using ultrasmall large-pore silica nanoparticles for glioblastoma. J. Control. Release 2023, 357, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Habibi, N.; Wu, D.; Lahann, J.; Mitragotri, S. Effect of nanoparticle composition, size, shape, and stiffness on penetration across the blood–brain barrier. ACS Biomater. Sci. Eng. 2020, 6, 4916–4928. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Yang, H.; Dutta, D.; Stanley, J.T.; Holloway, P.H.; Tan, W.; Moudgil, B.M.; Mericle, R.A. TAT conjugated, FITC doped silica nanoparticles for bioimaging applications. Chem. Commun. 2004, 24, 2810–2811. [Google Scholar] [CrossRef]
- Dong, S.; Roman, M. Fluorescently labeled cellulose nanocrystals for bioimaging applications. J. Am. Chem. Soc. 2007, 129, 13810–13811. [Google Scholar] [CrossRef]
- Souza, T.G.; Ciminelli, V.S.; Mohallem, N.D.S. A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. In Proceedings of the Journal of Physics: Conference Series, Bento Gonçalves, Brazil, 29 November–2 December 2015; p. 012039. [Google Scholar]
- Huang, X.; Shi, X.; Hansen, M.E.; Setiady, I.; Nemeth, C.L.; Celli, A.; Huang, B.; Mauro, T.; Koval, M.; Desai, T.A. Nanotopography enhances dynamic remodeling of tight junction proteins through cytosolic liquid complexes. ACS Nano 2020, 14, 13192–13202. [Google Scholar] [CrossRef]
- Nugent, M.A. The future of the COVID-19 pandemic: How good (or bad) can the SARS-CoV-2 spike protein get? Cells 2022, 11, 855. [Google Scholar] [CrossRef]
- Peisahovics, F.; Rohaim, M.A.; Munir, M. Structural topological analysis of spike proteins of SARS-CoV-2 variants of concern highlight distinctive amino acid substitution patterns. Eur. J. Cell Biol. 2022, 101, 151275. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Campbell, M. Tight junction modulation at the blood-brain barrier: Current and future perspectives. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183298. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Finbloom, J.A.; Huynh, C.; Huang, X.; Desai, T.A. Bioinspired nanotopographical design of drug delivery systems. Nat. Rev. Bioeng. 2023, 1, 139–152. [Google Scholar] [CrossRef]
- Lockman, P.R.; Koziara, J.M.; Mumper, R.J.; Allen, D.D. Nanoparticle surface charges alter blood–brain barrier integrity and permeability. J. Drug Target. 2004, 12, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; He, L.; Ma, B.; Chen, T. Tailoring particle size of mesoporous silica nanosystem to antagonize glioblastoma and overcome blood–brain barrier. ACS Appl. Mater. Interfaces 2016, 8, 6811–6825. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Du, D.; Li, L.; Xu, J.; Dutta, P.; Lin, Y. In vitro study of receptor-mediated silica nanoparticles delivery across blood–brain barrier. ACS Appl. Mater. Interfaces 2017, 9, 20410–20416. [Google Scholar] [CrossRef]

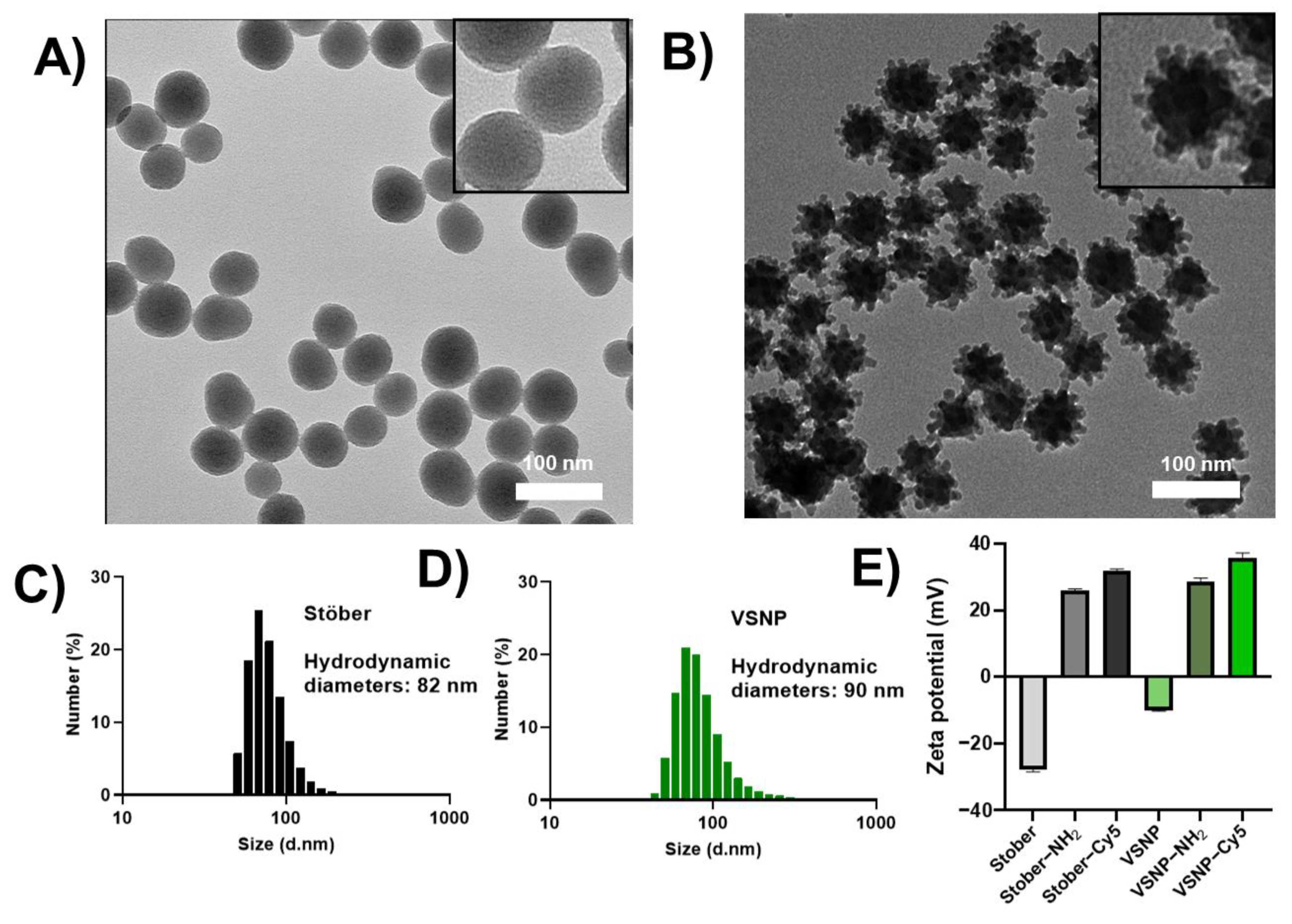

| Sample | Average Size nm (Measured via TEM) | BET Surface Area (m2g−1) |
|---|---|---|
| Stöber | 59 ± 6 | 78 |
| VSNP | 62 ± 10 | 117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Cao, Y.; Qu, Z.; Janjua, T.I.; Popat, A. Virus-like Silica Nanoparticles Improve Permeability of Macromolecules across the Blood–Brain Barrier In Vitro. Pharmaceutics 2023, 15, 2239. https://doi.org/10.3390/pharmaceutics15092239
Feng Y, Cao Y, Qu Z, Janjua TI, Popat A. Virus-like Silica Nanoparticles Improve Permeability of Macromolecules across the Blood–Brain Barrier In Vitro. Pharmaceutics. 2023; 15(9):2239. https://doi.org/10.3390/pharmaceutics15092239
Chicago/Turabian StyleFeng, Yuran, Yuxue Cao, Zhi Qu, Taskeen Iqbal Janjua, and Amirali Popat. 2023. "Virus-like Silica Nanoparticles Improve Permeability of Macromolecules across the Blood–Brain Barrier In Vitro" Pharmaceutics 15, no. 9: 2239. https://doi.org/10.3390/pharmaceutics15092239