Translational Pharmacokinetic/Pharmacodynamic Modeling and Simulation of Oxaliplatin and Irinotecan in Colorectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection of Pharmacokinetics and Pharmacodynamics
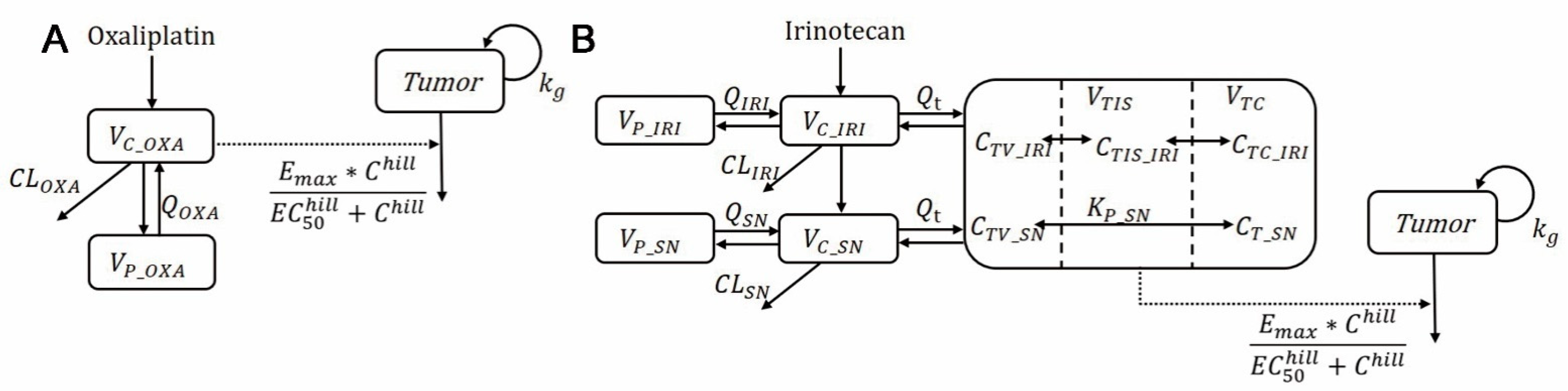
2.2. Modeling of In Vitro Pharmacodynamic and In Vivo Plasma Pharmacokinetics in Mice and Humans
2.3. Simulation of Tumor Shrinkage in Humans
2.4. Model-Based Virtual Clinical Trial
2.5. Statistical Analysis
3. Results
3.1. Development of an In Vitro PD Model
3.2. Development of the Oxaliplatin and Irinotecan PK Model
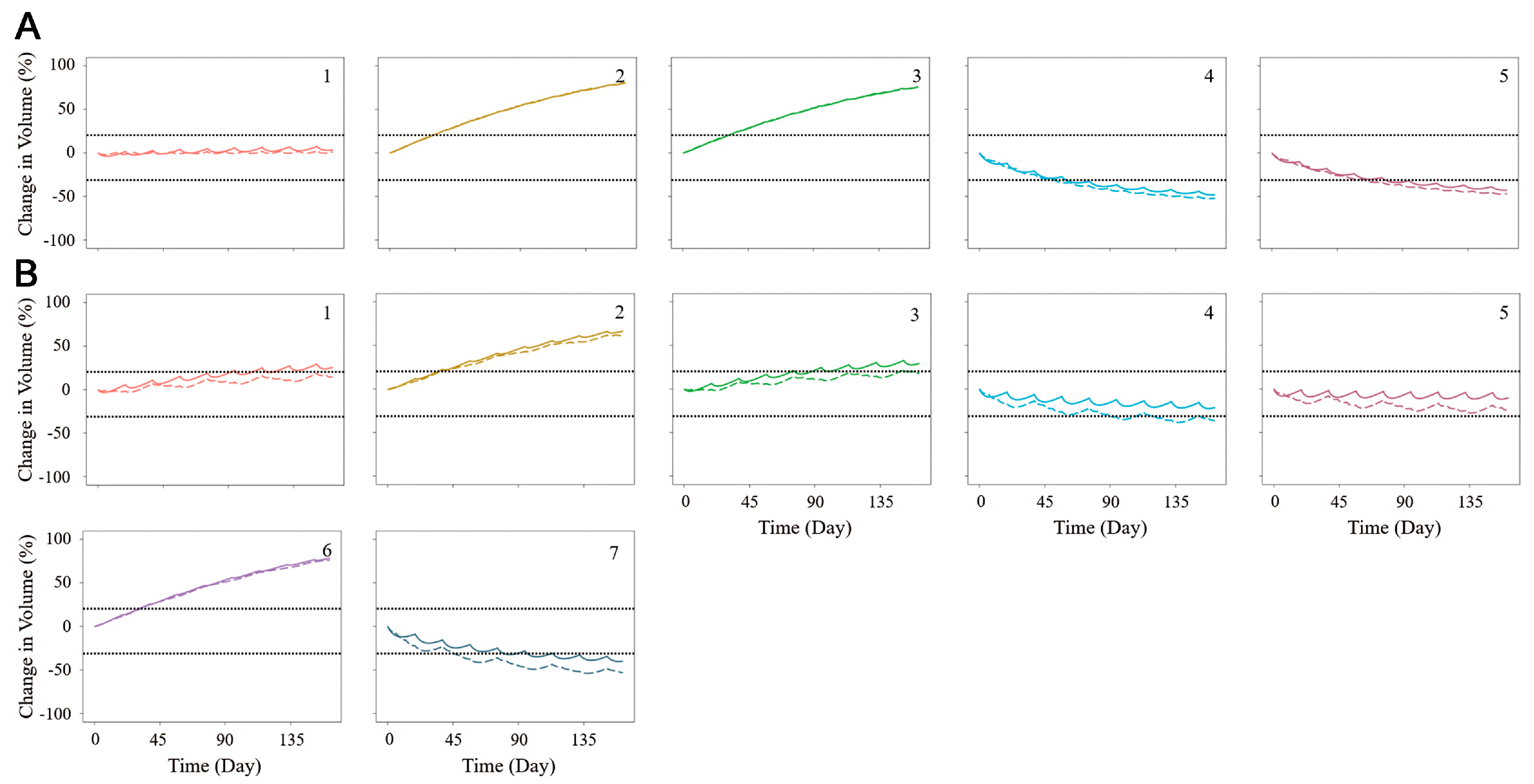
3.3. The Combination of the PK/PD Model and the Simulation of Tumor Shrinkage
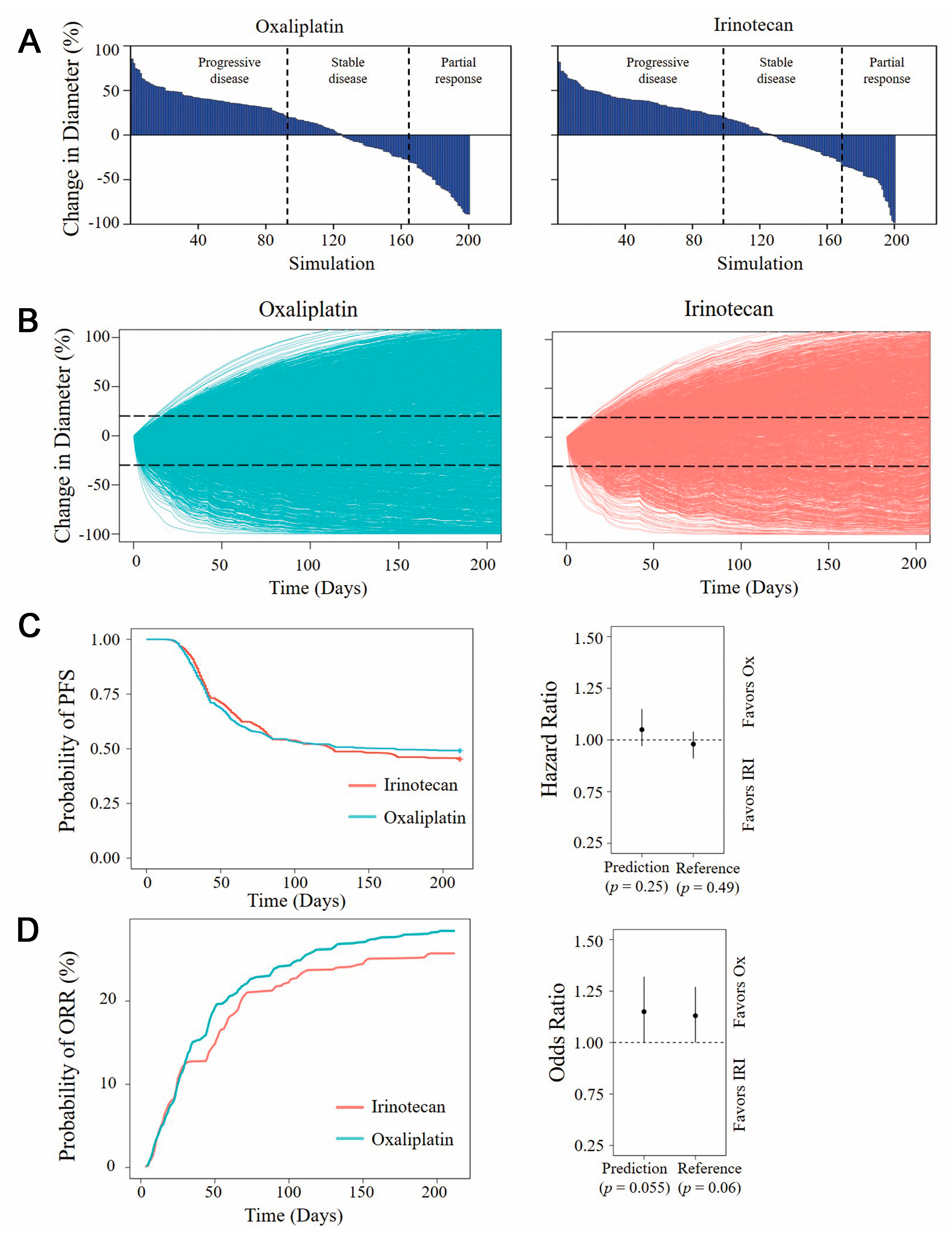
3.4. Model-Based Virtual Clinical Trial
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Ocana, A.; Garcia-Alonso, S.; Amir, E.; Pandiella, A. Refining Early Antitumoral Drug Development. Trends Pharmacol. Sci. 2018, 39, 922–925. [Google Scholar] [CrossRef]
- Khalil, A.S.; Jaenisch, R.; Mooney, D.J. Engineered tissues and strategies to overcome challenges in drug development. Adv. Drug Deliv. Rev. 2020, 158, 116–139. [Google Scholar] [CrossRef] [PubMed]
- Begley, C.G.; Ellis, L.M. Drug development: Raise standards for preclinical cancer research. Nature 2012, 483, 531–533. [Google Scholar] [CrossRef]
- Rossi, G.; Manfrin, A.; Lutolf, M.P. Progress and potential in organoid research. Nat. Rev. Genet. 2018, 19, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, K. Cancer research using organoid technology. J. Mol. Med. 2021, 99, 501–515. [Google Scholar] [CrossRef]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernandez-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Heo, I.; Clevers, H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol. Med. 2017, 23, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.H.N.; Siu, H.C.; Law, S.; Ho, S.L.; Yue, S.S.K.; Tsui, W.Y.; Chan, D.; Chan, A.S.; Ma, S.; Lam, K.O.; et al. A Comprehensive Human Gastric Cancer Organoid Biobank Captures Tumor Subtype Heterogeneity and Enables Therapeutic Screening. Cell Stem. Cell 2018, 23, 882–897.e811. [Google Scholar] [CrossRef] [PubMed]
- Ooft, S.N.; Weeber, F.; Dijkstra, K.K.; McLean, C.M.; Kaing, S.; van Werkhoven, E.; Schipper, L.; Hoes, L.; Vis, D.J.; van de Haar, J.; et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci. Transl. Med. 2019, 11, eaay2574. [Google Scholar] [CrossRef]
- Arena, S.; Corti, G.; Durinikova, E.; Montone, M.; Reilly, N.M.; Russo, M.; Lorenzato, A.; Arcella, P.; Lazzari, L.; Rospo, G.; et al. A Subset of Colorectal Cancers with Cross-Sensitivity to Olaparib and Oxaliplatin. Clin. Cancer Res. 2020, 26, 1372–1384. [Google Scholar] [CrossRef]
- Hsu, K.S.; Adileh, M.; Martin, M.L.; Makarov, V.; Chen, J.; Wu, C.; Bodo, S.; Klingler, S.; Sauve, C.G.; Szeglin, B.C.; et al. Colorectal Cancer Develops Inherent Radiosensitivity That Can Be Predicted Using Patient-Derived Organoids. Cancer Res. 2022, 82, 2298–2312. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Cao, Z.; Wang, Z.; Xin, J.; Kong, B.; Xu, J.; Zhang, L.; Chen, P. Patient-Derived Organoid Model in the Prediction of Chemotherapeutic Drug Response in Colorectal Cancer. ACS Biomater. Sci. Eng. 2022, 8, 3515–3525. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, H.; Madabushi, R.; Liu, Q.; Huang, S.M.; Zineh, I. Model-Informed Drug Development: Current US Regulatory Practice and Future Considerations. Clin. Pharmacol. Ther. 2019, 105, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhu, J.; Jiang, M.; Sang, L.; Hao, K.; He, H. The Combination of Cell Cultured Technology and In Silico Model to Inform the Drug Development. Pharmaceutics 2021, 13, 704. [Google Scholar] [CrossRef]
- Danhof, M.; de Lange, E.C.; Della Pasqua, O.E.; Ploeger, B.A.; Voskuyl, R.A. Mechanism-based pharmacokinetic-pharmacodynamic (PK-PD) modeling in translational drug research. Trends Pharmacol. Sci. 2008, 29, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Wensink, G.E.; Elias, S.G.; Mullenders, J.; Koopman, M.; Boj, S.F.; Kranenburg, O.W.; Roodhart, J.M.L. Patient-derived organoids as a predictive biomarker for treatment response in cancer patients. NPJ Precis. Oncol. 2021, 5, 30. [Google Scholar] [CrossRef]
- Sarrami-Foroushani, A.; Lassila, T.; MacRaild, M.; Asquith, J.; Roes, K.C.B.; Byrne, J.V.; Frangi, A.F. In-silico trial of intracranial flow diverters replicates and expands insights from conventional clinical trials. Nat. Commun. 2021, 12, 3861. [Google Scholar] [CrossRef]
- Pappalardo, F.; Russo, G.; Tshinanu, F.M.; Viceconti, M. In Silico clinical trials: Concepts and early adoptions. Brief. Bioinform. 2019, 20, 1699–1708. [Google Scholar] [CrossRef]
- Lehrach, H. Virtual Clinical Trials, an Essential Step in Increasing the Effectiveness of the Drug Development Process. Public Health Genom. 2015, 18, 366–371. [Google Scholar] [CrossRef]
- Ermakov, S.; Forster, P.; Pagidala, J.; Miladinov, M.; Wang, A.; Baillie, R.; Bartlett, D.; Reed, M.; Leil, T.A. Virtual Systems Pharmacology (ViSP) software for simulation from mechanistic systems-level models. Front. Pharmacol. 2014, 5, 232. [Google Scholar] [CrossRef]
- Singh, A.P.; Chen, W.; Zheng, X.; Mody, H.; Carpenter, T.J.; Zong, A.; Heald, D.L. Bench-to-bedside translation of chimeric antigen receptor (CAR) T cells using a multiscale systems pharmacokinetic-pharmacodynamic model: A case study with anti-BCMA CAR-T. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sove, R.J.; Jafarnejad, M.; Rahmeh, S.; Jaffee, E.M.; Stearns, V.; Roussos Torres, E.T.; Connolly, R.M.; Popel, A.S. Conducting a Virtual Clinical Trial in HER2-Negative Breast Cancer Using a Quantitative Systems Pharmacology Model With an Epigenetic Modulator and Immune Checkpoint Inhibitors. Front. Bioeng. Biotechnol. 2020, 8, 141. [Google Scholar] [CrossRef]
- Wang, H.; Ma, H.; Sove, R.J.; Emens, L.A.; Popel, A.S. Quantitative systems pharmacology model predictions for efficacy of atezolizumab and nab-paclitaxel in triple-negative breast cancer. J. Immunother. Cancer 2021, 9, e002100. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Pan, W.; Zheng, H.; Zheng, H.; Wang, Z.; Li, J.J.; Deng, C.; Yan, J. Accuracy of Using a Patient-Derived Tumor Organoid Culture Model to Predict the Response to Chemotherapy Regimens in Stage IV Colorectal Cancer: A Blinded Study. Dis. Colon Rectum 2021, 64, 833–850. [Google Scholar] [CrossRef]
- Schwartz, L.H.; Litiere, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, C.; Wu, Y.; Zhang, X.; Fan, J.; Cao, Y. A Multiscale Physiologically-Based Pharmacokinetic Model for Doxorubicin to Explore its Mechanisms of Cytotoxicity and Cardiotoxicity in Human Physiological Contexts. Pharm. Res. 2018, 35, 174. [Google Scholar] [CrossRef] [PubMed]
- Ait-Oudhia, S.; Straubinger, R.M.; Mager, D.E. Systems pharmacological analysis of paclitaxel-mediated tumor priming that enhances nanocarrier deposition and efficacy. J. Pharmacol. Exp. Ther. 2013, 344, 103–112. [Google Scholar] [CrossRef]
- Chabot, G.G. Clinical pharmacokinetics of irinotecan. Clin. Pharmacokinet. 1997, 33, 245–259. [Google Scholar] [CrossRef]
- Kawai, S.; Takeshima, N.; Hayasaka, Y.; Notsu, A.; Yamazaki, M.; Kawabata, T.; Yamazaki, K.; Mori, K.; Yasui, H. Comparison of irinotecan and oxaliplatin as the first-line therapies for metastatic colorectal cancer: A meta-analysis. BMC Cancer 2021, 21, 116. [Google Scholar] [CrossRef]
- de Witte, C.J.; Espejo Valle-Inclan, J.; Hami, N.; Lohmussaar, K.; Kopper, O.; Vreuls, C.P.H.; Jonges, G.N.; van Diest, P.; Nguyen, L.; Clevers, H.; et al. Patient-Derived Ovarian Cancer Organoids Mimic Clinical Response and Exhibit Heterogeneous Inter- and Intrapatient Drug Responses. Cell Rep. 2020, 31, 107762. [Google Scholar] [CrossRef]
- Singh, A.P.; Zheng, X.; Lin-Schmidt, X.; Chen, W.; Carpenter, T.J.; Zong, A.; Wang, W.; Heald, D.L. Development of a quantitative relationship between CAR-affinity, antigen abundance, tumor cell depletion and CAR-T cell expansion using a multiscale systems PK-PD model. MAbs 2020, 12, 1688616. [Google Scholar] [CrossRef]
- Song, L.; Xue, J.; Zhang, J.; Li, S.; Liu, D.; Zhou, T. Mechanistic prediction of first-in-human dose for bispecific CD3/EpCAM T-cell engager antibody M701, using an integrated PK/PD modeling method. Eur. J. Pharm. Sci. 2021, 158, 105584. [Google Scholar] [CrossRef]
- Ayyar, V.S.; Jaiprasart, P.; Geist, B.; Huang Devine, Z.; Case, M.; Hazra, A.; Hsu, C.H.; Chintala, M.; Wang, W. Translational PK/PD and model-informed development of JNJ-67842125, a F(ab) reversal agent for JNJ-64179375, a long-acting thrombin inhibitor. Br. J. Pharmacol. 2021, 178, 3943–3958. [Google Scholar] [CrossRef]
- Sou, T.; Hansen, J.; Liepinsh, E.; Backlund, M.; Ercan, O.; Grinberga, S.; Cao, S.; Giachou, P.; Petersson, A.; Tomczak, M.; et al. Model-Informed Drug Development for Antimicrobials: Translational PK and PK/PD Modeling to Predict an Efficacious Human Dose for Apramycin. Clin. Pharmacol. Ther. 2021, 109, 1063–1073. [Google Scholar] [CrossRef]
- Cao, Y.; Jusko, W.J. Applications of minimal physiologically-based pharmacokinetic models. J. Pharmacokinet. Pharmacodyn. 2012, 39, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Sove, R.J.; Verma, B.K.; Wang, H.; Ho, W.J.; Yarchoan, M.; Popel, A.S. Virtual clinical trials of anti-PD-1 and anti-CTLA-4 immunotherapy in advanced hepatocellular carcinoma using a quantitative systems pharmacology model. J. Immunother. Cancer 2022, 10, e005414. [Google Scholar] [CrossRef]
- Roenfeldt, K. Better than Average: Calculating Geometric Means Using SAS; Henry. M. Foundation for the Advancement of Military Medicine: North Bethesda, MD, USA, 2018. [Google Scholar]
- Fuchs, C.S.; Moore, M.R.; Harker, G.; Villa, L.; Rinaldi, D.; Hecht, J.R. Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J. Clin. Oncol. 2003, 21, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Solorzano, I.; Ibeas-Rollan, R.; Monzo-Planella, M.; Moreno-Solorzano, J.; Martinez-Rodenas, F.; Pou-Sanchis, E.; Hernandez-Borlan, R.; Navarro-Vigo, M.; Ortigosa-Rodriguez, S.; Gel-Moreno, B. Two Doses of oxaliplatin with capecitabine (XELOX) in metastatic colorectal cancer. Clin. Color. Cancer 2007, 6, 634–640. [Google Scholar] [CrossRef]
- Niu, J.; Straubinger, R.M.; Mager, D.E. Pharmacodynamic Drug-Drug Interactions. Clin. Pharmacol. Ther. 2019, 105, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.A.; Wicha, S.G.; Okour, M. Drug Combination Modeling: Methods and Applications in Drug Development. J. Clin. Pharmacol. 2023, 63, 151–165. [Google Scholar] [CrossRef] [PubMed]


| Drug | Title 2 | Title 3 |
|---|---|---|
| Irinotecan | 350 mg/m2 | Once every 3 weeks |
| 125 mg/m2 | Once a week for 4 consecutive weeks, followed by a two-week rest period | |
| Oxaliplatin | 130 mg/m2 | Once every 3 weeks |
| 85 mg/m2 | Once every 2 weeks |
| Patient No. | Oxaliplatin | SN-38 | |||||
|---|---|---|---|---|---|---|---|
| kg | Emax | EC50 | Hill | Emax | EC50 | Hill | |
| 1 | 0.03 | 0.095 | 308 | 0.614 | 0.076 | 15.50 | 0.349 |
| 2 | 0.095 | 246 | 0.977 | 0.040 | 12.40 | 0.396 | |
| 3 | 0.105 | 352 | 0.891 | 0.047 | 10.70 | 0.324 | |
| 4 | 0.056 | 622 | 0.397 | 0.049 | 7.04 | 0.266 | |
| 5 | 0.038 | 354 | 0.384 | 0.103 | 15.50 | 0.325 | |
| 6 | - | - | - | 0.023 | 6.10 | 0.435 | |
| 7 | - | - | - | 0.027 | 4.17 | 0.200 | |
| Parameters | Definition | Unit | Mouse | IIV (RSE%) | Human | IIV (RSE%) | Sources |
|---|---|---|---|---|---|---|---|
| Estimates (RSE%) | Estimates (RSE%) | ||||||
| VC_OXA | Apparent volumes of distribution in the central compartment of oxaliplatin | L | - | - | 49.9 (46.3) | 1.05 (30.9) | Estimated |
| VP_OXA | Apparent volumes of distribution in the peripheral compartment of oxaliplatin | L | - | - | 538 (29.3) | - | Estimated |
| CLOXA | Systematic clearance of oxaliplatin | L/h | - | - | 5.96 (42.5) | 0.597 (63.6) | Estimated |
| QOXA | Clearance between central and peripheral compartments of oxaliplatin | L/h | - | - | 49.3 (29.7) | - | Estimated |
| VC_IRI | Apparent volumes of distribution in the central compartment of irinotecan | L | 0.0349 (32) | 0.873 (20.4) | 72.1 (6.78) | 1.62 (39.3) | Estimated |
| VP_IRI | Apparent volumes of distribution in the peripheral compartment of irinotecan | L | 0.0493 (25.8) | - | 93.4 (15.2) | - | Estimated |
| VTIS | Volumes of tumor interstitial space | mL | 0.1 (fixed) | - | 2 (fixed) | - | Assumed |
| VTC | Volumes of tumor cells | mL | 0.4 (fixed) | - | 8 (fixed) | - | Assumed |
| VT | Volumes of tumor | mL | 0.5 (fixed) | - | 10 (fixed) | - | [26] |
| VC_SN | Apparent volumes of distribution in the central compartment of SN-38 | L | 0.00122 (15.9) | 0.494 (59.3) | 11.2 (34.5) | 0.139 (71.9) | Estimated |
| VP_SN | Apparent volumes of distribution in the peripheral compartment of SN-38 | L | 0.108 (33.1) | - | 706 (52.7) | - | Estimated |
| CLIRI | Systematic clearance of irinotecan | L/h | 0.0527 (19.7) | 0.627 (20.4) | 22.8 (5.69) | 0.149 (27.4) | Estimated |
| CLM_SN | Metabolic rate from irinotecan to SN-38 | L/h | 1.65 × 10−4 (93.8) | 0.851 (35.7) | 0.216 (51.9) | 0.666 (29) | Estimated |
| CLSN | Systematic clearance of SN-38 | L/h | 0.0402 (19.8) | 0.234 (78.9) | 42.8 (32.5) | 0.5 (50.6) | Estimated |
| QIRI | Clearance between central and peripheral compartments of irinotecan | L/h | 0.0156 (40.8) | 0.732 (26) | 24.6 (28.8) | 0.681 (35.8) | Estimated |
| QSN | Clearance between central and peripheral compartments of SN-38 | L/h | 0.0369 (38.2) | 0.606 (34.3) | 43.5 (30.7) | 0.478 (32.4) | Estimated |
| QT | Clearance between central and tumor compartments | L/h | 3.38 × 10−3 (fixed) | - | 0.06 (fixed) | - | [26,27] |
| PSIRI | Permeation rate of irinotecan in tumor cells | cm3/h | 0.448 (>100) | 1.9 (36.5) | 52 (fixed) | - | Estimated in mice/scaled in human |
| KP_IRI | Tumor/plasma partition coefficient of irinotecan | - | 3.43 (74.4) | 1.33 (37.1) | 3.43 (fixed) | - | Estimated/constant in spieces |
| KP_SN | Tumor/plasma partition coefficient of SN-38 | - | 7.32 (71.6) | 1.64 (30) | 7.32 (fixed) | - | Estimated/constant in spieces |
| fuIRI | Fraction unbound of irinotecan in plasma | - | 0.35 (fixed) | - | 0.35 (fixed) | - | [28] |
| fuSN | Fraction unbound of SN-38 in plasma | - | 0.05 (fixed) | - | 0.05 (fixed) | - | [28] |
| Parameter | Description | Unit | Mean Value | Variable Range (%) |
|---|---|---|---|---|
| Kg | Tumor growth rate | h−1 | 0.367 × 10−3 | 30 |
| Emax_SN | The maximum killing effect of SN-38 | - | 0.046 | 30 |
| EC50_SN | SN-38 concentration of half maximum effect | μmol/L | 9.2 | 30 |
| hill_SN | Hill efficient of SN-38 | - | 0.32 | 30 |
| Emax_OXA | The maximum killing effect of oxaliplatin | - | 0.073 | 30 |
| EC50_OXA | Oxaliplatin concentration of half maximum effect | μmol/L | 358 | 30 |
| hill_OXA | Hill efficient of oxaliplatin | - | 0.61 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Zhang, Y.; Zhao, Y.; Zhang, J.; Hao, K.; He, H. Translational Pharmacokinetic/Pharmacodynamic Modeling and Simulation of Oxaliplatin and Irinotecan in Colorectal Cancer. Pharmaceutics 2023, 15, 2274. https://doi.org/10.3390/pharmaceutics15092274
Zhu J, Zhang Y, Zhao Y, Zhang J, Hao K, He H. Translational Pharmacokinetic/Pharmacodynamic Modeling and Simulation of Oxaliplatin and Irinotecan in Colorectal Cancer. Pharmaceutics. 2023; 15(9):2274. https://doi.org/10.3390/pharmaceutics15092274
Chicago/Turabian StyleZhu, Jinwei, Yicui Zhang, Yixin Zhao, Jingwei Zhang, Kun Hao, and Hua He. 2023. "Translational Pharmacokinetic/Pharmacodynamic Modeling and Simulation of Oxaliplatin and Irinotecan in Colorectal Cancer" Pharmaceutics 15, no. 9: 2274. https://doi.org/10.3390/pharmaceutics15092274
APA StyleZhu, J., Zhang, Y., Zhao, Y., Zhang, J., Hao, K., & He, H. (2023). Translational Pharmacokinetic/Pharmacodynamic Modeling and Simulation of Oxaliplatin and Irinotecan in Colorectal Cancer. Pharmaceutics, 15(9), 2274. https://doi.org/10.3390/pharmaceutics15092274






