APIs were manufactured by Merck. MCC (Avicel® PH102, Rahway, NJ, USA) and croscarmellose sodium (Ac-Di-Sol®) were supplied by Dow/DuPont, now IFF, Midland, MI, USA. Lactose monohydrate 312 was supplied by Kerry, Norwich, NY, USA. PVP (Kollidon® K25) was supplied by BASF, Ludwigshafen, Germany. Magnesium stearate (HyqualTM) was manufactured by Mallinckrodt, St. Louis, MO, USA.
2.1. Formulations
In order to understand the impact of excipient and API properties, several formulations were evaluated in this study:
(1) A placebo formulation of conventional wet granulation excipients microcrystalline cellulose (MCC) and lactose (
Table 1); (2) a formulation of a hydrophilic API, MK-A, with a high level of lactose monohydrate, known to readily granulate (
Table 2); and (3) two formulations of a hydrophobic API, MK-B (
Table 3 and
Table 4). These formulations were studied because of prior experience with batch wet granulation at pilot and commercial scale for clinical development, prior twin-screw wet granulation evaluation with the Leistritz 18 mm, and the availability of bulk quantities of the APIs. A separate MK-B formulation (
Table 4) with more MCC was also studied with the intention of improving the performance of the formulation over the reference formulation. MCC can absorb water, so it was expected that high-quality granules could be made at lower granulation fluid levels (GFL), also known as liquid-to-solid ratios (L/S). L/S is reported as the ratio of the total binder liquid feed rate to the dry solid feed rate.
2.2. Continuous Granulation and Drying
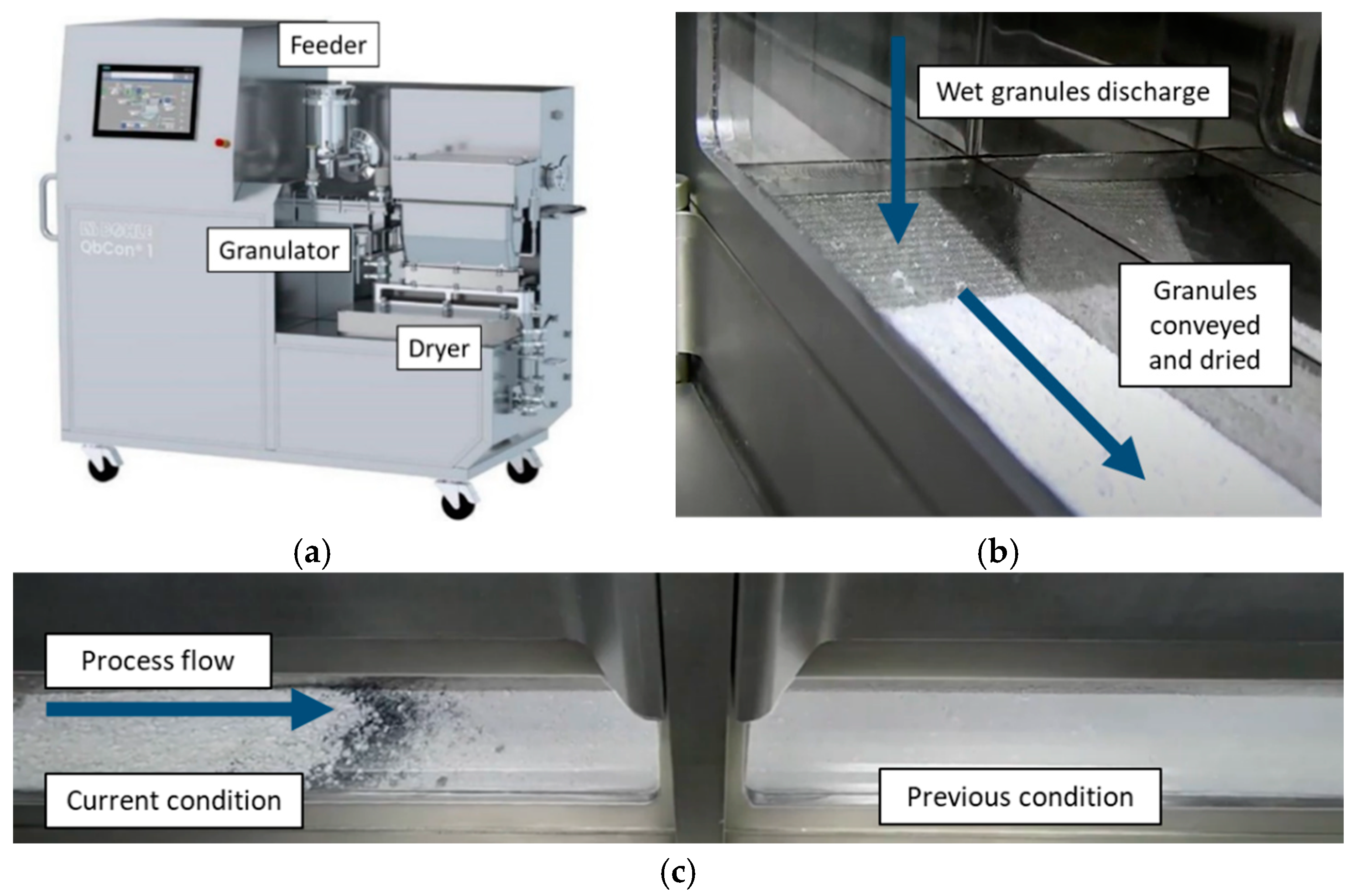
This work focuses on continuous granulation and drying (QbCon 1, L.B. Bohle, Ennigerloh, Germany) process performance, output granule properties, and downstream tabletability. The QbCon 1 granulation process used the screw and barrel profiles shown in
Figure 2 below. Pre-blends were prepared by tumble blending in approximately 10 kg batches within a 40 L tote blender (LMA-40, L.B.Bohle, Ennigerloh, Germany) for 10 min at 26 rpm. The pre-blends were charged into an integrated loss-in-weight feeder (GZD150.12, Gericke, Regensdorf, Switzerland) feeding into the granulator barrel under gravimetric control at the set points indicated. Binder solutions were prepared at the proper concentration to achieve the desired PVP concentration at the target L/S level, from 11–29%
w/
w. The binder solution was metered into the barrel using an integrated pump. The liquid and solid feed rates and screw speed for granulation were adjusted to different conditions. The wet granules were discharged for several minutes until they appeared visually consistent and then they were fed into the continuous dryer. The dryer air temperature, air flow rate, and vibration acceleration were then adjusted so that the dried granules were less than 2% loss on drying (LOD). The vibration rate is controlled as a dryer acceleration setting, with adjustments based on a qualitative assessment of fluidization and bed height. The process setpoints and resulting LODs are listed in
Table 5.
Residence time distribution (RTD) through the VFBD was measured by a tracer study using blue granules made using the 20% w/w MCC placebo formulation where blue dye was added to the feed of the granulator and collected. The blue granules were manually added to the dryer and the discharge color was measured using a camera and image analysis software (ExtruVis 3, MeltPrep GmbH, Graz, Austria). Residence time distributions (RTDs) in the dryer were measured across a typical range of acceleration settings, from 4–8 m/s2, with constant dryer airflow.
Dried granules were tested for LOD (HR73 Halogen moisture analyzer, Mettler-Toledo HR 73, Columbus, OH, USA) with the endpoint at weight change <1 mg over a 140 s run time, with a temperature of 90 °C. After the LOD was confirmed, dried granules were discharged to waste for approximately 5 min and then collected in bulk for downstream processing.
These results were compared to granules that were previously made by other granulation processes: (1) Continuous twin-screw wet granulation (Leistritz 18 mm, Somerville, NJ, USA) granulated at 0.9 kg/h with 5% L/S at 450 rpm for MK-A and a 2 kg/h solid feed rate with 15–25% L/S at 200 rpm for MK-B F2, followed by batch tray drying (Isotemp® oven, Fisher Scientific, Dubuque, IA, USA); (2) Previously made batch granulated granules. The reference MK-A batch granulated formulation was granulated using FBG with a 450–550 cfm inlet air flow, a 240–270 g/min spray rate, a 60 °C inlet air temperature, and dried <1.5% LOD. This batch was made at a 70 kg scale using a Niro® MP-4 (GEA, Columbia, MD, USA). The reference MK-B batch process formulation was granulated using HSWG at a 5 kg scale with wet binder addition to 17% L/S using a 25 L bowl (Diosna Dierks & Söhne GmbH, Osnabrück, Germany) followed by fluid-bed drying (Niro® MP-1, GEA, Columbia, MD, USA) to <2% LOD using 60 °C inlet air with 425–550 cfm airflow. The processing conditions for batch granulation and drying were based on previously developed large-scale conditions for these products.
2.3. Product Performance Characterization
2.3.1. Granule Size Distributions
The dried, unmilled granules were measured in triplicate using dynamic image analysis (QICPIC with RODOS, Sympatec, Inc., Pennington, NJ, USA) with vibratory dry feed (VIBRI), 2 bar dispersion pressure, and an M7 measuring range. The reported particle size attributes are based on volume mean distributions.
2.3.2. Scanning Electron Microscopy (SEM)
Environmental scanning electron microscopy (SEM) was performed at low vacuum with a 20 kV accelerating voltage and 3.0 nm spot size using a Large-Field Detector on a Quanta FEG 250 (FEI, Hillsboro, OR, USA). The samples were not sputter coated.
2.3.3. Bulk/Tap Densities
Bulk and tap density were measured using approximately 10 g of granules in a 25 mL graduated cylinder. Tapping was performed 500 times (Autotap, Anton Paar QuantaTec Inc., Boynton Beach, FL, USA). From these data, the Carr index (Equation (1)) was calculated to describe the flow behavior of the granules.
2.3.4. Tabletability
Tableting blends of unmilled test granules were prepared in glass bottles using tumble blending (TURBULA T2F, Willy A. Bachofen AG, Muttenz, Switzerland) for 5 min at 46 rpm followed by lubrication with #30 sieved magnesium stearate for 3 min at 46 rpm.
Tablets were manufactured using a 3/8″-diameter, round, flat-faced punch. The tablet blend was compressed at different compaction forces using a compaction simulator (Roland Research Devices, Inc., Ewing, NJ, USA) and the resulting tablets were tested for weight, thickness, and hardness (Dr. Schleuniger, Sotax, Inc., Westborough, MA, USA). Formulation tabletability was estimated based on the tablet tensile strength (Equation (2)) versus compaction pressure [
21].