Pyrenebutyrate Pt(IV) Complexes with Nanomolar Anticancer Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Apparatus and Materials
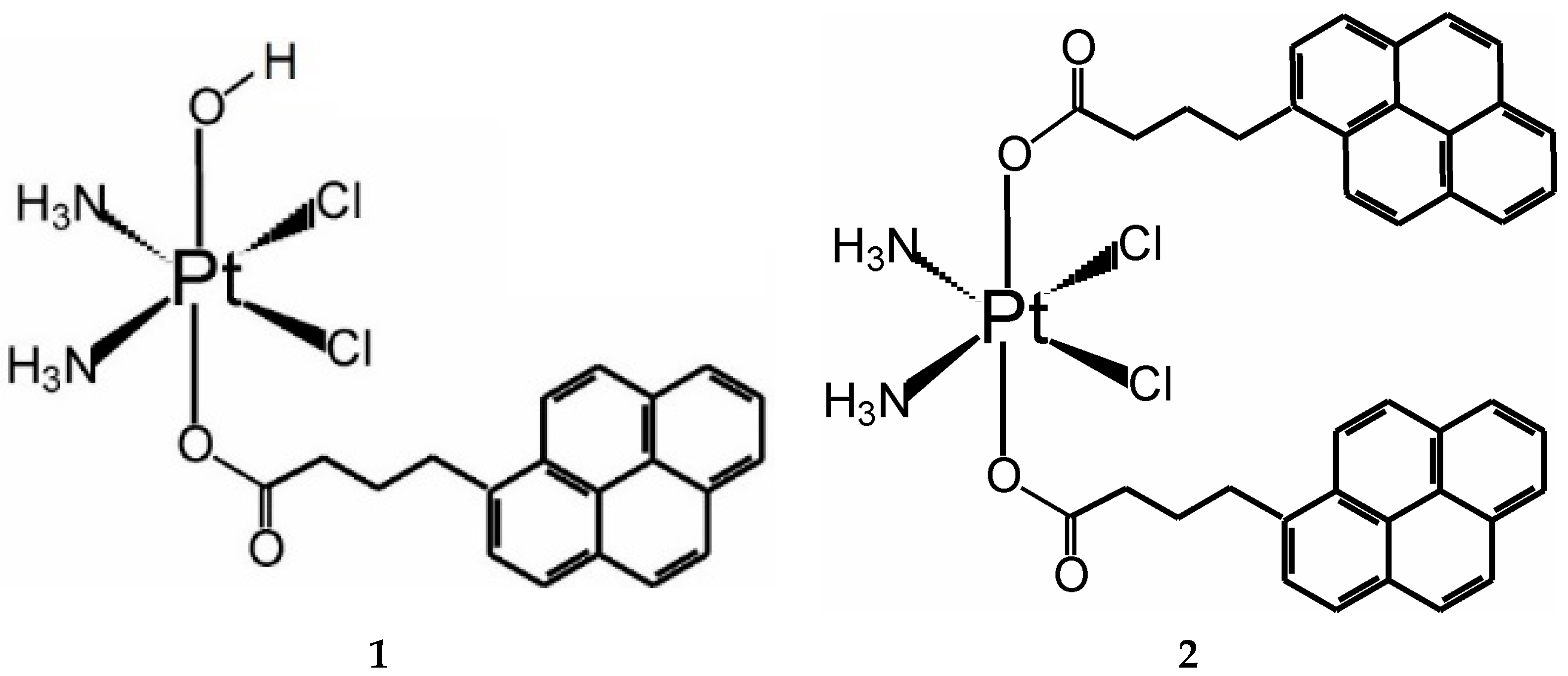
2.2. Synthesis
2.3. Cell Lines and Culture Conditions
2.4. MTT Test for Cytotoxicity Assessment
2.5. Proteome Analysis
2.6. ICP-MS Analysis
2.7. Total Protein Analysis
2.8. Lactate Dehydrogenase (LDH) Activity Assay
2.9. Cell Fractionation for the ICP-MS Analysis
3. Results
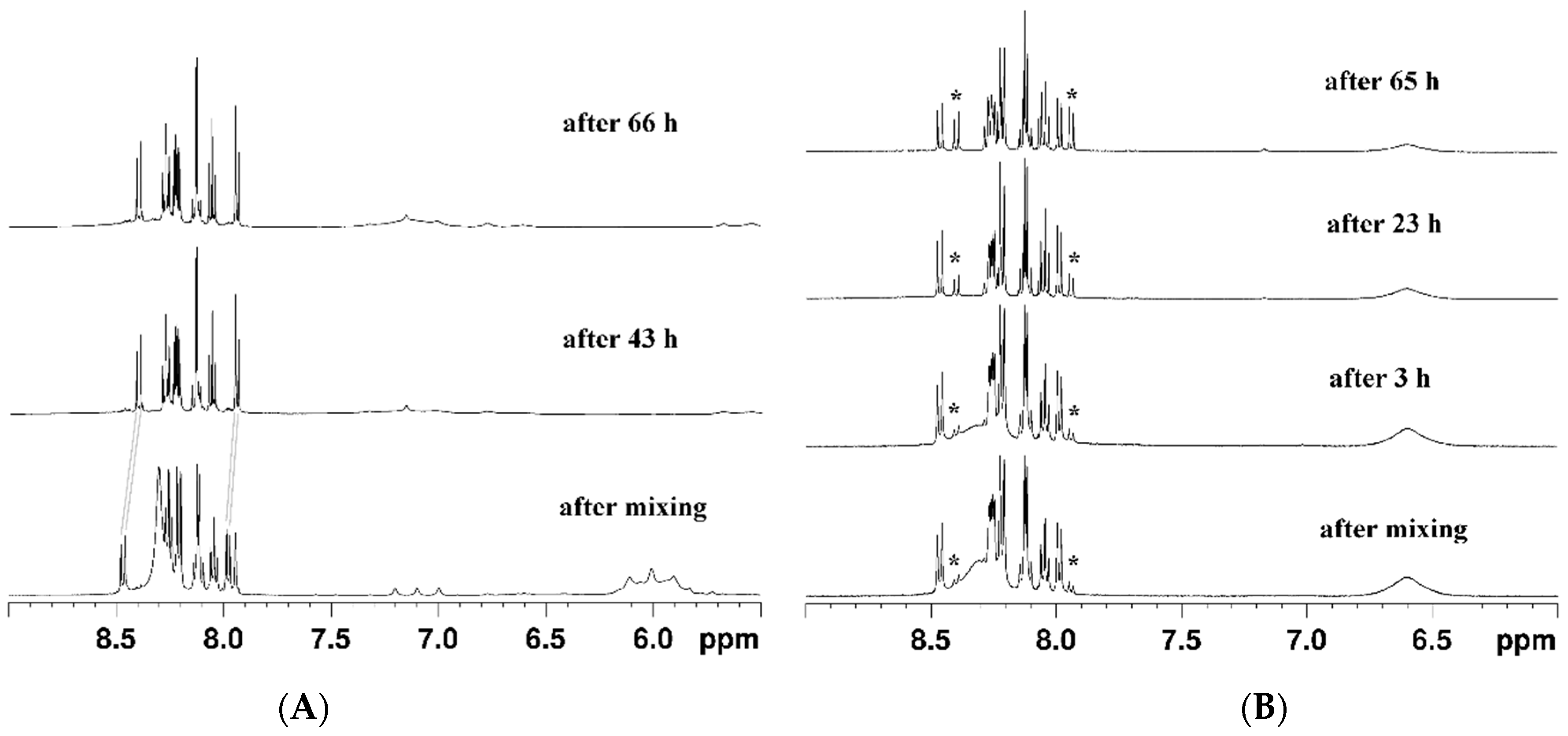
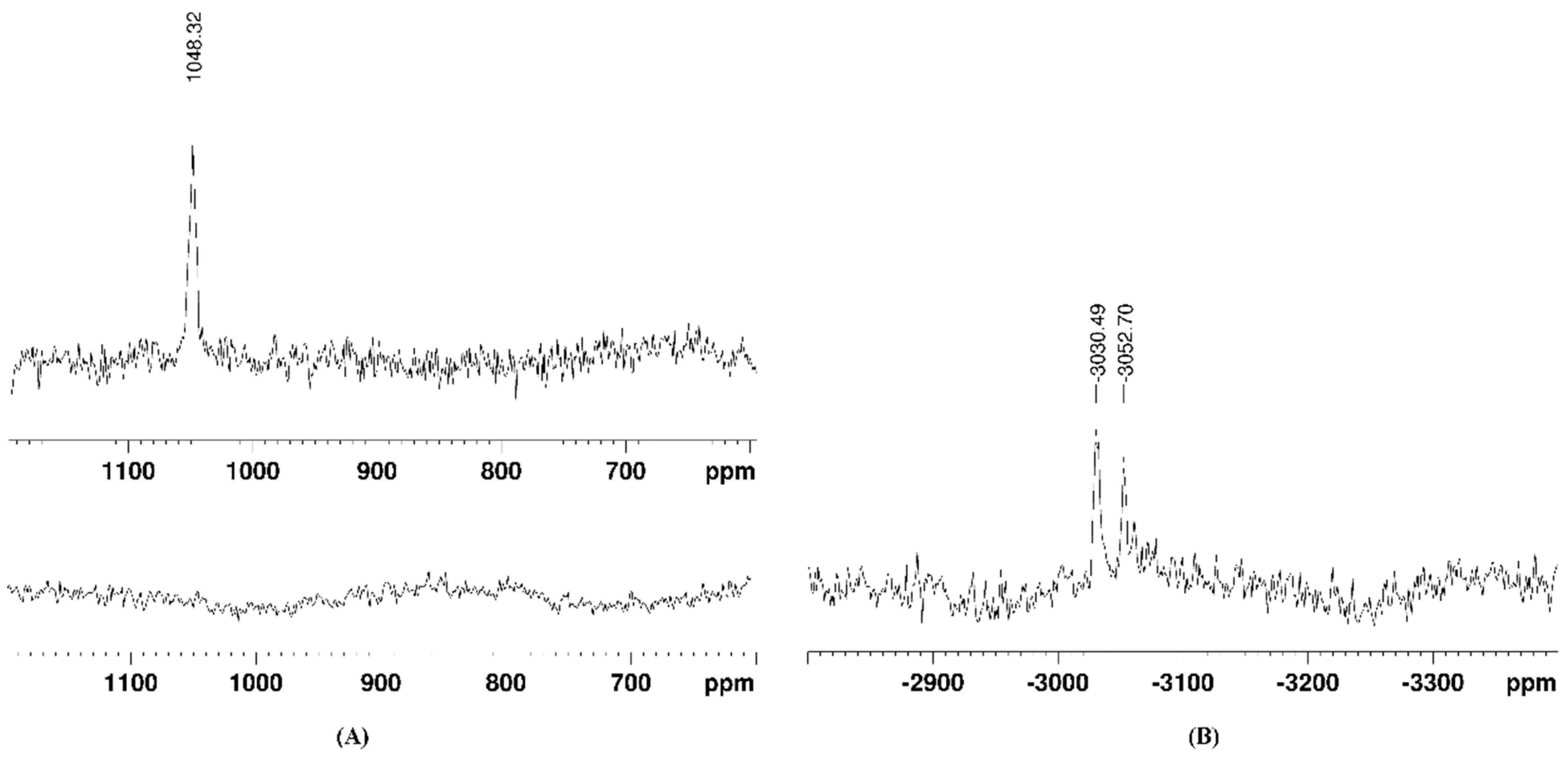
3.1. Synthesis and Stability of the Complexes
3.2. Anticancer Activity
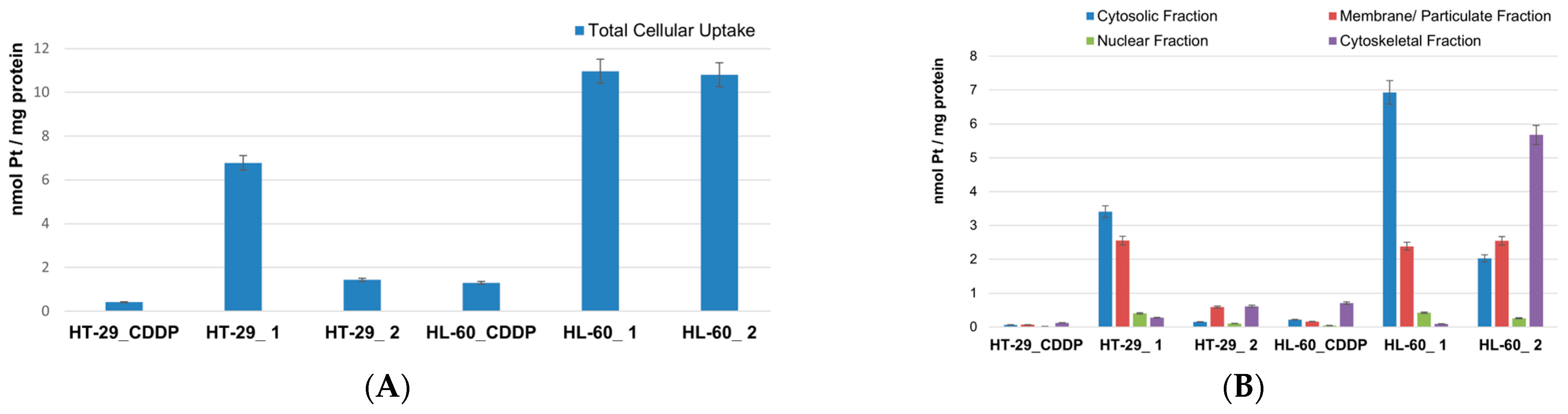
3.3. Platinum Uptake and LDH Activity
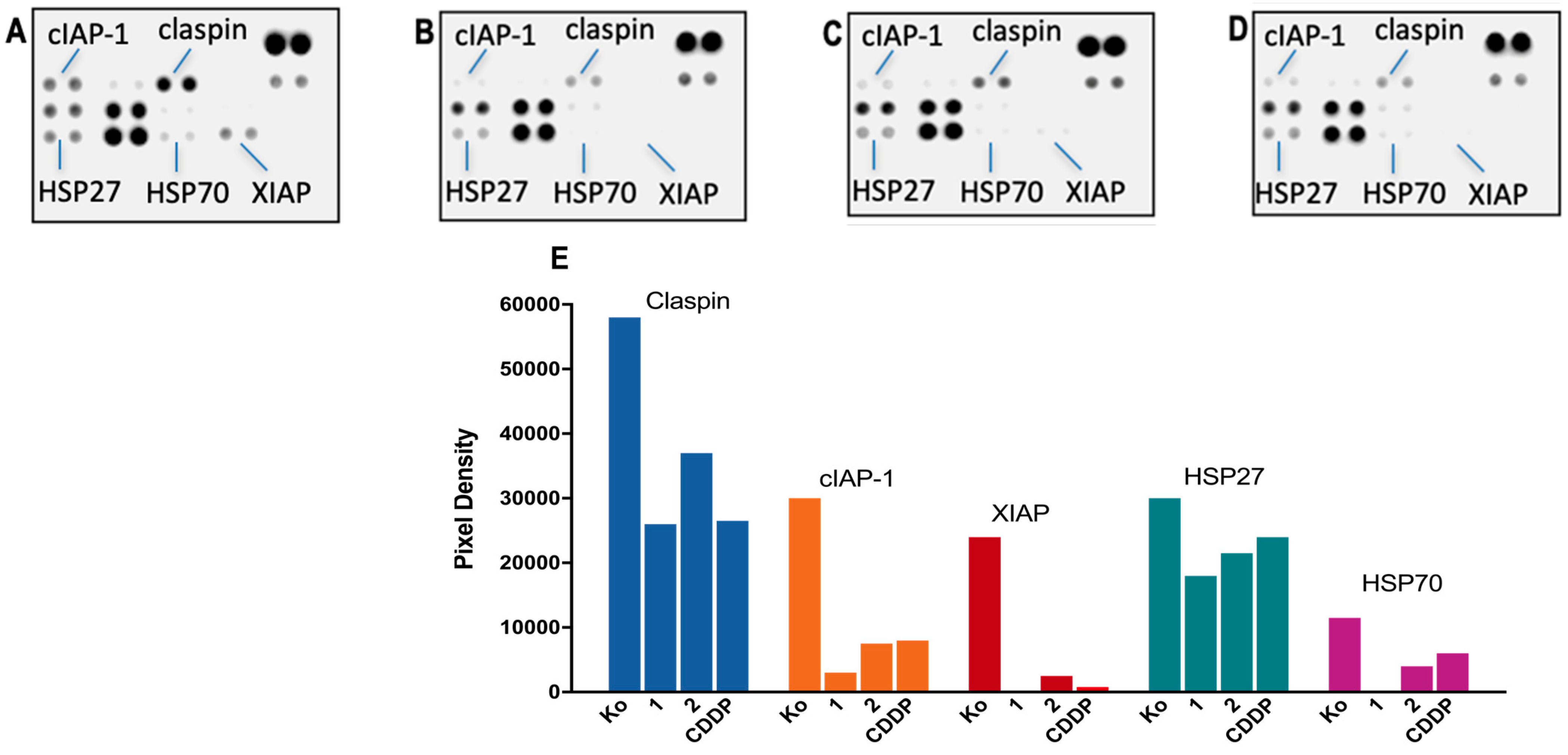
3.4. Proteome Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galanski, M. Recent developments in the field of anticancer platinum complexes. Recent Pat. Anti-Cancer Drug Discov. 2006, 1, 285–295. [Google Scholar] [CrossRef]
- Hall, M.D.; Mellor, H.R.; Callaghan, R.; Hambley, T.W. Basis for design and development of platinum(IV) anticancer complexes. J. Med. Chem. 2007, 50, 3403–3411. [Google Scholar] [CrossRef]
- Reisner, E.; Arion, V.B.; Keppler, B.K.; Pombeiro, A.J.L. Electron-transfer activated metal-based anticancer drugs. Inorg. Chim. Acta 2008, 361, 1569–1583. [Google Scholar] [CrossRef]
- Vouillamoz-Lorenz, S.; Buclin, T.; Lejeune, F.; Bauer, J.; Leyvraz, S.; Decosterd, L.A. Pharmacokinetics of satraplatin (JM216), an oral platinum(IV) complex under daily oral administration for 5 or 14 days. Anticancer Res. 2003, 23, 2757–2765. [Google Scholar]
- Sternberg, C.N.; Whelan, P.; Hetherington, J.; Paluchowska, B.; Slee, P.H.; Vekemans, K.; Van Erps, P.; Theodore, C.; Koriakine, O.; Oliver, T.; et al. Phase III trial of satraplatin, an oral platinum plus prednisone vs. prednisone alone in patients with hormone-refractory prostate cancer. Oncology 2005, 68, 2–9. [Google Scholar] [CrossRef]
- Almotairy, A.R.Z.; Montagner, D.; Morrison, L.; Devereux, M.; Howe, O.; Erxleben, A. Pt(IV) pro-drugs with an axial HDAC inhibitor demonstrate multimodal mechanisms involving DNA damage and apoptosis independent of cisplatin resistance in A2780/A2780cis cells. J. Inorg. Biochem. 2020, 210, 111125. [Google Scholar] [CrossRef]
- Tolan, D.; Almotairy, A.R.Z.; Howe, O.; Devereux, M.; Montagner, D.; Erxleben, A. Cytotoxicity and ROS production of novel Pt(IV)oxaliplatin derivatives with indole propionic acid. Inorg. Chim. Acta 2019, 492, 262–267. [Google Scholar] [CrossRef]
- Almotairy, A.R.Z.; Gandin, V.; Morrison, L.; Marzano, C.; Montagner, D.; Erxleben, A. Antitumor platinum(IV) derivatives of carboplatin and the histone deacetylase inhibitor 4-phenylbutyric acid. J. Inorg. Biochem. 2017, 177, 1–7. [Google Scholar] [CrossRef]
- Tolan, D.; Gandin, V.; Morrison, L.; El-Nahas, A.; Marzano, C.; Montagner, D.; Erxleben, A. Oxidative Stress Induced by Pt(IV) Pro-drugs Based on the Cisplatin Scaffold and Indole Carboxylic Acids in Axial Position. Sci. Rep. 2016, 6, 29367. [Google Scholar] [CrossRef]
- Raveendran, R.; Braude, J.P.; Wexselblatt, E.; Novohradsky, V.; Stuchlikova, O.; Brabec, V.; Gandin, V.; Gibson, D. Pt(IV) derivatives of cisplatin and oxaliplatin with phenylbutyrate axial ligands are potent cytotoxic agents that act by several mechanisms of action. Chem. Sci. 2016, 7, 2381–2391. [Google Scholar] [CrossRef]
- Harringer, S.; Hejl, M.; Enyedy, É.A.; Jakupec, M.A.; Galanski, M.S.; Keppler, B.K.; Dyson, P.J.; Varbanov, H.P. Multifunctional Pt(iv) prodrug candidates featuring the carboplatin core and deferoxamine. Dalton Trans. 2021, 50, 8167–8178. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Bonnitcha, P.; Wexselblatt, E.; Klein, A.V.; Najajreh, Y.; Gibson, D.; Hambley, T.W. Facile preparation of mono-, di- and mixed-carboxylato platinum(IV) complexes for versatile anticancer prodrug design. Chemistry 2013, 19, 1672–1676. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Z.; Deng, Z.; Zhu, G. Recent advances in the synthesis, stability, and activation of platinum(IV) anticancer prodrugs. Coord. Chem. Rev. 2021, 442, 213991. [Google Scholar] [CrossRef]
- Ravera, M.; Gabano, E.; McGlinchey, M.J.; Osella, D. A view on multi-action Pt(IV) antitumor prodrugs. Inorg. Chim. Acta 2019, 492, 32–47. [Google Scholar] [CrossRef]
- Novohradsky, V.; Zerzankova, L.; Stepankova, J.; Vrana, O.; Raveendran, R.; Gibson, D.; Kasparkova, J.; Brabec, V. Antitumor platinum(IV) derivatives of oxaliplatin with axial valproato ligands. J. Inorg. Biochem. 2014, 140, 72–79. [Google Scholar] [CrossRef]
- Abedi, A.; Amani, V.; Safari, N.; Ostad, S.N.; Notash, B. From proton transferred to cyclometalated platinum(IV) complex: Crystal structure and biological activity. J. Organomet. Chem. 2015, 799–800, 30–37. [Google Scholar] [CrossRef]
- Khoury, A.; Sakoff, J.A.; Gilbert, J.; Scott, K.F.; Karan, S.; Gordon, C.P.; Aldrich-Wright, J.R. Cyclooxygenase-Inhibiting Platinum(IV) Prodrugs with Potent Anticancer Activity. Pharmaceutics 2022, 14, 787. [Google Scholar] [CrossRef]
- Spector, D.V.; Pavlov, K.G.; Akasov, R.A.; Vaneev, A.N.; Erofeev, A.S.; Gorelkin, P.V.; Nikitina, V.N.; Lopatukhina, E.V.; Semkina, A.S.; Vlasova, K.Y.; et al. Pt(IV) Prodrugs with Non-Steroidal Anti-inflammatory Drugs in the Axial Position. J. Med. Chem. 2022, 65, 8227–8244. [Google Scholar] [CrossRef]
- Curci, A.; Denora, N.; Iacobazzi, R.M.; Ditaranto, N.; Hoeschele, J.D.; Margiotta, N.; Natile, G. Synthesis, characterization, and in vitro cytotoxicity of a Kiteplatin-Ibuprofen Pt(IV) prodrug. Inorg. Chim. Acta 2018, 472, 221–228. [Google Scholar] [CrossRef]
- Spector, D.; Krasnovskaya, O.; Pavlov, K.; Erofeev, A.; Gorelkin, P.; Beloglazkina, E.; Majouga, A. Pt(IV) prodrugs with nsaids as axial ligands. Int. J. Mol. Sci. 2021, 22, 3817. [Google Scholar] [CrossRef]
- Gibson, D. Multi-action Pt(IV) anticancer agents; do we understand how they work? J. Inorg. Biochem. 2019, 191, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Petruzzella, E.; Sirota, R.; Solazzo, I.; Gandin, V.; Gibson, D. Triple action Pt(IV) derivatives of cisplatin: A new class of potent anticancer agents that overcome resistance. Chem. Sci. 2018, 9, 4299–4307. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, N.; Ma, R.; Li, C.; Xu, Z.; Tse, M.K.; Zhu, G. Monochalcoplatin: An Actively Transported, Quickly Reducible, and Highly Potent PtIV Anticancer Prodrug. Angew. Chem.-Int. Ed. 2018, 57, 9098–9102. [Google Scholar] [CrossRef] [PubMed]
- Gabano, E.; Rangone, B.; Perin, E.; Caron, G.; Ermondi, G.; Vallaro, M.; Gandin, V.; Marzano, C.; Barbanente, A.; Margiotta, N.; et al. Pt(iv) complexes based on cyclohexanediamines and the histone deacetylase inhibitor 2-(2-propynyl)octanoic acid: Synthesis, characterization, cell penetration properties and antitumor activity. Dalton Trans. 2021, 50, 4663–4672. [Google Scholar] [CrossRef]
- Deo, K.M.; Sakoff, J.; Gilbert, J.; Zhang, Y.; Aldrich Wright, J.R. Synthesis, characterisation and influence of lipophilicity on cellular accumulation and cytotoxicity of unconventional platinum(IV) prodrugs as potent anticancer agents. Dalton Trans. 2019, 48, 17228–17240. [Google Scholar] [CrossRef]
- Varbanov, H.; Valiahdi, S.M.; Legin, A.A.; Jakupec, M.A.; Roller, A.; Galanski, M.S.; Keppler, B.K. Synthesis and characterization of novel bis(carboxylato) dichloridobis(ethylamine)platinum(IV) complexes with higher cytotoxicity than cisplatin. Eur. J. Med. Chem. 2011, 46, 5456–5464. [Google Scholar] [CrossRef]
- Margiotta, N.; Savino, S.; Marzano, C.; Pacifico, C.; Hoeschele, J.D.; Gandin, V.; Natile, G. Cytotoxicity-boosting of kiteplatin by Pt(IV) prodrugs with axial benzoate ligands. J. Inorg. Biochem. 2016, 160, 85–93. [Google Scholar] [CrossRef]
- Barbanente, A.; Gandin, V.; Ceresa, C.; Marzano, C.; Ditaranto, N.; Hoeschele, J.D.; Natile, G.; Arnesano, F.; Pacifico, C.; Intini, F.P.; et al. Improvement of Kiteplatin Efficacy by a Benzoato Pt(IV) Prodrug Suitable for Oral Administration. Int. J. Mol. Sci. 2022, 23, 7081. [Google Scholar] [CrossRef]
- Höfer, D.; Varbanov, H.P.; Legin, A.; Jakupec, M.A.; Roller, A.; Galanski, M.; Keppler, B.K. Tetracarboxylatoplatinum(IV) complexes featuring monodentate leaving groups—A rational approach toward exploiting the platinum(IV) prodrug strategy. J. Inorg. Biochem. 2015, 153, 259–271. [Google Scholar] [CrossRef]
- Hoffmeister, B.R.; Hejl, M.; Jakupec, M.A.; Galanski, M.; Keppler, B.K. Bis- and Tris(carboxylato)platinum(IV) Complexes with Mixed Am(m)ine Ligands in the trans Position Exhibiting Exceptionally High Cytotoxicity. Eur. J. Inorg. Chem. 2015, 2015, 1700–1708. [Google Scholar] [CrossRef]
- Hoffmeister, B.R.; Hejl, M.; Adib-Razavi, M.S.; Jakupec, M.A.; Galanski, M.; Keppler, B.K. Bis- and tetrakis(carboxylato)platinum(IV) complexes with mixed axial ligands-Synthesis, characterization, and cytotoxicity. Chem. Biodivers. 2015, 12, 559–574. [Google Scholar] [CrossRef]
- Song, X.Q.; Liu, R.P.; Wang, S.Q.; Li, Z.; Ma, Z.Y.; Zhang, R.; Xie, C.Z.; Qiao, X.; Xu, J.Y. Anticancer Melatplatin Prodrugs: High Effect and Low Toxicity, MT1-ER-Target and Immune Response in Vivo. J. Med. Chem. 2020, 63, 6096–6106. [Google Scholar] [CrossRef] [PubMed]
- Ahmedova, A.; Momekova, D.; Yamashina, M.; Shestakova, P.; Momekov, G.; Akita, M.; Yoshizawa, M. Anticancer Potencies of PtII-and PdII-linked M2L4 Coordination Capsules with Improved Selectivity. Chem. Asian J. 2016, 11, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Ahmedova, A.; Mihaylova, R.; Momekova, D.; Shestakova, P.; Stoykova, S.; Zaharieva, J.; Yamashina, M.; Momekov, G.; Akita, M.; Yoshizawa, M. M2L4 coordination capsules with tunable anticancer activity upon guest encapsulation. Dalton Trans. 2016, 45, 13214–13221. [Google Scholar] [CrossRef]
- Ahmedova, A.; Mihaylova, R.; Stoykova, S.; Mihaylova, V.; Paunova-Krasteva, T.; Mihaylov, L.; Stoitsova, S.; Nihtianova, D.; Momekov, G.; Momekova, D.; et al. Enhanced cellular uptake of platinum by a tetracationic Pt(II) nanocapsule and its implications to cancer treatment. Eur. J. Pharm. Sci. 2020, 155, 105545–105555. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Gomez, M.B.; Gomez, M.M.; Palacios, M.A. Control of interferences in the determination of Pt, Pd and Rh in airborne particulate matter by inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2000, 404, 285–294. [Google Scholar] [CrossRef]
- Stoscheck, C.M. [6] Quantitation of protein. In Methods in Enzymology. Guide to Protein Purification; Deutscher, M.P., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 1990; pp. 50–68. [Google Scholar]
- Ang, W.H.; Pilet, S.; Scopelliti, R.; Bussy, F.; Juillerat-Jeanneret, L.; Dyson, P.J. Synthesis and characterization of platinum(IV) anticancer drugs with functionalized aromatic carboxylate ligands: Influence of the ligands on drug efficacies and uptake. J. Med. Chem. 2005, 48, 8060–8069. [Google Scholar] [CrossRef]
- Kerrison, S.J.S.; Sadler, P.J. 195Pt NMR studies of platinum(II) dimethylsuphoxide complexes. Inorg. Chim. Acta 1985, 104, 197–201. [Google Scholar] [CrossRef]
- Pickering, R.W. Toxicity of polyaromatic hydrocarbons other than benzo(a)pyrene: A review. J. Toxicol.-Cut. Ocul. Toxicol. 2000, 19, 55–67. [Google Scholar]
- Bonsignore, R.; Notaro, A.; Salvo, A.M.P.; Spinello, A.; Fiasconaro, G.; Terenzi, A.; Giacalone, F.; Keppler, B.K.; Giuliano, M.; Gruttadauria, M.; et al. DNA-Binding and Anticancer Activity of Pyrene-Imidazolium Derivatives. ChemistrySelect 2016, 1, 6755–6761. [Google Scholar] [CrossRef]
- Mattsson, J.; Govindaswamy, P.; Furrer, J.; Sei, Y.; Yamaguchi, K.; Suss-Fink, G.; Therrien, B. Encapsulation of Aromatic Molecules in Hexanuclear Arene Ruthenium Cages: A Strategy to Build Up Organometallic Carceplex Prisms with a Dangling Arm Standing Out. Organometallics 2008, 27, 4346–4356. [Google Scholar] [CrossRef]
- Pitto-Barry, A.; Zava, O.; Dyson, P.J.; Deschenaux, R.; Therrien, B. Enhancement of Cytotoxicity by Combining Pyrenyl-Dendrimers and Arene Ruthenium Metallacages. Inorg. Chem. 2012, 51, 7119–7124. [Google Scholar] [CrossRef] [PubMed]
- Pitto-Barry, A.; Barry, N.P.E.; Zava, O.; Deschenaux, R.; Dyson, P.J.; Therrien, B. Double Targeting of Tumours with Pyrenyl-Modified Dendrimers Encapsulated in an Arene–Ruthenium Metallaprism. Chem. Eur. J. 2011, 17, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Toshihide, T.; Michie, K.; Akiko, T.; Yukio, S.; Mayumi, N.; Mitsuhiro, K.; Naomi, S.; Stefan, M.; Shiroh, F. Direct and Rapid Cytosolic Delivery Using Cell-Penetrating Peptides Mediated by Pyrenebutyrate. ACS Chem. Biol. 2006, 1, 299–304. [Google Scholar]
- Jablonski, A.E.; Kawakami, T.; Ting, A.Y.; Payne, C.K. Pyrenebutyrate Leads to Cellular Binding, Not Intracellular Delivery, of Polyarginine Quantum Dots. J. Phys. Chem. Lett. 2010, 1, 1312–1315. [Google Scholar] [CrossRef][Green Version]
- Lee, K.G.Z.; Babak, M.V.; Weiss, A.; Dyson, P.J.; Nowak-Sliwinska, P.; Montagner, D.; Ang, W.H. Development of an Efficient Dual-Action GST-Inhibiting Anticancer Platinum(IV) Prodrug. ChemMedChem 2018, 13, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, G.; Olszewski, U.; Ach, F.; Ulsperger, E.; Baumgartner, G.; Zeillinger, R.; Bednarski, P. In vitro evaluation of oxoplatin: An oral platinum(IV) anticancer agent. Met.-Based Drugs 2009, 2009, 12. [Google Scholar] [CrossRef]
- Velcheva, V.; Hegetschweiler, K.; Momekov, G.; Ivanova, S.; Ugrinov, A.; Morgenstern, B.; Gencheva, G. Platinum(IV) Complexes of the 1,3,5-Triamino Analogue of the Biomolecule Cis-Inositol Designed as Innovative Antineoplastic Drug Candidates. Pharmaceutics 2022, 14, 2057. [Google Scholar] [CrossRef]
- Smits, V.A.J.; Cabrera, E.; Freire, R.; Gillespie, D.A. Claspin-checkpoint adaptor and DNA replication factor. FEBS J 2019, 286, 441–455. [Google Scholar] [CrossRef]
- Gill, C.; Dowling, C.; O’Neill, A.J.; Watson, R.W. Effects of cIAP-1, cIAP-2 and XIAP triple knockdown on prostate cancer cell susceptibility to apoptosis, cell survival and proliferation. Mol. Cancer 2009, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, J.; Gaucher-Di-Stasio, C.; Weidmann, S.; Guzzo, J.; Garrido, C. Quantification of HSP27 and HSP70 molecular chaperone activities. In Molecular Chaperones. Methods in Molecular Biology; Calderwood, S., Prince, T., Eds.; Humana Press: Clifton, NJ, USA, 2011; Volume 787, pp. 137–143. [Google Scholar]
| Cell Line | CDDP | Complex 1 | Fold-Increase (FI) | Complex 2 | Pyrene |
|---|---|---|---|---|---|
| HEK-293 a | 13.8 ± 3.3 | 0.2 ± 0.1 | 69 | n.d. (>200) | - |
| CASKI b | 16.2 ± 1.8 | 0.15 ± 0.06 | 108 | n.d. (>200) | - |
| MDA-MB-231 c | 48.3 ± 3.1 | 0.7 ± 0.2 | 69 | n.d. (>200) | - |
| MCF-7 d | 55.5 ± 4.3 | 0.3 ± 0.15 | 185 | n.d. (>200) | - |
| HT-29 e | 36.6 ± 1.5 | 3.4 ± 0.7 | 10.8 | 90.0 ± 10.2 | >100 * |
| T-24 f | 10.4 ± 0.8 | 0.06 ± 0.01 | 173 | 4.9 ± 1.1 | >100 * |
| HL-60 g | 9.8 ± 1.1 | 0.07 ± 0.01 | 140 | 18.1 ± 3.9 | >100 * |
| HL-60/Dox h | 32.9 ± 1.2 | 0.1 ± 0.01 | 329 | 2.2 ± 0.5 | >100 * |
| RF | 3.4 | 1.4 | 0.12 | ||
| HL-60/CDDP i | 135.2 ± 2.9 | 0.05 ± 0.01 | 2704 | 1.9 ± 0.8 | - |
| RF | 13.8 | 0.7 | 0.06 | ||
| REH j | 1.1 ± 0.8 | 0.2 ± 0.03 | 5.5 | 3.1 ± 0.9 | - |
| SKW-3 k | 8.3 ± 0.9 | 0.06 ± 0.01 | 138 | 9.8 ± 2.0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmedova, A.; Mihaylova, R.; Stoykova, S.; Mihaylova, V.; Burdzhiev, N.; Elincheva, V.; Momekov, G.; Momekova, D. Pyrenebutyrate Pt(IV) Complexes with Nanomolar Anticancer Activity. Pharmaceutics 2023, 15, 2310. https://doi.org/10.3390/pharmaceutics15092310
Ahmedova A, Mihaylova R, Stoykova S, Mihaylova V, Burdzhiev N, Elincheva V, Momekov G, Momekova D. Pyrenebutyrate Pt(IV) Complexes with Nanomolar Anticancer Activity. Pharmaceutics. 2023; 15(9):2310. https://doi.org/10.3390/pharmaceutics15092310
Chicago/Turabian StyleAhmedova, Anife, Rositsa Mihaylova, Silviya Stoykova, Veronika Mihaylova, Nikola Burdzhiev, Viktoria Elincheva, Georgi Momekov, and Denitsa Momekova. 2023. "Pyrenebutyrate Pt(IV) Complexes with Nanomolar Anticancer Activity" Pharmaceutics 15, no. 9: 2310. https://doi.org/10.3390/pharmaceutics15092310
APA StyleAhmedova, A., Mihaylova, R., Stoykova, S., Mihaylova, V., Burdzhiev, N., Elincheva, V., Momekov, G., & Momekova, D. (2023). Pyrenebutyrate Pt(IV) Complexes with Nanomolar Anticancer Activity. Pharmaceutics, 15(9), 2310. https://doi.org/10.3390/pharmaceutics15092310








