M1 Macrophage-Biomimetic Targeted Nanoparticles Containing Oxygen Self-Supplied Enzyme for Enhancing the Chemotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Cell Lines, and Animals
2.1.1. Materials
2.1.2. Cell Lines and Animals
2.2. Preparation and Characterization of DOX/CAT@PLGA and DOX/CAT@PLGA-M1
2.2.1. Preparation of DOX/CAT@PLGA
2.2.2. M1 Macrophage Membrane Extraction and Characterization
2.2.3. Preparation of DOX/CAT@PLGA-M1
2.2.4. Characterization of DOX/CAT@PLGA and DOX/CAT@PLGA-M1
2.2.5. Stability of NPs and In Vitro Drug Release
2.2.6. Hemolytic Assay
2.2.7. Catalase Activity Assay
2.3. In Vitro Cell Cytotoxicity
2.4. In Vitro Cellular Uptake
2.5. Wound Healing Assay
2.6. In Vivo Assessments of Biodistribution
2.7. Antitumor Effects and Biosafety of NPs In Vivo
2.8. Statistical Analysis
3. Results and Discussion
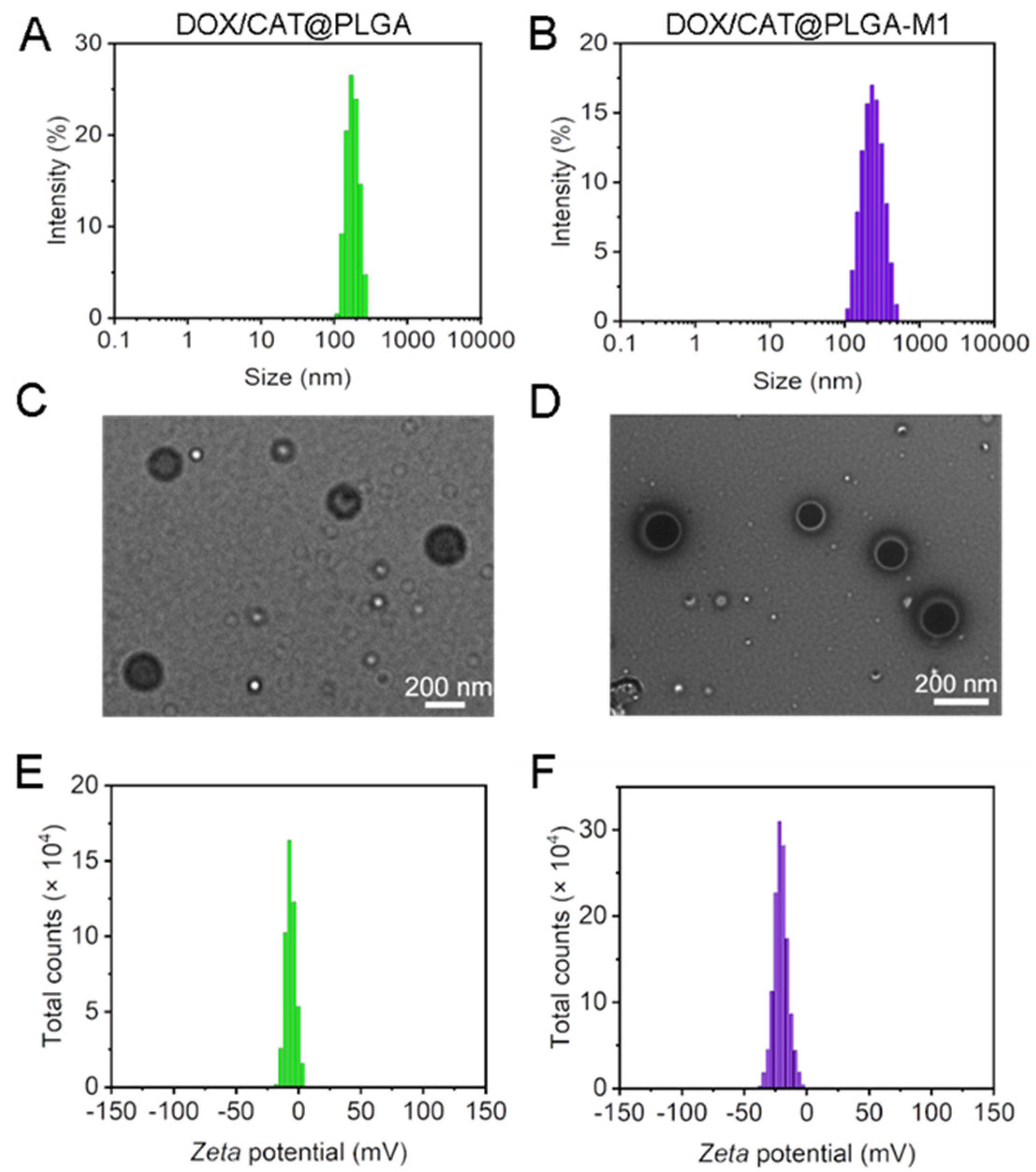
3.1. Characterization of DOX/CAT@PLGA and DOX/CAT@PLGA-M1
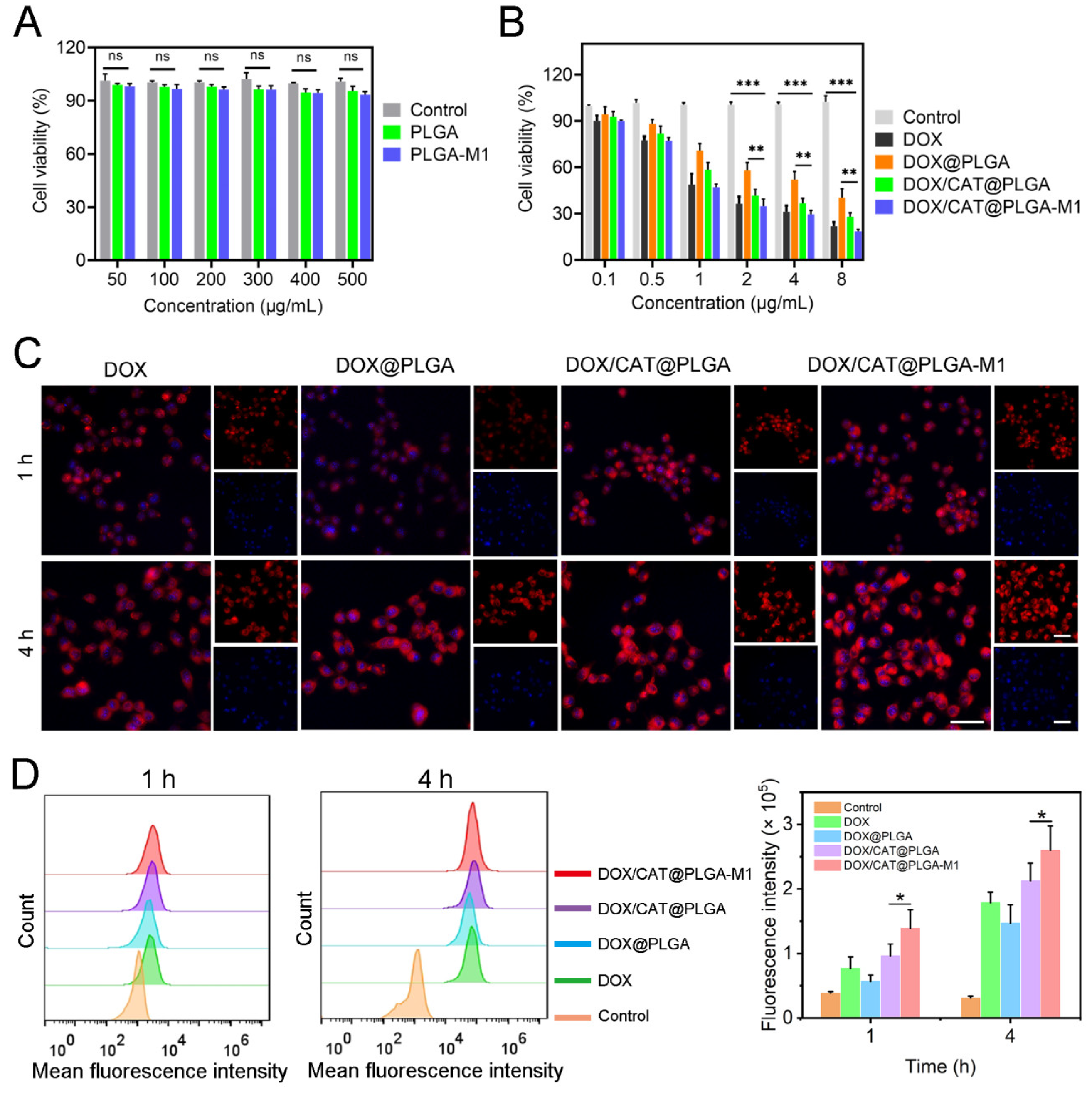
3.2. In Vitro Cellular Level Study
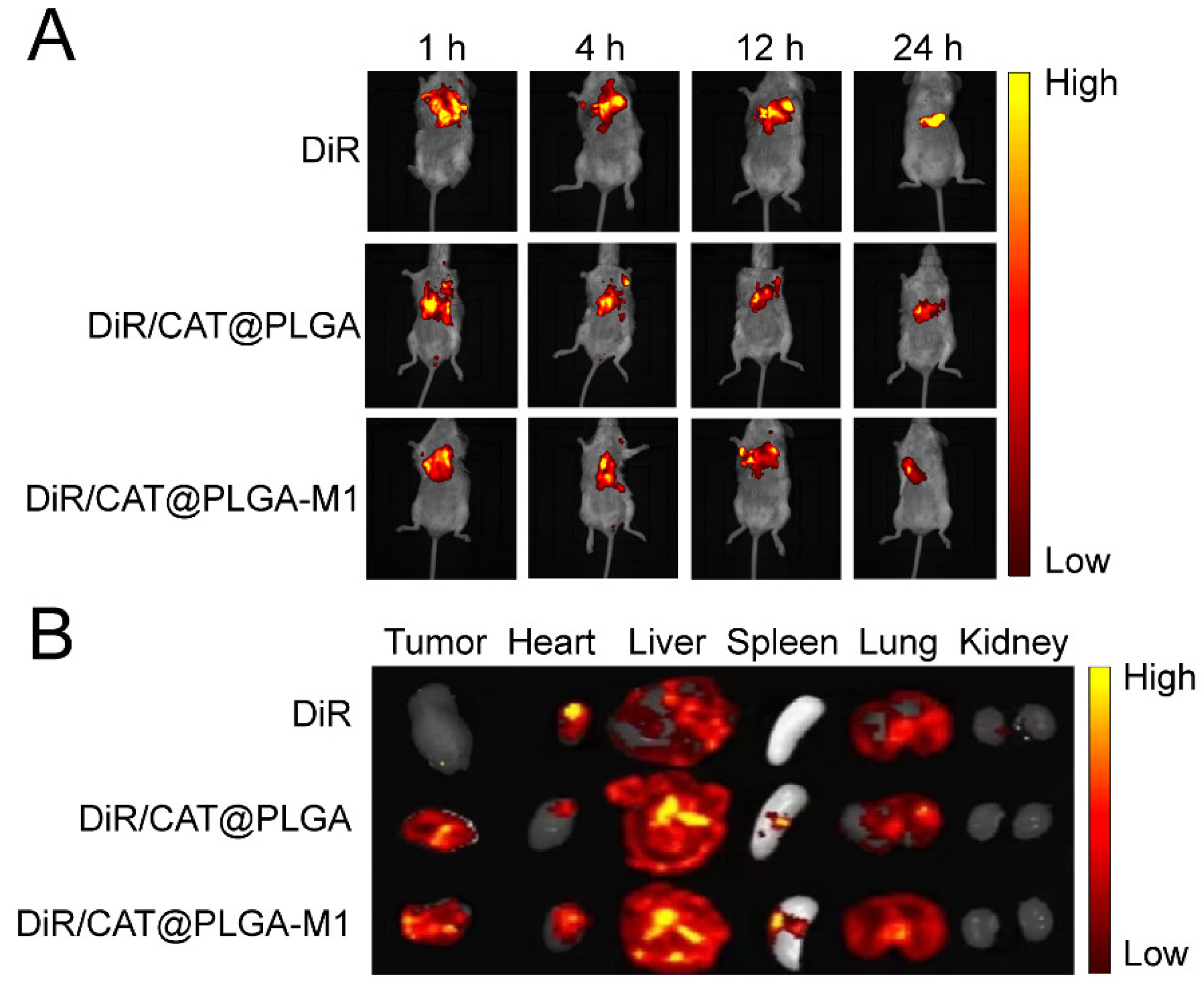
3.3. In Vivo Biodistribution
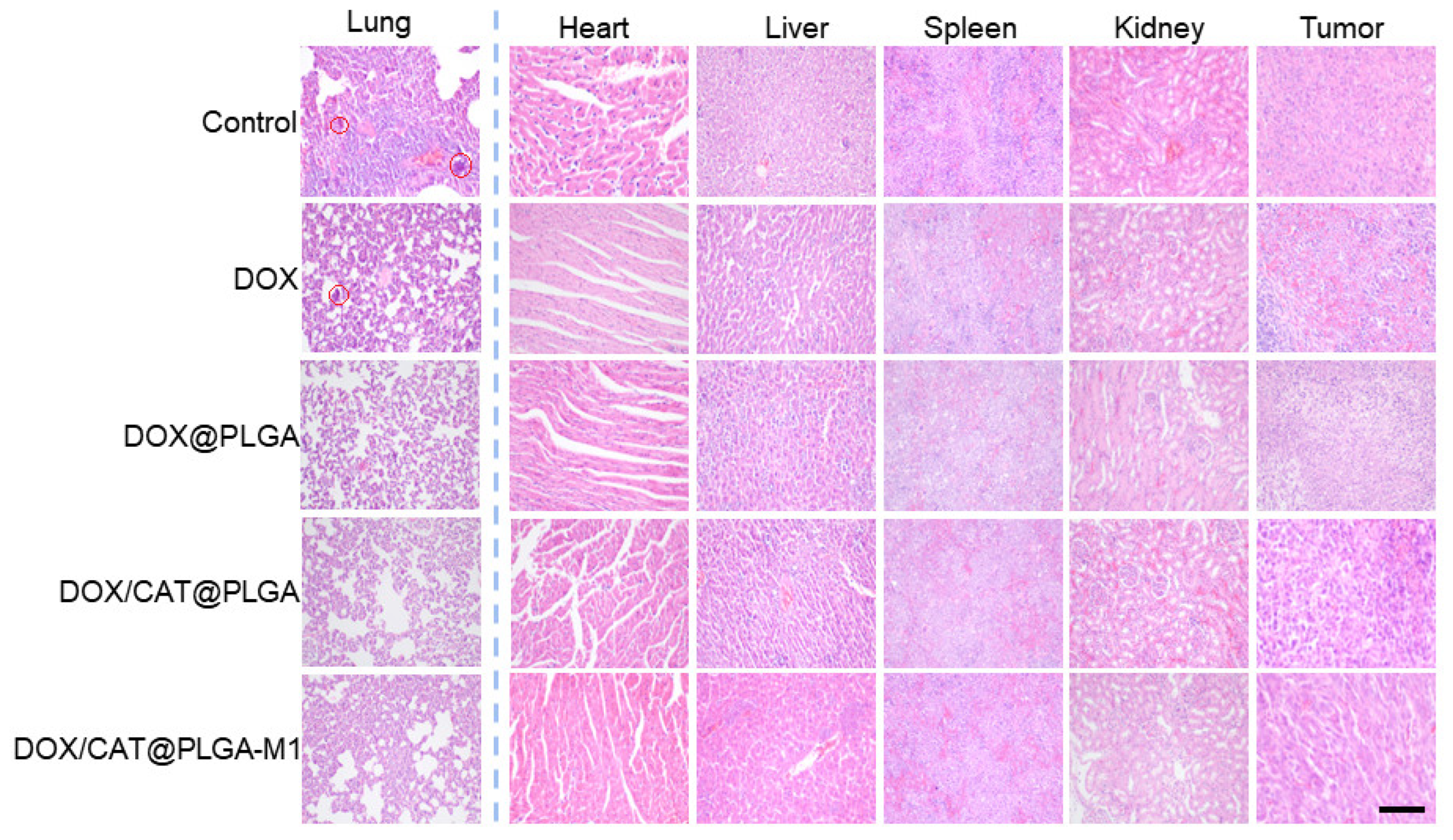
3.4. In Vivo Antitumor Efficacy
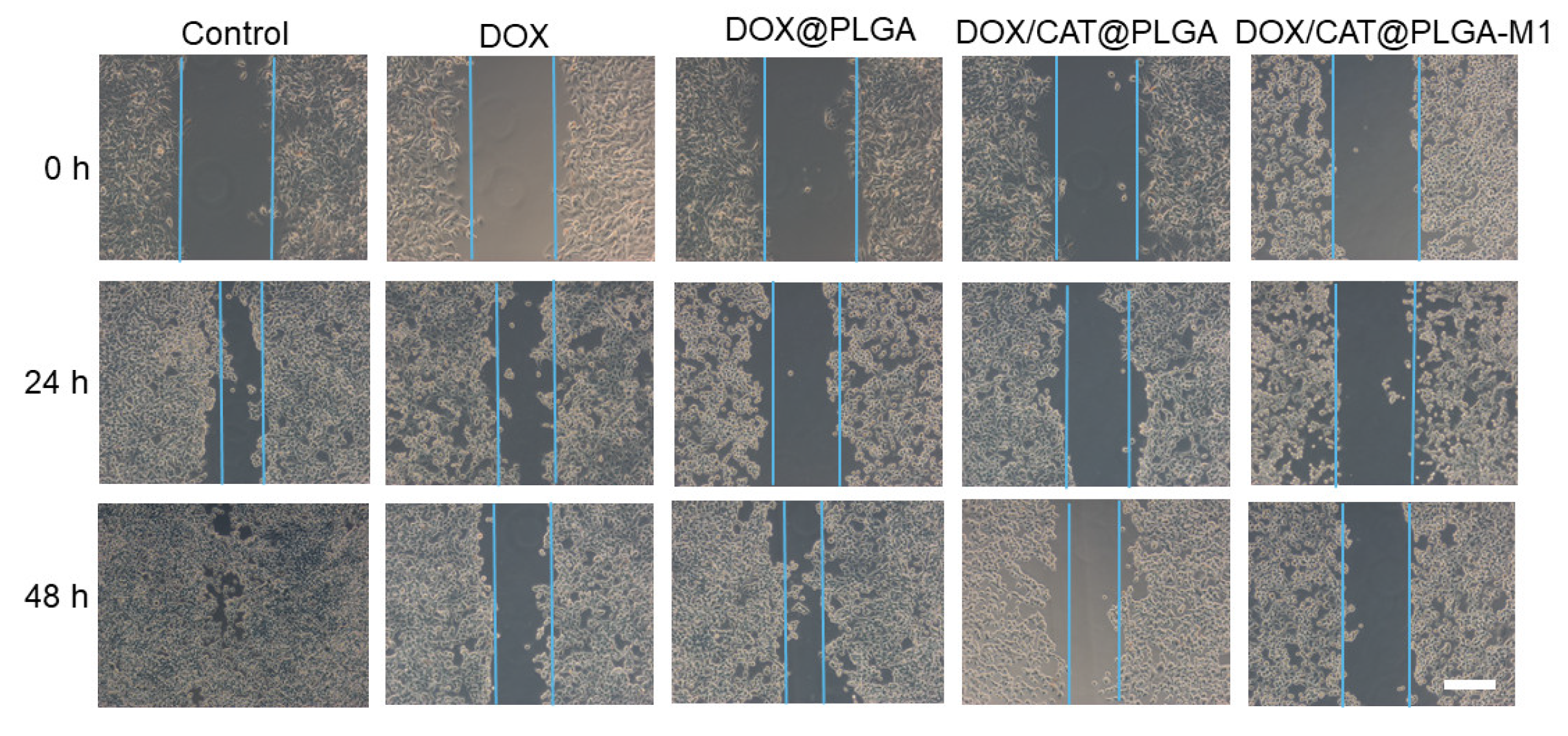
3.5. The Inhibition of Metastasis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, R.; Kim, E.J.; Han, J.; Lee, H.; Shin, W.S.; Kim, H.M.; Bhuniya, S.; Kim, J.S.; Hong, K.S. Hypoxia-directed and activated theranostic agent: Imaging and treatment of solid tumor. Biomaterials 2016, 104, 119–128. [Google Scholar] [CrossRef]
- Feng, L.; Cheng, L.; Dong, Z.; Tao, D.; Barnhart, T.E.; Cai, W.; Chen, M.; Liu, Z. Theranostic Liposomes with hypoxia-activated prodrug to effectively destruct hypoxic tumors post-photodynamic therapy. ACS Nano 2017, 11, 927–937. [Google Scholar] [CrossRef]
- Gong, H.; Chao, Y.; Xiang, J.; Han, X.; Song, G.; Feng, L.; Liu, J.; Yang, G.; Chen, Q.; Liu, Z. Hyaluronidase to enhance nanoparticle-based photodynamic tumor therapy. Nano Lett. 2016, 16, 2512–2521. [Google Scholar] [CrossRef]
- Yang, N.; Xiao, W.; Song, X.; Wang, W.; Dong, X. Recent advances in tumor microenvironment hydrogen peroxide-responsive materials for cancer photodynamic therapy. Nano-Micro Lett. 2020, 12, 15. [Google Scholar] [CrossRef]
- Zhou, R.; Ohulchanskyy, T.Y.; Xu, H.; Ziniuk, R.; Qu, J. Catalase nanocrystals loaded with methylene blue as oxygen self-supplied, imaging-guided platform for photodynamic therapy of hypoxic tumors. Small 2021, 17, e2103569. [Google Scholar] [CrossRef]
- Wang, H.; Chao, Y.; Liu, J.; Zhu, W.; Wang, G.; Xu, L.; Liu, Z. Photosensitizer-crosslinked in-situ polymerization on catalase for tumor hypoxia modulation & enhanced photodynamic therapy. Biomaterials 2018, 181, 310–317. [Google Scholar]
- Zhang, Y.; Liu, S.; Yao, Y.; Chen, Y.; Zhou, S.; Yang, X.; Wang, K.; Liu, J. Invasion and defense interactions between enzyme-active liquid coacervate protocells and living cells. Small 2020, 16, e2002073. [Google Scholar] [CrossRef]
- Cheng, X.; He, L.; Xu, J.; Fang, Q.; Yang, L.; Xue, Y.; Wang, X.; Tang, R. Oxygen-producing catalase-based prodrug nanoparticles overcoming resistance in hypoxia-mediated chemo-photodynamic therapy. Acta Biomater. 2020, 112, 234–249. [Google Scholar] [CrossRef]
- Lu, Z.; Gao, J.; Fang, C.; Zhou, Y.; Li, X.; Han, G. Porous Pt nanospheres incorporated with GOx to enable synergistic oxygen-inductive starvation/electrodynamic tumor therapy. Adv. Sci. 2020, 7, 2001223. [Google Scholar] [CrossRef]
- Shi, C.; Li, M.; Zhang, Z.; Yao, Q.; Shao, K.; Xu, F.; Xu, N.; Li, H.; Fan, J.; Sun, W.; et al. Catalase-based liposomal for reversing immunosuppressive tumor microenvironment and enhanced cancer chemo-photodynamic therapy. Biomaterials 2020, 233, 119755. [Google Scholar] [CrossRef]
- Cao, X.; Tan, T.; Zhu, D.; Yu, H.; Liu, Y.; Zhou, H.; Jin, Y.; Xia, Q. Paclitaxel-loaded macrophage membrane camouflaged albumin nanoparticles for targeted cancer therapy. Int. J. Nanomed. 2020, 15, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Ji, S.; Chang, T.; Yu, Z.; Zhang, J.; Hu, M.; Cheng, X.; Huo, Q. Construction of bionic nanoparticles camouflaged with macrophage membranes for drug delivery in breast cancer. J. Drug Deliv. Sci. Technol. 2023, 84, 104433. [Google Scholar] [CrossRef]
- Liu, R.; An, Y.; Jia, W.; Wang, Y.; Wu, Y.; Zhen, Y.; Cao, J.; Gao, H. Macrophage-mimic shape changeable nanomedicine retained in tumor for multimodal therapy of breast cancer. J. Control. Release 2020, 321, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Ying, K.; Zhu, Y.; Wan, J.; Zhan, C.; Wang, Y.; Xie, B.; Xu, P.; Pan, H.; Wang, H. Macrophage membrane-biomimetic adhesive polycaprolactone nanocamptothecin for improving cancer-targeting efficiency and impairing metastasis. Bioact. Mater. 2023, 20, 449–462. [Google Scholar] [CrossRef]
- Hu, C.; Lei, T.; Wang, Y.; Cao, J.; Yang, X.; Qin, L.; Liu, R.; Zhou, Y.; Tong, F.; Umeshappa, C.S.; et al. Phagocyte-membrane-coated and laser-responsive nanoparticles control primary and metastatic cancer by inducing anti-tumor immunity. Biomaterials 2020, 255, 120159. [Google Scholar] [CrossRef] [PubMed]
- Poudel, K.; Banstola, A.; Gautam, M.; Soe, Z.; Phung, C.D.; Pham, L.M.; Jeong, J.H.; Choi, H.G.; Ku, S.K.; Tran, T.H.; et al. Macrophage-membrane-camouflaged disintegrable and excretable nanoconstruct for deep tumor penetration. ACS Appl. Mater. Interfaces 2020, 12, 56767–56781. [Google Scholar] [CrossRef]
- Lv, W.; Xu, J.; Wang, X.; Li, X.; Xu, Q.; Xin, H. Bioengineered boronic ester modified dextran polymer nanoparticles as reactive oxygen species responsive nanocarrier for ischemic stroke treatment. ACS Nano 2018, 12, 5417–5426. [Google Scholar] [CrossRef]
- Cao, H.; Dan, Z.; He, X.; Zhang, Z.; Yu, H.; Yin, Q.; Li, Y. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano 2016, 10, 7738–7748. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, J.; Wang, Y.; Peng, C.; Hu, H.; Qiao, M.; Zhao, X.; Chen, D. Tumor-specific nitric oxide generator to amplify peroxynitrite based on highly penetrable nanoparticles for metastasis inhibition and enhanced cancer therapy. Biomaterials 2022, 283, 121448. [Google Scholar] [CrossRef]
- Li, J.; Cai, H.; Sun, H.; Qu, J.; Zhao, B.; Hu, X.; Li, W.; Qian, Z.; Yu, X.; Kang, F.; et al. Extracts of cordyceps sinensis inhibit breast cancer growth through promoting M1 macrophage polarization via NF-kappaB pathway activation. J. Ethnopharmacol. 2020, 260, 112969. [Google Scholar] [CrossRef]
- Wang, Q.S.; Li, K.; Gao, L.N.; Zhang, Y.; Lin, K.M.; Cui, Y.L. Intranasal delivery of berberine via in situ thermoresponsive hydrogels with non-invasive therapy exhibits better antidepressant-like effects. Biomater. Sci. 2020, 8, 2853–2865. [Google Scholar] [CrossRef]
- Krishnan, V.; Venkatasubbu, G.D.; Kalaivani, T. Investigation of hemolysis and antibacterial analysis of curcumin-loaded mesoporous SiO2 nanoparticles. Appl. Nanosci. 2021, 13, 811–818. [Google Scholar] [CrossRef]
- Góth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Xiao, T.; He, M.; Xu, F.; Fan, Y.; Jia, B.; Shen, M.; Wang, H.; Shi, X. Macrophage membrane-camouflaged responsive polymer nanogels enable magnetic resonance imaging-guided chemotherapy/chemodynamic therapy of orthotopic glioma. ACS Nano 2021, 15, 20377–20390. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Zhang, M.; Zhang, Z.; Shen, W.; Liu, L.; Zhang, Q. Anti-tumor drug delivery system based on cyclodextrin-containing pH-responsive star polymer: In vitro and in vivo evaluation. Int. J. Pharm. 2014, 474, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, J.; Liang, C.; Feng, L.; Dong, Z.; Song, X.; Song, G.; Liu, Z. Drug-induced co-assembly of albumin/catalase as smart nano-theranostics for deep intra-tumoral penetration, hypoxia relieve, and synergistic combination therapy. J. Control. Release 2017, 263, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Chaleshtori, M.; Shojaei, S.; Mohammadi-Yeganeh, S.; Hashemi, S.M. Transfer of miRNA in tumor-derived exosomes suppresses breast tumor cell invasion and migration by inducing M1 polarization in macrophages. Life Sci. 2021, 282, 119800. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Gu, B.; Wu, S.; Liu, L.; Gao, Y.; Yao, Y.; Yang, D.; Du, J.; Yang, C. M1 Macrophage-Biomimetic Targeted Nanoparticles Containing Oxygen Self-Supplied Enzyme for Enhancing the Chemotherapy. Pharmaceutics 2023, 15, 2243. https://doi.org/10.3390/pharmaceutics15092243
Zhang J, Gu B, Wu S, Liu L, Gao Y, Yao Y, Yang D, Du J, Yang C. M1 Macrophage-Biomimetic Targeted Nanoparticles Containing Oxygen Self-Supplied Enzyme for Enhancing the Chemotherapy. Pharmaceutics. 2023; 15(9):2243. https://doi.org/10.3390/pharmaceutics15092243
Chicago/Turabian StyleZhang, Jiayi, Bing Gu, Shimiao Wu, Lin Liu, Ying Gao, Yucen Yao, Degong Yang, Juan Du, and Chunrong Yang. 2023. "M1 Macrophage-Biomimetic Targeted Nanoparticles Containing Oxygen Self-Supplied Enzyme for Enhancing the Chemotherapy" Pharmaceutics 15, no. 9: 2243. https://doi.org/10.3390/pharmaceutics15092243
APA StyleZhang, J., Gu, B., Wu, S., Liu, L., Gao, Y., Yao, Y., Yang, D., Du, J., & Yang, C. (2023). M1 Macrophage-Biomimetic Targeted Nanoparticles Containing Oxygen Self-Supplied Enzyme for Enhancing the Chemotherapy. Pharmaceutics, 15(9), 2243. https://doi.org/10.3390/pharmaceutics15092243




