A Guide to Best Practice in Sensory Analysis of Pharmaceutical Formulations †
Abstract
:1. Introduction
2. Useful Non-Sensory Analytical Data
2.1. Dissolution
2.2. Rheology/Texture Analysis
3. Sensory Data from Non-Human Studies
3.1. Electronic Tongue
3.1.1. Molecule Selection
3.1.2. Taste Masking
3.1.3. Stability Assessment
3.2. Electronic Nose
3.3. Rodent Brief-Access Taste Aversion BATA Model
3.4. Colour/Appearance
4. Human Sensory Analysis
4.1. Risk Assessment
4.1.1. Introduction
4.1.2. Study Type
4.1.3. Risk Assessment Flow Chart
4.2. Sensory Study Design and Execution Decision Tool
4.2.1. Introduction
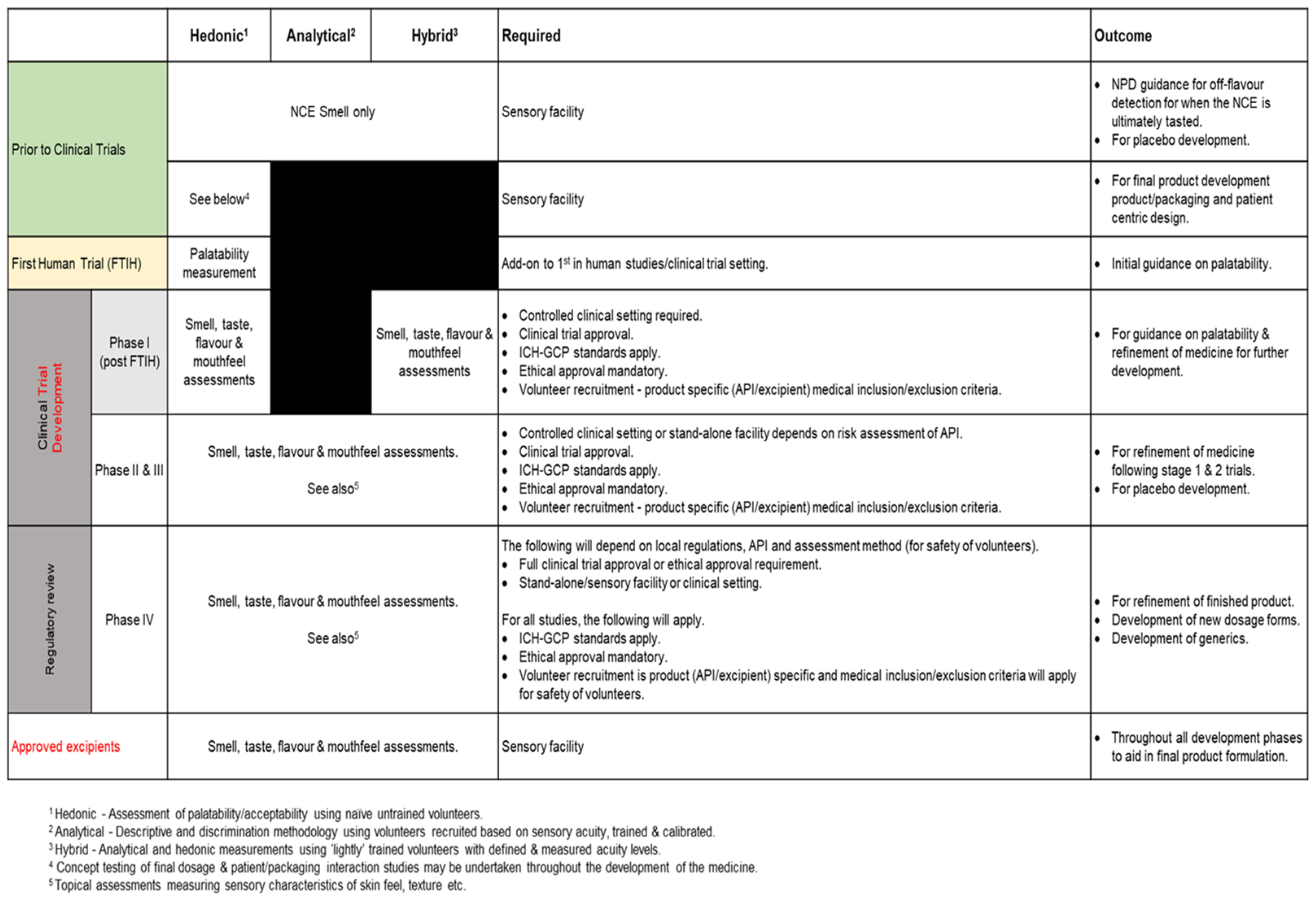
4.2.2. What Studies Can Be Undertaken at Each Stage of Product Development?
- Prior to Clinical Trials
- First Time in Human
- Product Development Phase—Post First in Human/Phase I
- Product Development—Phase II and III
- Product Development Phase—Phase IV
- Excipients
4.2.3. How to Conduct Sensory Studies
- Method Selection
- Panel Selection
4.2.4. Documentation and Performance of the Study
- The Study Aims and Objectives
- The Number and Type of Volunteer Assessors
- Informed Consent Procedure
- How Samples will Be Prepared and Handled
- Facilities where the Test Will Take Place
- Instructions to the Volunteer Assessors
- How the Data Will Be Captured
- How the Data Will Be Analysed
- Data Reporting
4.2.5. Paediatric Specific Aspects
- The age/developmental status of the child. ADME (absorption, distribution, metabolism, and excretion) aspects change with age. Young children may be more vulnerable to any adverse effects from any API that is absorbed. Older children are more likely to be similar to adults in this regard. A thorough knowledge of the toxicological effects (including, where possible, developmental effects) of the API/formulation is required in order to evaluate the nature/magnitude of any increased risk. For this reason, most early studies are undertaken in adults. Unless this evaluation clearly demonstrates that the study is low risk in children, it will also need to be undertaken under full clinical supervision.
- The capability of the child—this will influence whether they can be expected to understand and follow the study instructions (influencing how samples should be given) and how to record their responses. Different methods of gathering responses to ensure that scientifically valid data are generated are appropriate at different stages of development (often, but not always, linked with age).
- Informed consent—ideally this will be provided by the child themselves, although it may be necessary for this to be sought from the parent/carer. The topic of informed consent in this demographic is a large one. A detailed discussion is outside the scope of this paper.
5. Discussion/Conclusions/Recommendations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Survey of Regulators—Summary of Responses
- Q1. Do you agree that a “swirl and spit” sensory study is not a Clinical Trial of an Investigational Medicinal Product (IMP) as defined by the EU Directive 2001/20/EC?
- Q2. Do such “swirl and spit” studies require an authorization from, or a notification to, your national agency?
- Q3. Is there another entity or governing body (apart from ethical approval) to whom such studies should be referred to or would manage/control/oversee these studies in your member state?
- Q4. If your agency does monitor such studies would your requirements differ if the material being tested is a new chemical entity or an established product?
- Q5. Does your national agency have any requirements for what data should be provided and/or the format in which the data should be provided that is derived from human sensory evaluation studies? In addition: Q7 What evidence would your national agency ask for if a sponsor wished to make a claim about the sensory aspects of their product (for example that the product has superior palatability to a previous formulation)?
- Q6. Would your national agency consider data from palatability assessments performed with a panel of healthy adult volunteers to be adequate data to support evidence of palatability in the targeted population (e.g., paediatric) in the drug development dossier?
- Q7. See Q5 above.
- Q8. Would a similar approach be taken for other non-oral organoleptic sensory studies, e.g., for topical/transdermal products, nasal/inhaled products?
- Q9. Some sensory data are available using non-human studies, for example, e-tongue and rodent BATA (brief-access taste aversion) studies.
- Q10. In any sensory study there will be a proportion of respondents who find the product to be palatable and a proportion that do not. Does your agency have any guidance on the overall proportion of respondents that must find the product to be palatable for it to be approved?
Appendix B. Number of Volunteer Assessors Required
| The Questions | Method Type | Panel Selection | Minimum of Volunteers Typically Used | Number in the ISO Norms Typically Used for FMCG |
|---|---|---|---|---|
| What is the acceptability of the whole product or some aspect of the product? Is one product better than another? Using the preference test | hedonic | Recruited based on inclusion/exclusion criteria. No training required or experience needed. | Less than 10 for pre-screening of very different formulations. Generally, 30 (for acceptability of the whole product). | 100 (for acceptability of the whole product). 60 (for a ranking test) ISO:8587 * 24–30 (for a paired comparison test) ISO:5495. |
| Is one sample different to another? | Analytical—discrimination test | Recruited based on inclusion/exclusion criteria. Volunteers require minimum defined levels of sensory acuity. Method training then required. | Less than 10 for pre-screening of very different formulations. | 12–15 (for a ranking test) ISO:8587. * 24–30 (for a paired comparison test) ISO:5495. |
| How are products different to each other | Analytical—descriptive test | Recruited based on inclusion/exclusion criteria. Volunteers require high levels of sensory acuity. Training then required to profile sensory characteristics of the products. | Less than 5 for pre-screening of very different formulations. Generally, 8–12. | 8–15 Quantitative Descriptive Profile (ISO:13299). |
References
- European Medicine Agency. Guideline on Pharmaceutical Development of Medicines for Paediatric Use; EMA/CHMP/QWP/805880/2012 Rev. 2; EMA: London, UK, 2013. [Google Scholar]
- World Health Organization. Toolkit for Research and Development of Paediatric Antiretroviral Drugs and Formulations; Module 5: Acceptability; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Periodic Survey of Fellows 44, American Academy of Pediatrics Division of Health Policy Research EXECUTIVE SUMMARY Patient Compliance with Prescription Regimens. 2019. Available online: https://medcoatusa.com/wp-content/uploads/2019/04/periodic-survey.pdf (accessed on 12 September 2023).
- Baguley, D.; Lim, E.; Bevan, A.; Pallet, A.; Faust, S. Prescribing for children-taste and palatability affect adherence to antibiotics: A review. Arch. Dis. Child. 2012, 97, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Hofmanová, J.; Mason, J.; Batchelor, H. Sensory aspects of acceptability of bitter-flavoured 7.5 mm film-coated tablets in adults, preschool and school children. Int. J. Pharm. 2020, 585, 119511. [Google Scholar] [CrossRef]
- Herziger, B.; Jeschke, S.; Müller, R.; Neininger, M.; Bertsche, T.; Bertsche, A. Drug-handling problems and expectations of the ideal pediatric drug—Reported by children and their parents. Eur. J. Pediatr. 2022, 181, 2161–2171. [Google Scholar]
- Nordenmalm, S.; Kimland, E.; Ligas, F.; Lehmann, B.; Claverol, J.; Nafria, B.; Tötterman, A.; Pelle, B. Children’s views on taking medicines and participating in clinical trials. Arch. Dis. Child. 2019, 104, 900–905. [Google Scholar] [CrossRef]
- International Council for Harmonisation. ICH E11(R1) Addendum: Clinical Investigation of Medicinal Products in the Pediatric Population. Pearl IRB 2021, 11, R1. Available online: www.ema.europa.eu/en/documents/scientific-guideline/ich-e11r1-guideline-clinical-investigation-medicinal-products-pediatric-population-revision-1_en.pdf (accessed on 12 September 2023).
- Tuleu, C.; Hughes, D.; Clapham, D.; Vallet, T.; Ruiz, F. Acceptability of generic versus innovator oral medicines: Not only a matter of taste. Drug Discov. Today 2021, 26, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Venables, R.; Stirling, H.; Marriott, J. Oral formulation-related barriers to medicines administration in children with chronic conditions: Views of parents and young people. Arch. Dis. Child. 2014, 99, A169–A170. [Google Scholar] [CrossRef]
- Venables, R.; Stirling, H.; Batchelor, H.; Marriott, J. Problems with oral formulations prescribed to children: A focus group study of healthcare professionals. Int. J. Clin. Pharm. 2015, 15, 15. [Google Scholar] [CrossRef]
- Venables, R.; Batchelor, H.; Hodson, J.; Stirling, H.; Marriott, J. Determination of formulation factors that affect oral medicines acceptability in a domiciliary paediatric population. Int. J. Pharm. 2015, 480, 55–62. [Google Scholar]
- Gittings, S.; Turnbull, N.; Roberts, C.; Gershkovich, P. Dissolution methodology for taste masked oral dosage forms. J. Control. Release 2014, 173, 32–42. [Google Scholar]
- Coupland, J.; Hayes, J. Physical approaches to masking bitter taste: Lessons from food and pharmaceuticals. Pharm. Res. 2014, 31, 2921–2939. [Google Scholar] [CrossRef]
- TASTE-MASKING–Pharmaceutical Taste-Masking Technologies. Available online: https://drug-dev.com/taste-masking-pharmaceutical-taste-masking-technologies/ (accessed on 11 May 2023).
- Gupta, D.; Bhatia, D.; Dave, V.; Sutariya, V.; Gupta, S.V. Salts of Therapeutic Agents: Chemical, Physicochemical, and Biological Considerations. Molecules 2018, 23, 1719. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Bong, L.J.; Gittings, S.; Iachelini, A.; Bennett, J.; Cram, A.; Garnett, M.; Roberts, C.J.; Gershkovich, P. Development and optimisation of simulated salivary fluid for biorelevant oral cavity dissolution. Eur. J. Pharm. Biopharm. 2021, 160, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, H.; Venables, R.; Marriott, J.; Mills, T. The application of tribology in assessing texture perception of oral liquid medicines. Int. J. Pharm. 2015, 479, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Krop, E.M. Marrying oral tribology to sensory perception: A systematic review. Curr. Opin. Food Sci. 2019, 27, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Imbart, S.; Laplanche, A.; Ruzic, C.; Lavarde, M.; Marull-Tufeu, S.; Bernard, C.; Pensé-Lhéritier, A.-M.; Aoussat, A. Design of a Sensorial-Instrumental Correlation Methodology for a Category of Cosmetic Products: O/W Emulsions. Cosmetics 2022, 9, 84. [Google Scholar] [CrossRef]
- Clapham, D.; Bennett, J.; Cram, A.; Discihnger, A.; Inghelbrecht, S.; Pensé-Lhéritier, A.-M.; Ruiz, F.; Salunke, S.; Schiele, J.; Soto, J. Proposed tool to compare and assess the applicability of taste assessment techniques for pharmaceuticals. J. Pharm. Sci. 2022, 111, 1219–1223. [Google Scholar] [CrossRef]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. A comparative study on two electronic tongues for pharmaceutical formulation development. J. Pharm. Biomed. Anal. 2011, 55, 272–281. [Google Scholar] [CrossRef]
- Pein, M.; Kirsanov, D.; Ciosek, P.; del Valle, M.; Yaroshenko, I.; Wesoły, M.; Zabadaj, M.; Gonzalez-Calabuig, A.; Wróblewski, W.; Legin, A. Independent comparison study of six different electronic tongues applied for pharmaceutical analysis. J. Pharm. Biomed. Anal. 2015, 114, 321–329. [Google Scholar] [CrossRef]
- Cho, S.; Moazzem, M. Recent Applications of Potentiometric Electronic Tongue and Electronic Nose in Sensory Evaluation. Prev. Nutr. Food Sci. 2022, 27, 354–364. [Google Scholar] [CrossRef]
- Taste Analysis-ASTREE Electronic Tongue. Available online: https://www.alpha-mos.com/taste-analysis-astree-electronic-tongue (accessed on 11 May 2023).
- Insent Electronic-Tongue 1993 Launch. Available online: https://www.insentjp.com/ (accessed on 8 September 2023).
- Aliani, M.; Eideh, A.; Kapourchali, F.; Alharbi, R.; Fahmi, R. Evaluation of Bitterness by the Electronic Tongue: Correlation between Sensory Tests and Instrumental Methods: Chapter 9. In Bitterness: Perception, Chemistry and Food Processing, 1st ed.; Aliani, M., Eskin, M., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2017; pp. 195–207. [Google Scholar]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. Development of a taste-masked generic ibuprofen suspension: Top-down approach guided by electronic tongue measurements. J. Pharm. Sci. 2011, 100, 4460–4470. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, J.; Hong, X.; Han, X.; Liu, B.; Li, X.; Zhang, H.; Gao, J.; Liu, N.; Gao, X.; et al. Taste Masking Study Based on an Electronic Tongue: The Formulation Design of 3D Printed Levetiracetam Instant-Dissolving Tablets. Pharm. Res. 2021, 38, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Guedes, M.; Marques, M.; Guedes, P.; Contri, R.; Guerreiro, I. The use of electronic tongue and sensory panel on taste evaluation of pediatric medicines: A systematic review. Pharm. Dev. Tech. 2021, 26, 119–137. [Google Scholar] [CrossRef] [PubMed]
- An Evaluation of The Electronic Tongue for the Taste Assessment of Drugs and Pharmaceutical Formulations. Available online: https://discovery.ucl.ac.uk/id/eprint/1469900/4/XOLANI%20DERECK%20GONDONGWE%20combined%20thesis%20v5%202.pdf.REDACTED.pdf (accessed on 11 May 2023).
- Kovacs, Z.; Szöllősi, D.; Zaukuu, J.Z.; Bodor, Z.; Vitális, F.; Aouadi, B.; Zsom-Muha, V.; Gillay, Z. Factors Influencing the Long-Term Stability of Electronic Tongue and Application of Improved Drift Correction Methods. Biosensors 2020, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, T.; Migoń, D.; Gębicki, J.; Kamysz, W. Critical review of electronic nose and tongue instruments prospects in pharmaceutical analysis. Anal. Chim. Acta 2019, 1077, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, E.; Bai, J.; Plotto, A.; Dea, S. Electronic Noses and Tongues: Applications for the Food and Pharmaceutical Industries. Sensors 2011, 11, 4744–4766. [Google Scholar] [CrossRef]
- Soto, J.; Winzenburg, G.; Turner, R.; Desset-Brèthes, S.; Sheng, Y.; Orlu-Gul, M.; Tuleu, C. Assessing the bitter taste of medicines: A comparison between rat taste panels (via the brief-access taste aversion (BATA) model) and human taste panels. Int. J. Pharm. 2016, 511, 1127–1128. [Google Scholar] [CrossRef]
- Soto, J.; Keeley, A.; Keating, A.; Mohamed-Ahmed, A.; Sheng, Y.; Winzenburg, G.; Turner, R.; Desset-Brèthes, S.; Orlu, M.; Tuleu, C. Rats can predict aversiveness of Active Pharmaceutical Ingredients. Eur. J. Pharm. Biopharm. 2018, 113, 77–84. [Google Scholar] [CrossRef]
- Andrews, D.; Ives, R.; Wassell, E.; Fairman, D. A novel approach to assess and improve palatability of an inhaled asset using the rat brief access taste aversion assay and an in silico model of salivary flow. Int. J. Pharm. 2018, 536, 516–517. [Google Scholar] [CrossRef]
- Mohamed-Ahmed, A.; Soto, J.; Ernest, T.; Tuleu, C. Non-human tools for the evaluation of bitter taste in the design and development of medicines: A systematic review. Drug Discov. Today 2016, 21, 1170–1180. [Google Scholar] [CrossRef]
- European Pharmacopoeia Method 2.2.2 Degree of Colouration of Liquids. 2021. Available online: https://www.drugfuture.com/Pharmacopoeia/EP7/DATA/20202E.PDF (accessed on 8 September 2023).
- United States Pharmacopeia. USP44 Monograph 631 Colour and Achromaticity USP; United States Pharmacopeia: Rockville, MD, USA, 2021. [Google Scholar] [CrossRef]
- Munsell Color System. Available online: https://en.wikipedia.org/wiki/Munsell_color_system (accessed on 11 May 2023).
- USP44 Monograph 1061 Color-Instrumental Measurement USP. 2021. Available online: http://www.uspbpep.com/usp31/v31261/usp31nf26s1_c1061.asp (accessed on 12 September 2023).
- Hetrick, E.; Vannoy, J.; Montgomery, L.L.; Pack, B.W. Integrating tristimulus colorimetry into pharmaceutical development for color selection and physical appearance control: A quality-by-design approach. J. Pharm. Sci. 2013, 102, 2608–2621. [Google Scholar] [CrossRef]
- Sakiroff, L.; Chennell, P.; Yessaad, M.; Pereira, B.; Bouattour, Y.; Sautou, V. Evaluation of color changes during stability studies using spectrophotometric chromaticity measurements versus visual examination. Sci. Rep. 2022, 12, 8959. [Google Scholar] [CrossRef]
- European Pharmacopoeia Method 2.2.1 Clarity and Degree of Opalescence of Liquids. 2021. Available online: https://file.wuxuwang.com/yaopinbz/EP9/EP9.2_01__2.pdf (accessed on 8 September 2023).
- USP 44 Monograph 855 Nephelometry, Turbidimetry and Visual Comparison USP. 2021. 〈855〉 Nephelometry and Turbidimetry. Available online: usp.org (accessed on 8 September 2023).
- Lawler, D. Turbidity, Turbidimetry and Nephelometry in Encyclopaedia of Analytical Science, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 152–163. [Google Scholar]
- Rowe, R. Gloss measurement on film coated tablets. J. Pharm. Pharmacol. 1985, 37, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Bawuah, P.; Pääkkönen, P.; Peiponen, K. Gloss measurement in detection of surface quality of pharmaceutical tablets: A case study of screening of genuine and counterfeit antimalaria tablets. J. Eur. Opt. Soc. 2017, 13, 18. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on the Demonstration of Palatability of Veterinary Medicinal Products. Rev 1. 2022. Available online: europa.eu (accessed on 8 September 2023).
- Mennella, J.; Beauchamp, G. Optimizing oral medications for children. Clin. Ther. 2008, 30, 2120–2132. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.; Spector, A.; Reed, D.; Coldwell, S. The bad taste of medicines: Overview of basic research on bitter taste. Clin. Ther. 2013, 35, 1225–1246. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.; Bobowski, N. The sweetness and bitterness of childhood: Insights from basic research on taste preferences. Physiol. Behav. 2015, 20, 015. [Google Scholar] [CrossRef]
- Lawless, H.; Heymann, H. Sensory Evaluation of Food: Principles and Practices, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Medication Routes of Administration. Available online: https://www.ncbi.nlm.nih.gov/books/NBK568677/#:~:text=Oral%20administration%20of%20medication%20is,absorbed%20across%20the%20intestinal%20epithelium (accessed on 8 September 2023).
- European Parliament. Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the Approximation of the Laws, Regulations and Administrative Provisions of the Member States Relating to the Implementation of Good Clinical Practice in the Conduct of Clinical Trials on Medicinal Products for Human Use. 2001. Available online: https://health.ec.europa.eu/system/files/2016-11/dir_2001_20_en_0.pdf (accessed on 12 September 2023).
- Is It a Clinical Trial of a Medicinal Product? Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/949145/Algorithm_Clean__1_.pdf (accessed on 8 September 2023).
- Workman, A.; Palmer, J.; Adappa, N. The role of bitter and sweet taste receptors in upper airway immunity. Curr. Allergy Asthma Rep. 2015, 15, 72. [Google Scholar] [CrossRef]
- Kokrashvili, Z.; Mosinger, B.; Margolskee, R. Taste signalling elements expressed in gut enterendrochrine cells regulate nutrient responsive secretion of gut hormones. Gut Horm. Am. J. Clin. Nutr. 2009, 90, 822S–825S. [Google Scholar] [CrossRef]
- Behrens, M.; Meyerhof, W. Gustatory and extragustatory functions of mammalian taste receptors. Physiol. Behav. 2011, 105, 4–13. [Google Scholar] [CrossRef]
- Finger, T.; Kinnamon, S. Taste isn’t just for tastebuds any more F1000. BiolRep 2011, 3, 54. [Google Scholar]
- Rozengurt, E.; Sternini, C. Taste receptor signalling in the mammalian gut. Curr. Opin. Pharmacol. 2007, 7, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Salunke, S.; Agrawal, A.; Walsh, J.; Nunn, A.; Hughes, K.; Kuehl, P.; Caivano, G.; Clapham, D.; Thompson, K.; Rumodndor, A.; et al. Selecting Appropriate Excipients for Paediatric Dosage Form—Paediatric Excipients Risk Assessment (PERA) Framework—Part 1. 2023; in press. [Google Scholar]
- Rogers, L. Sensory Panel Management—A Practical Handbook for Recruitment, Training and Performance. A Volume in Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2017; 374p, ISBN 10: 008101001X. [Google Scholar]
- Lim, J. Hedonic scaling: A review of methods and theory. Food Qual. Prefer. 2011, 22, 733–747. [Google Scholar] [CrossRef]
- Pimentel, T.; da Cruz, A.; Deliza, R. Sensory evaluation: Sensory rating and scoring methods. In Encyclopaedia of Food and Health, 11th ed.; Cabellero, B., Finglas, P., Toldra, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Everitt, M. CHAPTER 8—Consumer-Targeted Sensory Quality; Barbosa-Cánovas, G., Mortimer, A., Lineback, D., Spiess, W., Buckle, K., Colonna, P., Eds.; Global Issues in Food Science and Technology; Academic Press: Cambridge, MA, USA, 2009; pp. 117–128. [Google Scholar]
- Popper, R.; Kroll, J. Chapter 9—Consumer testing of food products using children. In Food Science, Technology and Nutrition, Developing Children’s Food Products; Kilcast, D., Angus, F., Eds.; Woodhead Publishing: Sawston, UK, 2011; pp. 163–168. [Google Scholar]
- Davies, E.H.; Tuleu, C. Medicines for children: A matter of taste. J. Pediatr. 2008, 153, 599–604. [Google Scholar] [CrossRef]
- Shields, B.; Palermo, T.; Powers, J.; Grewe, S.; Smith, G. Predictors of a child′s ability to use visual analogue scale. Child’s Care Health Dev. 2003, 29, 281–290. [Google Scholar] [CrossRef]
- Rosenstein, D.; Oster, H. Differential facial responses to four basic tastes in newborns. Child Dev. 1988, 59, 1555–1568. [Google Scholar] [CrossRef]
- Yamamoto, T.; Mizuta, H.; Ueji, K. Analysis of facial expressions in response to basic taste stimuli using artificial intelligence to predict perceived hedonic ratings. PLoS ONE 2021, 16, e0258707. [Google Scholar]
- Medecine Acceptability. Available online: https://www.clinsearch.net/home/service-ax.php (accessed on 17 May 2023).
- Letter of Support for an Acceptability Score Test in Relative Acceptability Testing for Oral Medicines in Children under 12 Years of Age. Available online: http://www.ema.europa.eu/en/documents/other/letter-support-acceptability-score-test-relative-acceptability-testing-oral-medicines-children-under_en.pdf (accessed on 17 May 2023).
- Klingmann, V.; Vallet, T.; Münch, J.; Stegemann, R.; Wolters, L.; Bosse, H.M.; Ruiz, F. Dosage Forms Suitability in Pediatrics: Acceptability of Analgesics and Antipyretics in a German Hospital. Pharmaceutics 2022, 14, 337. [Google Scholar] [CrossRef]
- Perez, F.; Vallet, T.; Bravo, Z.; Callahan, K.; Ruiz, F. Acceptability of Mebendazole Chewable Tablet in Children Aged 2 to 4 Years in Peru. Pharmaceutics 2022, 14, 27. [Google Scholar] [CrossRef]
- Pokharkar, V.; Sajith, M.; Vallet, T.; Akshantal, S.; Shah, R.; Ruiz, F.; Salunke, S. Acceptability of different oral dosage forms in paediatric patients in hospital setting. Arch. Dis. Child. 2021, 107, 796–801. [Google Scholar] [CrossRef]
- Vallet, T.; Bensouda, Y.; Saito, J.; Mathiesen, L.; Pokharkar, V.; Klingmann, V.; Peak, M.; Elhamdaoui, O.; Yamatani, A.; Ivanovic, I.; et al. Exploring acceptability drivers of oral antibiotics in children: Findings from an international observational study. Pharmaceutics 2021, 13, 1721. [Google Scholar] [CrossRef] [PubMed]
- Saito, J.; Miyamoto, S.; Yamada, M.; Yamatani, A.; Ruiz, F.; Vallet, T. Adherence and acceptability of an oral antibiotic used for the prevention of pediatric urinary tract infection in Japan. Pharmaceutics 2021, 13, 345. [Google Scholar] [CrossRef] [PubMed]
- Emeryk, A.; Vallet, T.; Wawryk-Gawda, E.; Jędrzejewski, A.; Durmont, F.; Ruiz, F. Acceptability of a sublingual drug formulation for respiratory tract infections in children aged 3 to 5 years. Pharmaceutics 2021, 13, 294. [Google Scholar] [CrossRef]
- Vallet, T.; Elhamdaoui, O.; Berraho, A.; Cherkaoui, L.O.; Kriouile, Y.; Mahraoui, C.; Mouane, N.; Pense-Lheritier, A.M.; Ruiz, F.; Bensouda, Y. Medicines acceptability in hospitalized children: An ongoing need for age-appropriate formulations. Pharmaceutics 2020, 12, 766. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, F.; Vallet, T.; Dufaÿ Wojcicki, A.; Belissa, É.; Fontan, J.E.; de Pontual, L.; Nathanson, S.; Chevallier, A.; Laribe-Caget, S.; Boudy, V. Dosage form suitability in vulnerable populations: A focus on paracetamol acceptability from infants to centenarians. PLoS ONE 2019, 14, e0221261. [Google Scholar] [CrossRef]
- Vallet, T.; Ruiz, F.; Lavarde, M.; Pensé-Lhéritier, A.M.; Aoussat, A. Standardised evaluation of medicine acceptability in paediatric population: Reliability of a model. J. Pharm. Pharmacol. 2018, 70, 42–50. [Google Scholar] [CrossRef]
- Ruiz, F.; Vallet, T.; Pensé-Lhéritier, A.M.; Aoussat, A. Standardized method to assess medicines’ acceptability: Focus on paediatric population. J. Pharm. Pharmacol. 2017, 69, 406–416. [Google Scholar] [CrossRef]
- Guinard, J. Sensory and consumer testing with children. Trends Food Sci. Technol. 2000, 11, 273–283. [Google Scholar] [CrossRef]
- Popper, R.; Kroll, J. Conducting sensory research with children. J. Sens. Stud. 2005, 20, 75–87. [Google Scholar] [CrossRef]
- Squires, L.; Lombardi, D.; Sjostedt, P.; Thompson, C.A. Systematic Literature Review on the Assessment of Palatability and Swallowability in the Development of Oral Dosage Forms for Pediatric Patients. Ther. Innov. Regula Sci. 2013, 47, 533–541. [Google Scholar] [CrossRef]
- Thompson, C.; Lombardi, D.; Sjostedt, P.; Squires, L. Best Practice Recommendations Regarding the Assessment of Palatability and Swallowability in the Development of Oral Dosage Forms for Pediatric Patients. Ther. Innov. Regul. Sci. 2015, 49, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Angelilli, M.; Toscani, M.; Matsui, D.; Rieder, M. Palatability of oral antibiotics among children in an urban primary care center. Arch. Pediatr. Adolesc. Med. 2000, 154, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Keeley, A. Enhancing the Understanding of Palatability Assessment Used in the Development of Paediatric Medicines. Ph.D. Dissertation, University College London, London, UK, 2019. [Google Scholar]



| Factor | Study Type | ||
|---|---|---|---|
| Pre-Planned Clinical Trial with Additional Sensory Endpoint | Sensory Specific Clinical Trial | Standalone Sensory Study | |
| API exposure | Full exposure to product/API possible | Full exposure to product/API possible | Design limits exposure |
| Participant risk | Full clinical monitoring Higher risk studies possible | Full clinical monitoring possible, ethically only low to moderate risk studies possible | Low risk studies only |
| Participant type/number | Probably naïve/could be many | May be trained/very few | Full range of participant type/few |
| Focus | Sensory data secondary | Sensory data primary | Sensory data primary |
| Sensory data type | Simple/hedonic only | Simple/hedonic Limited hybrid | Full range |
| Sample throughput | Low | Low | Moderate to high |
| Cost | High | High | Moderate to Low |
| Complexity | High | High | Moderate |
| Flexibility | Low | Low | High |
| Frequency | Low | Low/moderate | High |
| Design ease | Low | Low/moderate | High |
| Question | Possible Methods | Comments |
|---|---|---|
| What is the sensory acceptability of the product as a whole? | Acceptance tests, paired comparison, or ranking tests. | A range of methods of capturing data are available. |
| What is the acceptability of some specific aspects of the product? | For specific aspect assessments use bimodal visual analogue scales (VASs) ** | Data captured by volunteer marking a scale that has “too little” at one end of the scale, “too much” at the other, and “just right” in the middle. |
| Is one product better than another? | Paired preference test (two samples) Or Ranking test (more than two samples) | When the difference between products is expected to vary according to only one attribute (e.g., bitterness), orientated questions such as “Which sample is the most bitter?” may be used. |
| What level of a specific characteristic(s) are perceived? | Profiling methods or Rating scales | Continuous methods such unimodal VAS scales (e.g., when measuring the level of bitter taste, the assessor marks on a scale their perception of the level of the bitter taste from none to extreme, or low to high, etc.). Categorical methods e.g., assessor ticks predefined boxes. |
| Is one sample different to another? | Triangle or Duo Trio test | Most useful when comparing samples that are nominally the same, e.g., samples from two batches of the same product, or new and aged samples of the same product. These sensory methodologies avoid volunteer assessor bias by simply asking if the samples are different. |
| ISO Standard Number 1,2 | Standard Name and Description |
|---|---|
| ISO 6658:2017 | Sensory analysis—Methodology General guidance on the use of sensory analysis. It describes tests for the examination of foods and other products by sensory analysis and includes some general information on the techniques to be used if statistical analysis of the results is required. |
| ISO 8589:2007 | Sensory analysis Provides general guidance for the design of test rooms intended for the sensory analysis of products. |
| ISO 13300-1:2006 | Sensory analysis General guidance for the staff of a sensory evaluation laboratory—Part 1: Staff responsibilities. |
| ISO 13300-2:2006 | Sensory analysis General guidance for the staff of a sensory evaluation laboratory—Part 2: Recruitment and training of panel leaders. |
| ISO 11136:2014 | Sensory analysis—Methodology General guidance for conducting hedonic tests with consumers in a controlled area. |
| ISO 13299:2016 | Sensory analysis—Methodology General guidance for establishing a sensory profile. |
| ISO 5492:2008 | Sensory analysis—Vocabulary Defines terms relating to sensory analysis. Applies to all industries concerned with the evaluation of products by the sense organs. The terms are given under the following headings: (1) general terminology; (2) terminology relating to the senses; (3) terminology relating to organoleptic attributes; and (4) terminology relating to methods. |
| ISO 4121:2003 | Sensory analysis Guidelines for the use of quantitative response scales. Provides guidelines describing quantitative response scales (where the response obtained indicates the intensity of perception) and their use when assessing samples. |
| ISO 8586:2012 | Sensory analysis General guidelines for the selection, training and monitoring of selected and expert volunteer assessors. |
| ISO 11132:2021 | Sensory analysis—Methodology Guidelines for the measurement of the performance of a quantitative descriptive sensory panel. |
| ISO 3972:2011 | Sensory analysis—Methodology Method of investigating sensitivity of taste. |
| ISO 5496:2006 | Sensory analysis—Methodology Initiation and training of volunteer assessors in the detection and recognition of odours. Describes several types of method for determining the aptitude of volunteer assessors and for training volunteer assessors to identify and describe odoriferous products. |
| ISO 4120:2021 | Sensory analysis—Methodology Triangle test. Specifies a procedure for determining whether a perceptible sensory difference or similarity exists between samples of two products. |
| ISO 13301:2018 | Sensory analysis—Methodology General guidance for measuring odour, flavour and taste detection thresholds by a three-alternative forced-choice (3-AFC) procedure. |
| ISO 8588:2017 | Sensory analysis—Methodology “A”—“not A” test. Specifies a procedure for determining whether a perceptible sensory difference exists between samples of two products. The method applies whether a difference exists in a single sensory attribute or in several. |
| ISO 10399:2017 | Sensory analysis—Methodology Duo-trio test. Specifies a procedure for determining whether a perceptible sensory difference or similarity exists between samples of two products. |
| ISO 8587:2006 | Sensory analysis—Methodology Ranking. Describes a method for sensory evaluation with the aim of placing a series of test samples in rank order. |
| ISO 5495:2005 | Sensory analysis—Methodology Paired comparison test. Describes a procedure for determining whether there exists a perceptible sensory difference or a similarity between samples of two products concerning the intensity of a sensory attribute. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clapham, D.; Belissa, E.; Inghelbrecht, S.; Pensé-Lhéritier, A.-M.; Ruiz, F.; Sheehan, L.; Shine, M.; Vallet, T.; Walsh, J.; Tuleu, C. A Guide to Best Practice in Sensory Analysis of Pharmaceutical Formulations. Pharmaceutics 2023, 15, 2319. https://doi.org/10.3390/pharmaceutics15092319
Clapham D, Belissa E, Inghelbrecht S, Pensé-Lhéritier A-M, Ruiz F, Sheehan L, Shine M, Vallet T, Walsh J, Tuleu C. A Guide to Best Practice in Sensory Analysis of Pharmaceutical Formulations. Pharmaceutics. 2023; 15(9):2319. https://doi.org/10.3390/pharmaceutics15092319
Chicago/Turabian StyleClapham, David, Emilie Belissa, Sabine Inghelbrecht, Anne-Marie Pensé-Lhéritier, Fabrice Ruiz, Liz Sheehan, Margaret Shine, Thibault Vallet, Jennifer Walsh, and Catherine Tuleu. 2023. "A Guide to Best Practice in Sensory Analysis of Pharmaceutical Formulations" Pharmaceutics 15, no. 9: 2319. https://doi.org/10.3390/pharmaceutics15092319
APA StyleClapham, D., Belissa, E., Inghelbrecht, S., Pensé-Lhéritier, A.-M., Ruiz, F., Sheehan, L., Shine, M., Vallet, T., Walsh, J., & Tuleu, C. (2023). A Guide to Best Practice in Sensory Analysis of Pharmaceutical Formulations. Pharmaceutics, 15(9), 2319. https://doi.org/10.3390/pharmaceutics15092319






