A NIR-Activated and Mild-Temperature-Sensitive Nanoplatform with an HSP90 Inhibitor for Combinatory Chemotherapy and Mild Photothermal Therapy in Cancel Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization
2.3. UV-Vis and Fluorescence Spectra Characterization
2.4. Molar Extinction Coefficient Measurement
2.5. Preparation of TSL Nanoparticles
2.6. Quantum Yield Test
2.7. Photothermal Conversion Efficiency Calculation
2.8. Cell Incubation Experiment
2.9. In vitro Confocal Laser Scanning Microscopy (CLSM)
2.10. In Vitro Dark & Light Cytotoxicity
3. Results and Discussion
3.1. Synthesis and Characterization of BDPII
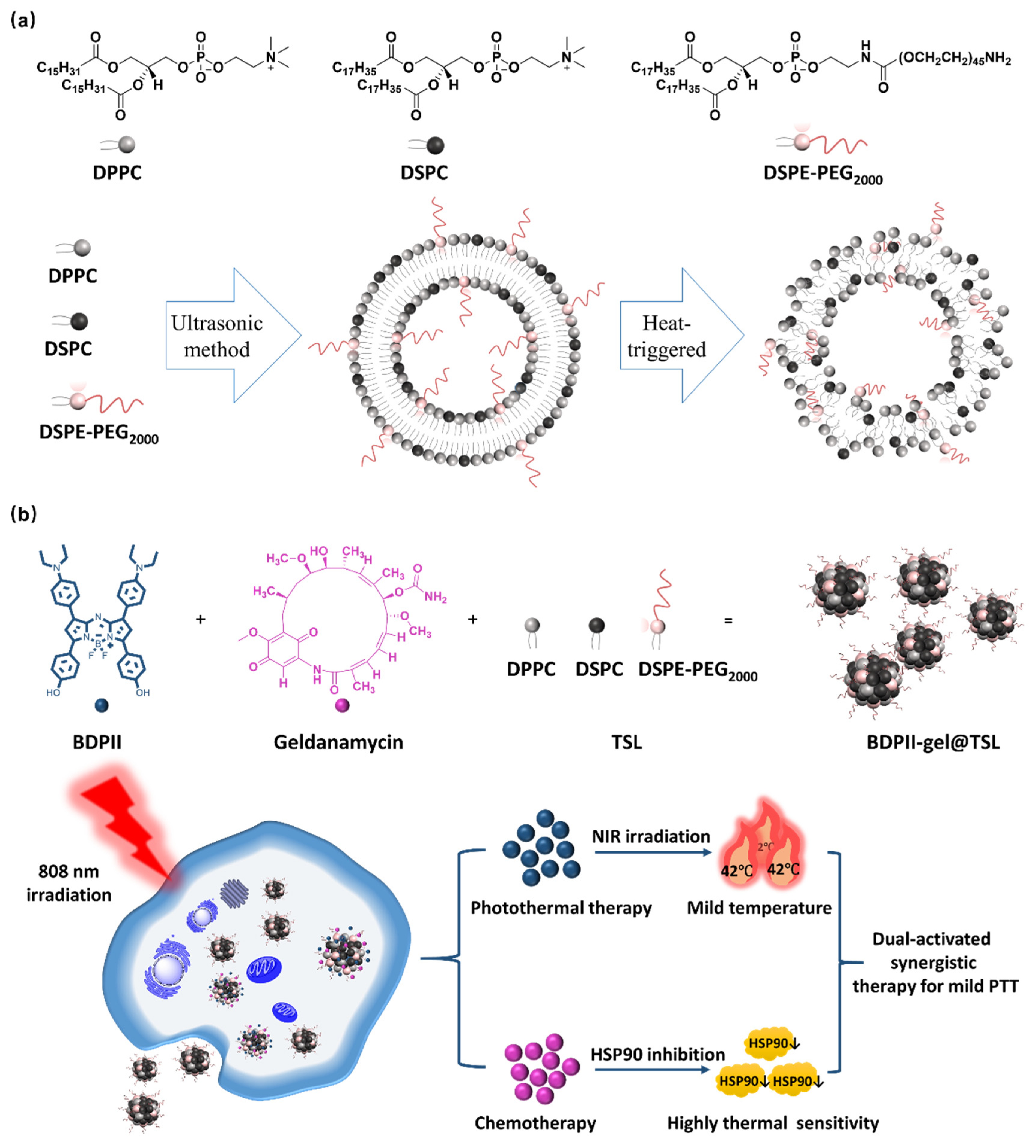
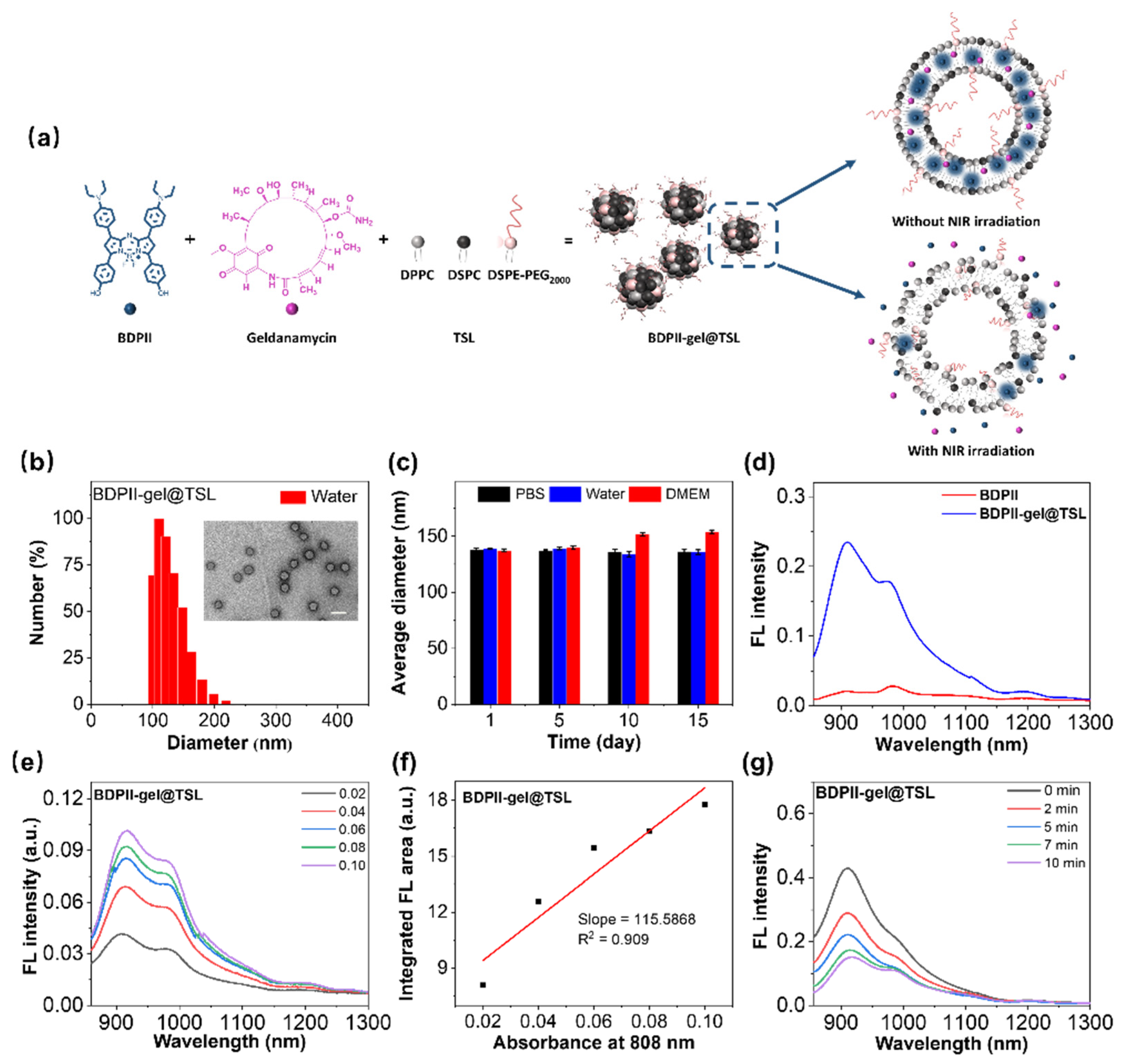
3.2. Preparation of BDPII-gel@TSL NPs
3.3. Photothermal Performance of BDPII-gel@TSL NPs
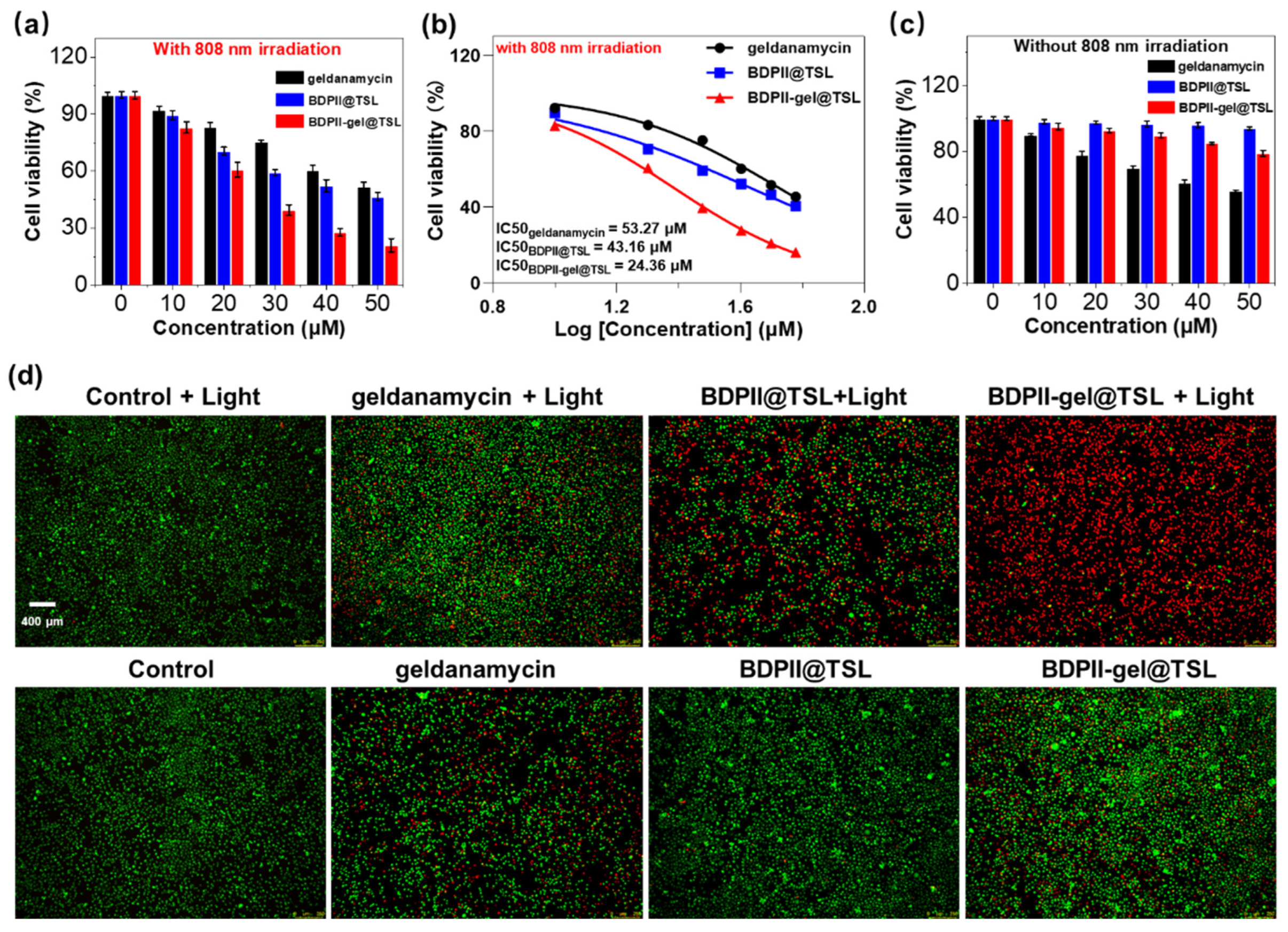
3.4. In Vitro Mild PTT Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, W.; Isenberg, S.; Roberts, D. Molecular Regulation of Tumor Angiogenesis and Perfusion via Redox Signaling. Chem. Rev. 2009, 40, 3099–3124. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yu, F.; Lv, C.; Choo, J.; Chen, L. Fluorescent chemical probes for accurate tumor diagnosis and targeting therapy. Chem. Soc. Rev. 2017, 46, 2237–2271. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, M.; Du, R.; Zheng, X.; Tang, C.; Wu, Y.; He, J.; Huang, W.; Wang, Y.; Zhang, Z. A polyethyleneimine-driven self-assembled nanoplatform for fluorescence and MR dual-mode imaging guided cancer chemotherapy. Chem. Eng. J. 2018, 350, 69–78. [Google Scholar] [CrossRef]
- Lee, S.; O’Halloran, V.; Nguyen, T. Polymer-caged nanobins for synergistic cisplatin-doxorubicin combination chemotherapy. J. Am. Chem. Soc. 2010, 132, 17130–17138. [Google Scholar] [CrossRef]
- Chabner, A.; Roberts, G., Jr. Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef]
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 2008, 8, 59–73. [Google Scholar] [CrossRef]
- Cheng, J.; Qin, Y.; Liu, L.; Ma, H.; Chen, S.; Zhang, Q.; Zhang, Z. Dual-Targeting Photosensitizer-Peptide Amphiphile Conjugate for Enzyme-Triggered Drug Delivery and Synergistic Chemo-Photodynamic Tumor Therapy. Adv. Mater. Interfaces 2020, 7, 2000935–2000944. [Google Scholar] [CrossRef]
- Kim, J.; Yung, C.; Kim, J.; Chen, X. Combination of nitric oxide and drug delivery systems: Tools for overcoming drug resistance in chemotherapy. J. Control. Release 2017, 263, 223–230. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Lu, K.; Liu, D.; Lin, W. Nanoscale metal–organic frameworks for the co-delivery of cisplatin and pooled siRNAs to enhance therapeutic efficacy in drug-resistant ovarian cancer cells. J. Am. Chem. Soc. 2014, 136, 5181–5184. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Y.; Tian, Y.; Xu, S.; Ren, E.; Bai, S.; Chen, X.; Chu, C.; Xu, Z.; Liu, G. Multi-responsive bottlebrush-like unimolecules self-assembled nano-riceball for synergistic sono-chemotherapy. Small Methods 2021, 5, 2000416–2000423. [Google Scholar] [CrossRef]
- Li, L.; Hagen, L.; Schipper, D.; Wijnberg, M.; Rhoon, C.; Eggermont, M.; Lindner, H.; Koning, A. Triggered content release from optimized stealth thermosensitive liposomes using mild hyperthermia. J. Control. Release 2010, 143, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Aw, J.; Xing, B. Nanostructures for NIR light-controlled therapies. Nanoscale 2017, 9, 3698–3718. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Sheng, Z.; Hu, D.; Liu, C.; Zheng, H.; Liu, B. Through scalp and skull NIR-II photothermal therapy of deep orthotopic brain tumors with precise photoacoustic imaging guidance. Adv. Mater. 2018, 30, 1802591–1802600. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, Y.; Wang, C.; Jiang, X.; Wu, J. Light-controlled oxygen production and collection for sustainable photodynamic therapy in tumor hypoxia. Biomaterials 2020, 269, 120621–120634. [Google Scholar] [CrossRef]
- Hong, W.; Jin, C.; Ming, S.; Wei, P.; Na, L. A Dual-Targeted Organic Photothermal Agent for Enhanced Photothermal Therapy. Angew. Chem. Int. Ed. 2018, 58, 1057–1061. [Google Scholar]
- Huang, X.; El-Sayed, H.; Qian, W.; El-Sayed, A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef]
- Perez, M.; Pino, D.; Mitchell, G.; Moros, M.; Fuente, J. Dissecting the molecular mechanism of apoptosis during photothermal therapy using gold nanoprisms. ACS Nano 2014, 9, 52–61. [Google Scholar] [CrossRef]
- Hu, J.; Cheng, J.; Zhang, Z. Recent advances in nanomaterials for enhanced photothermal therapy of tumors. Nanoscale 2018, 10, 22657–22672. [Google Scholar] [CrossRef]
- Jung, H.; Peter, S.; Amit, S.; Jinwoo, S.; Jonathan, L. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef]
- Austin, A.; Hoover, E.; Layton, C. Nanomaterial Applications in Photothermal Therapy for Cancer. Materials 2019, 12, 779–793. [Google Scholar]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.; Rodrigues, F.; Moreira, F.; Correia, J. Overview of the application of inorganic nanomaterials in cancer photothermal therapy. Biomater. Sci. 2020, 8, 2290–3020. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Sun, X.; Liang, G. Nanoagent-Promoted Mild-Temperature Photothermal Therapy for Cancer Treatment. Adv. Funct. Mater. 2021, 31, 2100738–2100752. [Google Scholar] [CrossRef]
- Day, S.; Morton, G.; West, L. Nanoparticles for thermal cancer therapy. J. Biomech. Eng. 2009, 131, 074001–074005. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics. Theranostics 2016, 6, 762–772. [Google Scholar] [CrossRef]
- Shu, Z.; Hua, C.; Li, W.; Chun, L.; Li, L.; Yu, S.; Xing, S. A simple strategy for simultaneously enhancing photostability and mitochondrial-targeting stability of near-infrared fluorophores for multimodal imaging-guided photothermal therapy. J. Mater. Chem. B 2021, 9, 1089–1095. [Google Scholar]
- Lv, Z.; He, S.; Wang, Y.; Zhu, X. Noble Metal Nanomaterials for NIR-Triggered Photothermal Therapy in Cancer. Adv. Healthc. Mater. 2021, 10, 2001806–2001812. [Google Scholar] [CrossRef]
- Mei, S.; Qiu, H.; Xiao, Y.; Yan, L.; Pei, L.; Wen, Z.; Qiang, Z. A Supramolecular Strategy to Engineering a Non-photobleaching and Near-Infrared Absorbing Nano-J-Aggregate for Efficient Photothermal Therapy. ACS Nano 2021, 15, 5032–5042. [Google Scholar]
- Sun, Q.; Tang, K.; Song, L.; Li, Y.; Pan, W.; Li, N.; Tang, B. Covalent organic framework based nanoagent for enhanced mild-temperature photothermal therapy. Biomater. Sci. 2021, 9, 7977–7983. [Google Scholar] [CrossRef]
- Gao, P.; Wang, H.; Cheng, Y. Strategies for efficient photothermal therapy at mild temperatures: Progresses and challenges. Chin. Chem. Lett. 2022, 33, 575–586. [Google Scholar] [CrossRef]
- Hu, H.; Li, D.; Dai, W.; Jin, Q.; Wang, D.; Ji, J.; Tang, B.Z.; Tang, Z. A NIR-II AIEgen-Based Supramolecular Nanodot for Peroxynitrite-Potentiated Mild-Temperature Photothermal Therapy of Hepatocellular Carcinoma. Adv. Funct. Mater. 2023, 33, 2213134–2213147. [Google Scholar] [CrossRef]
- Jung, B.-K.; Lee, K.; Hong, J.; Ghandehari, H.; Yun, C.-O. Mild hyperthermia induced by gold nanorod-mediated plasmonic photothermal therapy enhances transduction and replication of oncolytic adenoviral gene delivery. ACS Nano 2016, 10, 10533–10543. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Liu, J.; Shi, J. Nuclear-targeting gold nanorods for extremely low NIR activated photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 15952–15961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Du, J.; Guo, Z.; Yu, J.; Gao, Q.; Yin, W.; Zhu, S.; Gu, Z.; Zhao, Y. Efficient Near Infrared Light Triggered Nitric Oxide Release Nanocomposites for Sensitizing Mild Photothermal Therapy. Adv. Sci. 2018, 6, 1801122–1801132. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Chen, X.; Wang, X.; Liu, S.; Liu, J.; Xie, Z. Enhanced efficacy of photothermal therapy by combining a semiconducting polymer with an inhibitor of a heat shock protein. Mater. Chem. Front. 2019, 3, 127–136. [Google Scholar] [CrossRef]
- Liu, S.; Pan, X.; Liu, H. Two-dimensional nanomaterials for photothermal therapy. Angew. Chem. Int. Ed. 2020, 132, 5943–5953. [Google Scholar] [CrossRef]
- Gao, J.; Wang, F.; Wang, S.; Liu, L.; Liu, K.; Ye, Y.; Wang, Z.; Wang, H.; Chen, B.; Jiang, J. Hyperthermia-Triggered On-Demand Biomimetic Nanocarriers for Synergetic Photothermal and Chemotherapy. Adv. Sci. 2020, 7, 1903642–1903651. [Google Scholar] [CrossRef]
- Yang, G.; Li, M.; Song, T.; Chen, X.; Zhang, H.; Wei, X.; Li, N.; Li, T.; Qin, X.; Li, S. Polydopamine-Engineered Theranostic Nanoscouts Enabling Intracellular HSP90 mRNAs Fluorescence Detection for Imaging-Guided Chemo–Photothermal Therapy. Adv. Healthc. Mater. 2022, 11, 22016152201628. [Google Scholar] [CrossRef]
- Liu, C.; Li, L.; Guo, Z.-P.; Lin, L.; Li, Y.-H.; Tian, H.-Y. PLG-g-mPEG Mediated Multifunctional Nanoparticles for Photoacoustic Imaging Guided Combined Chemo/Photothermal Antitumor Therapy. Chin. J. Polym. Sci. 2023, 41, 538–546. [Google Scholar] [CrossRef]
- Kang, H.; Plescia, J.; Song, Y.; Meli, M.; Colombo, G.; Beebe, K.; Scroggins, B.; Neckers, L.; Altieri, C. Combinatorial drug design targeting multiple cancer signaling networks controlled by mitochondrial Hsp90. J. Clin. Investig. 2009, 119, 454–464. [Google Scholar] [CrossRef]
- Jaeger, M.; Whitesell, L. HSP90: Enabler of cancer adaptation. Annu. Rev. Cancer Biol. 2019, 3, 275–297. [Google Scholar] [CrossRef]
- Poggio, P.; Sorge, M.; Seclì, L.; Brancaccio, M. Extracellular HSP90 machineries build tumor microenvironment and boost cancer progression. Front. Cell Dev. Biol. 2021, 9, 735529–735537. [Google Scholar] [CrossRef] [PubMed]
- Birbo, B.; Madu, E.; Madu, O.; Jain, A.; Lu, Y. Role of HSP90 in Cancer. Int. J. Mol. Sci. 2021, 22, 10317–10336. [Google Scholar] [CrossRef]
- Yatvin, B.; Weinstein, N.; Dennis, H.; Blumenthal, R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science 1978, 202, 1290–1293. [Google Scholar] [CrossRef]
- Bassett, B.; Anderson, U.; Tacker, R. Use of temperature-sensitive liposomes in the selective delivery of methotrexate and cis-platinum analogues to murine bladder tumor. J. Urol. 1986, 135, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Unezaki, S.; Takahashi, N.; Iwatsuru, M. Enhanced delivery of doxorubicin to tumor by long-circulating thermosensitive liposomes and local hyperthermia. Biochim. Biophys. Acta (BBA)-Biomembr. 1993, 1149, 209–216. [Google Scholar] [CrossRef]
- Al-Ahmady, Z.; Kostarelos, K. Chemical components for the design of temperature-responsive vesicles as cancer therapeutics. Chem. Rev. 2016, 116, 3883–3918. [Google Scholar] [CrossRef]
- Cheng, H.; Kara, C.; Danielle, C.; Juan, G. Stable J-Aggregation of an aza-BODIPY-Lipid in a Liposome for Optical Cancer Imaging. Angew. Chem. Int. Ed. 2019, 58, 13394–13399. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Z.; Dai, H.; Lv, X.; Dong, X. Boosting O2 Photogeneration via Promoting Intersystem crossing and Electron donating Efficiency of Aza-BODIPY-Based Nanoplatforms for Hypoxicmild tumor Photodynamic Therapy. Small Methods 2020, 4, 2000013–2000024. [Google Scholar] [CrossRef]
- Chen, D.; Zhong, Z.; Ma, Q.; Shao, J.; Huang, W.; Dong, X. Aza-BODIPY-based nanomedicines in cancer phototheranostics. ACS Appl. Mater. Interfaces 2020, 12, 26914–26925. [Google Scholar] [CrossRef]
- Yw, A.; Dz, A.; Jie, W.B.; Dx, C.; Zx, B.; Xdj, A.; Jd, C. Near-infrared vinyl-containing aza-BODIPY nanoparticles as photosensitizer for phototherapy. Dye. Pigment. 2021, 198, 110026–110036. [Google Scholar]
- Zhao, M.; Zeng, Q.; Li, X.; Xing, D.; Zhang, T. Aza-BODIPY-based phototheranostic nanoagent for tissue oxygen auto-adaptive photodynamic/photothermal complementary therapy. Nano Res. 2022, 15, 716–727. [Google Scholar] [CrossRef]
- Qin, H.-L.; Panek, J.S. Total synthesis of the Hsp90 inhibitor geldanamycin. Org. Lett. 2008, 10, 2477–2479. [Google Scholar] [CrossRef]
- Pezzulo, A.; Tudas, A.; Stewart, G.; Buonfiglio, G.V.; Lindsay, D.; Taft, J.; Gansemer, D.; Zabner, J. HSP90 inhibitor geldanamycin reverts IL-13–and IL-17–induced airway goblet cell metaplasia. J. Clin. Investig. 2019, 129, 744–758. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, R.; Yang, H.; Xue, N.; Chen, X.; Yu, X. Rational design, synthesis, and biological evaluation of novel C6-modified geldanamycin derivatives as potent Hsp90 inhibitors and anti-tumor agents. Chin. Chem. Lett. 2023, 34, 107529–107535. [Google Scholar] [CrossRef]
- Yi, H.; Shun-Li, C.; Wei, G.; Xing, M.; Qunhui, Y. Understanding the Dynamic Behavior of an Anticancer Drug, Doxorubicin, on a Lipid Membrane Using Multiple Spectroscopic Techniques. J. Phys. Chem. B 2019, 23, 3756–3762. [Google Scholar]
- Yuan, Y.; Feng, Z.; Li, S.; Huang, Z.; Wan, Y.; Cao, C.; Lin, S.; Wu, L.; Zhou, J.; Liao, L. Molecular Programming of NIR-IIb-Emissive Semiconducting Small Molecules for In Vivo High—Contrast Bioimaging Beyond 1500 nm. Adv. Mater. 2022, 34, 2201263–2201272. [Google Scholar] [CrossRef]
- Feng, E.; Jiao, L.; Tang, S.; Chen, M.; Lv, S.; Liu, D.; Song, J.; Zheng, D.; Peng, X.; Song, F. Anti-photobleaching cyanine-based nanoparticles with simultaneous PET and ACQ effects for improved tumor photothermal therapy. Chem. Eng. J. 2022, 432, 134355–134363. [Google Scholar] [CrossRef]
- Yao, C.; Chen, Y.; Zhao, M.; Wang, S.; Wu, B.; Yang, Y.; Yin, D.; Yu, P.; Zhang, H.; Zhang, F. A Bright, Renal-Clearable NIR-II Brush Macromolecular Probe with Long Blood Circulation Time for Kidney Disease Bioimaging. Angew. Chem. Int. Ed. 2022, 61, e202114273–e202114280. [Google Scholar] [CrossRef]
- Bing, G.; Zong, S.; De, H.; Anran, L.; Shi, X.; Purima, N. Molecular Engineering of Conjugated Polymers for Biocompatible Organic Nanoparticles with Highly Efficient Photoacoustic and Photothermal Performance in Cancer Theranostics. ACS Nano 2017, 11, 10124–10134. [Google Scholar]
- Macdonald, D.; Liu, W.; Zheng, G. An MRI-Sensitive, Non-Photobleachable Porphysome Photothermal Agent. Angew. Chem. Int. Ed. 2014, 53, 6956–6959. [Google Scholar] [CrossRef]
- Zhao, M.; Xu, Y.; Xie, M.; Zou, L.; Wang, Z.; Liu, S.; Zhao, Q. Halogenated Aza-BODIPY for imaging-guided synergistic photodynamic and photothermal tumor therapy. Adv. Healthc. Mater. 2018, 7, 1800606–1800616. [Google Scholar] [CrossRef]
- Chen, D.; Tang, Q.; Zou, J.; Yang, X.; Huang, W.; Zhang, Q.; Shao, J.; Dong, X. pH-Responsive PEG–Doxorubicin-Encapsulated Aza-BODIPY Nanotheranostic Agent for Imaging-Guided Synergistic Cancer Therapy. Adv. Healthc. Mater. 2018, 7, 1701272–1701282. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, Y.; Li, P.; Li, B.; Jiang, H.; Guo, B.; Yuan, Q.; Gan, W. AIEgens for dual second harmonic generation and fluorescence “turn-on” imaging of membrane and photodynamic therapy in cancer cells. Mater. Chem. Front. 2023, 7, 502–513. [Google Scholar] [CrossRef]
- Bing, G.; Zong, S.; Kenry, K.; De, H.; Xiang, L.; Shi, X.; Cheng, L.; Hai, Z.; Bin, L. Biocompatible conjugated polymer nanoparticles for highly efficient photoacoustic imaging of orthotopic brain tumors in the second near-infrared window. Mater. Horiz. 2017, 4, 1151–1156. [Google Scholar]
- Tao, D.; Kai, W.; Jun, X.; Wei, F.; Han, M.; Lei, Y.; Xiao, W.; Fu, X. Significant Enhancement of Photothermal and Photoacoustic Efficiencies for Semiconducting Polymer Nanoparticles through Simply Molecular Engineering. Adv. Funct. Mater. 2018, 28, 1800135–1800146. [Google Scholar]
- Shi, L.; Liu, H.; Li, K.; Sharma, A.; Yu, K.; Ji, S.; Li, L.; Zhou, Q.; Zhang, H.; Kim, S. An AIE-Based Probe for Rapid and Ultrasensitive Imaging of Plasma Membranes in Biosystems. Angew. Chem. Int. Ed. 2020, 59, 9962–9966. [Google Scholar] [CrossRef]
- Sun, J.; Cai, X.; Wang, C.; Du, K.; Chen, W.; Feng, F.; Wang, S. Cascade reactions by nitric oxide and hydrogen radical for anti-hypoxia photodynamic therapy using an activatable photosensitizer. J. Am. Chem. Soc. 2021, 143, 868–878. [Google Scholar] [CrossRef]
- Antaris, L.; Chen, H.; Cheng, K.; Sun, Y.; Hong, G.; Qu, C.; Diao, S.; Deng, Z.; Hu, X.; Zhang, B. A small-molecule dye for NIR-II imaging. Nat. Mater. 2016, 15, 235–242. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Zhuang, Z.; He, J.; Wen, W.; Qiu, P.; Wang, K. Visualizing astrocytes in the deep mouse brain in vivo. J. Biophotonics 2019, 12, e201800420–e201800430. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Z.; Liu, S.; Zhang, H.; Wong, T.; Lam, W.; Kwok, T.; Qian, J.; Tang, Z. Design of AIEgens for near-infrared IIb imaging through structural modulation at molecular and morphological levels. Nat. Commun. 2020, 11, 1255–1265. [Google Scholar] [CrossRef]
- Han, C.; Duan, C.; Yang, W.; Xie, M.; Xu, H. Allochroic thermally activated delayed fluorescence diodes through field-induced solvatochromic effect. Sci. Adv. 2017, 3, e1700904–e1700914. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fan, Y.; Li, D.; Sun, C.; Lei, Z.; Lu, L.; Wang, T.; Zhang, F. Anti-quenching NIR-II molecular fluorophores for in vivo high-contrast imaging and pH sensing. Nat. Commun. 2019, 10, 1058–1069. [Google Scholar] [CrossRef]
- Gao, D.; Liu, A.; Zhang, Y.; Zhu, Y.; Wei, D.; Sun, J.; Luo, H.; Fan, H. Temperature triggered high-performance carbon dots with robust solvatochromic effect and self-quenching-resistant deep red solid state fluorescence for specific lipid droplet imaging. Chem. Eng. J. 2021, 415, 128984–128997. [Google Scholar] [CrossRef]
- Liu, C.; Li, S.; Ma, R.; Ji, C.; Müllen, K.; Yin, M. NIR-triggered dual sensitization of nanoparticles for mild tumor phototherapy. Nano Today 2022, 42, 101363–101372. [Google Scholar] [CrossRef]
- Lin, Z.; Peng, R.; Li, Z.; Wang, Y.; Lu, C.; Shen, Y.; Wang, J.; Shi, G. 17-ABAG, a novel geldanamycin derivative, inhibits LNCaP-cell proliferation through heat shock protein 90 inhibition. Int. J. Mol. Med. 2015, 36, 424–432. [Google Scholar] [CrossRef] [PubMed]
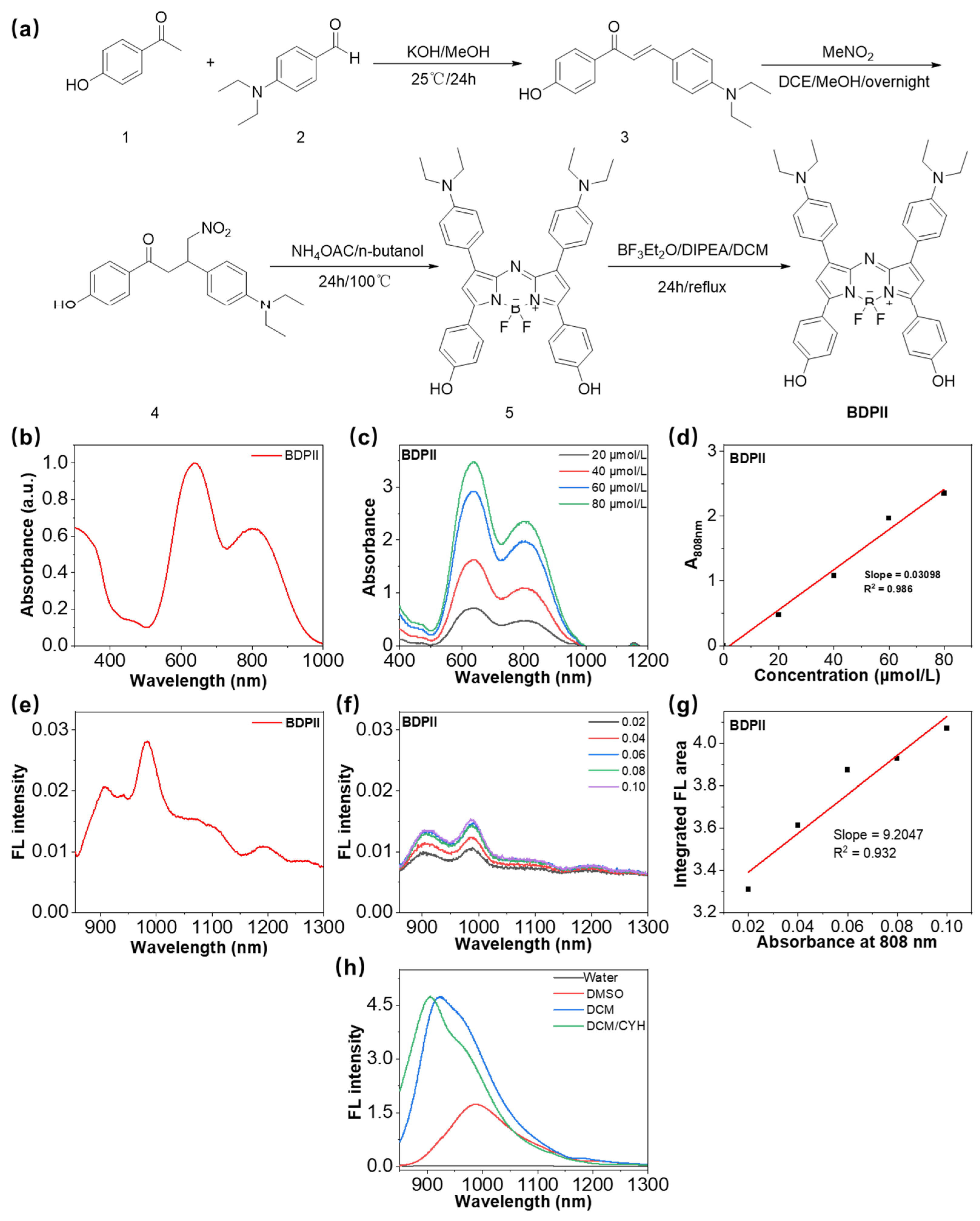

| Compound | λex (nm) | λem (nm) | Φf (%) |
|---|---|---|---|
| BDPII | 639/808 | 908/985 | 0.56 |
| BDPII@TSL | 644/775 | 912/979 | 4.17 |
| BDPII-gel@TSL | 647/775 | 909/977 | 6.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Jiang, H.; Li, B.; Liu, Y.; Guo, B.; Gan, W. A NIR-Activated and Mild-Temperature-Sensitive Nanoplatform with an HSP90 Inhibitor for Combinatory Chemotherapy and Mild Photothermal Therapy in Cancel Cells. Pharmaceutics 2023, 15, 2252. https://doi.org/10.3390/pharmaceutics15092252
Peng Y, Jiang H, Li B, Liu Y, Guo B, Gan W. A NIR-Activated and Mild-Temperature-Sensitive Nanoplatform with an HSP90 Inhibitor for Combinatory Chemotherapy and Mild Photothermal Therapy in Cancel Cells. Pharmaceutics. 2023; 15(9):2252. https://doi.org/10.3390/pharmaceutics15092252
Chicago/Turabian StylePeng, Yingying, Hanlin Jiang, Bifei Li, Yue Liu, Bing Guo, and Wei Gan. 2023. "A NIR-Activated and Mild-Temperature-Sensitive Nanoplatform with an HSP90 Inhibitor for Combinatory Chemotherapy and Mild Photothermal Therapy in Cancel Cells" Pharmaceutics 15, no. 9: 2252. https://doi.org/10.3390/pharmaceutics15092252
APA StylePeng, Y., Jiang, H., Li, B., Liu, Y., Guo, B., & Gan, W. (2023). A NIR-Activated and Mild-Temperature-Sensitive Nanoplatform with an HSP90 Inhibitor for Combinatory Chemotherapy and Mild Photothermal Therapy in Cancel Cells. Pharmaceutics, 15(9), 2252. https://doi.org/10.3390/pharmaceutics15092252








