Recent Advances in Electrospun Nanofiber-Based Strategies for Diabetic Wound Healing Application
Abstract
:1. Introduction
2. Diabetic Ulcers (DUs)
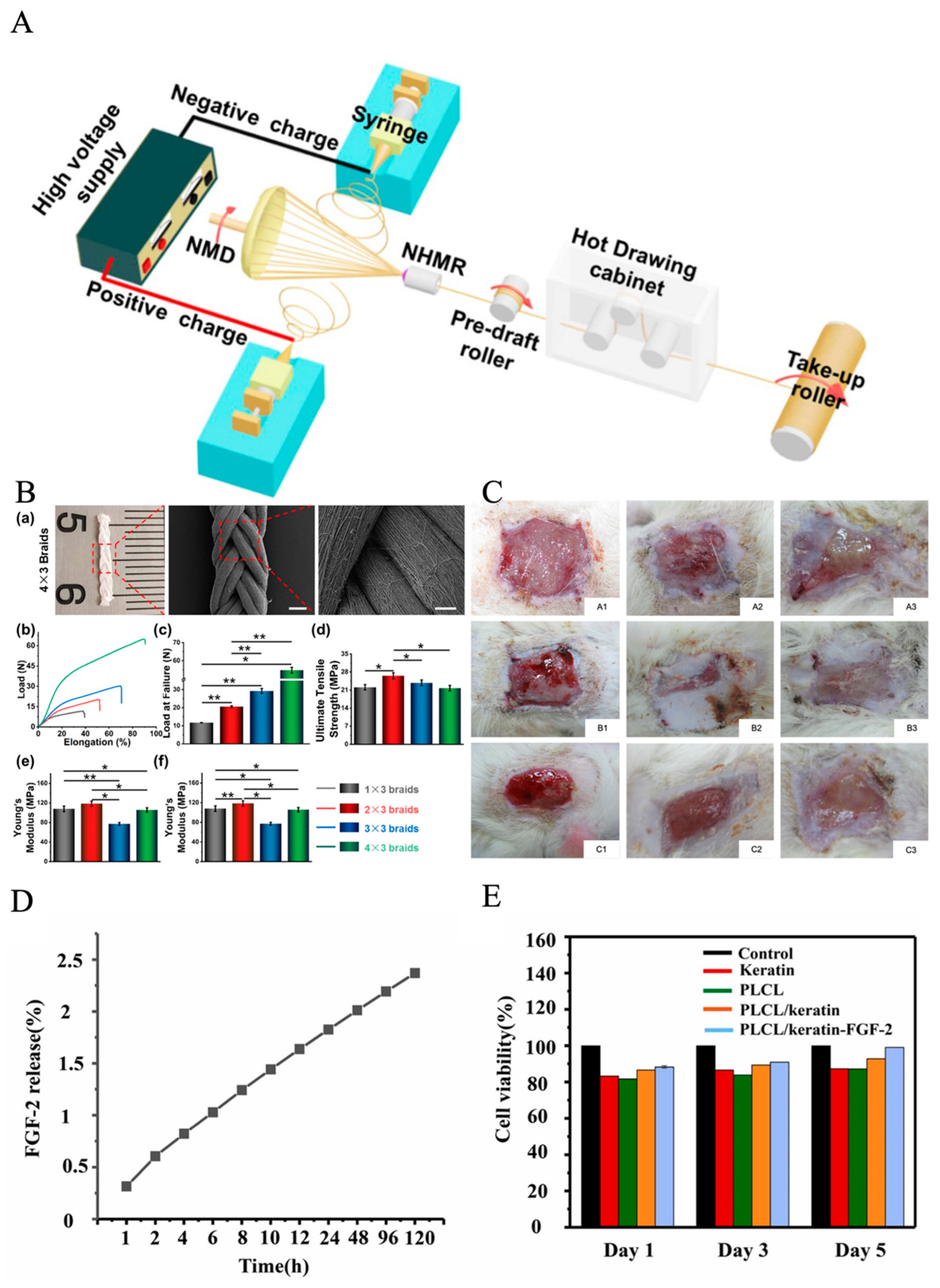
3. Mechanisms and Apparatus for Electrospinning Technology
4. Multi-Component Polymers in Electrospun Nanofibers for DU Treatment
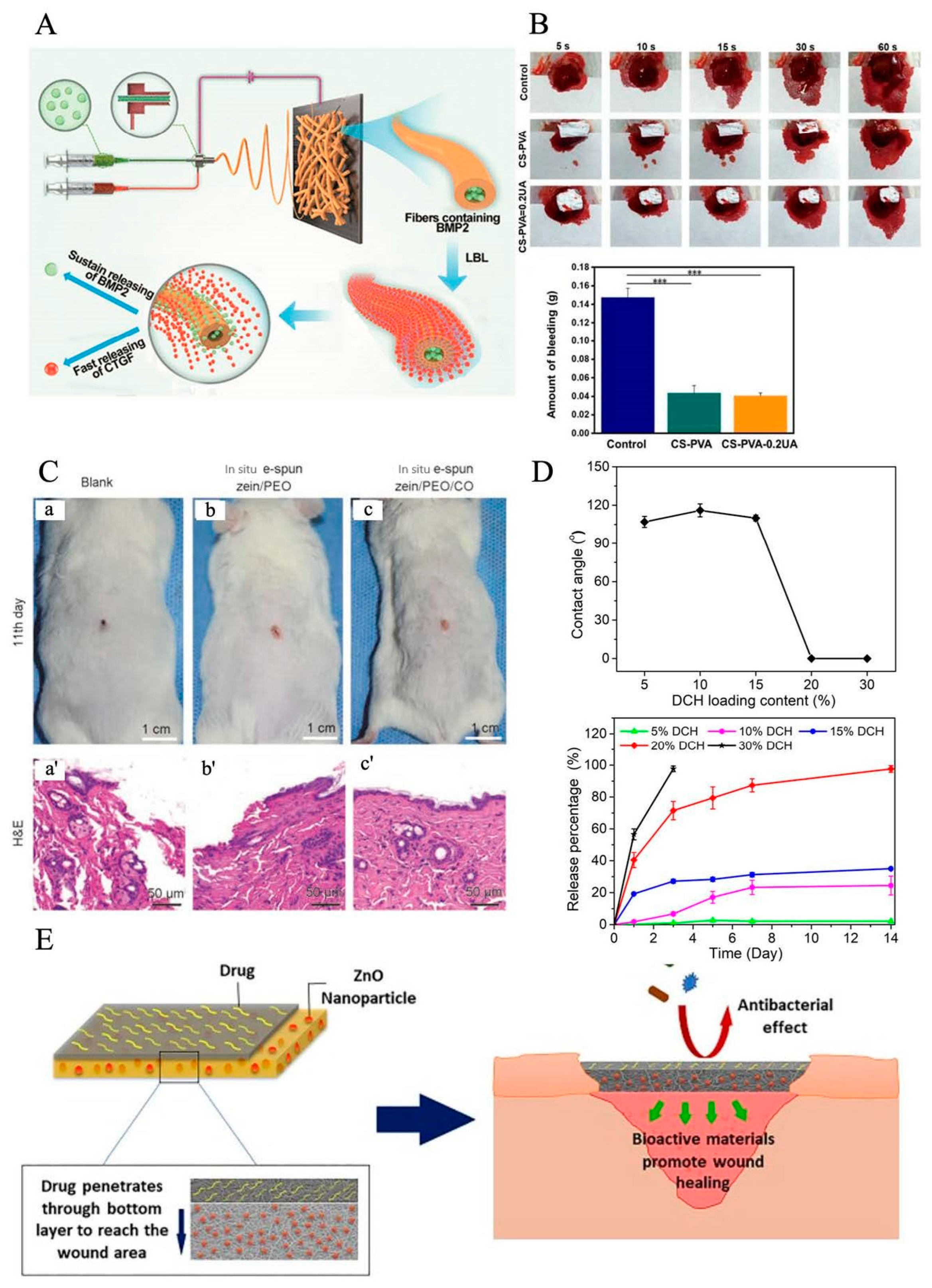
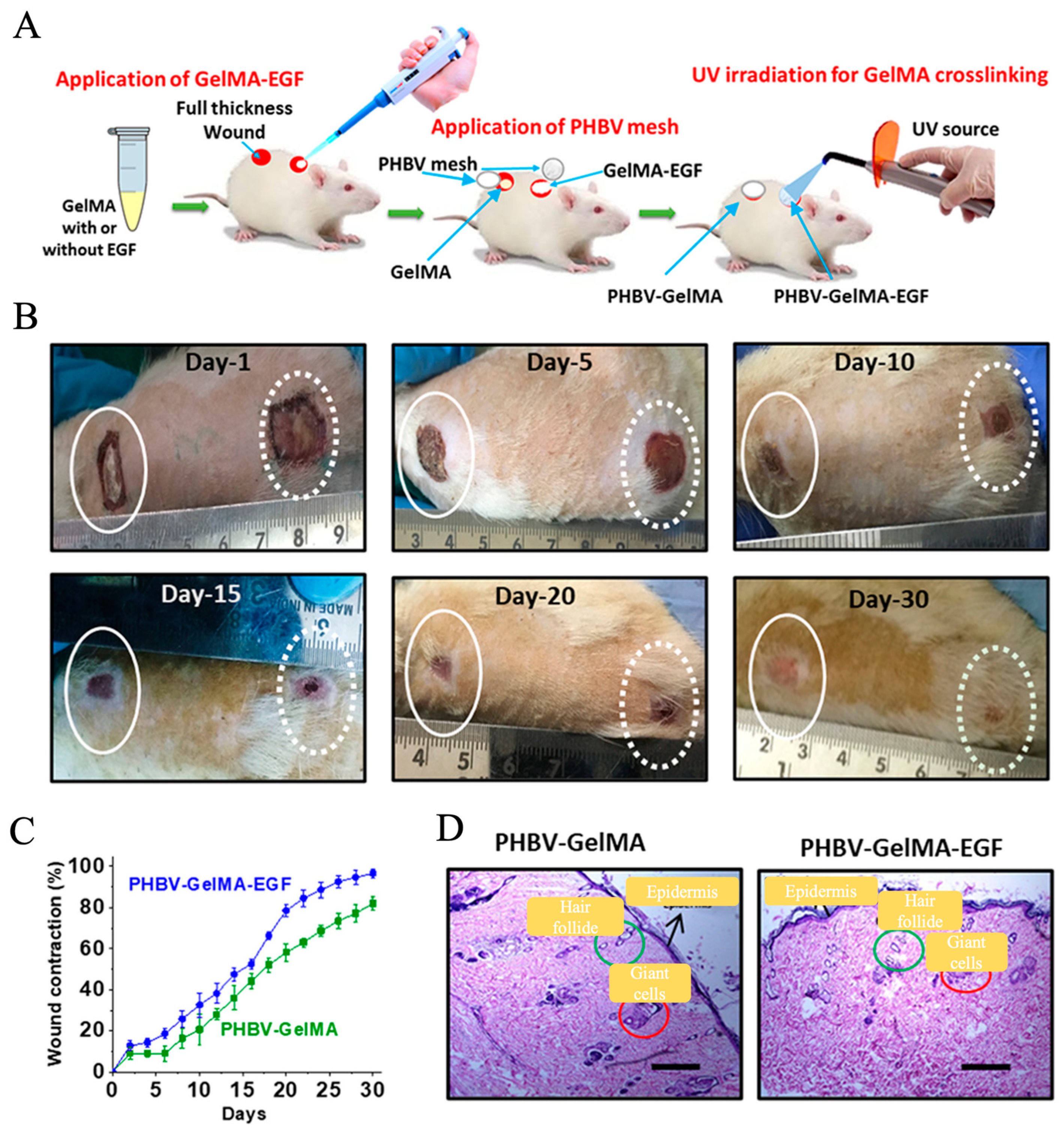
5. Electrospun Nanofiber Dressings Loaded with Bioactive Agents
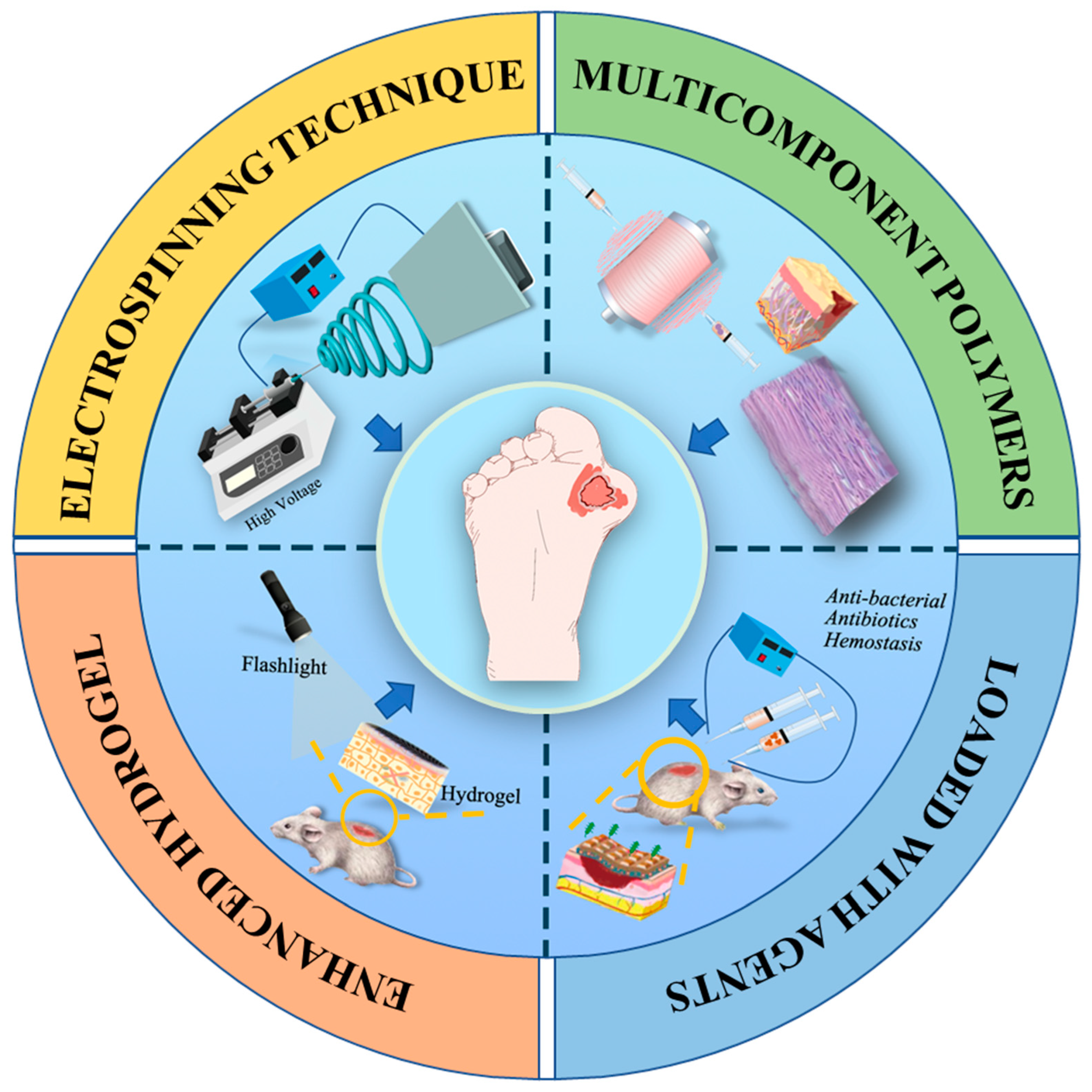
6. Electrospun Nanofiber/Hydrogel Composite Dressing
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Yazdanpanah, L.; Nasiri, M.; Adarvishi, S. Literature review on the management of diabetic foot ulcer. World J. Diabetes 2015, 6, 37. [Google Scholar] [CrossRef]
- Hajhosseini, B.; Gurtner, G.C.; Sen, C.K. And at last, the Wound is Healed… or, is it?! In Search of an Objective Way to Predict the Recurrence of Diabetic Foot Ulcers. Plast. Reconstr. Surg.–Glob. Open 2019, 7, 34–35. [Google Scholar] [CrossRef]
- Abazari, M.; Ghaffari, A.; Rashidzadeh, H.; Badeleh, S.M.; Maleki, Y. A Systematic Review on Classification, Identification, and Healing Process of Burn Wound Healing. Int. J. Lower Extr. Wound 2022, 21, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.; Rayman, G.; Jeffcoate, W. Cost of diabetic foot disease to the National Health Service in England. Diabetic Med. 2014, 31, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. Actice 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Glover, K.; Stratakos, A.C.; Varadi, A.; Lamprou, D.A. 3D scaffolds in the treatment of diabetic foot ulcers: New trends vs conventional approaches. Int. J. Pharmaceut. 2021, 599, 120423. [Google Scholar] [CrossRef] [PubMed]
- Grennan, D. Diabetic Foot Ulcers. JAMA 2019, 321, 114. [Google Scholar] [CrossRef]
- Gul, A.; Gallus, I.; Tegginamath, A.; Maryska, J.; Yalcinkaya, F. Electrospun Antibacterial Nanomaterials for Wound Dressings Applications. Membranes 2021, 11, 908. [Google Scholar] [CrossRef]
- Su, J.J.; Li, J.K.; Liang, J.H.; Zhang, K.; Li, J.A. Hydrogel Preparation Methods and Biomaterials for Wound Dressing. Life 2021, 11, 1016. [Google Scholar] [CrossRef]
- Li, T.; Sun, M.; Wu, S. State-of-the-Art Review of Electrospun Gelatin-Based Nanofiber Dressings for Wound Healing Applications. Nanomaterials 2022, 12, 784. [Google Scholar] [CrossRef] [PubMed]
- Gandavadi, D.; Sundarrajan, S.; Ramakrishna, S. Bio-Based Nanofibers Involved in Wastewater Treatment. Macromol. Mater. Eng. 2019, 304, 1900345. [Google Scholar] [CrossRef]
- Soares, R.M.D.; Siqueira, N.M.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. Mater. Sci. Eng. C 2018, 92, 969–982. [Google Scholar] [CrossRef]
- He, Y.; Liu, W.; Guan, L.; Chen, J.; Duan, L.; Jia, Z.; Huang, J.; Li, W.; Liu, J.; Xiong, J. A 3D-printed PLCL scaffold coated with collagen type I and its biocompatibility. BioMed Res. Int. 2018, 2018, 5147156. [Google Scholar] [CrossRef]
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, 2100477. [Google Scholar] [CrossRef]
- Yang, Y.T.; Du, Y.Z.; Zhang, J.; Zhang, H.L.; Guo, B.L. Structural and Functional Design of Electrospun Nanofibers for Hemostasis and Wound Healing. Adv. Fiber Mater. 2022, 4, 1027–1057. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Streubel, P.N.; Duan, B. Living nanofiber yarn-based woven biotextiles for tendon tissue engineering using cell tri-culture and mechanical stimulation. Acta Biomater. 2017, 62, 102–115. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Rathore, P.; Schiffman, J.D. Beyond the Single-Nozzle: Coaxial Electrospinning Enables Innovative Nanofiber Chemistries, Geometries, and Applications. ACS Appl. Mater. Interfaces 2021, 13, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, P.; Zhang, Y.; Zhang, H.; Qin, X. Flexible and conductive nanofiber-structured single yarn sensor for smart wearable devices. Sens. Actuat. B-Chem. 2017, 252, 697–705. [Google Scholar] [CrossRef]
- Sajkiewicz, P.; Kolbuk, D. Electrospinning of gelatin for tissue engineering-molecular conformation as one of the overlooked problems. J. Biomat. Sci.-Polym. Ed. 2014, 25, 2009–2022. [Google Scholar] [CrossRef] [PubMed]
- Shahriar, S.M.S.; Mondal, J.; Hasan, M.N.; Revuri, V.; Lee, D.Y.; Lee, Y.-K. Electrospinning Nanofibers for Therapeutics Delivery. Nanomaterials 2019, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- El husseny, M.W.A.; Mamdouh, M.; Shaban, S.; Ibrahim Abushouk, A.; Zaki, M.M.M.; Ahmed, O.M.; Abdel-Daim, M.M. Adipokines: Potential Therapeutic Targets for Vascular Dysfunction in Type II Diabetes Mellitus and Obesity. J. Diabetes Res. 2017, 2017, 8095926. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, L.; Shahbazian, H.; Nazari, I.; Hesam, S.; Ahmadi, F.; Cheraghian, B.; Arti, H.R.; Mohammadianinejad, S.E. Risk factors associated with diabetic foot ulcer-free survival in patients with diabetes. Diabetes Metab. Synd. Clin. Res. Rev. 2018, 12, 1039–1043. [Google Scholar] [CrossRef]
- Chang, M.; Nguyen, T.T. Strategy for Treatment of Infected Diabetic Foot Ulcers. Accounts Chem. Res. 2021, 54, 1080–1093. [Google Scholar] [CrossRef]
- Karri, V.V.S.R.; Kuppusamy, G.; Talluri, S.V.; Yamjala, K.; Mannemala, S.S.; Malayandi, R. Current and emerging therapies in the management of diabetic foot ulcers. Curr. Med. Res. Opin. 2016, 32, 519–542. [Google Scholar] [CrossRef]
- Markova, A.; Mostow, E.N. US Skin Disease Assessment: Ulcer and Wound Care. Dermatol. Clin. 2012, 30, 107–111. [Google Scholar] [CrossRef]
- Gianino, E.; Miller, C.; Gilmore, J. Smart Wound Dressings for Diabetic Chronic Wounds. Bioengineering 2018, 5, 51. [Google Scholar] [CrossRef]
- Madhukiran, D.R.; Jha, A.; Kumar, M.; Ajmal, G.; Bonde, G.V.; Mishra, B. Electrospun nanofiber-based drug delivery platform: Advances in diabetic foot ulcer management. Expert Opin. Drug Del. 2021, 18, 25–42. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Fiordaliso, F.; Clerici, G.; Maggioni, S.; Caminiti, M.; Bisighini, C.; Novelli, D.; Minnella, D.; Corbelli, A.; Morisi, R.; De Iaco, A.; et al. Prospective study on microangiopathy in type 2 diabetic foot ulcer. Diabetologia 2016, 59, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Li, Q.; Liu, Q.; Song, B.; Wu, J. Recent advances of the nanocomposite hydrogel as a local drug delivery for diabetic ulcers. Front. Bioeng. Biotech. 2022, 10, 1039495. [Google Scholar] [CrossRef]
- Association, A.D. Consensus Development Conference on Diabetic Foot Wound Care: 7-8 April 1999, Boston, Massachusetts. American Diabetes Association. Diabetes Care 1999, 22, 1354–1360. [Google Scholar] [CrossRef]
- Nataraj, M.; Maiya, A.G.; Karkada, G.; Hande, M.; Rodrigues, G.S.; Shenoy, R.; Prasad, S.S. Application of Topical Oxygen Therapy in Healing Dynamics of Diabetic Foot Ulcers—A Systematic Review. Rev. Diabet. Stud. 2019, 15, 74–82. [Google Scholar] [CrossRef]
- Liang, Y.P.; He, J.H.; Guo, B.L. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Li, Q.R.; Wang, D.X.; Jiang, Z.P.; Li, R.; Xue, T.Y.; Lin, C.; Deng, Y.Z.; Jin, Y.; Sun, B.Z. Advances of hydrogel combined with stem cells in promoting chronic wound healing. Front. Chem. 2022, 10, 15. [Google Scholar] [CrossRef]
- Li, Y.R.; Dong, T.; Li, Z.W.; Ni, S.L.; Zhou, F.; Alimi, O.A.; Chen, S.J.; Duan, B.; Kuss, M.; Wu, S.H. Review of advances in electrospinning-based strategies for spinal cord regeneration. Mater. Today Chem. 2022, 24, 20. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, S.J.; Cho, W.; Gu, B.K.; Kim, C.H. Neuropeptide Substance-P-Conjugated Chitosan Nanofibers as an Active Modulator of Stem Cell Recruiting. Int. J. Mol. Sci. 2016, 17, 68. [Google Scholar] [CrossRef]
- Pierce, G.F.; Mustoe, T.A.; Altrock, B.W.; Deuel, T.F.; Thomason, A. Role of platelet-derived growth factor in wound healing. J. Cell Biochem. 1991, 45, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, K.; Chakraborty, D.; Roychowdhury, V.; Ghosh, S.; Dutta, S. Recent Advancement of Functional Hydrogels toward Diabetic Wound Management. ACS Omega 2022, 7, 43364–43380. [Google Scholar] [CrossRef]
- Srivastava, P.; Sondak, T.; Sivashanmugam, K.; Kim, K.-S. A Review of Immunomodulatory Reprogramming by Probiotics in Combating Chronic and Acute Diabetic Foot Ulcers (DFUs). Pharmaceutics 2022, 14, 2436. [Google Scholar] [CrossRef] [PubMed]
- Grosu-Bularda, A.; Teodoreanu, R.N.; Mihai, C.; Lita, F.F.; Hodea, F.V.; Lascar, I.; Chiotoroiu, A. Diabetic foot ulcers, a comprehensive approach—Review. Ind. Textila 2022, 73, 213–221. [Google Scholar] [CrossRef]
- Ibrahim, A.; Berkache, M.; Morency-Potvin, P.; Juneau, D.; Koenig, M.; Bourduas, K.; Freire, V. Diabetic foot infections: How to investigate more efficiently? A retrospective study in a quaternary university center. Insights Into Imaging 2022, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Morbach, S.; Furchert, H.; Gröblinghoff, U.; Hoffmeier, H.; Kersten, K.; Klauke, G.-T.; Klemp, U.; Roden, T.; Icks, A.; Haastert, B.; et al. Long-Term Prognosis of Diabetic Foot Patients and Their Limbs: Amputation and death over the course of a decade. Diabetes Care 2012, 35, 2021–2027. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.J.; Martin, P. Wound repair at a glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef]
- Lima, T.D.D.; Passos, M.F. Skin wounds, the healing process, and hydrogel-based wound dressings: A short review. J. Biomat. Sci.-Polym. Ed. 2021, 32, 1910–1925. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Rajkuberan Chandrasekaran, M.K.; Bupesh, G.; Chacko, S.; Gawade, O.; Hasan, S.; George, E.; Vijayakumar, T.S.; Sundaram, M.; Sagadevan, S. Prospective features of functional 2D nanomaterial graphene oxide in the wound healing process. J. Drug Deliv. Sci. Technol. 2023, 82, 104352. [Google Scholar] [CrossRef]
- Anton, F. Process and Apparatus for Preparing Artificial Threads. U.S. Patents US-1975504-A, 2 October 1934. [Google Scholar]
- Qi, Y.; Wang, C.E.; Wang, Q.Y.; Zhou, F.; Li, T.; Wang, B.; Su, W.D.; Shang, D.W.; Wu, S.H. A simple, quick, and cost-effective strategy to fabricate polycaprolactone/silk fibroin nanofiber yarns for biotextile-based tissue scaffold application. Eur. Polym. J. 2023, 186, 12. [Google Scholar] [CrossRef]
- Chen, F.; Li, X.; Mo, X.; He, C.; Wang, H.; Ikada, Y. Electrospun chitosan-P (LLA-CL) nanofibers for biomimetic extracellular matrix. J. Biomat. Sci.-Polym. Ed. 2008, 19, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chang, Z.; Wang, X.; Li, Q. Synthesis of Poly (l-lactide-co-ε-caprolactone) Copolymer: Structure, Toughness, and Elasticity. Polymers 2021, 13, 1270. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Juncos Bombin, A.D.; Dunne, N.J.; McCarthy, H.O. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater. Sci. Eng. C 2020, 114, 110994. [Google Scholar] [CrossRef] [PubMed]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Tan, N.C.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Chen, K.; Hu, H.; Zeng, Y.; Pan, H.; Wang, S.; Zhang, Y.; Shi, L.; Tan, G.X.; Pan, W.S.; Liu, H. Recent advances in electrospun nanofibers for wound dressing. Eur. Polym. J. 2022, 178, 14. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Tung, S.-H. One-Step Electrospinning to Produce Nonsolvent-Induced Macroporous Fibers with Ultrahigh Oil Adsorption Capability. Macromolecules 2017, 50, 2528–2534. [Google Scholar] [CrossRef]
- Suresh, S.; Gryshkov, O.; Glasmacher, B. Impact of setup orientation on blend electrospinning of poly-ε-caprolactone-gelatin scaffolds for vascular tissue engineering. Int. J. Artif. Organs 2018, 41, 801–810. [Google Scholar] [CrossRef]
- Dong, B.; Smith, M.E.; Wnek, G.E. Encapsulation of multiple biological compounds within a single electrospun fiber. Small 2009, 5, 1508–1512. [Google Scholar] [CrossRef]
- Han, D.; Steckl, A.J. Coaxial Electrospinning Formation of Complex Polymer Fibers and their Applications. ChemPlusChem 2019, 84, 1453–1497. [Google Scholar] [CrossRef]
- Feng, X.; Li, J.; Zhang, X.; Liu, T.; Ding, J.; Chen, X. Electrospun polymer micro/nanofibers as pharmaceutical repositories for healthcare. J. Control. Release 2019, 302, 19–41. [Google Scholar] [CrossRef]
- Yin, A.; Luo, R.; Li, J.; Mo, X.; Wang, Y.; Zhang, X. Coaxial electrospinning multicomponent functional controlled-release vascular graft: Optimization of graft properties. Colloid Surf. B-Biointerfaces 2017, 152, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Li, L.; Zhao, B.A.; Li, X.; Liang, W.; Lang, M.; Cheng, B.; Li, J. Antibacterial, antioxidant and anti-inflammatory PLCL/gelatin nanofiber membranes to promote wound healing. Int. J. Biol. Macromol. 2022, 194, 914–923. [Google Scholar] [CrossRef]
- Han, W.-H.; Wang, M.-Q.; Yuan, J.-X.; Hao, C.-C.; Li, C.-J.; Long, Y.-Z.; Ramakrishna, S. Electrospun Aligned Nanofibers: A Review. Arab. J. Chem. 2022, 15, 104193. [Google Scholar] [CrossRef]
- Xu, S.C.; Qin, C.C.; Yu, M.; Dong, R.H.; Yan, X.; Zhao, H.; Han, W.P.; Zhang, H.D.; Long, Y.Z. A battery-operated portable handheld electrospinning apparatus. Nanoscale 2015, 7, 12351–12355. [Google Scholar] [CrossRef] [PubMed]
- Persano, L.; Camposeo, A.; Pisignano, D. Advancing the Science and Technology of Electrospinning and Functional Nanofibers. Macromol. Mater. Eng. 2017, 302, 1700237. [Google Scholar] [CrossRef]
- Aldana, A.A.; Abraham, G.A. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int. J. Pharmaceut. 2017, 523, 441–453. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Nukavarapu, S.P.; James, R.; Nair, L.S.; Laurencin, C.T. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 2008, 29, 4100–4107. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.; Netravali, A.N.; Joo, Y.L. Mechanical properties and biodegradability of electrospun soy protein Isolate/PVA hybrid nanofibers. Polym. Degrad. Stabil. 2012, 97, 747–754. [Google Scholar] [CrossRef]
- Jamnongkan, T.; Wattanakornsiri, A.; Pansila, P.P.; Migliaresi, C.; Kaewpirom, S. Effect of poly (vinyl alcohol)/chitosan ratio on electrospun-nanofiber morphologies. Trans. Tech. Publ. 2012, 463–464, 734–738. [Google Scholar] [CrossRef]
- Boncu, T.E.; Ozdemir, N. Electrospinning of ampicillin trihydrate loaded electrospun PLA nanofibers I: Effect of polymer concentration and PCL addition on its morphology, drug delivery and mechanical properties. Int. J. Polym. Mater. Polym. 2022, 71, 669–676. [Google Scholar] [CrossRef]
- Alven, S.; Peter, S.; Mbese, Z.; Aderibigbe, B.A. Polymer-Based Wound Dressing Materials Loaded with Bioactive Agents: Potential Materials for the Treatment of Diabetic Wounds. Polymers 2022, 14, 35. [Google Scholar] [CrossRef]
- Daristotle, J.L.; Erdi, M.; Lau, L.W.; Zaki, S.T.; Srinivasan, P.; Balabhadrapatruni, M.; Ayyub, O.B.; Sandler, A.D.; Kofinas, P. Biodegradable, Tissue Adhesive Polyester Blends for Safe, Complete Wound Healing. ACS Biomater. Sci. Eng. 2021, 7, 3908–3916. [Google Scholar] [CrossRef]
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocoll. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Lin, J.; Li, C.; Zhao, Y.; Hu, J.; Zhang, L.-M. Co-electrospun Nanofibrous Membranes of Collagen and Zein for Wound Healing. ACS Appl. Mater. Interfaces 2012, 4, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Massoumi, H.; Nourmohammadi, J.; Marvi, M.S.; Moztarzadeh, F. Comparative study of the properties of sericin-gelatin nanofibrous wound dressing containing halloysite nanotubes loaded with zinc and copper ions. Int. J. Polym. Mater. Polym. 2019, 68, 1142–1153. [Google Scholar] [CrossRef]
- Fernandez, A.; Torres-Giner, S.; Lagaron, J.M. Novel route to stabilization of bioactive antioxidants by encapsulation in electrospun fibers of zein prolamine. Food Hydrocoll. 2009, 23, 1427–1432. [Google Scholar] [CrossRef]
- Kitazono, E.; Kaneko, H.; Miyoshi, T.; Miyamoto, K. Tissue Engineering using Nanofiber. J. Syn. Org. Chem. Jpn. 2004, 62, 514–519. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Shan, Y.; Xiong, J.; Hu, Z.; Zhang, Y.; Gao, J. In vivo study of silk fibroin/gelatin electrospun nanofiber dressing loaded with astragaloside IV on the effect of promoting wound healing and relieving scar. J. Drug Deliv. Sci. Technol. 2019, 52, 272–281. [Google Scholar] [CrossRef]
- Anand, S.; Rajinikanth, P.S.; Arya, D.K.; Pandey, P.; Gupta, R.K.; Sankhwar, R.; Chidambaram, K. Multifunctional Biomimetic Nanofibrous Scaffold Loaded with Asiaticoside for Rapid Diabetic Wound Healing. Pharmaceutics 2022, 14, 273. [Google Scholar] [CrossRef] [PubMed]
- Sofokleous, P.; Stride, E.; Edirisinghe, M. Preparation, Characterization, and Release of Amoxicillin from Electrospun Fibrous Wound Dressing Patches. Pharm. Res. 2013, 30, 1926–1938. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Kau, Y.C.; Chou, C.Y.; Chen, J.K.; Wu, R.C.; Yeh, W.L. Electrospun PLGA/collagen nanofibrous membrane as early-stage wound dressing. J. Membr. Sci. 2010, 355, 53–59. [Google Scholar] [CrossRef]
- Wu, S.; Liu, J.; Cai, J.; Zhao, J.; Duan, B.; Chen, S. Combining electrospinning with hot drawing process to fabricate high performance poly (L-lactic acid) nanofiber yarns for advanced nanostructured bio-textiles. Biofabrication 2021, 13, 045018. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Tang, Q.; Yu, Y.; Li, J.; Luo, J.; Li, M. Electrospun core–sheath fibers for integrating the biocompatibility of silk fibroin and the mechanical properties of PLCL. Polym. Adv. Technol. 2014, 25, 1596–1603. [Google Scholar] [CrossRef]
- Liu, S.; Li, C.; Zachman, M.J.; Zeng, Y.; Yu, H.; Li, B.; Wang, M.; Braaten, J.; Liu, J.; Meyer, H.M.; et al. Atomically dispersed iron sites with a nitrogen–carbon coating as highly active and durable oxygen reduction catalysts for fuel cells. Nat. Energy 2022, 7, 652–663. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, S.; Du, C.; Wang, R.; Han, C.; Che, Y.; Feng, W.; Wang, C.; Gao, S.; Zhao, W. Novel PLCL nanofibrous/keratin hydrogel bilayer wound dressing for skin wound repair. Colloid Surf. B-Biointerfaces 2023, 222, 113119. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.T.; Chung, O.H.; Dela Cruz, J.; Park, J.S. Fabrication and characterization of electrospun curcumin-loaded polycaprolactone-polyethylene glycol nanofibers for enhanced wound healing. Macromol. Res. 2014, 22, 1288–1296. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Chen, X.; Xu, Q.; Lu, F.; Nie, J. Electrospun Water-Soluble Carboxyethyl Chitosan/Poly(vinyl alcohol) Nanofibrous Membrane as Potential Wound Dressing for Skin Regeneration. Biomacromolecules 2008, 9, 349–354. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, M.; Zhang, Y.; Cao, Q.; Wang, X.; Han, Y.; Sun, G.; Li, Y.; Zhou, J. Novel lignin–chitosan–PVA composite hydrogel for wound dressing. Mater. Sci. Eng. C 2019, 104, 110002. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.K.; Kidoaki, S.; Matsuda, T. Electrospun nano-to microfiber fabrics made of biodegradable copolyesters: Structural characteristics, mechanical properties and cell adhesion potential. Biomaterials 2005, 26, 3929–3939. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.I.; Mok, J.Y.; Jeon, I.H.; Park, K.H.; Thuy, T.T.N.; Park, J.S.; Hwang, H.M.; Song, M.S.; Lee, D.; Chai, K.Y. Effect of Electrospun Non-Woven Mats of Dibutyryl Chitin/Poly(Lactic Acid) Blends on Wound Healing in Hairless Mice. Molecules 2012, 17, 2992–3007. [Google Scholar] [CrossRef] [PubMed]
- Mirbagheri, M.S.; Akhavan-Mahdavi, S.; Hasan, A.; Kharazmi, M.S.; Jafari, S.M. Chitosan-based electrospun nanofibers for diabetic foot ulcer management; recent advances. Carbohyd. Polym. 2023, 313, 120512. [Google Scholar] [CrossRef]
- Song, Y.-S.; Kim, B.-Y.; Yang, D.H.; Lee, D.Y. Poly(ε-caprolactone)/gelatin nanofibrous scaffolds for wound dressing. Appl. Nanosci. 2022, 12, 3261–3270. [Google Scholar] [CrossRef]
- Powell, H.M.; Supp, D.M.; Boyce, S.T. Influence of electrospun collagen on wound contraction of engineered skin substitutes. Biomaterials 2008, 29, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, E.; Tozzi, S.; Tsukada, M.; Taddei, P. Structural study on methacrylamide-grafted Tussah silk fibroin fibres. Int. J. Biol. Macromol. 2016, 88, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Moomand, K.; Lim, L.-T. Effects of solvent and n-3 rich fish oil on physicochemical properties of electrospun zein fibres. Food Hydrocoll. 2015, 46, 191–200. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, X.; Li, Y.; Que, F.; Kang, X.; Liu, Y.; Feng, F.; Zhang, H. Characterization of gelatin/zein nanofibers by hybrid electrospinning. Food Hydrocoll. 2018, 75, 72–80. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, L.; An, T.; Xian, M.; Luckanagul, J.A.; Su, Z.; Lin, Y.; Wang, Q. A hydrogen sulfide-releasing alginate dressing for effective wound healing. Acta Biomater. 2020, 104, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, X.; Wang, L.; Yan, X.; Ma, D.; Liu, Z.; Liu, X. Sesamol incorporated cellulose acetate-zein composite nanofiber membrane: An efficient strategy to accelerate diabetic wound healing. Int. J. Biol. Macromol. 2020, 149, 627–638. [Google Scholar] [CrossRef]
- Cam, M.E.; Crabbe-Mann, M.; Alenezi, H.; Hazar-Yavuz, A.N.; Ertas, B.; Ekentok, C.; Ozcan, G.S.; Topal, F.; Guler, E.; Yazir, Y.; et al. The comparision of glybenclamide and metformin-loaded bacterial cellulose/gelatin nanofibres produced by a portable electrohydrodynamic gun for diabetic wound healing. Eur. Polym. J. 2020, 134, 109844. [Google Scholar] [CrossRef]
- Selvaraj, S.; Fathima, N.N. Fenugreek Incorporated Silk Fibroin Nanofibers—A Potential Antioxidant Scaffold for Enhanced Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 5916–5926. [Google Scholar] [CrossRef] [PubMed]
- Moutsatsou, P.; Coopman, K.; Georgiadou, S. Biocompatibility Assessment of Conducting PANI/Chitosan Nanofibers for Wound Healing Applications. Polymers 2017, 9, 687. [Google Scholar] [CrossRef]
- Yin, L.-H.; Wang, H.; Zhang, Z.; Huang, S.; Wang, J.-K.; Qing, Z.-S.; Sun, Q.; Yu, Z.H. A research on the performance of SF/COL/PLCL Electrospun three-dimensions nano fiber scaffold. J. Funct. Mater. 2014, 45, 8102–8107. [Google Scholar]
- Wang, X.; Cheng, F.; Gao, J.; Wang, L. Antibacterial wound dressing from chitosan/polyethylene oxide nanofibers mats embedded with silver nanoparticles. J. Biomater. Appl. 2015, 29, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Gilotra, S.; Chouhan, D.; Bhardwaj, N.; Nandi, S.K.; Mandal, B.B. Potential of silk sericin based nanofibrous mats for wound dressing applications. Mater. Sci. Eng. C 2018, 90, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.; Prabhakaran, M.P.; Kai, D.; Ramakrishna, S. Human cardiomyocyte interaction with electrospun fibrinogen/gelatin nanofibers for myocardial regeneration. J. Biomat. Sci.-Polym. Ed. 2013, 24, 1660–1675. [Google Scholar] [CrossRef]
- Yang, F.; Miao, Y.; Wang, Y.; Zhang, L.-M.; Lin, X. Electrospun Zein/Gelatin Scaffold-Enhanced Cell Attachment and Growth of Human Periodontal Ligament Stem Cells. Materials 2017, 10, 1168. [Google Scholar] [CrossRef]
- Chiou, B.-S.; Jafri, H.; Avena-Bustillos, R.; Gregorski, K.S.; Bechtel, P.J.; Imam, S.H.; Glenn, G.M.; Orts, W.J. Properties of electrospun pollock gelatin/poly (vinyl alcohol) and pollock gelatin/poly (lactic acid) fibers. Int. J. Biol. Macromol. 2013, 55, 214–220. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.; Duan, B.; Wu, L.; Li, S.; Yuan, X. Preparation of electrospun chitosan/poly(vinyl alcohol) membranes. Colloid Polym. Sci. 2007, 285, 855–863. [Google Scholar] [CrossRef]
- Wu, S.H.; Qi, Y.; Shi, W.; Kuss, M.; Chen, S.J.; Duan, B. Electrospun conductive nanofiber yarns for accelerating mesenchymal stem cells differentiation and maturation into Schwann cell-like cells under a combination of electrical stimulation and chemical induction. Acta Biomater. 2022, 139, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial biomaterials for skin wound dressing. Asian J. Pharm. Sci. 2022, 17, 353–384. [Google Scholar] [CrossRef]
- Son, Y.J.; Kim, W.J.; Yoo, H.S. Therapeutic applications of electrospun nanofibers for drug delivery systems. Arch. Pharm. Res. 2014, 37, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Fahimirad, S.; Ajalloueian, F. Naturally-derived electrospun wound dressings for target delivery of bio-active agents. Int. J. Pharmaceut. 2019, 566, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, H.; Yang, Y.; Yoon, J.; Wang, S.; Zhang, X. Supramolecular antibacterial materials for combatting antibiotic resistance. Adv. Mater. 2019, 31, 1805092. [Google Scholar] [CrossRef]
- Shalaby, T.I.; Fekry, N.M.; Sodfy, A.S.E.; Sheredy, A.G.E.; Moustafa, M.E.S.S.A. Preparation and characterisation of antibacterial silver-containing nanofibres for wound healing in diabetic mice. Int. J. Nanoparticles 2015, 8, 82–98. [Google Scholar] [CrossRef]
- Yu, Q.; Qiao, G.-H.; Wang, M.; Yu, L.; Sun, Y.; Shi, H.; Ma, T.-L. Stem Cell-Based Therapy for Diabetic Foot Ulcers. Front. Cell Dev. Biol. 2022, 10, 812262. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wong, K.K.; Ho, C.M.; Lok, C.N.; Yu, W.Y.; Che, C.M.; Chiu, J.F.; Tam, P.K. Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem 2007, 2, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Jannesari, M.; Varshosaz, J.; Morshed, M.; Zamani, M. Composite poly(vinyl alcohol)/poly(vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int. J. Nanomed. 2011, 6, 11. [Google Scholar]
- Qin, M.; Liu, D.Q.; Dai, Z.; Meng, X.; Liu, G.S.; Liu, H.; Huang, X.W.; Yan, X.; Chen, S.J. One Step Fabrication and Application of Antibacterial Electrospun Zein/Cinnamon Oil Membrane Wound Dressing via In situ Electrospinning Process. Chem. Res. Chin. Univ. 2021, 37, 464–469. [Google Scholar] [CrossRef]
- Charernsriwilaiwat, N.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Lysozyme-loaded, electrospun chitosan-based nanofiber mats for wound healing. Int. J. Pharmaceut. 2012, 427, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, B.; Carlson, M.A.; Gombart, A.F.; Reilly, D.A.; Xie, J. Recent advances in electrospun nanofibers for wound healing. Nanomedicine 2017, 12, 1335–1352. [Google Scholar] [CrossRef]
- Cui, S.; Sun, X.; Li, K.; Gou, D.; Zhou, Y.; Hu, J.; Liu, Y. Polylactide nanofibers delivering doxycycline for chronic wound treatment. Mater. Sci. Eng. C 2019, 104, 109745. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Q.F.; Yu, J.; Shao, N.N.; Lu, H.T.; Guo, J.S.; Qiu, X.P.; Zhou, D.F.; Huang, Y.B. Absorbable Thioether Grafted Hyaluronic Acid Nanofibrous Hydrogel for Synergistic Modulation of Inflammation Microenvironment to Accelerate Chronic Diabetic Wound Healing. Adv. Healthc. Mater. 2020, 9, e2000198. [Google Scholar] [CrossRef] [PubMed]
- Dayya, D.; O’Neill, O.J.; Huedo-Medina, T.B.; Habib, N.; Moore, J.; Iyer, K. Debridement of Diabetic Foot Ulcers. Adv. Wound Care 2022, 11, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Massara, M.; De Caridi, G.; Serra, R.; Barilla, D.; Cutrupi, A.; Volpe, A.; Cutrupi, F.; Alberti, A.; Volpe, P. The role of procalcitonin as a marker of diabetic foot ulcer infection. Int. Wound J. 2017, 14, 31–34. [Google Scholar] [CrossRef]
- BarathManiKanth, S.; Kalishwaralal, K.; Sriram, M.; Pandian, S.R.K.; Youn, H.-S.; Eom, S.; Gurunathan, S. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J. Nanobiotechnol. 2010, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Lau, P.; Bidin, N.; Islam, S.; Shukri, W.; Zakaria, N.; Musa, N.; Krishnan, G. Influence of gold nanoparticles on wound healing treatment in rat model: Photobiomodulation therapy. Lasers Surg. Med. 2017, 49, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Narayanan, K.; Thakar, M.B.; Jagani, H.V.; Venkata Rao, J. Biosynthesis and wound healing activity of copper nanoparticles. IET Nanobiotechnol. 2014, 8, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.W.; Cui, S.S.; Song, X.Y.; Hu, J.L.; Zhou, Y.F.; Liu, Y.C. An antimicrobial peptide-immobilized nanofiber mat with superior performances than the commercial silver-containing dressing. Mater. Sci. Eng. C 2021, 119, 111608. [Google Scholar] [CrossRef]
- Jafari, A.; Amirsadeghi, A.; Hassanajili, S.; Azarpira, N. Bioactive antibacterial bilayer PCL/gelatin nanofibrous scaffold promotes full-thickness wound healing. Int. J. Pharmaceut. 2020, 583, 119413. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Jiang, L.B.; Wu, S.J.; Yang, S.W.; Ren, L.; Cheng, B.; Xia, J. A Shape-Programmable Hierarchical Fibrous Membrane Composite System to Promote Wound Healing in Diabetic Patients. Small 2022, 18, e2107544. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhao, M.; Li, Y.; Li, K.; Chen, S.; Zhao, W.; Wu, S.; Han, Y. Electrospun Chitosan–Polyvinyl Alcohol Nanofiber Dressings Loaded with Bioactive Ursolic Acid Promoting Diabetic Wound Healing. Nanomaterials 2022, 12, 2933. [Google Scholar] [CrossRef]
- Kharat, Z.; Goushki, M.A.; Sarvian, N.; Asad, S.; Dehghan, M.M.; Kabiri, M. Chitosan/PEO nanofibers containing Calendula officinalis extract: Preparation, characterization, in vitro and in vivo evaluation for wound healing applications. Int. J. Pharmaceut. 2021, 609, 121132. [Google Scholar] [CrossRef] [PubMed]
- Almasian, A.; Najafi, F.; Eftekhari, M.; Ardekani, M.R.S.; Sharifzadeh, M.; Khanavi, M. Polyurethane/carboxymethylcellulose nanofibers containing Malva sylvestris extract for healing diabetic wounds: Preparation, characterization, in vitro and in vivo studies. Mater. Sci. Eng. C 2020, 114, 111039. [Google Scholar] [CrossRef]
- Peng, Y.; Ma, Y.; Bao, Y.; Liu, Z.; Chen, L.; Dai, F.; Li, Z. Electrospun PLGA/SF/artemisinin composite nanofibrous membranes for wound dressing. Int. J. Biol. Macromol. 2021, 183, 68–78. [Google Scholar] [CrossRef]
- Xie, Z.W.; Aphale, N.V.; Kadapure, T.D.; Wadajkar, A.S.; Orr, S.; Gyawali, D.; Qian, G.Y.; Nguyen, K.T.; Yang, J. Design of antimicrobial peptides conjugated biodegradable citric acid derived hydrogels for wound healing. J. Biomed. Mater. Res. A 2015, 103, 3907–3918. [Google Scholar] [CrossRef]
- Fu, Y.; Guan, J.; Guo, S.; Guo, F.; Niu, X.; Liu, Q.; Zhang, C.; Nie, H.; Wang, Y. Human urine-derived stem cells in combination with polycaprolactone/gelatin nanofibrous membranes enhance wound healing by promoting angiogenesis. J. Transl. Med. 2014, 12, 274. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Leong, K.W.; Yoo, H.S. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials 2008, 29, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shen, Q.; Lin, Y.; Xu, S.; Meng, Q. Evaluation of the potential of chimeric spidroins/poly(L-lactic-co-ε-caprolactone) (PLCL) nanofibrous scaffolds for tissue engineering. Mater. Sci. Eng. C 2020, 111, 110752. [Google Scholar] [CrossRef] [PubMed]
- Abazari, M.F.; Gholizadeh, S.; Karizi, S.Z.; Birgani, N.H.; Abazari, D.; Paknia, S.; Derakhshankhah, H.; Allahyari, Z.; Amini, S.M.; Hamidi, M. Recent Advances in Cellulose-Based Structures as the Wound-Healing Biomaterials: A Clinically Oriented Review. Appl. Sci. 2021, 11, 7769. [Google Scholar] [CrossRef]
- Liu, N.; Miao, Y.; Qi, F.; Gu, J. The possibility of different proportions of electrospun nanomaterials PLCL/Poloxamer as a skin tissue substitute. Fudan Univ. J. Med. Sci. 2014, 41, 34–39. [Google Scholar]
- Wang, K.; Hazra, R.S.; Ma, Q.; Jiang, L.; Liu, Z.; Zhang, Y.; Wang, S.; Han, G. Multifunctional silk fibroin/PVA bio-nanocomposite films containing TEMPO-oxidized bacterial cellulose nanofibers and silver nanoparticles. Cellulose 2022, 29, 1647–1666. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chang, S.-H.; Chen, W.-J.; Hung, K.-C.; Lin, Y.-H.; Liu, S.-J.; Hsieh, M.-J.; Pang, J.-H.S.; Juang, J.-H. Augmentation of diabetic wound healing and enhancement of collagen content using nanofibrous glucophage-loaded collagen/PLGA scaffold membranes. J. Colloid Interf. Sci. 2015, 439, 88–97. [Google Scholar] [CrossRef]
- Derakhshan, M.A.; Nazeri, N.; Khoshnevisan, K.; Heshmat, R.; Omidfar, K. Three-layered PCL-collagen nanofibers containing melilotus officinalis extract for diabetic ulcer healing in a rat model. J. Diabetes Metab. Disord. 2022, 21, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Afshar, S.; Rashedi, S.; Nazockdast, H.; Ghazalian, M. Preparation and characterization of electrospun poly(lactic acid)-chitosan core-shell nanofibers with a new solvent system. Int. J. Biol. Macromol. 2019, 138, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mei, S.; Dong, Y.; She, F.; Li, P.; Li, Y.; Kong, L. Multi-Functional Core-Shell Nanofibers for Wound Healing. Nanomaterials 2021, 11, 1546. [Google Scholar] [CrossRef] [PubMed]
- Mwiiri, F.K.; Daniels, R. Influence of PVA molecular weight and concentration on electrospinnability of birch bark extract-loaded nanofibrous scaffolds intended for enhanced wound healing. Molecules 2020, 25, 4799. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, A.W.; Kim, I.S.; Ogasawara, H.; Ni, Q.-Q. Characterizations and application of CA/ZnO/AgNP composite nanofibers for sustained antibacterial properties. Mater. Sci. Eng. C 2019, 105, 110077. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Singh, H.; Joshi, M.; Sharma, A.; Garg, T.; Goyal, A.K.; Rath, G. Recent Advances in Polymeric Electrospun Nanofibers for Drug Delivery. Crit. Rev. Ther. Drug 2014, 31, 187–217. [Google Scholar] [CrossRef]
- Ramadass, S.K.; Nazir, L.S.; Thangam, R.; Perumal, R.K.; Manjubala, I.; Madhan, B.; Seetharaman, S. Type I collagen peptides and nitric oxide releasing electrospun silk fibroin scaffold: A multifunctional approach for the treatment of ischemic chronic wounds. Colloid Surf. B-Biointerfaces 2019, 175, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Khazaeli, P.; Alaei, M.; Khaksarihadad, M.; Ranjbar, M. Preparation of PLA/chitosan nanoscaffolds containing cod liver oil and experimental diabetic wound healing in male rats study. J. Nanobiotechnol. 2020, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Tang, P.F.; Tang, Y.H.; Wei, C.; Wang, Z.M.; Zhang, H.P. Breathable, Moisturizing, Anti-Oxidation SSD-PG-PVA/KGM Fibrous Membranes for Accelerating Diabetic Wound Tissue Regeneration. ACS Appl. Bio Mater. 2022, 5, 2894–2901. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Wang, Y.; Cui, W.G. Advanced electrospun hydrogel fibers for wound healing. Compos. Part B-Eng. 2021, 223, 14. [Google Scholar] [CrossRef]
- Sharma, A.; Panwar, V.; Mondal, B.; Prasher, D.; Bera, M.K.; Thomas, J.; Kumar, A.; Kamboj, N.; Mandal, D.; Ghosh, D. Electrical stimulation induced by a piezo-driven triboelectric nanogenerator and electroactive hydrogel composite, accelerate wound repair. Nano Energy 2022, 99, 107419. [Google Scholar] [CrossRef]
- Noori, S.; Kokabi, M.; Hassan, Z.M. Poly(vinyl alcohol)/chitosan/honey/clay responsive nanocomposite hydrogel wound dressing. J. Appl. Polym. Sci. 2018, 135, 109101. [Google Scholar] [CrossRef]
- Wang, S.G.; Sun, X.F.; Liu, X.W.; Gong, W.X.; Gao, B.Y.; Bao, N. Chitosan hydrogel beads for fulvic acid adsorption: Behaviors and mechanisms. Chem. Eng. J. 2008, 142, 239–247. [Google Scholar] [CrossRef]
- Qian, Z.; Wang, H.; Bai, Y.; Wang, Y.; Tao, L.; Wei, Y.; Fan, Y.; Guo, X.; Liu, H. Improving Chronic Diabetic Wound Healing through an Injectable and Self-Healing Hydrogel with Platelet-Rich Plasma Release. ACS Appl. Mater. Interfaces 2020, 12, 55659–55674. [Google Scholar] [CrossRef]
- Naseri-Nosar, M.; Ziora, Z.M. Wound dressings from naturally-occurring polymers: A review on homopolysaccharide-based composites. Carbohyd. Polym. 2018, 189, 379–398. [Google Scholar] [CrossRef]
- Winter, G.D. Epidermal wound healing under a new polyurethane foam dressing (Lyofoam). Plast. Reconstr. Surg. 1975, 56, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, J.; Zeng, H.; Yu, J. Functional Conductive Hydrogels for Bioelectronics. ACS Mater. Lett. 2020, 2, 1287–1301. [Google Scholar] [CrossRef]
- Zhang, S.M.; Ge, G.R.; Qin, Y.; Li, W.H.; Dong, J.L.; Mei, J.W.; Ma, R.X.; Zhang, X.Z.; Bai, J.X.; Zhu, C.; et al. Recent advances in responsive hydrogels for diabetic wound healing. Mater. Today Bio 2023, 18, 100508. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zeng, X.; Zheng, S.; Wang, Y.; Li, Z.; Zhang, H.; Nie, L.; Zhang, Y.; Zhao, Y.; Yang, X. Bio-adhesive catechol-modified chitosan wound healing hydrogel dressings through glow discharge plasma technique. Chem. Eng. J. 2022, 427, 130843. [Google Scholar] [CrossRef]
- Xuan, H.; Wu, S.; Fei, S.; Li, B.; Yang, Y.; Yuan, H. Injectable nanofiber-polysaccharide self-healing hydrogels for wound healing. Mater. Sci. Eng. C 2021, 128, 112264. [Google Scholar] [CrossRef] [PubMed]
- Ilomuanya, M.O.; Okafor, P.S.; Amajuoyi, J.N.; Onyejekwe, J.C.; Okubanjo, O.O.; Adeosun, S.O.; Silva, B.O. Polylactic acid-based electrospun fiber and hyaluronic acid-valsartan hydrogel scaffold for chronic wound healing. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 13. [Google Scholar] [CrossRef]
- Jirkovec, R.; Samkova, A.; Kalous, T.; Chaloupek, J.; Chvojka, J. Preparation of a Hydrogel Nanofiber Wound Dressing. Nanomaterials 2021, 11, 2178. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Hasan, A.; Dalvi, Y.B.; Rehman, S.R.U.; Varghese, R.; Unni, R.N.; Yalcin, H.C.; Alfkey, R.; Thomas, S.; Al Moustafa, A.-E. Growth factor loaded in situ photocrosslinkable poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/gelatin methacryloyl hybrid patch for diabetic wound healing. Mater. Sci. Eng. C 2021, 118, 111519. [Google Scholar] [CrossRef]
- Chouhan, D.; Lohe, T.-u.; Samudrala, P.K.; Mandal, B.B. In Situ Forming Injectable Silk Fibroin Hydrogel Promotes Skin Regeneration in Full Thickness Burn Wounds. Adv. Healthc. Mater. 2018, 7, 1801092. [Google Scholar] [CrossRef] [PubMed]
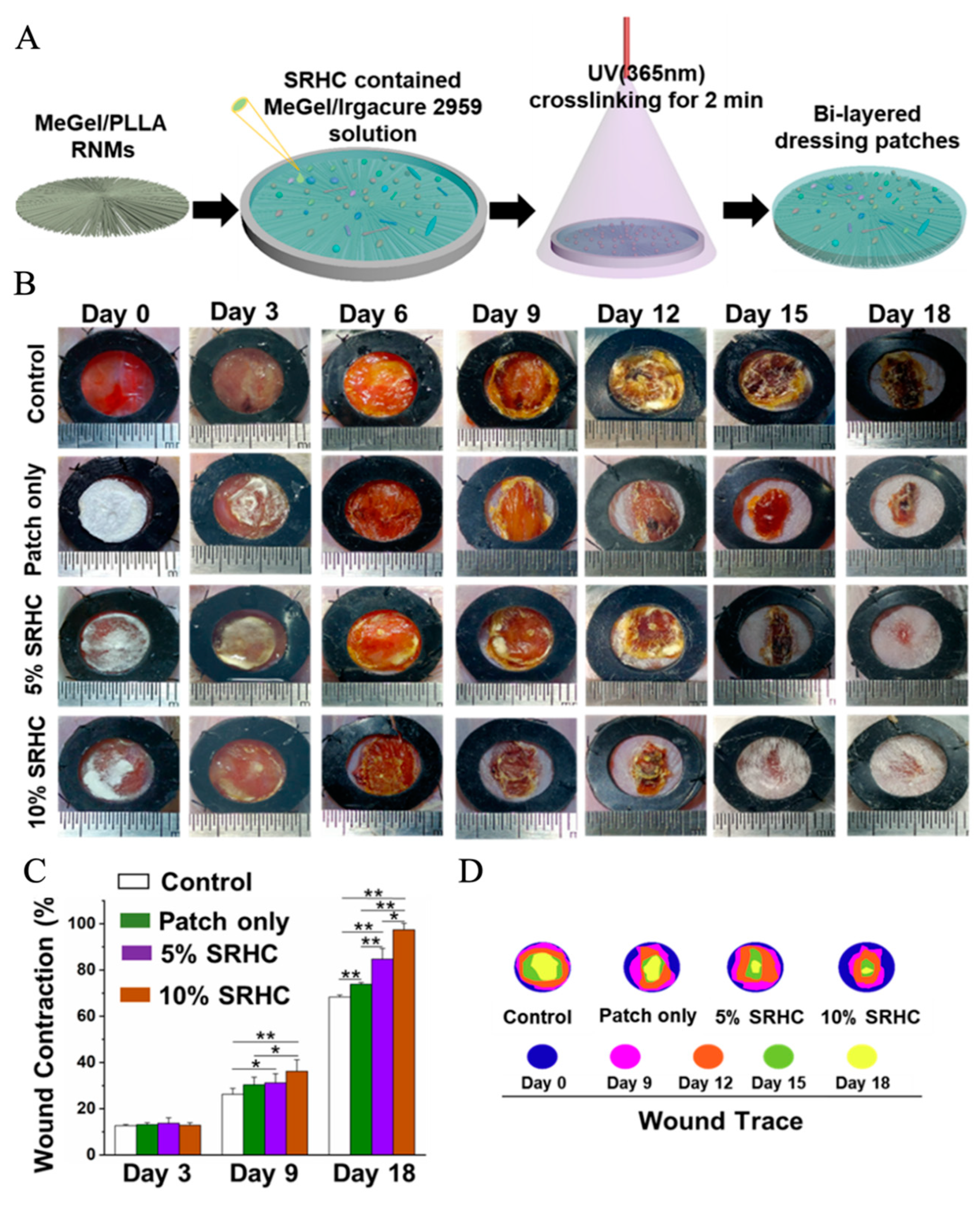
- Wu, S.; Zhao, W.; Sun, M.; He, P.; Lv, H.; Wang, Q.; Zhang, S.; Wu, Q.; Ling, P.; Chen, S. Novel bi-layered dressing patches constructed with radially-oriented nanofibrous pattern and herbal compound-loaded hydrogel for accelerated diabetic wound healing. Appl. Mater. Today 2022, 28, 101542. [Google Scholar] [CrossRef]
- Zhong, H.; Huang, J.; Luo, M.; Fang, Y.; Zeng, X.; Wu, J.; Du, J. Near-field electrospun PCL fibers/GelMA hydrogel composite dressing with controlled deferoxamine-release ability and retiform surface for diabetic wound healing. Nano Res. 2022, 116, 599–612. [Google Scholar] [CrossRef]

| Polymers | Solvent | Mean Fiber Diameter (nm) | Tensile Stress (MPa) | Animal Model | Biological Performance | Ref. |
|---|---|---|---|---|---|---|
| PCL/SF | HFIP | 453–950 | 100.5 ± 5.0 | 6-week-old Kunming mice | Cell proliferation; reduce inflammatory response | [51] |
| PLGA/Collagen | HFIP | 150–650 | 96 ± 13.0 | S.D. rats | Promoting cell proliferation; cell adhesion | [84] |
| DBC/PLA | TFA | NONE | NONE | Hairless mice | Increasing the skin remodeling for wound healing | [93] |
| CA/Zein/Sesame | Acetic acid water | 150–250 | NONE | Normal mice and Diabetic mice | Promoting keratinocyte growth | [102] |
| Gelatin/BC | Acetic acid/DMF | 220–390 | NONE | Sprague Dawley rats | Enhanced adhesion; proliferation and neurite extension | [103] |
| Silk fibroin/Fenugreek | HFIP | 309–439 | 1.90 ± 4.57 | Healthy male Wistar rats | Enhancing proliferation; re-epithelialization | [104] |
| PANI/CS | TFA/DCM | 111–160 | NONE | Staphylococcus aureus | Anti-inflammatory; antibacterial | [105] |
| Silk fibroin/Collagen/ PLCL | HFIP | 72–162 | NONE | NONE | Enhancing adhesion and proliferation; improving hydrophilic | [106] |
| Chitosan/PEO | 90% aqueous acetic acid | 250–330 | 6.4 ± 0.47 | NONE | Good potential for wound dressing | [107] |
| Silk sericin/PVA | Lukewarm water | 130–167 | NONE | Male mice | Antibacterial and antioxidant potential | [108] |
| Gelatin/Fibrinogen | HFIP | 150–300 | 0.0125–0.46 | Hongkong mouse | Revealing higher cell proliferation | [109] |
| Zein/Gelatin | HFIP | 69–950 | NONE | Male mice | Supporting cell adhesion and proliferation | [110] |
| PLA/Gelatin | HFIP | 230–360 | NONE | NONE | Supporting the adhesion and proliferation of cells | [111] |
| Chitosan/PVA | Acetic acid | 90–100 | NONE | Rabbit Schwann cells | Higher water uptake | [112] |
| CNTs/PPDO | HFIP | 214–216 | 225 ± 5.1 | Rabbits | Promoting cell growth; cell regeneration | [113] |
| Polymers | Solvent | Drug | Mean Fiber Diameter (nm) | Animal Model | Biological Performance | Ref. |
|---|---|---|---|---|---|---|
| CA | Acetone and DMF | AgNO3 | 80–130 | Swiss albino mice (25–30 g) | Antibacterial properties, antimicrobial activity | [118] |
| PEO | Mixed ethanol and deionized water | Zein | 156–540 | Kunming mice | Promoting the wound healing process | [122] |
| PLATMC | Deionized water | Gel MA/PAA | NONE | STZ-infected diabetic trauma mouse | Antibacterial | [135] |
| CS/PVA | Deionized water | UA | 100–200 | Qingdao mice | Anti-inflammation, antioxidation | [136] |
| PEG-PCL | Methylene chloride | EGF | NONE | Female C57BL/6 mice (14–16 g) | Enhanced keratinocytic expression | [142] |
| PLCL | Deionized water | Spidroins NTW1-4CT | 239–625 | Spidroins | Improving the cytocompatibility | [143] |
| CA/Gelatin | HFIP | ZM | NONE | Male Wistar rats | Biocompatible and antibacterial | [144] |
| PVA-CMC-PEG | HFIP | NA | NONE | New Zealand rabbits | Biocompatibility | [145] |
| Silk fibroin/PVA | Water | CP/GSNO | NONE | NONE | Promoting cell proliferation and antibacterial | [146] |
| PLGA/Collagen | HFIP | Glucophage | 203–410 | Eighteen Sprague–Dawley rats | Increasing collagen content | [147] |
| PCL/Collagen | Glacial acetic acid | Melilotus officinalis extract | 160–373 | Shaved rats | Regenerate tissues of the skin and prevent infections | [148] |
| PLA/Chitosan | TFA-AA/DMF | Curcumin | NONE | Mice | Anti-inflammatory and antioxidant | [149] |
| CCS | DI water | Ibuprofen | 410–510 | NONE | Promote cell migration and proliferation | [150] |
| PVA | Water | Triterpenes | NONE | NONE | Anti-inflammatory | [151] |
| CA | DMF/Acetone Water | ZnO/AgNPs | NONE | NONE | Antibacterial | [152] |
| Chitosan-PVA | Acetic acid | Lignin | 350-790 | MICE | Biocompatibility and antibacterial | [91] |
| PLGA | Acetone/Dichloromethane/ DMF | Amoxicillin/ Dopamine | NONE | NONE | Promoting cell proliferation | [153] |
| SF-PVA | Water | GSNO | 80–300 | NIH3T3 fibroblast cells | Enhancing blood supply | [154] |
| PLA/Chitosan | Deionized water | Cod liver oil | 50–150 | Kerman University of Medical Animal’s farm | Acceleration of the potential mechanisms | [155] |
| PVA-KGM | Acetic acid | SSD/PG | 250–400 | Dossy laboratory male mice (C57BL/6J) | Good biocompatibility degradability | [156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Zhu, Z.; Zhai, Y.; Chen, S. Recent Advances in Electrospun Nanofiber-Based Strategies for Diabetic Wound Healing Application. Pharmaceutics 2023, 15, 2285. https://doi.org/10.3390/pharmaceutics15092285
Li K, Zhu Z, Zhai Y, Chen S. Recent Advances in Electrospun Nanofiber-Based Strategies for Diabetic Wound Healing Application. Pharmaceutics. 2023; 15(9):2285. https://doi.org/10.3390/pharmaceutics15092285
Chicago/Turabian StyleLi, Kun, Zhijun Zhu, Yanling Zhai, and Shaojuan Chen. 2023. "Recent Advances in Electrospun Nanofiber-Based Strategies for Diabetic Wound Healing Application" Pharmaceutics 15, no. 9: 2285. https://doi.org/10.3390/pharmaceutics15092285




