The Contribution of Antimicrobial Peptides to Immune Cell Function: A Review of Recent Advances
Abstract
:1. Introduction
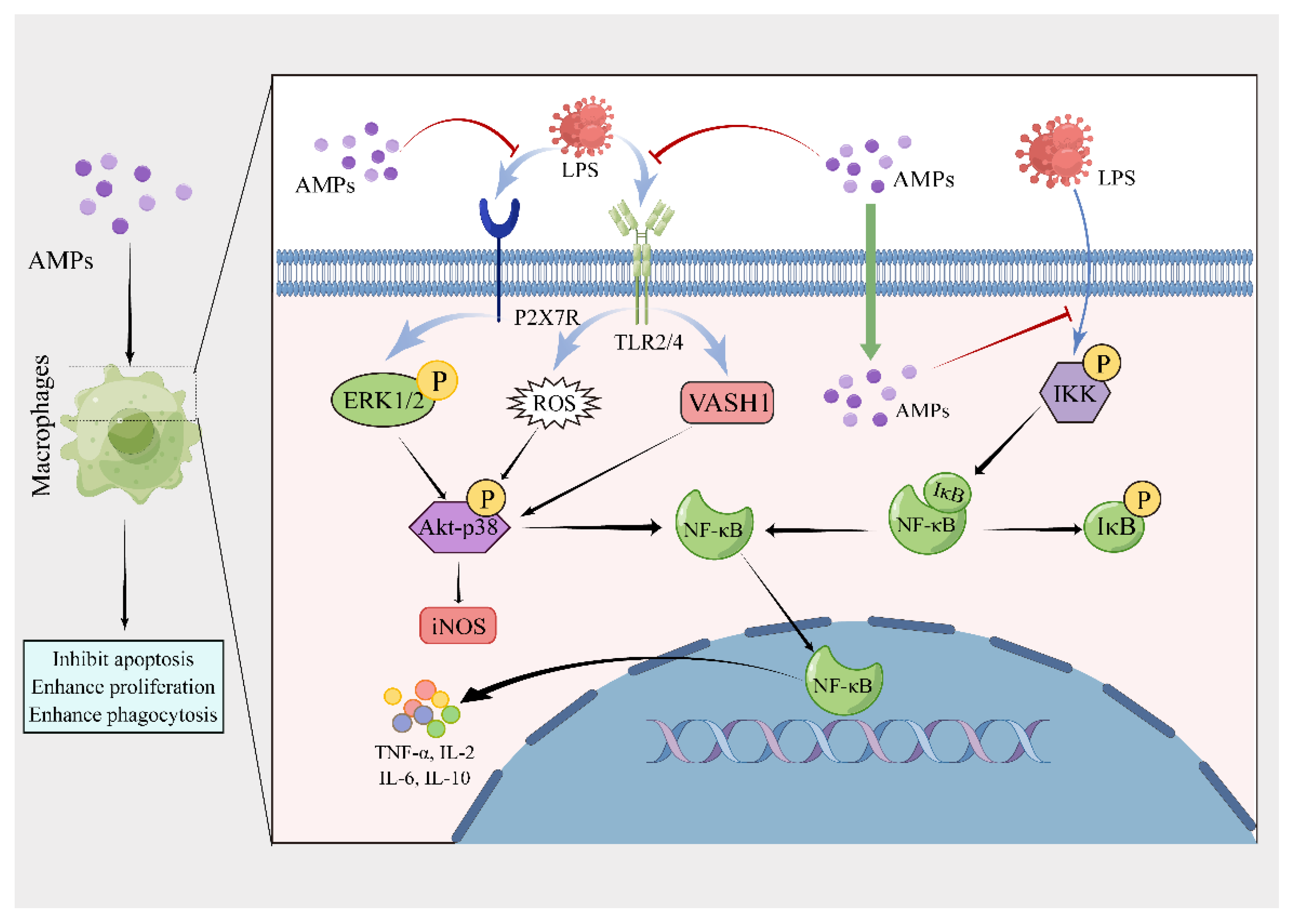
2. Regulatory Effect of AMPs on Macrophages
2.1. Regulation of Macrophages by Mammalian-Derived AMPs
2.2. Regulation of Macrophages by Amphibian-Derived AMPs
2.3. Regulation of Macrophages by Insect-Derived AMPs
2.4. Regulation of Macrophages by Plant-Derived AMPs
2.5. Regulation of Macrophages by Microbial-Derived AMPs
2.6. Regulation of Macrophages by Avian-Derived AMPs
2.7. Regulation of Macrophages by Other AMPs
3. Regulatory Effects on Monocytes by AMPs
3.1. Regulation of Monocytes by Mammalian-Derived AMPs
3.2. Regulation of Monocytes by Other AMPs
4. Regulatory Effects on Lymphocytes by AMPs
4.1. Regulation of Lymphocytes by Mammalian-Derived AMPs
4.2. Regulation of Lymphocytes by Amphibian-Derived AMPs
4.3. Regulation of Lymphocytes by Insect-Derived AMPs
4.4. Regulation of Lymphocytes by Microbial-Derived AMPs
4.5. Regulation of Lymphocytes by Other AMPs
5. Regulatory Effects of AMPs on Mast Cells
5.1. Regulation of Mast Cells by Mammalian-Derived AMPs
5.2. Regulation of Mast Cells by Amphibian-Derived AMPs
5.3. Regulation of Mast Cells by Insect-Derived AMPs
5.4. Regulation of Mast Cells by Other AMPs
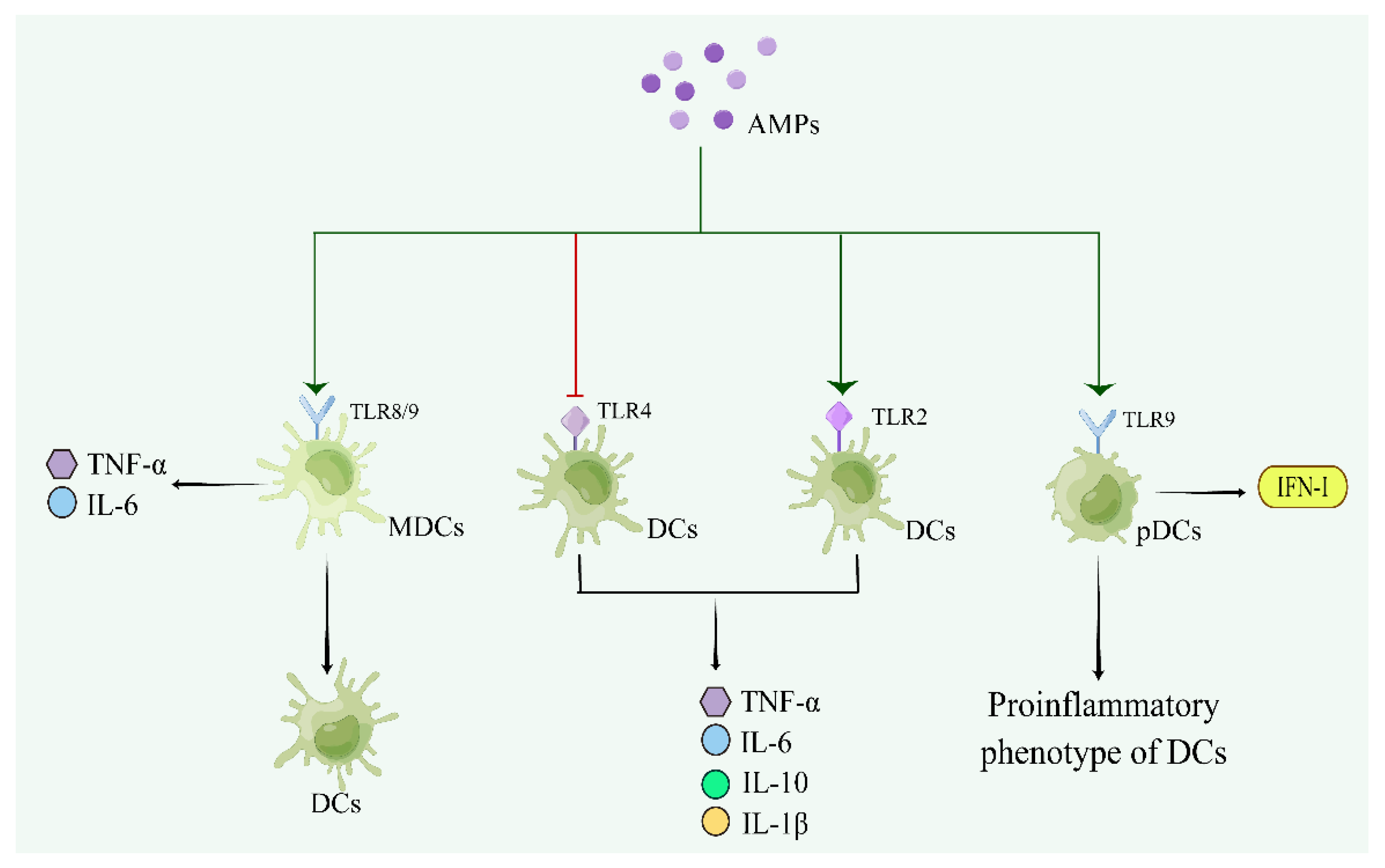
6. Regulatory Effects of AMPs on DCs
6.1. Regulation of DCs by Mammalian-Derived AMPs
6.2. Regulation of DCs by Microbial-Derived AMPs
6.3. Regulation of DCs by Other AMPs
7. Regulatory Effect of AMPs on Neutrophils
7.1. Regulation of Neutrophils by Mammalian-Derived AMPs
7.2. Regulation of Neutrophils by Insect-Derived AMPs
7.3. Regulation of Neutrophils by Other AMPs
8. Regulatory Effect of AMPs on Eosinophils
9. Application Prospects of AMPs
10. Summary and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial peptides: Application informed by evolution. Science 2020, 368, 5480. [Google Scholar] [CrossRef] [PubMed]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020, 20, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wu, D.; Gao, Y. Progress on the design and optimization of antimicrobial peptides. J. Biomed. Eng. 2022, 39, 1247–1253. [Google Scholar]
- Xu, B.; Qiu, S.; Shan, A. Classification, action mechanism and application in animal production of antibacterial peptides. Heilongjiang Anim. Sci. Vet. Med. 2017, 522, 72–76. [Google Scholar]
- Lee, T.H.; Hall, K.N.; Aguilar, M.I. Antimicrobial Peptide Structure and Mechanism of Action: A Focus on the Role of Membrane Structure. Curr. Top. Med. Chem. 2016, 16, 25–39. [Google Scholar] [CrossRef]
- Teixeira, V.; Feio, M.J.; Bastos, M. Role of lipids in the interaction of antimicrobial peptides with membranes. Prog. Lipid. Res. 2012, 51, 149–177. [Google Scholar] [CrossRef]
- Wang, J.; Dou, X.; Song, J.; Lyu, Y.; Zhu, X.; Xu, L.; Li, W.; Shan, A. Antimicrobial peptides: Promising alternatives in the post feeding antibiotic era. Med. Res. Rev. 2019, 39, 831–859. [Google Scholar] [CrossRef]
- Qin, D.; Bian, Y.; Ma, Z. Analysis of immunomodulatory function of antimicrobial peptides. Livest. Poult. Ind. 2021, 387, 17–18. [Google Scholar]
- Kang, Y.; Meng, J.; Wang, Y.; Zhang, S. Research progress of functional characteristics and mechanism of antimicrobial peptides. Food Sci. Technol. 2021, 46, 65–270. [Google Scholar]
- Wang, Y.; Xiao, N.; Wang, Y. Research of progress of cathelicidins family antimicrobial peptides in amphibians. Chin. J. Zool. 2021, 56, 303–319. [Google Scholar]
- Morioka, Y.; Yamasaki, K.; Leung, D.; Gallo, R.L. Cathelicidin antimicrobial peptides inhibit hyaluronan-induced cytokine release and modulate chronic allergic dermatitis. J. Immunol. 2008, 181, 3915–3922. [Google Scholar] [CrossRef]
- He, B. Adjuvant Activity and Application of Antimicrobial Peptide BSN-37. Doctoral Thesis, Henan Institute of Science and Technology, Xinxiang, China, 2022. [Google Scholar]
- Ramanathan, B.; Wu, H.; Ross, C.R.; Blecha, F. PR-39, a porcine antimicrobial peptide, inhibits apoptosis: Involvement of caspase-3. Dev. Comp. Immunol. 2004, 28, 163–169. [Google Scholar] [CrossRef]
- Wen, Y.; Guo, J.; Yu, X.; Cen, D.; Chen, Y.; Zhang, J.; Luo, M.; Tu, Z. Construction of enkaryotic expression vector encoding antimicrobial peptides PR39 and its expression and antimicrobial function in RAW264.7 cells. Acta Acad. Med. Mil. Tertiae 2008, 30, 2044–2046. [Google Scholar]
- Shibusawa, K.; Murakami, T.; Yomogida, S.; Tamura, H.; Nagaoka, I. Antimicrobial cathelicidin peptide CAP11 suppresses HMGB1 release from lipopolysaccharide-stimulated mononuclear phagocytes via the prevention of necrotic cell death. Int. J. Mol. Med. 2009, 23, 341–346. [Google Scholar]
- Liu, S.; Qin, B.; Zhu, L.; Zhao, Y.; Zhao, X.; Xia, X.; Hu, J.; Wang, D.; Wang, L.; An, Z. Progerss on antiviral mechanisms and applications of antimicrobial. Prog. Vet. Med. 2022, 43, 100–104. [Google Scholar]
- Ruan, Y.; Shen, T.; Wang, Y.; Hou, M.; Li, J.; Sun, T. Antimicrobial peptide LL-37 attenuates LTA induced inflammatory effect in macrophages. Int. Immunopharmacol. 2013, 15, 575–580. [Google Scholar] [CrossRef]
- Scott, M.G.; Davidson, D.J.; Gold, M.R.; Bowdish, D.; Hancock, R.E. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 2002, 169, 3883–3891. [Google Scholar] [CrossRef]
- Wan, M.; Soehnlein, O.; Tang, X.; van der Does, A.M.; Smedler, E.; Uhlén, P.; Lindbom, L.; Agerberth, B.; Haeggström, J.Z. Cathelicidin LL-37 induces time-resolved release of LTB4 and TXA2 by human macrophages and triggers eicosanoid generation in vivo. FASEB J. 2014, 28, 3456–3467. [Google Scholar] [CrossRef]
- Hu, Z.; Murakami, T.; Suzuki, K.; Tamura, H.; Reich, J.; Kuwahara-Arai, K.; Iba, T.; Nagaoka, I. Antimicrobial cathelicidin peptide LL-37 inhibits the pyroptosis of macrophages and improves the survival of polybacterial septic mice. Int. Immunol. 2016, 28, 245–253. [Google Scholar] [CrossRef]
- Hu, Z.; Murakami, T.; Suzuki, K.; Tamura, H.; Kuwahara-Arai, K.; Iba, T.; Nagaoka, I. Antimicrobial cathelicidin peptide LL-37 inhibits the LPS/ATP-induced pyroptosis of macrophages by dual mechanism. PLoS ONE 2014, 9, 85765. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yuan, H.; Jiao, S.; Yu, L.; Chang, Y. Effect of hCAP-18/LL-37 gene transfection on activation of RAW264.7 cells. Chin. J. Biol. 2011, 24, 153–156. [Google Scholar]
- Kim, J.; Yang, Y.L.; Jang, S.H.; Jang, Y.S. Human β-defensin 2 plays a regulatory role in innate antiviral immunity and is capable of potentiating the induction of antigen-specific immunity. Virol. J. 2018, 15, 124. [Google Scholar] [CrossRef]
- Bian, T.; Li, L.; Lyu, J.; Cui, D.; Lei, L.; Yan, F. Human β-defensin 3 suppresses Porphyromonas gingivalis lipopolysaccharide-induced inflammation in RAW 264.7 cells and aortas of ApoE-deficient mice. Peptides 2016, 82, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Sun, Y.; Qiu, X.; Huang, J.; Wang, A.; Zhang, Q.; Pang, S.; Huang, Q.; Zhou, R.; Li, L. The Intracellular Interaction of Porcine β-Defensin 2 with VASH1 Alleviates Inflammation via Akt Signaling Pathway. J. Immunol. 2022, 208, 2795–2805. [Google Scholar] [CrossRef]
- Huang, C.; Yang, X.; Huang, J.; Liu, X.; Yang, X.; Jin, H.; Huang, Q.; Li, L.; Zhou, R. Porcine Beta-Defensin 2 Provides Protection Against Bacterial Infection by a Direct Bactericidal Activity and Alleviates Inflammation via Interference with the TLR4/NF-κB Pathway. Front. Immunol. 2019, 10, 1673. [Google Scholar] [CrossRef]
- Motzkus, D.; Schulz-Maronde, S.; Heitland, A.; Schulz, A.; Forssmann, W.G.; Jübner, M.; Maronde, E. The novel beta-defensin DEFB123 prevents lipopolysaccharide-mediated effects in vitro and in vivo. FASEB J. 2006, 20, 1701–1702. [Google Scholar] [CrossRef]
- Dong, B.; Lin, Y.; Su, Z.; Sun, C.; Wang, J.; Fu, S.; Du, W.; Wu, T. Recombinant human β-defensin130 inhibited the growth of foodborne bacteria through membrane disruption and exerted anti-inflammatory activity. Food Sci. Biotechnol. 2022, 31, 893–904. [Google Scholar] [CrossRef]
- Nan, Y.H.; Bang, J.K.; Shin, S.Y. Design of novel indolicidin-derived antimicrobial peptides with enhanced cell specificity and potent anti-inflammatory activity. Peptides 2009, 30, 832–838. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Xia, X.; Zhu, C.; Qin, W.; Xu, Y.; Hang, B.; Sun, Y.; Chen, S.; Zhang, H.; et al. Antimicrobial Peptide JH-3 Effectively Kills Salmonella enterica Serovar Typhimurium Strain CVCC541 and Reduces Its Pathogenicity in Mice. Probiotics Antimicrob. Proteins 2019, 11, 1379–1390. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Xia, X.; Zhu, C.; Zhang, H.; Qin, W.; Xu, Y.; Hang, B.; Sun, Y.; Chen, S.; et al. Inhibitory Effects of Antimicrobial Peptide JH-3 on Salmonella enterica Serovar Typhimurium Strain CVCC541 Infection-Induced Inflammatory Cytokine Release and Apoptosis in RAW264.7 Cells. Molecules 2019, 24, 596. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Kim, Y.C.; Nan, Y.H.; Shin, S.Y. Cell selectivity, mechanism of action and LPS-neutralizing activity of bovine myeloid antimicrobial peptide-18 (BMAP-18) and its analogs. Peptides 2011, 32, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Price, R.L.; Bugeon, L.; Mostowy, S.; Makendi, C.; Wren, B.W.; Williams, H.D.; Willcocks, S.J. In vitro and in vivo properties of the bovine antimicrobial peptide, Bactenecin 5. PLoS ONE 2019, 14, 210508. [Google Scholar] [CrossRef] [PubMed]
- Malone, A.; Clark, R.F.; Hoskin, D.W.; Power Coombs, M.R. Regulation of macrophage-associated inflammatory responses by species-specific lactoferricin peptides. Front. Biosci. 2022, 27, 43. [Google Scholar] [CrossRef] [PubMed]
- van der Does, A.M.; Bogaards, S.J.; Ravensbergen, B.; Beekhuizen, H.; van Dissel, J.T.; Nibbering, P.H. Antimicrobial peptide hLF1-11 directs granulocyte-macrophage colony-stimulating factor-driven monocyte differentiation toward macrophages with enhanced recognition and clearance of pathogens. Antimicrob. Agents Chemother. 2010, 54, 811–816. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, B.; Liao, R.; Zhou, J. HEPC1 and HEPC2 gene transfection increases iron retention in RAW264.7 cells. Acta Acad. Med. Mil. Tertiae 2006, 28, 1772–1774. [Google Scholar]
- Hu, Y.; Yao, B. Advance in research of fish antimicrobial peptides. J. Wuhan Inst. Technol. 2020, 42, 8–17. [Google Scholar]
- Ma, X. Effects of the Antimicrobial Peptide Chensinin-1b and Its Analogue on Polarization of Macrophages. Ph.D. Thesis, Liaoning Normal University, Dalian, China, 2020. [Google Scholar]
- Yang, X.F.; Liu, X.; Yan, X.Y.; Shang, D.J. Effects of frog skin peptide temporin-1CEa and its analogs on ox-LDL induced macrophage-derived foam cells. Front. Pharmacol. 2023, 14, 1139532. [Google Scholar] [CrossRef]
- Wang, Y.; Ouyang, J.; Luo, X.; Zhang, M.; Jiang, Y.; Zhang, F.; Zhou, J.; Wang, Y. Identification and characterization of novel bi-functional cathelicidins from the black-spotted frog (Pelophylax nigromaculata) with both anti-infective and antioxidant activities. Dev. Comp. Immunol. 2021, 116, 103928. [Google Scholar] [CrossRef]
- Rajasekaran, G.; Kamalakannan, R.; Shin, S.Y. Enhancement of the anti-inflammatory activity of temporin-1Tl-derived antimicrobial peptides by tryptophan, arginine and lysine substitutions. J. Pept. Sci. 2015, 21, 779–785. [Google Scholar] [CrossRef]
- Qi, R.H.; Chen, Y.; Guo, Z.L.; Zhang, F.; Fang, Z.; Huang, K.; Yu, H.N.; Wang, Y.P. Identification and characterization of two novel cathelicidins from the frog Odorrana livida. Zool. Res. 2019, 40, 94–101. [Google Scholar] [PubMed]
- Chen, J.; Lin, Y.F.; Chen, J.H.; Chen, X.; Lin, Z.H. Molecular characterization of cathelicidin in tiger frog (Hoplobatrachus rugulosus): Antimicrobial activity and immunomodulatory activity. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 247, 109072. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wu, J.; Chen, Q.; Shen, Y.; Mi, K.; Yang, H.; Mu, L. A Frog-Derived Cathelicidin Peptide with Dual Antimicrobial and Immunomodulatory Activities Effectively Ameliorates Staphylococcus aureus-Induced Peritonitis in Mice. ACS Infect. Dis. 2022, 8, 2464–2479. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Ouyang, J.; Wang, Y.; Zhang, M.; Fu, L.; Xiao, N.; Gao, L.; Zhang, P.; Zhou, J.; Wang, Y. A novel anionic cathelicidin lacking direct antimicrobial activity but with potent anti-inflammatory and wound healing activities from the salamander Tylototriton kweichowensis. Biochimie 2021, 191, 37–50. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Li, D.; Lv, J.; Chen, J.; Sun, F.; Tian, Z.; Xia, P. The effects of CLP-19 on inflammatory factors regulation in RAW264.7 cells. Immunol. J. 2013, 29, 185–189. [Google Scholar]
- Kong, D.; Hua, X.; Zhou, R.; Cui, J.; Wang, T.; Kong, F.; You, H.; Liu, X.; Adu-Amankwaah, J.; Guo, G.; et al. Antimicrobial and Anti-Inflammatory Activities of MAF-1-Derived Antimicrobial Peptide Mt6 and Its D-Enantiomer D-Mt6 against Acinetobacter baumannii by Targeting Cell Membranes and Lipopolysaccharide Interaction. Microbiol. Spectr. 2022, 10, 131222. [Google Scholar] [CrossRef]
- Xu, X.; Sun, H.; Gao, J.; Cui, H.; Liao, Y.; Xia, Q. Antimicrobial peptide HI-3 from Hermetia illucens alleviates inflammation in lipopolysaccharide-stimulated RAW264.7 cells via suppression of the nuclear factor kappa-B signaling pathway. Microbiol. Immunol. 2023, 67, 32–43. [Google Scholar]
- Xu, X.; Sun, H.; Cui, H.; Hu, Z.; Xia, Q. Immunomodulatory effect of antimicrobial peptides HI-3 from Hermetia illucens L. on RAW264.7 cells. J. Environ. Entomol. 2023, 45, 464–472. [Google Scholar]
- Scott, M.G.; Rosenberger, C.M.; Gold, M.R.; Finlay, B.B.; Hancock, R.E. An alpha-helical cationic antimicrobial peptide selectively modulates macrophage responses to lipopolysaccharide and directly alters macrophage gene expression. J. Immunol. 2000, 165, 3358–3365. [Google Scholar] [CrossRef]
- Motobu, M.; Amer, S.; Yamada, M.; Nakamura, K.; Saido-Sakanaka, H.; Asaoka, A.; Yamakawa, M.; Hirota, Y. Effects of antimicrobial peptides derived from the beetle Allomyrina dichotoma defensin on mouse peritoneal macrophages stimulated with lipopolysaccharide. J. Vet. Med. Sci. 2004, 66, 319–322. [Google Scholar] [CrossRef]
- Bertrams, W.; Lindhauer, N.S.; Rieke, M.C.; Paas, A.; Hoffmann, K.; Greene, B.; Visekruna, A.; Vilcinskas, A.; Seidel, K.; Schmeck, B. Tribolium castaneum defensin 1 kills Moraxella catarrhalisin an in vitro infection model but does not harm commensal bacteria. Virulence 2021, 12, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.N.; Hong, J.; Zhang, P.; Hwang, J.S.; Kim, H. An Analog of the Antimicrobial Peptide CopA5 Inhibits Lipopolysaccharide-Induced Macrophage Activation. J. Microbiol. Biotechnol. 2017, 27, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Veloso Júnior, P.H.H.; Simon, K.S.; de Castro, R.J.A.; Coelho, L.C.; Erazo, F.A.H.; de Souza, A.C.B.; das Neves, R.C.; Lozano, V.F.; Schwartz, E.F.; Tavares, A.H.; et al. Peptides ToAP3 and ToAP4 decrease release of inflammatory cytokines through TLR-4 blocking. Biomed. Pharmacother. 2019, 118, 109152. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Valencia, M.A.; Espino-Solis, G.P.; Estrada, B.E.; Corzo, G. Immunomodulatory Responses of Two Synthetic Peptides against Salmonella Typhimurium Infection. Molecules 2021, 26, 5573. [Google Scholar] [CrossRef]
- Lin, Q.P.; Zhou, L.F.; Li, N.N.; Chen, Y.Q.; Li, B.C.; Cai, Y.F.; Zhang, S.Q. Lipopolysaccharide neutralization by the antibacterial peptide CM4. Eur. J. Pharmacol. 2008, 596, 160–165. [Google Scholar] [CrossRef]
- Wang, J.; Ma, K.; Ruan, M.; Wang, Y.; Li, Y.; Fu, Y.V.; Song, Y.; Sun, H.; Wang, J. A novel cecropin B-derived peptide with antibacterial and potential anti-inflammatory properties. PeerJ 2018, 6, 5369. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, E.; Shin, S.; Jeong, K.W.; Lee, J.Y.; Bae, S.Y.; Kim, S.H.; Lee, J.; Kim, S.R.; Lee, D.G.; et al. Structure and function of papiliocin with antimicrobial and anti-inflammatory activities isolated from the swallowtail butterfly, Papilio xuthus. J. Biol. Chem. 2011, 286, 41296–41311. [Google Scholar] [CrossRef]
- Kim, I.W.; Lee, J.H.; Seo, M.; Lee, H.J.; Baek, M.; Kim, M.A.; Shin, Y.P.; Kim, S.H.; Kim, I.; Hwang, J.S. Anti-Inflammatory Activity of Antimicrobial Peptide Periplanetasin-5 Derived from the Cockroach Periplaneta americana. J. Microbiol. Biotechnol. 2020, 30, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.K.; Lee, B.; Kim, S.T.; Yoo, J.S.; Sung, J.S. Designing a Novel Functional Peptide With Dual Antimicrobial and Anti-inflammatory Activities via in Silico Methods. Front. Immunol. 2022, 13, 821070. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, S.; Hu, M.; Liu, Z.; Yu, P.; Li, C.; Zhang, X. Antibacterial and Anti-Inflammatory Properties of Peptide KN-17. Microorganisms 2022, 10, 2114. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.D.; Park, J.; Park, Y. Therapeutic Potential of Antimicrobial Peptide PN5 against Multidrug-Resistant E. coli and Anti-Inflammatory Activity in a Septic Mouse Model. Microbiol. Spectr. 2022, 10, 0149422. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ye, Q.; Wang, K.; Zeng, X.; Huang, S.; Yu, H.; Ge, Q.; Qi, D.; Qiao, S. Enhancement of Macrophage Function by the Antimicrobial Peptide Sublancin Protects Mice from Methicillin-Resistant Staphylococcus aureus. J. Immunol. Res. 2019, 2019, 3979352. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.Y.; Lee, H.; Hwangbo, H.; Hong, S.H.; Cha, H.J.; Park, C.; Kim, D.H.; Kim, G.Y.; Kim, S.; Kim, H.S.; et al. A Novel Peptide Oligomer of Bacitracin Induces M1 Macrophage Polarization by Facilitating Ca2+ Influx. Nutrients 2020, 12, 1603. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Kim, Y.K.; Bilkis, T.; Suh, J.W.; Lee, D.Y.; Yoo, J.C. Reduction of Oxidative Stress through Activating the Nrf2 mediated HO-1 Antioxidant Efficacy Signaling Pathway by MS15, an Antimicrobial Peptide from Bacillus velezensis. Antioxidants 2020, 9, 934. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.N.; Gao, M.; Choi, H.; Jeong, G.S. Marine Microorganism-Derived Macrolactins Inhibit Inflammatory Mediator Effects in LPS-Induced Macrophage and Microglial Cells by Regulating BACH1 and HO-1/Nrf2 Signals through Inhibition of TLR4 Activation. Molecules 2020, 25, 656. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhou, Y.X.; Tang, X.X.; Liu, X.X.; Yi, Z.W.; Fang, M.J.; Wu, Z.; Jiang, F.Q.; Qiu, Y.K. Macrolactins from Marine-Derived Bacillus subtilis B5 Bacteria as Inhibitors of Inducible Nitric Oxide and Cytokines Expression. Mar. Drugs 2016, 14, 195. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y. Surfactin inhibits immunostimulatory function of macrophages through blocking NK-kappaB, MAPK and Akt pathway. Int. Immunopharmacol. 2009, 9, 886–893. [Google Scholar] [CrossRef]
- Jeon, S.M.; Kim, Y.J.; Nguyen, T.Q.; Cui, J.; Thi Bich Hanh, B.; Silwal, P.; Kim, J.K.; Kim, J.M.; Oh, D.C.; Jang, J.; et al. Ohmyungsamycin promotes M1-like inflammatory responses to enhance host defence against Mycobacteroides abscessus infections. Virulence 2022, 13, 1966–1984. [Google Scholar] [CrossRef]
- Śmiałek, J.; Bzowska, M.; Hinz, A.; Mężyk-Kopeć, R.; Sołtys, K.; Mak, P. Bacteriocin BacSp222 and Its Succinylated Forms Exhibit Proinflammatory Activities Toward Innate Immune Cells . J. Inflamm. Res. 2022, 15, 4601–4621. [Google Scholar] [CrossRef]
- Yoo, S.; Kim, M.Y.; Cho, J.Y. Beauvericin, a cyclic peptide, inhibits inflammatory responses in macrophages by inhibiting the NF-κB pathway. Korean J. Physiol. Pharmacol. 2017, 21, 449–456. [Google Scholar] [CrossRef]
- Gammelsrud, A.; Solhaug, A.; Dendelé, B.; Sandberg, W.J.; Ivanova, L.; Kocbach Bølling, A.; Lagadic-Gossmann, D.; Refsnes, M.; Becher, R.; Eriksen, G.; et al. Enniatin B-induced cell death and inflammatory responses in RAW 267.4 murine macrophages. Toxicol. Appl. Pharmacol. 2012, 261, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Coorens, M.; Schneider, V.A.F.; de Groot, A.M.; van Dijk, A.; Meijerink, M.; Wells, J.M.; Scheenstra, M.R.; Veldhuizen, E.J.A.; Haagsman, H.P. Cathelicidins Inhibit Escherichia coli-Induced TLR2 and TLR4 Activation in a Viability-Dependent Manner. J. Immunol. 2017, 199, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Scheenstra, M.R.; van Harten, R.M.; Haagsman, H.P.; Veldhuizen, E.J.A. The immunomodulatory effect of cathelicidin-B1 on chicken macrophages. Vet. Res. 2020, 51, 122. [Google Scholar] [CrossRef] [PubMed]
- Bommineni, Y.R.; Pham, G.H.; Sunkara, L.T.; Achanta, M.; Zhang, G. Immune regulatory activities of fowlicidin-1, a cathelicidin host defense peptide. Mol. Immunol. 2014, 59, 55–63. [Google Scholar] [CrossRef]
- Kim, W.H.; Lillehoj, H.S.; Min, W. Evaluation of the Immunomodulatory Activity of the Chicken NK-Lysin-Derived Peptide cNK-2. Sci. Rep. 2017, 7, 45099. [Google Scholar] [CrossRef]
- Kumar, S.D.; Shin, S.Y. Antimicrobial and anti-inflammatory activities of short dodecapeptides derived from duck cathelicidin: Plausible mechanism of bactericidal action and endotoxin neutralization. Eur. J. Med. Chem. 2020, 204, 112580. [Google Scholar] [CrossRef]
- Hong, Y.; Lee, J.; Vu, T.H.; Lee, S.; Lillehoj, H.S.; Hong, Y.H. Chicken avian β-defensin 8 modulates immune response via the mitogen-activated protein kinase signaling pathways in a chicken macrophage cell line. Poult. Sci. 2020, 99, 4174–4182. [Google Scholar] [CrossRef]
- Álvarez, C.A.; Santana, P.A.; Salinas-Parra, N.; Beltrán, D.; Guzmán, F.; Vega, B.; Acosta, F.; Mercado, L. Immune Modulation Ability of Hepcidin from Teleost Fish. Animals 2022, 12, 1586. [Google Scholar] [CrossRef]
- Liu, C.W.; Su, B.C.; Chen, J.Y. Tilapia Piscidin 4 (TP4) Reprograms M1 Macrophages to M2 Phenotypes in Cell Models of Gardnerella vaginalis-Induced Vaginosis. Front. Immunol. 2021, 12, 773013. [Google Scholar] [CrossRef]
- Su, B.C.; Chen, J.Y. Epinecidin-1: An orange-spotted grouper antimicrobial peptide that modulates Staphylococcus aureus lipoteichoic acid-induced inflammation in macrophage cells. Fish Shellfish. Immunol. 2020, 99, 362–367. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Rajanbabu, V.; Pan, C.Y.; Chen, J.Y. Transcriptome analysis reveals modulation of differentially expressed genes in LPS-treated mouse macrophages (RAW264.7 cells) by grouper (Epinephelus coioides) Epinecidin-1. Fish Shellfish Immunol. 2023, 139, 108880. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Lee, H.Y.; Jung, Y.S.; Park, J.S.; Hwang, J.S.; Bae, Y.S. Antimicrobial peptide scolopendrasin VII, derived from the centipede Scolopendra subspinipes mutilans, stimulates macrophage chemotaxis via formyl peptide receptor 1. BMB Rep. 2015, 48, 479–484. [Google Scholar] [CrossRef]
- Uen, W.C.; Choong, C.Y.; Tai, C.J.; Tai, C.J. Pardaxin Promoted Differentiation and Maturation of Leukemic Cells via Regulating TLR2/MyD88 Signal against Cell Proliferation. Evid. Based Complement. Alternat. Med. 2019, 2019, 7035087. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.X.; Hu, S.Y.; Lu, X.J.; Hu, J.R. Identification and characterization of two novel antimicrobial peptides from Japanese sea bass (Lateolabrax japonicus) with antimicrobial activity and MO/MΦ activation capability. Dev. Comp. Immunol. 2023, 145, 104726. [Google Scholar] [CrossRef]
- Wang, C.B.; Yan, X.; Wang, G.H.; Liu, W.Q.; Wang, Y.; Hao, D.F.; Liu, H.M.; Zhang, M. NKHs27, a sevenband grouper NK-Lysin peptide that possesses immunoregulatory and antimicrobial activity. Fish Shellfish Immunol. 2023, 136, 108715. [Google Scholar] [CrossRef] [PubMed]
- Haitao, Y.; Yifan, C.; Mingchao, S.; Shuaijuan, H. A Novel Polymeric Nanohybrid Antimicrobial Engineered by Antimicrobial Peptide MccJ25 and Chitosan Nanoparticles Exerts Strong Antibacterial and Anti-Inflammatory Activities. Front. Immunol. 2022, 12, 811381. [Google Scholar] [CrossRef]
- Ajish, C.; Yang, S.; Kumar, S.D.; Kim, E.Y.; Min, H.J.; Lee, C.W.; Shin, S.H.; Shin, S.Y. A novel hybrid peptide composed of LfcinB6 and KR-12-a4 with enhanced antimicrobial, anti-inflammatory and anti-biofilm activities. Sci. Rep. 2022, 12, 4365. [Google Scholar] [CrossRef]
- Shin, A.; Lee, E.; Jeon, D.; Park, Y.G.; Bang, J.K.; Park, Y.S.; Shin, S.Y.; Kim, Y. Peptoid-Substituted Hybrid Antimicrobial Peptide Derived from Papiliocin and Magainin 2 with Enhanced Bacterial Selectivity and Anti-inflammatory Activity. Biochemistry 2015, 54, 3921–3931. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Q.; Hu, M.; Zhao, B.; Liu, Z.; Li, C.; Zhang, X. Study on Optimizing Novel Antimicrobial Peptides with Bifunctional Activity to Prevent and Treat Peri-Implant Disease. Antibiotics 2022, 11, 1482. [Google Scholar] [CrossRef]
- Nan, Y.H.; Jeon, Y.J.; Park, I.S.; Shin, S.Y. Antimicrobial peptide P18 inhibits inflammatory responses by LPS- but not by IFN-gamma-stimulated macrophages. Biotechnol. Lett. 2008, 30, 1183–1187. [Google Scholar] [CrossRef]
- Nan, Y.H.; Shin, S.Y. Antimicrobial and anti-inflammatory activities of designed antimicrobial peptide P18 analogues. Protein Pept. Lett. 2008, 15, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Han, Y.; Ding, J.; Chen, X.; Huang, S.; Xing, X.; Wu, D.; Chen, J. Regulation of an Antimicrobial Peptide GL13K-Modified Titanium Surface on Osteogenesis, Osteoclastogenesis, and Angiogenesis Base on Osteoimmunology. ACS Biomater. Sci. Eng. 2021, 7, 4569–4580. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, L.; Wu, D.; Huang, W.; Lin, Y.; Zhou, B.; Chen, J. The Effects of Titanium Surfaces Modified with an Antimicrobial Peptide GL13K by Silanization on Polarization, Anti-Inflammatory, and Proinflammatory Properties of Macrophages. Biomed. Res. Int. 2020, 2020, 2327034. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.H.; Park, K.H.; Jeon, Y.J.; Park, Y.; Park, I.S.; Hahm, K.S.; Shin, S.Y. Antimicrobial and anti-inflammatory activities of a Leu/Lys-rich antimicrobial peptide with Phe-peptoid residues. Protein Pept. Lett. 2007, 14, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Jantaruk, P.; Roytrakul, S.; Sitthisak, S.; Kunthalert, D. Potential role of an antimicrobial peptide, KLK in inhibiting lipopolysaccharide-induced macrophage inflammation. PLoS ONE 2017, 12, 183852. [Google Scholar] [CrossRef]
- Nishihara, S.; Kawasaki, K. Enhanced cellular uptake of CpG DNA by α-helical antimicrobial peptide Kn2-7: Effects on macrophage responsiveness to CpG DNA. Biochem. Biophys. Res. Commun. 2020, 530, 100–106. [Google Scholar] [CrossRef]
- Wang, P.; Nan, Y.H.; Yang, S.T.; Kang, S.W.; Kim, Y.; Park, I.S.; Hahm, K.S.; Shin, S.Y. Cell selectivity and anti-inflammatory activity of a Leu/Lys-rich alpha-helical model antimicrobial peptide and its diastereomeric peptides. Peptides 2010, 31, 251–261. [Google Scholar] [CrossRef]
- Ajish, C.; Yang, S.; Kumar, S.D.; Lee, C.W.; Kim, D.M.; Cho, S.J.; Shin, S.Y. Cell selectivity and antibiofilm and anti-inflammatory activities and antibacterial mechanism of symmetric-end antimicrobial peptide centered on D-Pro-Pro. Biochem. Biophys. Res. Commun. 2023, 666, 21–28. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Xu, L.; Tu, F.; Rui, X.; Zhang, L.; Yan, Z.; Liu, Y.; Hu, R. Neutrophil membrane-coated nanoparticles exhibit increased antimicrobial activities in an anti-microbial resistant K. pneumonia infection model. Nanomedicine 2023, 48, 102640. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, D.; Cheng, Z.; Niu, Y.; Kong, L.; Lu, Z.; Bie, X. Designed symmetrical β-hairpin peptides for treating multidrug-resistant salmonella typhimurium infections. Eur. J. Med. Chem. 2022, 243, 114769. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Yu, J.; Li, C.; Yu, D.; Dai, R.; Li, Q.; Cao, C.Y. A dual functional polypeptide with antibacterial and anti-inflammatory properties for the treatment of periodontitis. Int. J. Biol. Macromol. 2023, 242, 124920. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Yang, H.; Wang, Z.; Jiang, N.; Zhang, A. Antimicrobial peptide CC34 attenuates intestinal inflammation via downregulation of the NF-κB signaling pathway. 3 Biotech 2021, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Ju, T.C.; Hsieh, C.Y.; Dong, W.C.; Chen, W.T.; Hua, K.F.; Chen, W.J. A synthetic cationic antimicrobial peptide inhibits inflammatory response and the NLRP3 inflammasome by neutralizing LPS and ATP. PLoS ONE 2017, 12, 182057. [Google Scholar] [CrossRef]
- Liu, R.; Ni, Y.; Song, J.; Xu, Z.; Qiu, J.; Wang, L.; Zhu, Y.; Huang, Y.; Ji, M.; Chen, Y. Research on the effect and mechanism of antimicrobial peptides HPRP-A1/A2 work against Toxoplasma gondii infection. Parasite Immunol. 2019, 41, 12619. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; He, L.; Wang, Y.; Ye, X.; Ma, L. Cbf-14, a cationic peptide derived from cathelin-domain, exhibits anti-inflammation activity via inhibiting PI3K-Akt/ROS/NF-κB signaling pathway. Peptides 2023, 166, 171040. [Google Scholar] [CrossRef] [PubMed]
- Shim, D.W.; Heo, K.H.; Kim, Y.K.; Sim, E.J.; Kang, T.B.; Choi, J.W.; Sim, D.W.; Cheong, S.H.; Lee, S.H.; Bang, J.K.; et al. Anti-Inflammatory Action of an Antimicrobial Model Peptide That Suppresses the TRIF-Dependent Signaling Pathway via Inhibition of Toll-Like Receptor 4 Endocytosis in Lipopolysaccharide-Stimulated Macrophages. PLoS ONE 2015, 10, 126871. [Google Scholar] [CrossRef]
- Kim, S.J.; Kang, T.B.; Kim, D.H.; Keum, M.; Lee, S.H.; Kim, J.H.; Lee, S.H.; Kim, J.; Kweon, H.J.; Park, J.W.; et al. 10-mer and 9-mer WALK Peptides with Both Antibacterial and Anti-Inflammatory Activities. Antibiotics 2022, 11, 1588. [Google Scholar] [CrossRef]
- Sun, Y.; Chan, J.; Bose, K.; Tam, C. Simultaneous control of infection and inflammation with keratin-derived antibacterial peptides targeting TLRs and co-receptors. Sci. Transl. Med. 2023, 15, 2909. [Google Scholar] [CrossRef]
- Guo, G.; Wu, J.; Fu, P.; Zhang, Y. Effects of secreting type antibaterial peptides from Musca domestica larvae on immune function in mice. Chin. J. Public Health 2012, 28, 619–620. [Google Scholar]
- Wang, G.; Li, X.; Wang, Z. APD2: The updated antimicrobial peptide database and its application in peptide design. Nucleic Acids. Res. 2009, 37, 933–937. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Z.; Jia, Y. Progress in research of antitumor properties of antimicrobial peptides derived from microbes. J. Biol. 2022, 39, 100–105, 119. [Google Scholar]
- Li, Y.; Yao, Q.; Ren, M.; Yang, F.; Zou, M.; Zhang, Y.; Lin, Q. Progress on action mechanisms of antimicrobial peptides. Prog. Vet. Med. 2019, 40, 98–103. [Google Scholar]
- Geng, J.; Wang, Y.; Chen, L. Effects of antimicrobial peptides from duck leukocytes on immunity of mice. J. Henan Agr. Sci. 2011, 40, 142–145. [Google Scholar]
- Liu, H.; Xue, Y.; Zhang, S.; Wang, J.; Zhao, M.; Chen, Y.; Bian, W. Research progress of antimicrobial peptides in fish (I). J. Aquacult. 2019, 40, 20–22, 25. [Google Scholar]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Z.; Wang, K. Research progress on source, classification, biological activity, mechanism and application of antimicrobial peptides. Chin. Med. Biotechnol. 2016, 11, 539–543. [Google Scholar]
- Shang, D.; Zhang, Q.; Dong, W.; Liang, H.; Bi, X. The effects of LPS on the activity of Trp-containing antimicrobial peptides against Gram-negative bacteria and endotoxin neutralization. Acta Biomater. 2016, 33, 153–165. [Google Scholar] [CrossRef]
- Wang, K.; Luo, Y.; Zhou, H.; Pi, C.; Peng, Y.; Su, X.; Li, Y. Effect of Anitimicrobial peptides feed additive on the phagocytic function of macrophages in mice. Prog. Vet. Med. 2009, 30, 52–54. [Google Scholar]
- Dong, R.; Zeng, F.; Zhao, C.; Fang, Y.; Wu, Z.; Liu, D.; Zheng, E.; Li, Z. Research progress of antimicrobial peptides from animals. Heilongjiang Anim. Sci. Vet. Med. 2017, 521, 84–87. [Google Scholar]
- Xu, L.; Zhang, R.; Dong, C. Research progress in antibacterial peptide LL-37. Mil. Med. Sci. 2022, 46, 551–557. [Google Scholar]
- Qian, Q.; Bai, Y.; Qian, L.; Li, M.; Gao, C.; Shen, H. Effect of antimicrobial peptides LL37 combined with peptidoglycan on the differentiation of monocytes. Chin. J. Lepr. Skin Dis. 2015, 31, 654–658. [Google Scholar]
- Pinegin, B.V.; Pashenkov, M.V.; Kulakov, V.V.; Murugin, V.V.; Zhmak, M.N. Complexes of DNA with the Antimicrobial Peptide LL37 Augment NK Cell Functions by Inducing Type I Interferon Production from Circulating Monocytes and Plasmacytoid Predendritic Cells. J. Interferon Cytokine Res. 2015, 35, 850–858. [Google Scholar] [CrossRef]
- Liu, X.; Qin, F.; Wei, W.; Wei, H.; Chao, W. Research progress of HNP1-3 in related disrases. Med. Equip. 2023, 36, 150–154. [Google Scholar]
- Hu, Q.; Wu, J.; Zhang, C.; Chen, T. Effect of HBD1 on activation and proliferation of CD4+T lymphocytes in umbilical cord blood of neonates. Curr. Immunol. 2017, 37, 404–411. [Google Scholar]
- Lioi, A.B.; Ferrari, B.M.; Dubyak, G.R.; Weinberg, A.; Sieg, S.F. Human β Defensin-3 Increases CD86 Expression on Monocytes by Activating the ATP-Gated Channel P2X7. J. Immunol. 2015, 195, 4438–4445. [Google Scholar] [CrossRef] [PubMed]
- Funderburg, N.; Lederman, M.M.; Feng, Z.; Drage, M.G.; Jadlowsky, J.; Harding, C.V.; Weinberg, A.; Sieg, S.F. Human β-defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc. Natl. Acad. Sci. USA 2007, 104, 18631–18635. [Google Scholar] [CrossRef]
- Chen, J.; Lv, Y.P.; Dai, Q.M.; Hu, Z.H.; Liu, Z.M.; Li, J.H. Host defense peptide LEAP-2 contributes to monocyte/macrophage polarization in barbel steed (Hemibarbus labeo). Fish Shellfish Immunol. 2019, 87, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.F.; Li, C.H.; Chen, J. Molecular characterization of the NK-lysin in a teleost fish, Boleophthalmus pectinirostris: Antimicrobial activity and immunomodulatory activity on monocytes/macrophages. Fish Shellfish Immunol. 2019, 92, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, G.; Dinesh Kumar, S.; Nam, J.; Jeon, D.; Kim, Y.; Lee, C.W.; Park, I.S.; Shin, S.Y. Antimicrobial and anti-inflammatory activities of chemokine CXCL14-derived antimicrobial peptide and its analogs. Biochim. Biophys. Acta Biomembr. 2019, 1861, 256–267. [Google Scholar] [CrossRef]
- Hurtado, P.; Peh, C.A. LL-37 promotes rapid sensing of CpG oligodeoxynucleotides by B lymphocytes and plasmacytoid dendritic cells. J. Immunol. 2010, 184, 1425–1435. [Google Scholar] [CrossRef]
- Mader, J.S.; Ewen, C.; Hancock, R.E.; Bleackley, R.C. The human cathelicidin, LL-37, induces granzyme-mediated apoptosis in regulatory T cells. J. Immunother. 2011, 34, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Mader, J.S.; Marcet-Palacios, M.; Hancock, R.E.; Bleackley, R.C. The human cathelicidin, LL-37, induces granzyme-mediated apoptosis in cytotoxic T lymphocytes. Exp. Cell. Res. 2011, 317, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Taefehshokr, N.; Isazadeh, A.; Oveisi, A.; Key, Y.A.; Taefehshokr, S. Reciprocal role of hBD2 and hBD3 on the adaptive immune response by measuring T lymphocyte proliferation in terms of CD4 and CCR6 expression. Horm. Mol. Biol. Clin. Investig. 2018, 35, 20080023. [Google Scholar] [CrossRef]
- Meisch, J.P.; Vogel, R.M.; Schlatzer, D.M.; Li, X.; Chance, M.R.; Levine, A.D. Human β-defensin 3 induces STAT1 phosphorylation, tyrosine phosphatase activity, and cytokine synthesis in T cells. J. Leukoc. Biol. 2013, 94, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ma, C.; Wan, X.; Xiao, Y.; Hu, L.; Li, J.; Wang, Z.; Lv, X.; Gao, R. Co-expression of porcine IL-4/6 and antimicrobial peptide fusion gene in yeast and its bioactivity. Chin. J. Prev. Vet. Med. 2018, 40, 834–841. [Google Scholar]
- Ma, C.; Wu, X.; Wan, X.; Xiao, Y.; Xiong, Q.; Liang, G.; Li, J.; Lv, X.; Wang, Z.; Gao, R. Construction of recombinant Pihia pastoris co-expressing pig IL-4/6 and fusion bovine cathelicidins and its synergistic effect on the immunity of mice. Chin. J. Sci. 2018, 38, 1519–1527. [Google Scholar]
- Jin, Y.; Liu, H.; Geng, J.; Yang, X.; Chen, L. Effect of antimicrobial bovine neutrophil extractonimmune functions in mice. J. Henan Agr. Univ. 2009, 43, 630–633. [Google Scholar]
- Pantic, J.M.; Radosavljevic, G.D.; Jovanovic, I.P.; Arsenijevic, N.N.; Conlon, J.M.; Lukic, M.L. In vivo administration of the frog skin peptide frenatin 2.1S induces immunostimulatory phenotypes of mouse mononuclear cells. Peptides 2015, 71, 269–275. [Google Scholar] [CrossRef]
- Liu, L.; Yang, K.; Hua, J.; Wang, X.; Liu, T. Antimicrobial peptides: Effects on small intestinal mucosal morphology and immune active cell number in hy-line brown young roosters. Chin. J. Anim. Nutr. 2013, 25, 190–197. [Google Scholar]
- Liu, X.; Fan, H.; Chen, L.; Li, H.; Qiao, J.; Wang, W. Effects of cecropin and synbiotics on the intestinal mucosal morphology and intestinal mucosal immune cells of AA broilers. China Anim. Husb. Vet. Med. 2017, 44, 3187–3194. [Google Scholar]
- Wang, L.; Chen, X.; Wang, S. Effects of antimicrobial peptide on grown performance and immune function of 817 broiler hybirds. China Anim. Husb. Vet. Med. 2017, 44, 2354–2359. [Google Scholar]
- Li, Z.; Zhao, S.; Xin, X.; Zhang, B.; Thomas, A.; Charles, A.; Lee, K.S.; Jin, B.R.; Gui, Z. Purification, Identification and Functional Analysis of a Novel Immunomodulatory Peptide from Silkworm Pupa Protein. Int. J. Pept. Res. Ther. 2020, 26, 243–249. [Google Scholar] [CrossRef]
- Yang, T.; Wang, S.; Huang, S.; Shang, L.; Yu, H.; Zeng, X.; Qiao, S. Comparative study of immunomodulatory effect of antimicrobial peptide Sublancin and astragalus polysaccharide of mice. Chin. J. Anim. Nutr. 2018, 30, 2337–2345. [Google Scholar]
- Xu, S. Effects of Compound Antibacterial Peptide “Taikanglibao” on the Index of Spleen and Cellular Immunity in Weaned Piglets. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2014. [Google Scholar]
- Han, F.F.; Gao, Y.H.; Luan, C.; Xie, Y.G.; Liu, Y.F.; Wang, Y.Z. Comparing bacterial membrane interactions and antimicrobial activity of porcine lactoferricin-derived peptides. J. Dairy Sci. 2013, 96, 3471–3487. [Google Scholar] [CrossRef]
- Sun, D.; Pan, B. Research status and progress of probiotics and synbiotics. Feed Res. 2008, 314, 64–66. [Google Scholar]
- Liu, M.; Liu, Y.; He, Y.; Guo, X.; Li, Y.; Li, P. Effects of dietary antimicrobial peptides on immune function and antioxidant capacity of laying hens in late laying period. Heilongjiang Anim. Sci. Vet. Med. 2023, 666, 108–114. [Google Scholar]
- Yang, Y.; Liang, H.; Wei, H. The preliminary study of antimicrobial peptides extracted from African ostrich skin on the immune oranges indexes and the number of T lymphocytes in immune oranges of chickens. Chin. Agr. Sci. Bull. 2009, 25, 46–48. [Google Scholar]
- Wang, J.; Li, T. The effect of antibacterial peptides on peripheral lymphocyte transformation in broilers. Acta Ecol. Anim. Domastici 2007, 28, 45–48. [Google Scholar]
- Bąbolewska, E.; Brzezińska-Błaszczyk, E. Human-derived cathelicidin LL-37 directly activates mast cells to proinflammatory mediator synthesis and migratory response. Cell. Immunol. 2015, 293, 67–73. [Google Scholar] [CrossRef]
- Yoshioka, M.; Fukuishi, N.; Kubo, Y.; Yamanobe, H.; Ohsaki, K.; Kawasoe, Y.; Murata, M.; Ishizumi, A.; Nishii, Y.; Matsui, N.; et al. Human cathelicidin CAP18/LL-37 changes mast cell function toward innate immunity. Biol. Pharm. Bull. 2008, 31, 212–216. [Google Scholar] [CrossRef]
- Murakami, T.; Suzuki, K.; Niyonsaba, F.; Tada, H.; Reich, J.; Tamura, H.; Nagaoka, I. MrgX2-mediated internalization of LL-37 and degranulation of human LAD2 mast cells. Mol. Med. Rep. 2018, 18, 4951–4959. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Y.; Zhang, Y.; Lai, Y.; Chen, W.; Xiao, Z.; Zhang, W.; Jin, M.; Yu, B. LL-37-induced human mast cell activation through G protein-coupled receptor MrgX2. Int. Immunopharmacol. 2017, 49, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Niyonsaba, F.; Ushio, H.; Hara, M.; Yokoi, H.; Tominaga, M.; Takamori, K.; Kajiwara, N.; Saito, H.; Nagaoka, I.; Ogawa, H.; et al. Antimicrobial peptides human beta-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J. Immunol. 2010, 184, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Niyonsaba, F.; Someya, A.; Hirata, M.; Ogawa, H.; Nagaoka, I. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur. J. Immunol. 2001, 31, 1066–1075. [Google Scholar] [CrossRef]
- Chen, X.; Niyonsaba, F.; Ushio, H.; Hara, M.; Yokoi, H.; Matsumoto, K.; Saito, H.; Nagaoka, I.; Ikeda, S.; Okumura, K.; et al. Antimicrobial peptides human beta-defensin (hBD)-3 and hBD-4 activate mast cells and increase skin vascular permeability. Eur. J. Immunol. 2007, 37, 434–444. [Google Scholar] [CrossRef]
- Gao, Y.; Chai, J.; Wu, J.; Zeng, Q.; Guo, R.; Chen, X.; Xu, X. Molecular Cloning and Characterization of a Novel Antimicrobial Peptide from the Skin of Kaloula pulchra. Curr. Pharm. Biotechnol. 2022, 23, 1873–1882. [Google Scholar]
- Keitel, U.; Schilling, E.; Knappe, D.; Al-Mekhlafi, M.; Petersen, F.; Hoffmann, R.; Hauschildt, S. Effect of antimicrobial peptides from Apis mellifera hemolymph and its optimized version Api88 on biological activities of human monocytes and mast cells. Innate. Immun. 2013, 19, 355–367. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Song, P.; Yue, H.; Sutthammikorn, N.; Umehara, Y.; Okumura, K.; Ogawa, H. Antimicrobial peptide derived from insulin-like growth factor-binding protein 5 activates mast cells via Mas-related G protein-coupled receptor X2. Allergy 2020, 75, 203–207. [Google Scholar] [CrossRef]
- Amponnawarat, A.; Chompunud Na Ayudhya, C.; Ali, H. Murepavadin, a Small Molecule Host Defense Peptide Mimetic, Activates Mast Cells via MRGPRX2 and MrgprB2. Front. Immunol. 2021, 12, 689410. [Google Scholar] [CrossRef]
- Kanazawa, K.; Okumura, K.; Ogawa, H.; Niyonsaba, F. An antimicrobial peptide with angiogenic properties, AG-30/5C, activates human mast cells through the MAPK and NF-κB pathways. Immunol. Res. 2016, 64, 594–603. [Google Scholar] [CrossRef]
- Che, D.; Jia, T.; Zhang, X.; Zhang, L.; Du, X.; Zheng, Y.; Zhou, T.; Song, X.; Geng, S. Dermcidin-derived polypeptides: DCD(86-103) induced inflammatory reaction in the skin by activation of mast cells via ST2. Immunol. Lett. 2022, 251–252, 29–37. [Google Scholar] [CrossRef]
- Pundir, P.; Catalli, A.; Leggiadro, C.; Douglas, S.E.; Kulka, M. Pleurocidin, a novel antimicrobial peptide, induces human mast cell activation through the FPRL1 receptor. Mucosal. Immunol. 2014, 7, 177–187. [Google Scholar] [CrossRef]
- Agier, J.; Brzezińska-Błaszczyk, E.; Różalska, S.; Wiktorska, M.; Kozłowska, E.; Żelechowska, P. Mast cell phenotypic plasticity and their activity under the influence of cathelicidin-related antimicrobial peptide (CRAMP) and IL-33 alarmins. Cell. Immunol. 2021, 369, 104424. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, A.; Braff, M.H.; Taylor, K.R.; Na, C.; Granstein, R.D.; McInturff, J.E.; Krutzik, S.; Modlin, R.L.; Gallo, R.L. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J. Immunol. 2007, 178, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.J.; Currie, A.J.; Reid, G.S.; Bowdish, D.M.; MacDonald, K.L.; Ma, R.C.; Hancock, R.E.; Speert, D.P. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J. Immunol. 2004, 172, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Bandholtz, L.; Ekman, G.J.; Vilhelmsson, M.; Buentke, E.; Agerberth, B.; Scheynius, A.; Gudmundsson, G.H. Antimicrobial peptide LL-37 internalized by immature human dendritic cells alters their phenotype. Scand. J. Immunol. 2006, 63, 410–419. [Google Scholar] [CrossRef]
- Findlay, E.G.; Currie, A.J.; Zhang, A.; Ovciarikova, J.; Young, L.; Stevens, H.; McHugh, B.J.; Canel, M.; Gray, M.; Milling, S.W.F.; et al. Exposure to the antimicrobial peptide LL-37 produces dendritic cells optimized for immunotherapy. Oncoimmunology 2019, 8, 1608106. [Google Scholar] [CrossRef]
- Yuan, H.; Xu, N.; Wang, F.; Chang, Y. The effect of eukaryotic expression products of the whole range peptide of hCAP-18/LL-37 on differentiation and maturation of DC. Chin. J. Lab. Diagn. 2010, 14, 346–348. [Google Scholar]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.H.; Homey, B.; Cao, W.; Wang, Y.H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef]
- Badal, D.; Dayal, D.; Singh, G.; Sachdeva, N. Role of DNA-LL37 complexes in the activation of plasmacytoid dendritic cells and monocytes in subjects with type 1 diabetes. Sci. Rep. 2020, 10, 8896. [Google Scholar] [CrossRef]
- Ganguly, D.; Chamilos, G.; Lande, R.; Gregorio, J.; Meller, S.; Facchinetti, V.; Homey, B.; Barrat, F.J.; Zal, T.; Gilliet, M. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 2009, 206, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Tewary, P.; de la Rosa, G.; Sharma, N.; Rodriguez, L.G.; Tarasov, S.G.; Howard, O.M.; Shirota, H.; Steinhagen, F.; Klinman, D.M.; Yang, D.; et al. β-Defensin 2 and 3 promote the uptake of self or CpG DNA, enhance IFN-α production by human plasmacytoid dendritic cells, and promote inflammation. J. Immunol. 2013, 191, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Ferris, L.K.; Mburu, Y.K.; Mathers, A.R.; Fluharty, E.R.; Larregina, A.T.; Ferris, R.L.; Falo, L.D., Jr. Human beta-defensin 3 induces maturation of human langerhans cell-like dendritic cells: An antimicrobial peptide that functions as an endogenous adjuvant. J. Investig. Dermatol. 2013, 133, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Biragyn, A.; Ruffini, P.A.; Leifer, C.A.; Klyushnenkova, E.; Shakhov, A.; Chertov, O.; Shirakawa, A.K.; Farber, J.M.; Segal, D.M.; Oppenheim, J.J.; et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science 2002, 298, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Biragyn, A.; Coscia, M.; Nagashima, K.; Sanford, M.; Young, H.A.; Olkhanud, P. Murine beta-defensin 2 promotes TLR-4/MyD88-mediated and NF-kappaB-dependent atypical death of APCs via activation of TNFR2. J. Leukoc. Biol. 2008, 83, 998–1008. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, J.; Cheng, M.; Zhang, X. Mouse β-defensin-14 for inducing the maturation of dendritic cells. Int. Immunopharmacol. 2018, 55, 133–141. [Google Scholar] [CrossRef]
- Baumann, A.; Démoulins, T.; Python, S.; Summerfield, A. Porcine cathelicidins efficiently complex and deliver nucleic acids to plasmacytoid dendritic cells and can thereby mediate bacteria-induced IFN-α responses. J. Immunol. 2014, 193, 364–371. [Google Scholar] [CrossRef]
- Pfalzgraff, A.; Heinbockel, L.; Su, Q.; Gutsmann, T.; Brandenburg, K.; Weindl, G. Synthetic antimicrobial and LPS-neutralising peptides suppress inflammatory and immune responses in skin cells and promote keratinocyte migration. Sci. Rep. 2016, 6, 31577. [Google Scholar] [CrossRef]
- Xu, W.; Liu, H.; Wang, X.; Yang, Q. Surfactin induces maturation of dendritic cells in vitro. Biosci. Rep. 2016, 36, 387. [Google Scholar] [CrossRef]
- Skerra, J.; Islam, H.; Wachtmeister, T.; Alter, C.; Huang, A.; Bhatia, S.; Köhrer, K.; Kirschning, C.; Weighardt, H.; Kalinke, U.; et al. The mycotoxin beauvericin exhibits immunostimulatory effects on dendritic cells via activating the TLR4 signaling pathway. Front. Immunol. 2022, 13, 856230. [Google Scholar]
- Xie, F.; Zan, Y.; Zhang, X.; Zhang, H.; Jin, M.; Zhang, W.; Zhang, Y.; Liu, S. Differential Abilities of Mammalian Cathelicidins to Inhibit Bacterial Biofilm Formation and Promote Multifaceted Immune Functions of Neutrophils. Int. J. Mol. Sci. 2020, 21, 1871. [Google Scholar] [CrossRef] [PubMed]
- Barlow, P.G.; Li, Y.; Wilkinson, T.S.; Bowdish, D.M.; Lau, Y.E.; Cosseau, C.; Haslett, C.; Simpson, A.J.; Hancock, R.E.; Davidson, D.J. The human cationic host defense peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J. Leukoc. Biol. 2006, 80, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Berends, E.T.; Nerlich, A.; Molhoek, E.M.; Gallo, R.L.; Meerloo, T.; Nizet, V.; Naim, H.Y.; von Köckritz-Blickwede, M. The antimicrobial peptide LL-37 facilitates the formation of neutrophil extracellular traps. Biochem. J. 2014, 464, 3–11. [Google Scholar] [CrossRef]
- Neumann, A.; Völlger, L.; Berends, E.T.; Molhoek, E.M.; Stapels, D.A.; Midon, M.; Friães, A.; Pingoud, A.; Rooijakkers, S.H.; Gallo, R.L.; et al. Novel role of the antimicrobial peptide LL-37 in the protection of neutrophil extracellular traps against degradation by bacterial nucleases. J. Innate. Immun. 2014, 6, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Mutua, V.; Gershwin, L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin. Rev. Allergy Immunol. 2021, 61, 194–211. [Google Scholar] [CrossRef]
- Zheng, Y.; Niyonsaba, F.; Ushio, H.; Nagaoka, I.; Ikeda, S.; Okumura, K.; Ogawa, H. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br. J. Dermatol. 2007, 157, 1124–1131. [Google Scholar] [CrossRef]
- Alalwani, S.M.; Sierigk, J.; Herr, C.; Pinkenburg, O.; Gallo, R.; Vogelmeier, C.; Bals, R. The antimicrobial peptide LL-37 modulates the inflammatory and host defense response of human neutrophils. Eur. J. Immunol. 2010, 40, 1118–1126. [Google Scholar] [CrossRef]
- Hosoda, H.; Nakamura, K.; Hu, Z.; Tamura, H.; Reich, J.; Kuwahara-Arai, K.; Iba, T.; Tabe, Y.; Nagaoaka, I. Antimicrobial cathelicidin peptide LL-37 induces NET formation and suppresses the inflammatory response in a mouse septic model. Mol. Med. Rep. 2017, 16, 5618–5626. [Google Scholar] [CrossRef]
- Nagaoka, I.; Suzuki, K.; Niyonsaba, F.; Tamura, H.; Hirata, M. Modulation of neutrophil apoptosis by antimicrobial peptides. ISRN Microbiol. 2012, 2012, 345791. [Google Scholar] [CrossRef]
- Kraemer, B.F.; Campbell, R.A.; Schwertz, H.; Cody, M.J.; Franks, Z.; Tolley, N.D.; Kahr, W.H.; Lindemann, S.; Seizer, P.; Yost, C.C.; et al. Novel anti-bacterial activities of β-defensin 1 in human platelets: Suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 2011, 7, 1002355. [Google Scholar] [CrossRef]
- Milad, N.; Pineault, M.; Bouffard, G.; Maranda-Robitaille, M.; Lechasseur, A.; Beaulieu, M.J.; Aubin, S.; Jensen, B.A.H.; Morissette, M.C. Recombinant human β-defensin 2 delivery improves smoking-induced lung neutrophilia and bacterial exacerbation. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2022, 323, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Kim, H.S.; Lee, H.Y.; Hwang, J.S.; Bae, Y.S. A novel antimicrobial peptide isolated from centipede Scolopendra subspinipes mutilans stimulates neutrophil activity through formyl peptide receptor 2. Biochem. Biophys. Res. Commun. 2017, 494, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Park, B.; Lee, M.; Jeong, Y.S.; Lee, H.Y.; Sohn, D.H.; Song, J.J.; Lee, J.H.; Hwang, J.S.; Bae, Y.S. A novel antimicrobial peptide acting via formyl peptide receptor 2 shows therapeutic effects against rheumatoid arthritis. Sci. Rep. 2018, 8, 14664. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Chen, L.; Guang, H.; Li, Z.; Yang, H.; Li, J.; You, D.; Yu, H.; Lai, R. Cathelicidin-BF, a snake cathelicidin-derived antimicrobial peptide, could be an excellent therapeutic agent for acne vulgaris. PLoS ONE 2011, 6, 22120. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qi, J.; Shan, B.; Gao, R.; Gao, F.; Xie, H.; Yuan, M.; Liu, H.; Jin, S.; Wu, F.; et al. Pretreatment with cathelicidin-BF ameliorates Pseudomonas aeruginosa pneumonia in mice by enhancing NETosis and the autophagy of recruited neutrophils and macrophages. Int. Immunopharmacol. 2018, 65, 382–391. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, Z.; Yang, P.; Wei, L.; Xu, W. Antimicrobial peptide cathelicidin-BF derived from Bungarus fasciatus enhances resistance of mice to S. aureus infection via activating innate immunity. J. Curr. Immunol. 2018, 38, 449–455. [Google Scholar]
- Ma, J.; Chen, J.; Xue, K.; Yu, C.; Dang, E.; Qiao, H.; Fang, H.; Pang, B.; Li, Q.; Sun, Z.; et al. LCN2 Mediates Skin Inflammation in Psoriasis through the SREBP2-NLRC4 Axis. J. Investig. Dermatol. 2022, 142, 2194–2204. [Google Scholar] [CrossRef]
- Williams, R.L.; Sroussi, H.Y.; Leung, K.; Marucha, P.T. Antimicrobial decapeptide KSL-W enhances neutrophil chemotaxis and function. Peptides 2012, 33, 1–8. [Google Scholar] [CrossRef]
- Woloszynek, J.C.; Hu, Y.; Pham, C.T. Cathepsin G-regulated release of formyl peptide receptor agonists modulate neutrophil effector functions. J. Biol. Chem. 2012, 287, 34101–34109. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Du, M.; Liu, N.; Shan, Q.; Zou, Y.; Wang, J.; Zhu, Y. A Novel β-Hairpin Peptide Z-d14CFR Enhances Multidrug-Resistant Bacterial Clearance in a Murine Model of Mastitis. Int. J. Mol. Sci. 2022, 23, 4617. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Zhang, J.; Zhai, T.; Hu, J.; Luo, H.; Zhou, H.; Zhang, Q.; Zhou, Z.; Liu, F. Cathelicidin aggravates myocardial ischemia/reperfusion injury via activating TLR4 signaling and P2X7R/NLRP3 inflammasome. J. Mol. Cell. Cardiol. 2020, 139, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Tjabringa, G.S.; Ninaber, D.K.; Drijfhout, J.W.; Rabe, K.F.; Hiemstra, P.S. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int. Arch. Allergy Immunol. 2006, 140, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Q.; Cheng, L. The role of inflammatory release from human eosinophils-induced by the antimicrobial peptide LL-37 in the pathogenesis of asthma. J. Med. Postgrad. 2017, 30, 70–76. [Google Scholar]
- Sun, J.; Dahlén, B.; Agerberth, B.; Haeggström, J.Z. The antimicrobial peptide LL-37 induces synthesis and release of cysteinyl leukotrienes from human eosinophils--implications for asthma. Allergy 2013, 68, 304–311. [Google Scholar] [CrossRef]
- Jiao, D.; Wong, C.K.; Tsang, M.S.; Chu, I.M.; Liu, D.; Zhu, J.; Chu, M.; Lam, C.W. Activation of Eosinophils Interacting with Bronchial Epithelial Cells by Antimicrobial Peptide LL-37: Implications in Allergic Asthma. Sci. Rep. 2017, 7, 1848. [Google Scholar] [CrossRef] [PubMed]
- Ilangala, A.B.; Lechanteur, A.; Fillet, M.; Piel, G. Therapeutic peptides for chemotherapy: Trends and challenges for advanced delivery systems. Eur. J. Pharm. Biopharm. 2021, 167, 140–158. [Google Scholar]
- Henninot, A.; Collins, J.C.; Nuss, J.M. The current state of peptide drug discovery: Back to the future? J. Med. Chem. 2018, 61, 1382–1414. [Google Scholar] [CrossRef] [PubMed]
- Muller, H.; Salzig, D.; Czermak, P. Considerations for the process development of insect-derived antimicrobial peptide production. Biotechnol. Prog. 2015, 31, 1–11. [Google Scholar]
- Kasser, L.; Rotter, M.; Coletta, L.; Salzig, D.; Czermak, P. Process intensification for the continuous production of an antimicrobial peptide in stably-transformed Sf-9 insect cells. Sci. Rep. 2022, 12, 1086. [Google Scholar] [CrossRef]
- Roldan-Tapia, M.; Anne, J.; Reyes, A.G.; Carrasco, U.; Millan-Pacheco, C.; Barrios-Gonzalez, J.; Mejia, A. Streptomyces as overexpression system for heterologous production of an antimicrobial peptide. Protein Pept. Lett. 2017, 24, 483–488. [Google Scholar] [CrossRef]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.M.; Bechinger, B.; Naas, T. Antimicrobial peptides: A potent alternative to antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [PubMed]
- Qi, L.; Jiang, N.; Zhang, A.; Quan, J.; Song, L.; Zhang, W. Research strategy of antimicrobial peptides. China Anim. Husb. Vet. Med. 2016, 43, 450–456. [Google Scholar]
- Zheng, R.; Yao, B.; Yu, H.; Wang, H.; Bian, J.; Feng, F. Novel family of antimicrobial peptides from the skin of Rana shuchinae. Peptides 2010, 31, 1674–1677. [Google Scholar]
- Dathe, M.; Wieprecht, T. Structural features of helical antimicrobial peptides: Their potential to modulate activity on model membranes and biological cells. Biochim. Biophys. Acta 1999, 1462, 71–87. [Google Scholar] [PubMed]
- Mingfu, N.; Qiang, G.; Yang, L.; Ying, H.; Chengshui, L.; Cuili, Q. The antimicrobial peptide MetchnikowinII enhances Ptfa antigen immune responses against avian Pasteurella multocida in chickens. J. Vet. Med. Sci. 2023. ahead of print. [Google Scholar] [CrossRef]
- Shi, S.; Dong, H.; Chen, X.; Xu, S.; Song, Y.; Li, M.; Yan, Z.; Wang, X.; Niu, M.; Zhang, M.; et al. Sustained release of alginate hydrogel containing antimicrobial peptide Chol-37(F34-R) in vitro and its effect on wound healing in murine model of Pseudomonas aeruginosa infection. J. Vet. Sci. 2023, 24, e44. [Google Scholar] [PubMed]
- Shi, S.; Shen, T.; Liu, Y.; Chen, L.; Wang, C.; Liao, C. Porcine Myeloid Antimicrobial Peptides: A Review of the Activity and Latest Advances. Front. Vet. Sci. 2021, 8, 664139. [Google Scholar]
- Shen, T.; Chen, L.; Liu, Y.; Shi, S.; Liu, Z.; Cai, K.; Liao, C.; Wang, C. Decanoic acid modification enhances the antibacterial activity of PMAP-23RI-Dec. Eur. J. Pharm. Sci. 2021, 157, 105609. [Google Scholar]
- Liu, Y.; Shi, S.; Shen, T.; Chen, L.; Liao, C.; Wang, C. Sequence design and structural optimization of porcine myeloid antimicrobial peptide PMAP-36: A review. Microbiol. China 2021, 48, 1331–1339. [Google Scholar]
- Chen, L.; Shen, T.; Liu, Y.; Zhou, J.; Shi, S.; Wang, Y.; Zhao, Z.; Yan, Z.; Liao, C.; Wang, C. Enhancing the antibacterial activity of antimicrobial peptide PMAP-37(F34-R) by cholesterol modification. BMC Vet. Res. 2020, 16, 419. [Google Scholar]
- Liu, Y.; Li, S.; Shen, T.; Chen, L.; Zhou, J.; Shi, S.; Wang, Y.; Zhao, Z.; Liao, C.; Wang, C. N-terminal Myristoylation Enhanced the Antimicrobial Activity of Antimicrobial Peptide PMAP-36PW. Front. Cell. Infect. Microbiol. 2020, 10, 450. [Google Scholar]
- Zhou, J.; Liu, Y.; Shen, T.; Chen, L.; Zhang, C.; Cai, K.; Liao, C.; Wang, C. Antimicrobial activity of the antibacterial peptide PMAP-36 and its analogues. Microb. Pathog. 2019, 136, 103712. [Google Scholar] [PubMed]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned from Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [PubMed]
- Zhou, J.; Liu, Y.; Shen, T.; Chen, L.; Zhang, C.; Cai, K.; Liu, Z.; Meng, X.; Zhang, L.; Liao, C.; et al. Enhancing the antibacterial activity of PMAP-37 by increasing its hydrophobicity. Chem. Biol. Drug Des. 2019, 94, 1986–1999. [Google Scholar] [PubMed]





| Peptide Name | Source | Amino Acid Sequence | Inflammatory Mediator | Signaling Pathway | Functions | Ref. |
|---|---|---|---|---|---|---|
| BSN-37 | Bovine | FRPPIRRPPIRPPFYPPFRPPIRPPIFPPIRPPFRPP | IL-2, IFN-γ, IL-4, and IL-10 ↑ | - | Promote expression of CD40/80, MHC Ⅰ and MHC Ⅱ | [13] |
| PR-39 | Porcine | RRRPRPPYLPRPRPPPFFPPRLPPRIPPGFPPRFPPRFP | - | - | Inhibit apoptosis and reduce caspase-3 activity, enhance the ability to kill and clear intracellular bacteria | [14,15] |
| CAP11 | Guinea pig | GLRKKFRKTRKRIQKLGRKIGKTGRKVWKAWREYGQIPYPCRI | - | - | Inhibit the binding of LPS, release of HMGB1, and the death of necrotic cells | [16] |
| LL-37 | Human | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | IL-8, MCP-1, LTB4, and COX-2 ↑; MIP-1α, IL-12, and IL-1β ↓ | Promote ERK1/2 and MAPK p38 | Promote proliferation and phagocytosis, neutralize LPS and inhibit the response of P2X7R to ATP, inhibit activation of caspase-1 and pyroptosis | [17,18,19,20,21,22,23] |
| HBD2 | Human | GIGDPVTCLKSGAICHPVFCPRRYKQIGTCGLPGTKCCKKP | INF-β, IFN-γ, IL-6, IL-1β, and TNF-α ↑ | - | - | [24] |
| HBD3 | Human | GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK | TNF-α and IL-6 ↓ | Inhibit MAPK p38 and ERK1/2 | - | [25] |
| PBD-2 | Porcine | DHYICAKKGGTCNFSPCPLFNRIEGTCYSGKAKCCIR | IL-12, IL-6, IL-1β, TNF-α, and NO ↓ | Inhibit NF-κB and Akt | - | [26,27] |
| DEFB123 | Human | GTQRCWNLYGKCRYRCSKKERVYVYCINNKMCCVKPK | TNF-α ↓ | Inhibit MAPK | - | [28] |
| HBD130 | Human | GVIPGQKQCIALKGVCRDKLCSTLDDTIGICNEGKKCCRRWWILEPYPTPVPKGKSP | NO, IL-1β, IL-6, and TNF-α ↓ | - | - | [29] |
| Indolicidin | Bovine | ILPWKWPWWPWRR-NH2 | NO and iNOS ↓ | - | - | [30] |
| JH-3 | Bovine | RRFKLLSHSLLVTLASHL | IL-2, IL-6, TNF-α, LDH, and TNF-Rα2 ↓ | Inhibit MAPK p38 | Inhibit activation of caspase-3 and caspase-8, reduce macrophages apoptosis induced by Salmonella, inhibit release of cytochrome C in the cytoplasm and the expression of caspase-8/9 | [31,32] |
| BMAP-18 | Bovine | GRFKRFRKKFKKLFKKLS-NH2 | NO and TNF-α ↓ | - | - | [33] |
| Bac5 | Bovine | RFRPPIRRPPIRPPFYPPFRPPIRPPIFPPIRPPFRPPLGPF | TNF-α ↑ | - | Activate macrophage-like THP-1 cells | [34] |
| Bovine lactoferricin | Bovine | NH2PHELYSCYSARGARGTRPGLNTRPARGMETLYSLYSLEUGLYALAPROSERILETHRCYSVALARGARGALAPHECOOH | TNF-α, IL-6, NO, and iNOS ↓ | Inhibit NF-κB and MAPK | - | [35] |
| HLF1-11 | Human | GRRRRSVQWCA | IL-10 ↑ | - | Promote monocyte differentiation into macrophages driven by GM-CSF | [36] |
| HEPC1 | Musculus | QSHLSMCRYCCNCCRNNKGCGFCCKF | - | - | Promote iron storage and inhibit iron release of Ana.1 and RAW264.7, and promote iron retention | [37] |
| HEPC2 | Musculus | NPAGCRFCCGCCPNMIGCGVCCRF | - | - | Promote iron storage and inhibit iron release of Ana.1 and RAW264.7, and promote iron retention | [38] |
| CHensinin-1b | Frog | SKVWRHWRRFWHRAHRKL | TNF-α, IL-6, IL-1β, and NO ↓; IL-10 and TGF-β1 ↑ | Inhibit NF-κB and MAPK | - | [39] |
| W3R6 | Frog | VWRRWRRFWRR | TNF-α, IL-6, IL-1β, and NO ↓; IL-10 and TGF-β1 ↑ | Inhibit NF-κB and MAPK | - | [39] |
| Temporin-1CEa | Frog | FVDLKKIANIINSIFGK-NH2 | TNF-α and IL-6 ↓ | Inhibit NF-κB and MAPK | - | [40] |
| LK2 (6) | Frog | FVKLKKIANIINSIFKK-NH2 | TNF-α and IL-6 ↓ | Inhibit NF-κB and MAPK | - | [40] |
| LK2 (6) A (L) | Frog | FVKLKKILNIINSIFKK-NH2 | TNF-α and IL-6 ↓ | Inhibit NF-κB and MAPK | - | [40] |
| PN-CATH1 | Frog | KKCNFFCKLKKKVKSVGSRNLIGSATHHHRIYRV | IL-6, IL-1β, and TNF-α ↓ | - | - | [41] |
| PN-CATH2 | Frog | EGCNILCLLKRKVKAVKNVVKNVVKSVVG | IL-6, IL-1β, and TNF-α ↓ | - | - | [41] |
| Temporin-1TI | Frog | FVQWFSKFLGRIL-NH2 | TNF-α, NO, and iNOS ↓ | - | - | [42] |
| OL-CATH2 | Frog | RKCNFLCKVKNKLKSVGSKSLIGSATHHGIYRV | TNF-α, IL-1β, and IL-6 ↓ | - | - | [43] |
| HR-CATH | Frog | ASKKGKCNLLCKLKQKLRSVGAGTHIGSVVLKG | - | - | Induce macrophage chemotaxis and enhance respiratory burst | [44] |
| Nv-CATH | Frog | NCNFLCKVKQRLRSVSSSHIGMAIPRPRG | NO, IL-6, TNF-α, and IL-1β ↓; CXCL1/2 and CCL2 ↑ | Inhibit NF-κB-NLRP3 and MAPK | - | [45] |
| TK-CATH | Salamander | GGQDTGKEGETGKKKKSDNWFMNLLNKFLELIGLKEAGDDSEPFCFTCIFDMFSQ | TNF-α, IL-6, and MCP-1 ↓ | Inhibit MAPK p38 | - | [46] |
| CLP-19 | Limulus | CRKPTFRRLKWKIKFKFKC | TNF-α ↓ | - | - | [47] |
| Mt6 | Housefly | KKFKKTAKWLIKSAWLLLKSLALKMK | IL-1β and TNF-α ↓ | Inhibit MAPK | - | [48] |
| D-Mt6 | Housefly | KKFKKTAKWLIKSAWLLLKSLALKMK | IL-1β and TNF-α ↓ | Inhibit MAPK | - | [48] |
| HI-3 | Hermetia illucens | - | IL-6, TNF-α, IL-1β, and NO ↓; IL-10 ↑ | Inhibit NF-κB | Enhance the phagocytosis and inhibit LPS-induced differentiation of RAW264.7, increase the superoxide dismutase activity and total antioxidant capacit | [49,50] |
| CEMA | Insect | KWKLFKKIGIGAVLKVLTTGLPALKLTK | IL-1β, IL-6, TNF-α, MIP-1α, and MIP-1β ↓ | - | Induce expression of gene-involved in cell adhesion and apoptosis | [51] |
| Peptide A | Beetle | RLYLRIGRR-NH2 | TNF-α and NO ↓ | Inhibit NF-κB | - | [52] |
| Peptide B | Beetle | RLRLRIGRR-NH2 | TNF-α ↓ | - | - | [52] |
| Defensin 1 | Beetle | YPLDQVEEQDEHQVAHIRVRRVTCDLLSAEAKGVKVNHAACAAHCLLKRKRGGYCNKRRICVCRN | IL-1β, IL-6, IL-8, IL-10, IL-12p70, IL-23, and TNF-α ↓ | - | - | [53] |
| CopA5 | Dung beetle | LLCIA | NO and TNF-α ↓ | Inhibit STAT1 | Inhibit the phagocytic activity of PEM | [54] |
| ToAP3 | Brazilian scorpion | FIGMIPGLIGGLISAIK | TNF-α and IL-1β ↓; IL-10 ↑ | - | Decrease co-stimulatory molecules (CD80 and CD86) | [55] |
| ToAP4 | Brazilian scorpion | MQIKHLITLFFLVLIVADQCSAFFSLIPSLIGGLVSAIKGGRRKREIAAQIEQYRDLQKREAELEELLDRLPMF | TNF-α and IL-1β ↓; IL-10 ↑ | - | Increase expression of MHC Ⅱ | [55] |
| FA1 | Scorpion | - | IL-10, IL-12p70, and TNF-α ↑ | - | Activate phagocytic activity | [56] |
| CM4 | Bombyx | RWKIFKKIEKVGQNIRDGIVKAGPAVAVVGQAATI | TNF-α, NO, and IL-6 ↓ | - | - | [57] |
| Cecropin DH | Chinese oak silk moth | KWKIFKKIEKVGRNIRNGIIKAVAVLGEAKAL | NO and TNF-α ↓ | - | - | [58] |
| Papiliocin | Swallowtail butterfly | RWKIFKKIEKVGRNVRDGIIKAGPAVAVVGQAATVVK-NH2 | NO, TNF-α, and MIP-2 ↓ | Inhibit NF-κB and TLR4 | - | [59] |
| Periplanetasin-5 | Cockroach | MKTFLRLYRSLINKVLH | TNF-α and IL-6 ↓ | Inhibit NF-κB and MAPKs | - | [60] |
| AK-N’ | Spider | FKGLAKLLKIGLKALAKVIQ | IL-6, IL-1β, and TNF-α ↓ | - | - | [61] |
| AK-N’ m | Spider | NKGLAKLLKIGLKALESVIQ | IL-6, IL-1β, and TNF-α ↓ | Inhibit TLR4 | - | [61] |
| KN-17 | Tianchan pupa | KWKVFKKIEKMGRNIRN | iNOS, TNF-α, and IL-1α ↓; Arg1 and TGF-β ↑ | Inhibit NF-κB | Enhance RAW264.7 to transform from M1 to M2 | [62] |
| PN5 | Pine needle | FKFLARTGKFL | IL-6 and TNF-α ↓ | Inhibit NF-κB and MAPKs | - | [63] |
| Sublancin | Bacillus subtilis 168 | GLGKAQCAALWLQCASGGTIGCGGGAVACQNYRQFCR | IL-1β, IL-6, TNF-α, and NO ↑ | Promote NF-κB, MAPK, and TLR4 | Enhance the phagocytosis and killing activity of RAW264.7 and mouse peritoneal macrophages against methicillin-resistant Staphylococcus aureus | [64] |
| CSP32 | Bacillus spp. | APLEXXIFHDN | NO, TNF-α, IL-1β, MCP-1, and PGE2 ↑ | Promote NF-κB and MAPK | Stimulate phagocytosis, induce the appearance of M1 type macrophages, increase the number of Ca2+ positive macrophages, upregulate phospholipase C and activate protein kinase Cε | [65] |
| MS15 | Bacillus | - | ROS and NO ↓ | - | Increase translation and transcription levels of catalase, glutathione peroxidase, and superoxide dismutase | [66] |
| Macrolactin | Bacillus subtilis | - | iNOS, COX-2 and IL-6 ↓ | - | - | [67,68] |
| Surfactin | Bacillus subtilis | ELIVDIL | - | Inhibit NF-κB, p38, JNK and Akt | Impair the antigen delivery function of macrophages | [69] |
| OMS A | Marine bacteria | VVTTVLVVWVFV | TNF-α, IL-1β, CCL5, IL-12p40, and iNOS ↑ | - | Downregulate arginase-1 expression | [70] |
| BacSp222 | Staphylococcus | - | NO, TNF-α, IFN-β, IL-1α, IL-10, IL-27 and MCP-1 ↑ | Promote NF-κB | - | [71] |
| BEA | Fungi | - | NO ↓ | Inhibit NF-κB | - | [72] |
| EnnB | Fungi | - | IL-1β ↑ | - | Caused a G0/G1-arrest, M2-like macrophage differentiation, apoptosis and necrosis | [73] |
| CATH-2 | Chicken | RFGRFLRKIRRFRPKVTITIQGSARF-NH2 | IL-6 and IL-1β ↓ | Inhibit TLR2 and TLR4 | - | [74] |
| CATH-B1 | Chicken | PITYLDAILAAVRLLNQRISGPCILRLREAQPRPGWVGTLQRRREVSFLVEDGPCPPGVDCRSCEPGALQHCVGTVSIEQ | IFN-β, IL-1β, IL-6, and IL-8 ↓; IL-10 ↑ | Inhibit TLR4 | Enhance phagocytosis | [75] |
| Fowlicidin-1(6–26) | Chicken | WPLVIRTVIAGYNLYRAIKKK-NH2 | IL-1β, CCL2, and CCL3 ↑ | - | Enhance the surface expression of MHC Ⅱ and CD86 on RAW264.7 | [76] |
| CNK-2 | Chicken | RRQRSICKQLLKKLRQQLSDALQNNDD | IL-1β ↓; CCL4 ↑ | Promote MAPK | - | [77] |
| DCATH 12-4 | Duck | LIKKIYRKWKRW-NH2 | NO, TNF-α, and iNOS ↓ | - | - | [78] |
| DCATH 12-5 | Duck | LWKKIYRKWKRW-NH2 | NO, TNF-α, and iNOS ↓ | - | - | [78] |
| AvBD8 | Chicken | MKILYFLLAVLLTVLQSSLGFMRVPNNEAQCEQAGGICSKDHCFHLHTRAFGHCQRGVPCCRTVYD | IL-1β, INF-γ, IL-12p40, CCL4, CXCL13, and CCL20 ↑ | Promote MAPK | - | [79] |
| Hep20 | Rainbow trout | ICIFCCGCCHRSKCGMCCKT | IL-10, IL-1β, and TNF-α ↑ | - | - | [80] |
| TP4 | Nile tilapia | H-FIHHIIGGLFSAGKAIHRLIRRRRR-OH | NO, TNF-α, IL-1β, and IL-6 ↓ | Promote MAPK, ERK, and IL-10-STAT3 | Enrich markers of M2 macrophages | [81] |
| EPI | Orange-spotted grouper | H-GFIFHIIKGLFHAGKMIHGLV-OH | IL-6, COX-2, iNOS, TNF-α, and ROS ↓ | Inhibit Akt and NF-κB | Reduce the cytotoxicity of RAW264.7 induced by LPS | [82] |
| Epi-1 | Orange spotted grouper | GFIFHIIKGLFHAGKMIHGLV | TNF-α, IL-6, and IL-1β ↓; TGF-β and Sytx1 ↑ | - | Induce expression of MHC related genes | [83] |
| Scolopendrasin VII | Scolopendra subspinipes mutilans | FCTCNVKGFNAKNKRGIIYP-NH2 | - | Promote ERK and Akt | Stimulate actin polymerization and the chemotactic migration of macrophages | [84] |
| Pardaxin | Marine fish species | GFFALIPKIISSPLFKTLLSAVGSALSSSGGQE | - | Promote MyD88 | Induce THP-1 and U937 cells to differentiate into macrophages with phagocytic ability, increase expression of MyD88, and reduce the phagocytic ability and superoxide anion production of leukemia cells | [85] |
| LjP-3 | Japanese sea bass | FFGMLIHGAIHAGKVIHJLIHG | IL-1β, TNF-α, and TGF-β ↓ | - | Promote macrophage chemotaxis and phagocytosis | [86] |
| LjP-2 | Japanese sea bass | FLKSIWRAAKGAIRGAKSGWRA | IL-1β, TNF-α, and TGF-β ↓ | - | Promote macrophage chemotaxis and phagocytosis | [87] |
| NKHs27 | Sevenband grouper | KLTSKLKSICDQIGLLKALCRKSVKTH | - | - | Enhance the respiratory burst and upregulate immune-related genes expression | [87] |
| CNMs | Synthesis | - | TNF-α, IL-6, IL-8, IL-1β, and NO ↓ | Inhibit NF-κB, MAPK, and TLR4 | - | [88] |
| LF-KR | Synthesis | RRWQWRPKRIVKLIKKWLR-NH2 | NO and TNF-α ↓ | - | - | [89] |
| PapMA | Synthesis | RWKIFKKIPKFLHSAKKF-NH2 | NO, TNF-α, IL-6, IL-1β, and MIP-1/2 ↓ | - | - | [90] |
| KR-1 | Synthesis | KKKKKKRAFARWRAFAR | iNOS, TNF-α, and IL-1β ↓ | Inhibit NF-κB | Increase the percentage of M2 phenotype in macrophages | [91] |
| KR-2 | Synthesis | KKKKKKRRFRRWRRFRR | iNOS, TNF-α, and IL-1β ↓ | Inhibit NF-κB | Increase the percentage of M2 phenotype in macrophages | [91] |
| P18 | Synthesis | KWKLFKKIPKFLHLAKKF-NH2 | iNOS, IL-1β, TNF-α, and NO↓ | - | - | [92] |
| P18-W6 | Synthesis | KKKLFWKIPKFLHLAKKF-NH2 | NO ↓ | - | - | [93] |
| P18-W8 | Synthesis | KIKLFKKWPKFLHLAKKF-NH2 | NO ↓ | - | - | [93] |
| P18-Nala9 | Synthesis | KWKLFKKIaKFLHLAKKF-NH2 | NO ↓ | - | - | [93] |
| GL13K | Synthesis | GL13KGKIIKLKASLKLLCONH2 | M1: TNF-α and IL-1β ↓; M2: IL-10 and TGF-β3 ↑ | - | Inhibit proliferation of M1 type macrophages | [94,95] |
| KLW-f | Synthesis | KWKKLLKKfLKKLKKLLK-NH2 | NO ↓ | - | - | [96] |
| KLK | Synthesis | KLKLLLLLKLK | NO, TNF-α, iNOS, COX-2, IL-1β, and PGE2 ↓ | Inhibit NF-κB | - | [97] |
| Kn2-7 | Synthesis | FIKRIARLLRKIF | IL-10, TNF-α ↑ | - | Enhance the uptake of CpG DNA | [98] |
| K9L8W | Synthesis | KLKKLLKKWLKLLKKLLK-NH2 | NO, TNF-α, and iNOS ↓ | - | - | [99] |
| Lf6-pP | Synthesis | RRWQWRpPRWQWRR-NH2 | TNF-α and IL-6 ↓ | - | - | [100] |
| KLA-NNPs | Synthesis | KLAKLAKKLAKLAK | - | - | Decrease phagocytosis and reduce the activity of intracellular bacteria and caspase-1 | [101] |
| WK2 | Synthesis | (WK)2CTKSGC(KW)2 | iNOS, IL-8, IL-4, IL-1β, and TNF-α ↓ | - | - | [102] |
| LL37-C15 | Synthesis | AGEDPHGYFLPGQFA-GG-LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | TNF-α, IL-1β, iNOS, and IL-6 ↓ | - | - | [103] |
| CC34 | Synthesis | GWLKKIGKKIERVGQHTRDAILPILSLIGGLLGK | TNF-α, IL-1β, IL-6, ROS, iNOS, and COX-2 ↓ | Inhibit NF-κB | - | [104] |
| GW-A2 | Synthesis | GAKYAKIIYNYLKKIANALW | NO, iNOS, COX-2, TNF-α, and IL-6 ↓ | Inhibit NF-κB and MAPK | - | [105] |
| HPRP-A1/A2 | Synthesis | Ac-FKKLKKLFSKLWNWK-amide 15 mer | - | - | Reduce the survival ability of tachyzoites and adherence and invasion in macrophages, disrupt the integrity of the tachyzoite membrane | [106] |
| Cbf-14 | Cathelin-like domain | RLLLRKFFRKLKKSV | ROS, NO, and iNOS ↓ | Inhibit NF-κB, PI3K, and MAPK | - | [107] |
| WALK11.3 | Antimicrobial model peptide | LKWLKKLLKKL-NH2 | NO, COX-2, IL-1β, IL-6, INF-β, and TNF-α ↓ | Inhibit TLR4 | - | [108] |
| WALK244.04 | Antimicrobial model peptide | LLKWLKKKWLK-NH2 | iNOS, COX-2, and IL-1β ↓ | Inhibit TRIF | - | [109] |
| WALK243.04 | Antimicrobial model peptide | LLKWLKKWL-NH2 | iNOS, COX-2, and IL-1β ↓ | Inhibit TRIF | - | [109] |
| KAMPs | Keratin 6a | - | IL-6, TNF-α, CXCL1, and CXCL10 ↓ | Inhibit NF-κB, IRF3 | Reduce cell surface availability of TLR2 and TLR4 | [110] |
| Peptide Name | Source | Amino Acid Sequence | Inflammatory Mediator | Signaling Pathway | Functions | Ref. |
|---|---|---|---|---|---|---|
| LL-37 | Human | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | IFN-γ ↑ | - | - | [122,123,124] |
| HBD1 | Human | DHYNCVSSGGQCLYSACPIFTKIQGTCYRGKAKCCK | - | - | Promote differentiation of monocyte derived from Cord blood of human newborns into immature DCs and maturation of monocyte sderived DCs | [126] |
| HBD3 | Human | GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK | - | Promote TLR1/2 and MyD88 | Induce activation of monocyte and expression of CD80, CD86, and CD40 on monocytes | [127,128] |
| LEAP-2 | Boleophthamus pectinirostris | MTPLWRILNSKPFGAYCQNNYECSTGLCRAGFCATMHRSATVSVTN | TNF-α and IL-1β ↓ | - | Enhance respiratory burst and bactericidal ability | [129] |
| BpNKLP40 | Boleophthamus pectinirostris | SIKAKLLAVCKNIGLLKSLCQKFVNKHLGVLIEELTTTDD | TNF-α, IL-1β, and IFN-γ ↑ TGF-β and IL-10 ↓ | - | - | [130] |
| CXCL14-C17-a2 | Synthesis | KRFIKWYKAWNKKWRKY-NH2 | NO, TNF-α, IL-6, and MCP-1 ↓ | - | - | [131] |
| CXCL14-C17-a3 | Synthesis | KRFKKWYKAWRKKWRKY-NH2 | NO, TNF-α, IL-6, and MCP-1 ↓ | - | - | [131] |
| Peptide Name | Source | Amino Acid Sequence | Inflammatory Mediator | Signaling Pathway | Functions | Ref. |
|---|---|---|---|---|---|---|
| LL37 | Human | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | - | - | Enhance delivery of CTLs and Tregs cells and induce apoptosis | [132,133,134] |
| HBD2 | Human | GIGDPVTCLKSGAICHPVFCPRRYKQIGTCGLPGTKCCKKP | - | - | Promote CD4+ T cell proliferation | [135] |
| HBD3 | Human | GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK | IL-2 and IL-10 ↑ | Inhibit STAT1 | Promote CD4+ T cells proliferation, activate T cells and enhance T cells effect function | [135,136] |
| BSN-37 | Bovine | FRPPIRRPPIRPPFYPPFRPPIRPPIFPPIRPPFRPP | IL-2, IL-10, and IL-6 ↑ | - | Enhance proliferation and activation ability of mouse spleen lymphocytes | [13] |
| Frenatin 2.1S | Loach | GLVGTLLGHIGKAILG-NH2 | - | - | Enhance activation and homing ability of Th1 cells and NKT cell in the abdominal cavity of mice and expression of MHC II molecules on macrophages, increase the percentage of M1 macrophages | [140] |
| Cecropin | Tianchan pupa | GWLLKLGKRIERIGQHTRDATIQGLGIAQQAANVAATAR-NH2 | - | - | Increase the number of lymphocytes in the jejunal epithelium | [141,142,143] |
| Immunopeptide | Silkworm chrysalis | DHAV | IL-12 and IL-6 ↑ | Promote NF-κB | Promote the transformation of mouse Th cells to Th1 type | [144] |
| Sublancin | Bacillus subtilis | GLGKAQCAALWLQCASGGTIGCGGGAVACQNYRQFCR | - | - | Increase the ratio of CD4+/CD8+ in splenocyte | [145] |
| Taikanglibao | Synthesis | - | - | - | Improve the spleen index of weaned piglets, promote lymphocyte proliferation and reduce lymphocyte apoptosis | [146] |
| LF-6 | Recombination | KWRQWQSKWRRTNPWFWIRR | IL-1, IL-2 and IL-6 ↓ | - | - | [147] |
| Peptide Name | Source | Amino Acid Sequence | Inflammatory Mediator | Signaling Pathway | Functions | Ref. |
|---|---|---|---|---|---|---|
| LL-37 | Human | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | IL-4, IL-5, and IL-1β ↑ | Promote MAPKs, P13K, and Akt | Activate MrgX2-induced degranulation of human mast cells and release of de novo synthesized mediators | [152,153,154,155] |
| HBD2 | Human | GIGDPVTCLKSGAICHPVFCPRRYKQIGTCGLPGTKCCKKP | Histamine and PGD2 ↑ | - | Mobilize intracellular Ca2+ and release histamine | [157] |
| HBD3 | Human | GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK | PGD2 ↑ | Promote MAPK p38 and EPK1/2 | Increase intracellular Ca2+ concentration and degranulation | [158] |
| HBD4 | Human | EFELDRICGYGTARCRKKCRSQEYRIGRCPNTYACCLRKWDESLLNRTKP | PGD2 ↑ | Promote MAPK p38 and EPK1/2 | Increase intracellular Ca2+ concentration and degranulation. Enhance vascular permeability | [158] |
| Brevinin-2KP | Frog | MFTMKKSLLLLFFLGTVSLSLCEQERGADEDDGGEMTEELKRGVITDALKGAAKTVAAELLKKAHCKLTNSC | Histamine ↑ | - | Promote degranulation and histamine release of mast cells | [159] |
| Api88 | Honeybee | Gu-ONNRPVYIPRPRPPHPRL-NH2 | TNF-α ↓ | - | Trigger degranulation and intracellular Ca2+ mobilization in human MC | [160] |
| AMP-IBP5 | Enzyme lysate | AVYLPNCDRKGFYKRKQCKPSR | - | Promote MAPK p38 and NF-κB | Increase the content of Ca2+ in mast cells, induce degranulation of mouse peritoneal mast cells | [161] |
| Murepavadin | Simulator | TWLKKRRWKKAKPP | IL-8 and CCL3 ↑ | - | Induce mobilization and degranulation of LAD2 cells that express MrgprX2 endogenously and increase vascular permeability | [162] |
| AG-30/5C | Blood vessel | NH2-MLSLIFLHRLKSMRKRLDRKLRLWHRKNYP-COOH | LCT4, PGD2, PGE2, TNF-α, IL-8, MCP-1, MCP-3, MIP-1α, and MIP-1β ↑ | Promote MAPK p38 and NF-κB | Activate mast cells degranulation and produce lipid mediators, enhance the chemotaxis of mast cells | [163] |
| Dermcidin | Sweat glands | - | CCL1, CCL2, IL-6, and TNF-α ↑ | - | Activate mast cells | [164] |
| Pleurocidins | Fish | - | CCL2, CCL4, and MCP-1 ↑ | - | - | [165] |
| Pleurocidins NCR-04 | Fish | GWGSFFKKAAHVGKHVGKAALTHYL-NH2 | PGD2 ↑ | - | Promote LAD2 adhesion, migration, degranulation and release cysteine, LTs and PGD2 | [165] |
| Peptide Name | Source | Amino Acid Sequence | Inflammatory Mediator | Signaling Pathway | Functions | Ref. |
|---|---|---|---|---|---|---|
| LL-37 | Human | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | IL-4, IL-6, IL-12, IL-10, and TNF-α ↑ | - | Increase expression of HLA-DR and CD86 in immature DCs, enhance expansion and differentiation of DCs with CD103+/CD141+, and the antigen presenting ability of DCs, activate MDCs and promote differentiation | [168,169,170,171,172,173,174] |
| HBD2 | Human | GIGDPVTCLKSGAICHPVFCPRRYKQIGTCGLPGTKCCKKP | INF-α and IFN-γ ↑ | - | Activate plasmacytoid DCs by enhancing the uptake of CpG and self DNA | [175] |
| HBD3 | Human | GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK | INF-α ↑ | - | Activate pDCs by enhancing the uptake of CpG and self DNA, phenotypic maturation of primary human skin migration DC induced by Langerhans cell like DC and human skin explants | [175,176] |
| MDF2β | Murine | CHTNGGYCVRAICPPSARRPGSCFPEKNPCCKYM | TNF-α and TNFR2 ↑ | - | Induce co-stimulatory molecule upregulation and DCs maturation | [177,178] |
| MBD-14 | Murine | FLPKTLRKFFCRIRGGRCAVLNCLGKEEQIGRCSNSGRKCCRKKK | - | - | Increase expression of CD40 and MHC Ⅱ on the surface of DCs, reduce the endocytosis ability | [179] |
| PMAP-23 | Porcine | RIIDLLWRVRRPQKPKFVTVWVR | INF-α ↑ | - | - | [180] |
| PMAP-36 | Porcine | GRFRRLRKKTRKRLKKIGKVLKWIPPIVGSIPLGCG | INF-α ↑ | - | - | [180] |
| Surfactin | Bacilllus subtilis | ELIVDIL | IL-6 and TNF-α ↑ | Promote NF-κB | - | [181] |
| BEA | Fungi | - | IL-12 ↑ | - | Active BMDCs | [182] |
| Pep19-2.5 | Synthesis | GCKKYRRFRWKFKGKFWFWG | IL-6 ↓ | - | Inhibit DCs migration | [183] |
| Peptide Name | Source | Amino Acid Sequence | Inflammatory Mediator | Signaling Pathway | Functions | Ref. |
|---|---|---|---|---|---|---|
| LL-37 | Human | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | TNF-α and IL-1β ↓ IL-8 and ROS ↑ | Promote MAPK p38 and ERK | Inhibit neutrophil apoptosis, promote neutrophil to release NETs, and enhance the resistance of NETs to Staphylococcus aureus nuclease degradation | [185,186,187,189,190,191] |
| HBD-3 | Human | GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKK | - | - | Inhibit neutrophils apoptosis | [192] |
| HBD-1 | Human | DHYNCVSSGGQCLYSACPIFTKIQGTCYRGKAKCCK | - | - | Induce NET formation in PMNs | [193] |
| HBD-2 | Human | GIGDPVTCLKSGAICHPVFCPRRYKQIGTCGLPGTKCCKKP | - | - | Limit the infiltration of neutrophils in the lungs | [194] |
| Scolopendrasin X | Centipede | MKKFHCLKKICKGLCAKL-CONH2 | TNF-α and IL-6 ↓ | - | Increase Ca2+ and superoxide anion in neutrophils, and migrate through G protein and phospholipase C pathway | [195] |
| Scolopendrasin IX | Centipede | MCKYFIKIVSKSAKK-CONH2 | TNF-α, IL-6, IL-10, and CCL2 ↓ | - | Increase Ca2+ and superoxide anion in neutrophils, and migrate through G protein and phospholipase C pathway, recruit neutrophils | [196] |
| Cathelicidin BF | Bungarus fasciatus | KFFRKLKKSVKKRAKEFFKKPRVIGVSIPF | CCL2, CXCL1, and CXCL2 ↑ | - | Stimulate formation of NETs in neutrophils in vitro in a dose-dependent manner | [197,198,199] |
| LCN2 | Neutrophils | - | IL-1α, IL-6, IL-8, and TNF-α ↑ | Promote ERK1/2 and MAPK p38 | - | [200] |
| KSLW | Synthesis | KKVVFWVKFK-NH2 | - | - | Increase polymerization of F-Actin in neutrophils | [201] |
| Ac2-26 | Synthesis | acetyl-AMVSEFLKQARFLENQEQEYVQAVK | CXCL2 ↑ | - | - | [202] |
| Zd-14CFR | Synthesis | RGCRCNSKSFCVCR-NH2 | TNF-α and IL-1β ↓ | - | Decrease neutrophils infiltration | [203] |
| Peptide Name | Source | Amino Acid Sequence | Inflammatory Mediator | Signaling Pathway | Functions | Ref. |
|---|---|---|---|---|---|---|
| LL-37 | Human | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | IL-6, CXCL8, and CCL4 ↑ | Promote ERK1/2 | Trigger eosinophil nucleus to release Cys-LTs through FPRs, and release ECP | [205,206,207,208] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Niu, J.; Wang, X.; Niu, M.; Liao, C. The Contribution of Antimicrobial Peptides to Immune Cell Function: A Review of Recent Advances. Pharmaceutics 2023, 15, 2278. https://doi.org/10.3390/pharmaceutics15092278
Li H, Niu J, Wang X, Niu M, Liao C. The Contribution of Antimicrobial Peptides to Immune Cell Function: A Review of Recent Advances. Pharmaceutics. 2023; 15(9):2278. https://doi.org/10.3390/pharmaceutics15092278
Chicago/Turabian StyleLi, Hanxiao, Junhui Niu, Xiaoli Wang, Mingfu Niu, and Chengshui Liao. 2023. "The Contribution of Antimicrobial Peptides to Immune Cell Function: A Review of Recent Advances" Pharmaceutics 15, no. 9: 2278. https://doi.org/10.3390/pharmaceutics15092278
APA StyleLi, H., Niu, J., Wang, X., Niu, M., & Liao, C. (2023). The Contribution of Antimicrobial Peptides to Immune Cell Function: A Review of Recent Advances. Pharmaceutics, 15(9), 2278. https://doi.org/10.3390/pharmaceutics15092278






