The Recent Applications of PLGA-Based Nanostructures for Ischemic Stroke
Abstract
:1. Introduction
2. A Brief Introduction to PLGA and PLGA-Based Nanostructures
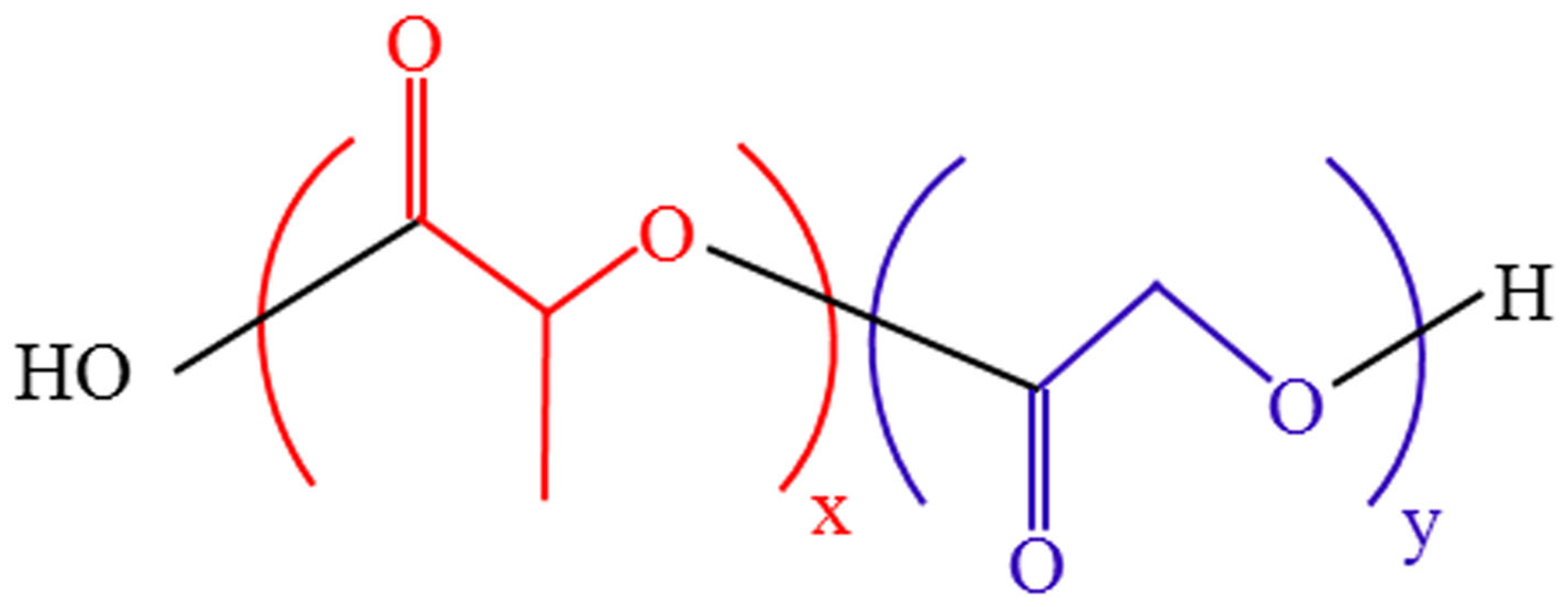
2.1. Synthesis and Properties of PLGA
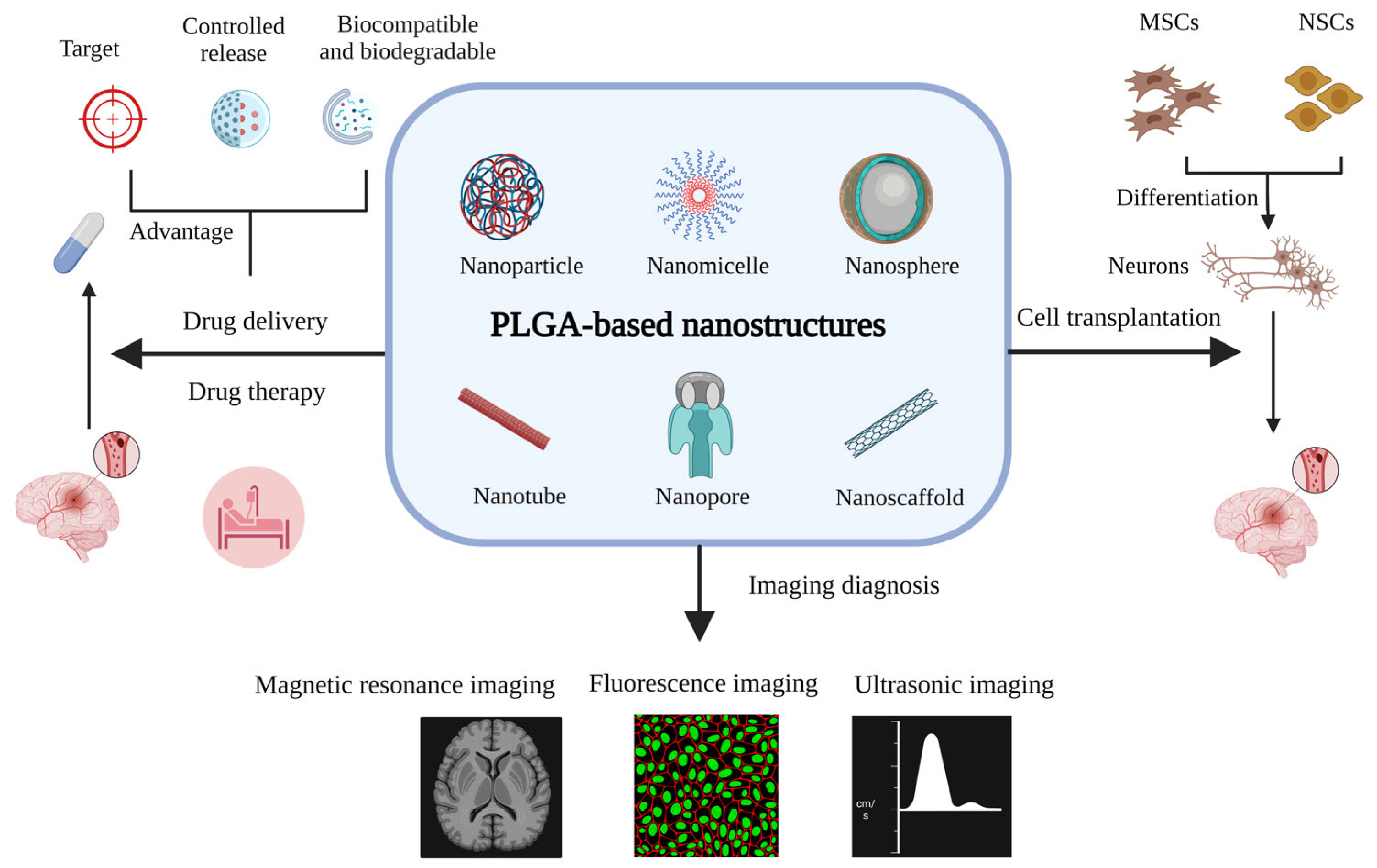
2.2. PLGA-Based Nanostructures and Their Formulation
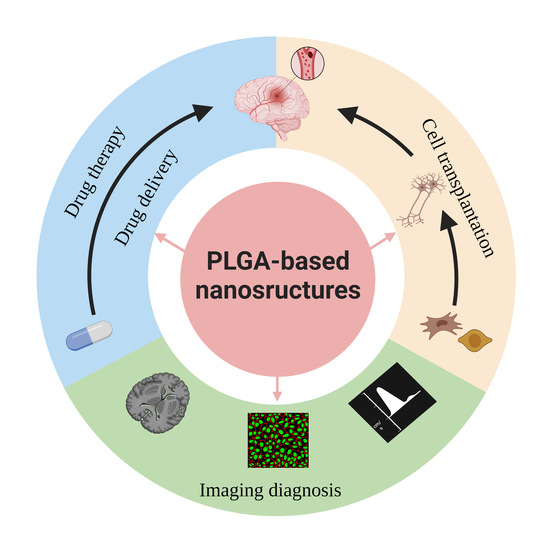
3. Applications of PLGA-Based Nanostructures in Drug Delivery and the Treatment of Ischemic Stroke
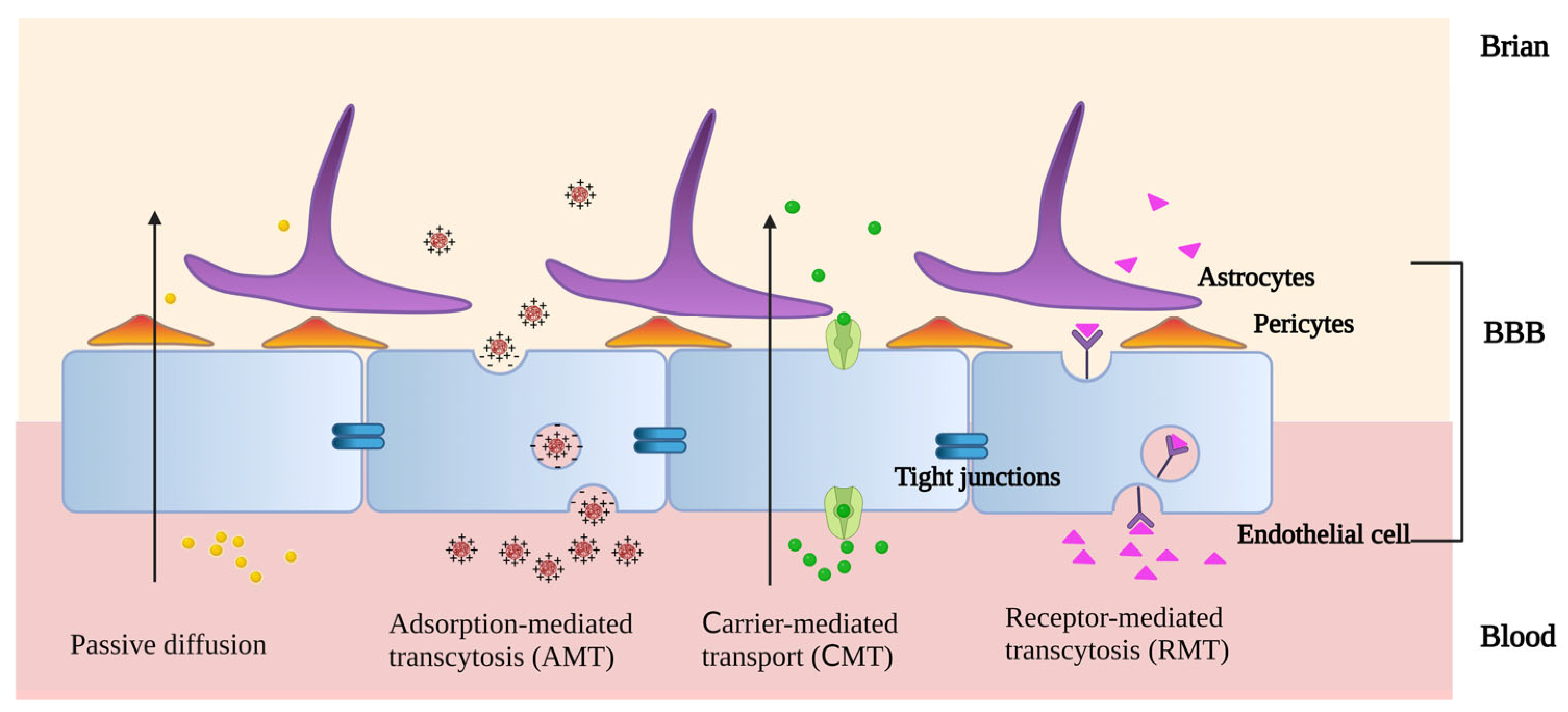
3.1. Mechanism of PLGA-Based Nanostructures Crossing the BBB
3.2. Applications of PLGA-Based Nanocarriers in Ischemic Stroke Treatment
3.2.1. Thrombolytic Therapy
3.2.2. Anti-Oxidative Stress and Anti-Apoptosis
3.2.3. Anti-Inflammation
3.2.4. Inhibition of Neuroexcitatory Toxicity
3.2.5. Supplementing with Neurotrophic Factor
4. Applications of PLGA-Based Nanostructures in Cell Transplantation for Ischemic Stroke
5. Applications of PLGA-Based Nanostructures in Imaging Diagnosis of Ischemic Stroke
6. Shortcomings and Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T.; et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 2014, 383, 245–254. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Dai, Y.; Guo, E.; Zhang, C.; Wang, Y. Ischaemic stroke etiological classification system: The agreement analysis of CISS, SPARKLE and TOAST. Stroke Vasc. Neurol. 2019, 4, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Gao, S.; Wang, Y.J.; Xu, A.D.; Li, Y.S.; Wang, D. Classifying Ischemic Stroke, from TOAST to CISS. CNS Neurosci. Ther. 2012, 18, 452–456. [Google Scholar] [CrossRef]
- Farhoudi, M.; Sadigh-Eteghad, S.; Mahmoudi, J.; Farjami, A.; Mahmoudian, M.; Salatin, S. The Therapeutic Benefits of Intravenously Administrated Nanoparticles in Stroke and Age-related Neurodegenerative Diseases. Curr. Pharm. Des. 2022, 28, 1985–2000. [Google Scholar] [CrossRef]
- Sa, P.; Singh, P.; Dilnawaz, F.; Sahoo, S.K. Application of Therapeutic Nanoplatforms as a Potential Candidate for the Treatment of CNS Disorders: Challenges and Possibilities. Curr. Pharm. Des. 2022, 28, 2742–2757. [Google Scholar] [CrossRef] [PubMed]
- Kalani, A.; Chaturvedi, P.; Kamat, P.K.; Maldonado, C.; Bauer, P.; Joshua, I.G.; Tyagi, S.C.; Tyagi, N. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int. J. Biochem. Cell Biol. 2016, 79, 360–369. [Google Scholar] [CrossRef]
- Han, L.; Jiang, C. Evolution of blood-brain barrier in brain diseases and related systemic nanoscale brain-targeting drug delivery strategies. Acta Pharm. Sin. B 2021, 11, 2306–2325. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, M.; Chen, Q.; Wang, J. Hemorrhagic Transformation after Tissue Plasminogen Activator Reperfusion Therapy for Ischemic Stroke: Mechanisms, Models, and Biomarkers. Mol. Neurobiol. 2015, 52, 1572–1579. [Google Scholar] [CrossRef]
- Li, C.; Sun, T.; Jiang, C. Recent advances in nanomedicines for the treatment of ischemic stroke. Acta Pharm. Sin. B 2021, 11, 1767–1788. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Alharbi, F.D.; Alhibs, A.S.; Alanazi, N.B.; Alshehri, B.Y.; Saleh, M.A.; Alshehri, F.S.; Algarni, M.A.; Almugaiteeb, T.; Uddin, M.N.; et al. PLGA-Based Nanomedicine: History of Advancement and Development in Clinical Applications of Multiple Diseases. Pharmaceutics 2022, 14, 2728. [Google Scholar] [CrossRef]
- Zhou, J.; Zhai, Y.; Xu, J.; Zhou, T.; Cen, L. Microfluidic preparation of PLGA composite microspheres with mesoporous silica nanoparticles for finely manipulated drug release. Int. J. Pharm. 2021, 593, 120173. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, X.; Jiang, Y.; Qi, L.; Zhuge, D.; Xu, T.; Guo, Y.; Deng, M.; Zhang, W.; Tian, D.; et al. Targeted delivery of fat extract by platelet membrane-cloaked nanocarriers for the treatment of ischemic stroke. J. Nanobiotechnol. 2022, 20, 249. [Google Scholar] [CrossRef] [PubMed]
- Zhi, K.; Raji, B.; Nookala, A.R.; Khan, M.M.; Nguyen, X.H.; Sakshi, S.; Pourmotabbed, T.; Yallapu, M.M.; Kochat, H.; Tadrous, E.; et al. PLGA Nanoparticle-Based Formulations to Cross the Blood-Brain Barrier for Drug Delivery: From R&D to cGMP. Pharmaceutics 2021, 13, 500. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zeng, Y.-S.; Zeng, C.-G.; Du, B.-l.; He, L.-M.; Quan, D.-P.; Zhang, W.; Wang, J.-M.; Wu, J.-L.; Li, Y.; et al. Synaptic transmission of neural stem cells seeded in 3-dimensional PLGA scaffolds. Biomaterials 2009, 30, 3711–3722. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Chen, C.-W. Neuroregeneration of Induced Pluripotent Stem Cells in Polyacrylamide-Chitosan Inverted Colloidal Crystal Scaffolds with Poly(lactide-co-glycolide) Nanoparticles and Transactivator of Transcription von Hippel-Lindau Peptide. Tissue Eng. Part A 2017, 23, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Khang, G.; Kim, H.L.; Hong, M.; Lee, D. Neurogenesis of bone marrow-derived mesenchymal stem cells onto beta-mercaptoethanol-loaded PLGA film. Cell Tissue Res. 2012, 347, 713–724. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, D.; Zhang, Y.; Wu, W.; Ran, H.; Wang, Z. Construction and evaluation of Fe₃O₄-based PLGA nanoparticles carrying rtPA used in the detection of thrombosis and in targeted thrombolysis. ACS Appl. Mater. Interfaces 2014, 6, 5566–5576. [Google Scholar] [CrossRef]
- Dhuri, K.; Vyas, R.N.; Blumenfeld, L.; Verma, R.; Bahal, R. Nanoparticle Delivered Anti-miR-141-3p for Stroke Therapy. Cells 2021, 10, 1011. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Tsai, Y.-H.; Kuo, Y.-M. Characterization of the pattern of ischemic stroke induced by artificial particle embolization in the rat brain. Biomaterials 2011, 32, 6381–6388. [Google Scholar] [CrossRef]
- Amani, H.; Kazerooni, H.; Hassanpoor, H.; Akbarzadeh, A.; Pazoki-Toroudi, H. Tailoring synthetic polymeric biomaterials towards nerve tissue engineering: A review. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3524–3539. [Google Scholar] [CrossRef]
- Gao, Q.; Lan, P.; Shao, H.; Hu, X. Direct Synthesis with Melt Polycondensation and Microstructure Analysis of Poly(L-lactic acid-co-glycolic acid). Polym. J. 2002, 34, 786–793. [Google Scholar] [CrossRef]
- Deasy, P.B.; Finan, M.P.; Meegan, M.J. Preparation and characterization of lactic/glycolic acid polymers and copolymers. J. Microencapsul. 1989, 6, 369–378. [Google Scholar] [CrossRef]
- Lü, J.-M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar] [CrossRef]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; McEnnis, K. Glass Transition Temperature of PLGA Particles and the Influence on Drug Delivery Applications. Polymers 2022, 14, 993. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Mahar, R.; Chakraborty, A.; Nainwal, N.; Bahuguna, R.; Sajwan, M.; Jakhmola, V. Application of PLGA as a Biodegradable and Biocompatible Polymer for Pulmonary Delivery of Drugs. AAPS PharmSciTech 2023, 24, 39. [Google Scholar] [CrossRef]
- Pinto, M.; Silva, V.; Barreiro, S.; Silva, R.; Remião, F.; Borges, F.; Fernandes, C. Brain drug delivery and neurodegenerative diseases: Polymeric PLGA-based nanoparticles as a forefront platform. Ageing Res. Rev. 2022, 79, 101658. [Google Scholar] [CrossRef]
- Montelione, N.; Loreni, F.; Nenna, A.; Catanese, V.; Scurto, L.; Ferrisi, C.; Jawabra, M.; Gabellini, T.; Codispoti, F.A.; Spinelli, F.; et al. Tissue Engineering and Targeted Drug Delivery in Cardiovascular Disease: The Role of Polymer Nanocarrier for Statin Therapy. Biomedicines 2023, 11, 789. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Elkasabgy, N.A. Biomaterials for Tissue Engineering Applications and Current Updates in the Field: A Comprehensive Review. AAPS PharmSciTech 2022, 23, 267. [Google Scholar] [CrossRef]
- Hashemi, M.; Shamshiri, A.; Saeedi, M.; Tayebi, L.; Yazdian-Robati, R. Aptamer-conjugated PLGA nanoparticles for delivery and imaging of cancer therapeutic drugs. Arch. Biochem. Biophys. 2020, 691, 108485. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; Rehman, A.U. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Siegel, S.J.; Kahn, J.B.; Metzger, K.; Winey, K.I.; Werner, K.; Dan, N. Effect of drug type on the degradation rate of PLGA matrices. Eur. J. Pharm. Biopharm. 2006, 64, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.S.; Wang, N. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers. Part II: Biodegradation. J. Biomater. Sci. Polym. Ed. 2001, 12, 21–34. [Google Scholar] [CrossRef]
- Mu, L.; Feng, S.S. PLGA/TPGS nanoparticles for controlled release of paclitaxel: Effects of the emulsifier and drug loading ratio. Pharm. Res. 2003, 20, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Shariati, A.; Chegini, Z.; Ghaznavi-Rad, E.; Zare, E.N.; Hosseini, S.M. PLGA-Based Nanoplatforms in Drug Delivery for Inhibition and Destruction of Microbial Biofilm. Front. Cell. Infect. Microbiol. 2022, 12, 926363. [Google Scholar] [CrossRef]
- Félix Lanao, R.P.; Jonker, A.M.; Wolke, J.G.; Jansen, J.A.; van Hest, J.C.; Leeuwenburgh, S.C. Physicochemical properties and applications of poly(lactic-co-glycolic acid) for use in bone regeneration. Tissue Eng. Part B Rev. 2013, 19, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Schliecker, G.; Schmidt, C.; Fuchs, S.; Kissel, T. Characterization of a homologous series of D,L-lactic acid oligomers; a mechanistic study on the degradation kinetics in vitro. Biomaterials 2003, 24, 3835–3844. [Google Scholar] [CrossRef]
- Partikel, K.; Korte, R.; Stein, N.C.; Mulac, D.; Herrmann, F.C.; Humpf, H.U.; Langer, K. Effect of nanoparticle size and PEGylation on the protein corona of PLGA nanoparticles. Eur. J. Pharm. Biopharm. 2019, 141, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Chauhan, M.; Shekhar, S.; Kumar, A.; Mehata, A.K.; Nayak, A.K.; Dutt, R.; Garg, V.; Kailashiya, V.; Muthu, M.S.; et al. RGD-decorated PLGA nanoparticles improved effectiveness and safety of cisplatin for lung cancer therapy. Int. J. Pharm. 2023, 633, 122587. [Google Scholar] [CrossRef]
- McBride, D.A.; Kerr, M.D.; Johnson, W.T.; Nguyen, A.; Zoccheddu, M.; Yao, M.; Prideaux, E.B.; Dorn, N.C.; Wang, W.; Svensson, M.N.D.; et al. Immunomodulatory Microparticles Epigenetically Modulate T Cells and Systemically Ameliorate Autoimmune Arthritis. Adv. Sci. 2023, 10, e2202720. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.; Satpathy, S.; Naik, P.K.; Kazi, M.; Hussain, M.D. Folate receptor-targeted PLGA-PEG nanoparticles for enhancing the activity of genistein in ovarian cancer. Artif. Cells Nanomed. Biotechnol. 2022, 50, 228–239. [Google Scholar] [CrossRef]
- Naskar, S.; Das, S.K.; Sharma, S.; Kuotsu, K. A Review on Designing Poly (Lactic-co-glycolic Acid) Nanoparticles as Drug Delivery Systems. Pharm. Nanotechnol. 2021, 9, 36–50. [Google Scholar] [CrossRef]
- Haggag, Y.A.; Abosalha, A.K.; Tambuwala, M.M.; Osman, E.Y.; El-Gizawy, S.A.; Essa, E.A.; Donia, A.A. Polymeric nanoencapsulation of zaleplon into PLGA nanoparticles for enhanced pharmacokinetics and pharmacological activity. Biopharm. Drug Dispos. 2021, 42, 12–23. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, B.; Li, F.; Wang, J.; Zhi, J.; Luo, E.; Liu, Z.; Zhao, G. Intravenous thrombolysis guided by a telemedicine consultation system for acute ischaemic stroke patients in China: The protocol of a multicentre historically controlled study. BMJ Open 2015, 5, e006704. [Google Scholar] [CrossRef]
- Spindler, L.M.; Feuerhake, A.; Ladel, S.; Günday, C.; Flamm, J.; Günday-Türeli, N.; Türeli, E.; Tovar, G.E.M.; Schindowski, K.; Gruber-Traub, C. Nano-in-Micro-Particles Consisting of PLGA Nanoparticles Embedded in Chitosan Microparticles via Spray-Drying Enhances Their Uptake in the Olfactory Mucosa. Front. Pharmacol. 2021, 12, 732954. [Google Scholar] [CrossRef]
- Bazgir, M.; Zhang, W.; Zhang, X.; Elies, J.; Saeinasab, M.; Coates, P.; Youseffi, M.; Sefat, F. Degradation and Characterisation of Electrospun Polycaprolactone (PCL) and Poly(lactic-co-glycolic acid) (PLGA) Scaffolds for Vascular Tissue Engineering. Materials 2021, 14, 4773. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gan, L.; Ren, L.; Lin, Y.; Ma, C.; Lin, X. Factors influencing the blood-brain barrier permeability. Brain Res. 2022, 1788, 147937. [Google Scholar] [CrossRef]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gan, L.; Cao, F.; Wang, H.; Gong, P.; Ma, C.; Ren, L.; Lin, Y.; Lin, X. The barrier and interface mechanisms of the brain barrier, and brain drug delivery. Brain Res. Bull. 2022, 190, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Djiokeng Paka, G.; Doggui, S.; Zaghmi, A.; Safar, R.; Dao, L.; Reisch, A.; Klymchenko, A.; Roullin, V.G.; Joubert, O.; Ramassamy, C. Neuronal Uptake and Neuroprotective Properties of Curcumin-Loaded Nanoparticles on SK-N-SH Cell Line: Role of Poly(lactide-co-glycolide) Polymeric Matrix Composition. Mol. Pharm. 2016, 13, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Muniswamy, V.J.; Raval, N.; Gondaliya, P.; Tambe, V.; Kalia, K.; Tekade, R.K. ‘Dendrimer-Cationized-Albumin’ encrusted polymeric nanoparticle improves BBB penetration and anticancer activity of doxorubicin. Int. J. Pharm. 2019, 555, 77–99. [Google Scholar] [CrossRef]
- Falanga, A.P.; Melone, P.; Cagliani, R.; Borbone, N.; D’Errico, S.; Piccialli, G.; Netti, P.A.; Guarnieri, D. Design, Synthesis and Characterization of Novel Co-Polymers Decorated with Peptides for the Selective Nanoparticle Transport across the Cerebral Endothelium. Molecules 2018, 23, 1655. [Google Scholar] [CrossRef] [PubMed]
- Latronico, T.; Rizzi, F.; Panniello, A.; Laquintana, V.; Arduino, I.; Denora, N.; Fanizza, E.; Milella, S.; Mastroianni, C.M.; Striccoli, M.; et al. Luminescent PLGA Nanoparticles for Delivery of Darunavir to the Brain and Inhibition of Matrix Metalloproteinase-9, a Relevant Therapeutic Target of HIV-Associated Neurological Disorders. ACS Chem. Neurosci. 2021, 12, 4286–4301. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, X.; Mu, H.; Meng, Q.; Jiang, Y.; Wang, Y.; Lu, X.; Wang, A.; Liu, S.; Zhang, Y.; et al. RVG29-modified docetaxel-loaded nanoparticles for brain-targeted glioma therapy. Int. J. Pharm. 2018, 543, 179–189. [Google Scholar] [CrossRef]
- Bai, S.; Liao, J.; Zhang, B.; Zhao, M.; You, B.; Li, P.; Ran, H.; Wang, Z.; Shi, R.; Zhang, G. Multimodal and multifunctional nanoparticles with platelet targeting ability and phase transition efficiency for the molecular imaging and thrombolysis of coronary microthrombi. Biomater. Sci. 2020, 8, 5047–5060. [Google Scholar] [CrossRef]
- Kaya, S.; Callan, B.; Hawthorne, S. Non-Invasive, Targeted Nanoparticle-Mediated Drug Delivery across a Novel Human BBB Model. Pharmaceutics 2023, 15, 1382. [Google Scholar] [CrossRef]
- Qin, C.; Yang, S.; Chu, Y.H.; Zhang, H.; Pang, X.W.; Chen, L.; Zhou, L.Q.; Chen, M.; Tian, D.S.; Wang, W. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Zamanlu, M.; Eskandani, M.; Barar, J.; Jaymand, M.; Pakchin, P.S.; Farhoudi, M. Enhanced thrombolysis using tissue plasminogen activator (tPA)-loaded PEGylated PLGA nanoparticles for ischemic stroke. J. Drug Deliv. Sci. Technol. 2019, 53, 101165. [Google Scholar] [CrossRef]
- Chen, H.A.; Ma, Y.H.; Hsu, T.Y.; Chen, J.P. Preparation of Peptide and Recombinant Tissue Plasminogen Activator Conjugated Poly(Lactic-Co-Glycolic Acid) (PLGA) Magnetic Nanoparticles for Dual Targeted Thrombolytic Therapy. Int. J. Mol. Sci. 2020, 21, 2690. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xu, J.; Zhang, W.; Wang, J.; Fang, N.; Luo, Y.; Xu, L.; Liu, J.; Zhang, Y.; Ran, H.; et al. A Synergistic and Efficient Thrombolytic Nanoplatform: A Mechanical Method of Blasting Combined with Thrombolytic Drugs. Int. J. Nanomed. 2022, 17, 5229–5246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Ren, J.; Xue, Z.; Qi, X.; Si, Q. Cyclic RGD functionalized PLGA nanoparticles loaded with noncovalent complex of indocyanine green with urokinase for synergistic thrombolysis. Front. Bioeng. Biotechnol. 2022, 10, 945531. [Google Scholar] [CrossRef] [PubMed]
- Sharifyrad, M.; Gohari, S.; Fathi, M.; Danafar, H.; Hosseini, M.J.; Mostafavi, H.; Manjili, H.K. The efficacy and neuroprotective effects of edaravone-loaded mPEG-b-PLGA polymeric nanoparticles on human neuroblastoma SH-SY5Y cell line as in vitro model of ischemia. J. Drug Deliv. Sci. Technol. 2022, 73, 103378. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, Q.; Yang, S.; Wang, L.; Xu, X.; Li, L.; Al-Jamal, W.T. Intravenous Administration of Scutellarin Nanoparticles Augments the Protective Effect against Cerebral Ischemia-Reperfusion Injury in Rats. Mol. Pharm. 2022, 19, 1410–1421. [Google Scholar] [CrossRef]
- Waters, E.S.; Kaiser, E.E.; Yang, X.; Fagan, M.M.; Scheulin, K.M.; Jeon, J.H.; Shin, S.K.; Kinder, H.A.; Kumar, A.; Platt, S.R.; et al. Intracisternal administration of tanshinone IIA-loaded nanoparticles leads to reduced tissue injury and functional deficits in a porcine model of ischemic stroke. IBRO Neurosci. Rep. 2021, 10, 18–30. [Google Scholar] [CrossRef]
- Saralkar, P.; Arsiwala, T.; Geldenhuys, W.J. Nanoparticle formulation and in vitro efficacy testing of the mitoNEET ligand NL-1 for drug delivery in a brain endothelial model of ischemic reperfusion-injury. Int. J. Pharm. 2020, 578, 119090. [Google Scholar] [CrossRef]
- Chung, C.H.; Chung, S.D.; Cheng, Y.H.; Yang, C.P.; Chien, C.T. Long-Lasting Exendin-4-Loaded PLGA Nanoparticles Ameliorate Cerebral Ischemia/Reperfusion Damage in Diabetic Rats. J. Pers. Med. 2022, 12, 390. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sarkar, S.; Jana, S.; Swarnakar, S.; Das, N. Neuro-protective role of nanocapsulated curcumin against cerebral ischemia-reperfusion induced oxidative injury. Brain Res. 2019, 1704, 164–173. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Ma, C.; Li, T.; Yang, L. Preparation of baicalin-loaded ligand-modified nanoparticles for nose-to-brain delivery for neuroprotection in cerebral ischemia. Drug Deliv. 2022, 29, 1282–1298. [Google Scholar] [CrossRef]
- Liu, S.; Xu, J.; Liu, Y.; You, Y.; Xie, L.; Tong, S.; Chen, Y.; Liang, K.; Zhou, S.; Li, F.; et al. Neutrophil-Biomimetic “Nanobuffer” for Remodeling the Microenvironment in the Infarct Core and Protecting Neurons in the Penumbra via Neutralization of Detrimental Factors to Treat Ischemic Stroke. ACS Appl. Mater. Interfaces 2022, 14, 27743–27761. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Lee, K.Y.; Kang, J.W.; Choi, S.G.; Kim, D.W.; Yi, Y.Y. Perampanel Reduces Brain Damage via Induction of M2 Microglia in a Neonatal Rat Stroke Model. Int. J. Nanomed. 2022, 17, 2791–2804. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.G.; Shin, J.; Lee, K.Y.; Park, H.; Kim, S.I.; Yi, Y.Y.; Kim, D.W.; Song, H.J.; Shin, H.J. PINK1 siRNA-loaded poly(lactic-co-glycolic acid) nanoparticles provide neuroprotection in a mouse model of photothrombosis-induced ischemic stroke. Glia 2023, 71, 1294–1310. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, G.H.; Jeong, J.H.; Lee, I.H.; Lee, Y.J.; Lee, N.S.; Jeong, Y.G.; Lee, J.H.; Yu, K.S.; Lee, S.H.; et al. Neuroprotective effect of estradiol-loaded poly(lactic-co-glycolic acid) nanoparticles on glutamate-induced excitotoxic neuronal death. J. Nanosci. Nanotechnol. 2014, 14, 8390–8397. [Google Scholar] [CrossRef]
- Chaturvedi, M.; Figiel, I.; Sreedhar, B.; Kaczmarek, L. Neuroprotection from tissue inhibitor of metalloproteinase-1 and its nanoparticles. Neurochem. Int. 2012, 61, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kang, S.H.; Kim, J.H.; Yu, K.S.; Lee, I.H.; Lee, Y.J.; Lee, J.H.; Lee, N.S.; Jeong, Y.G.; Kim, D.K.; et al. Protective effects of poly(lactic-co-glycolic acid) nanoparticles loaded with erythropoietin stabilized by sodium cholate against glutamate-induced neurotoxicity. J. Nanosci. Nanotechnol. 2014, 14, 8365–8371. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kim, J.J.; Bak, D.H.; Yu, K.S.; Lee, J.H.; Lee, N.S.; Jeong, Y.G.; Kim, D.K.; Kim, D.K.; Han, S.Y. Protective Effects of Indole-3-Carbinol-Loaded Poly(lactic-co-glycolic acid) Nanoparticles Against Glutamate-Induced Neurotoxicity. J. Nanosci. Nanotechnol. 2015, 15, 7922–7928. [Google Scholar] [CrossRef]
- Kamarudin, S.N.; Iezhitsa, I.; Tripathy, M.; Alyautdin, R.; Ismail, N.M. Neuroprotective effect of poly(lactic-co-glycolic acid) nanoparticle-bound brain-derived neurotrophic factor in a permanent middle cerebral artery occlusion model of ischemia in rats. Acta Neurobiol. Exp. 2020, 80, 1–18. [Google Scholar] [CrossRef]
- Obermeyer, J.M.; Tuladhar, A.; Payne, S.L.; Ho, E.; Morshead, C.M.; Shoichet, M.S. Local Delivery of Brain-Derived Neurotrophic Factor Enables Behavioral Recovery and Tissue Repair in Stroke-Injured Rats. Tissue Eng. Part A 2019, 25, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Herpich, F.; Rincon, F. Management of Acute Ischemic Stroke. Crit. Care Med. 2020, 48, 1654–1663. [Google Scholar] [CrossRef]
- Rabinstein, A.A.; Albers, G.W.; Brinjikji, W.; Koch, S. Factors that may contribute to poor outcome despite good reperfusion after acute endovascular stroke therapy. Int. J. Stroke 2019, 14, 23–31. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Katsanos, A.H.; Sandset, E.C.; Turc, G.; Nguyen, T.N.; Bivard, A.; Fischer, U.; Khatri, P. Thrombolysis for acute ischaemic stroke: Current status and future perspectives. Lancet Neurol. 2023, 22, 418–429. [Google Scholar] [CrossRef]
- Zenych, A.; Fournier, L.; Chauvierre, C. Nanomedicine progress in thrombolytic therapy. Biomaterials 2020, 258, 120297. [Google Scholar] [CrossRef] [PubMed]
- Zivin, J.A. Acute stroke therapy with tissue plasminogen activator (tPA) since it was approved by the U.S. Food and Drug Administration (FDA). Ann. Neurol. 2009, 66, 6–10. [Google Scholar] [CrossRef]
- Mican, J.; Toul, M.; Bednar, D.; Damborsky, J. Structural Biology and Protein Engineering of Thrombolytics. Comput. Struct. Biotechnol. J. 2019, 17, 917–938. [Google Scholar] [CrossRef]
- Zhang, R.; Wei, H.; Ren, Y.; Wu, Y.; Luo, Y.; Zhang, L.; Huo, Y.; Feng, J.; Monnier, P.P.; Qin, X. Outcomes and Treatment Complications of Intravenous Urokinase Thrombolysis in Acute Ischemic Stroke in China. Front. Neurol. 2021, 12, 685454. [Google Scholar] [CrossRef]
- Orellana-Urzúa, S.; Rojas, I.; Líbano, L.; Rodrigo, R. Pathophysiology of Ischemic Stroke: Role of Oxidative Stress. Curr. Pharm. Des. 2020, 26, 4246–4260. [Google Scholar] [CrossRef]
- Jurcau, A.; Ardelean, I.A. Molecular pathophysiological mechanisms of ischemia/reperfusion injuries after recanalization therapy for acute ischemic stroke. J. Integr. Neurosci. 2021, 20, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Mandalaneni, K.; Rayi, A.; Jillella, D.V. Stroke Reperfusion Injury. In StatPearls; Copyright © 2022; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Zhao, L.Q.; Parikh, A.; Xiong, Y.X.; Ye, Q.Y.; Ying, G.; Zhou, X.F.; Luo, H.Y. Neuroprotection of Oral Edaravone on Middle Cerebral Artery Occlusion in Rats. Neurotox. Res. 2022, 40, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhou, L.; Han, Y.; Yang, Q.; Li, X.; Xin, B.; Chi, M.; Wang, Y.; Guo, C. Scutellarin Attenuates Doxorubicin-Induced Cardiotoxicity by Inhibiting Myocardial Fibrosis, Apoptosis and Autophagy in Rats. Chem. Biodivers. 2023, 20, e202200450. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Wang, J.; Zhang, X.; Zhao, J.; Bai, N.; Vijayalakshmi, A.; Huo, Q. Scutellarin alleviates cerebral ischemia/reperfusion by suppressing oxidative stress and inflammatory responses via MAPK/NF-κB pathways in rats. Environ. Toxicol. 2022, 37, 2889–2896. [Google Scholar] [CrossRef]
- Deng, M.; Sun, J.; Peng, L.; Huang, Y.; Jiang, W.; Wu, S.; Zhou, L.; Chung, S.K.; Cheng, X. Scutellarin acts on the AR-NOX axis to remediate oxidative stress injury in a mouse model of cerebral ischemia/reperfusion injury. Phytomedicine 2022, 103, 154214. [Google Scholar] [CrossRef]
- Yang, X.; Yan, J.; Feng, J. Treatment with tanshinone IIA suppresses disruption of the blood-brain barrier and reduces expression of adhesion molecules and chemokines in experimental autoimmune encephalomyelitis. Eur. J. Pharmacol. 2016, 771, 18–28. [Google Scholar] [CrossRef]
- Yan, J.; Yang, X.; Han, D.; Feng, J. Tanshinone IIA attenuates experimental autoimmune encephalomyelitis in rats. Mol. Med. Rep. 2016, 14, 1601–1609. [Google Scholar] [CrossRef]
- Sherawat, K.; Mehan, S. Tanshinone-IIA mediated neuroprotection by modulating neuronal pathways. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 1647–1667. [Google Scholar] [CrossRef] [PubMed]
- Geldenhuys, W.J.; Funk, M.O.; Barnes, K.F.; Carroll, R.T. Structure-based design of a thiazolidinedione which targets the mitochondrial protein mitoNEET. Bioorganic Med. Chem. Lett. 2010, 20, 819–823. [Google Scholar] [CrossRef]
- Vijikumar, A.; Saralkar, P.; Saylor, S.D.; Sullivan, P.G.; Huber, J.D.; Geldenhuys, W.J. Novel mitoNEET ligand NL-1 improves therapeutic outcomes in an aged rat model of cerebral ischemia/reperfusion injury. Exp. Neurol. 2022, 355, 114128. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Cheng, L.; Ma, X.; Luo, X. Exendin-4 induces a novel extended effect of ischemic tolerance via crosstalk with IGF-1R. Brain Res. Bull. 2021, 169, 145–155. [Google Scholar] [CrossRef]
- Li, M.; Tang, H.; Li, Z.; Tang, W. Emerging Treatment Strategies for Cerebral Ischemia-Reperfusion Injury. Neuroscience 2022, 507, 112–124. [Google Scholar] [CrossRef]
- Kelly, P.J.; Lemmens, R.; Tsivgoulis, G. Inflammation and Stroke Risk: A New Target for Prevention. Stroke 2021, 52, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.T.; Wu, W.F.; Deng, Y.H.; Ge, J.W. Modulators of microglia activation and polarization in ischemic stroke (Review). Mol. Med. Rep. 2020, 21, 2006–2018. [Google Scholar] [CrossRef] [PubMed]
- Candelario-Jalil, E.; Dijkhuizen, R.M.; Magnus, T. Neuroinflammation, Stroke, Blood-Brain Barrier Dysfunction, and Imaging Modalities. Stroke 2022, 53, 1473–1486. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Puleo, M.G.; Velardo, M.C.; Corpora, F.; Daidone, M.; Pinto, A. Molecular Biology of Atherosclerotic Ischemic Strokes. Int. J. Mol. Sci. 2020, 21, 9372. [Google Scholar] [CrossRef]
- Shen, Z.; Xiang, M.; Chen, C.; Ding, F.; Wang, Y.; Shang, C.; Xin, L.; Zhang, Y.; Cui, X. Glutamate excitotoxicity: Potential therapeutic target for ischemic stroke. Biomed. Pharmacother. 2022, 151, 113125. [Google Scholar] [CrossRef]
- Koh, P.O. Estradiol ameliorates the reduction in parvalbumin expression induced by ischemic brain injury. Neurosci. Lett. 2014, 574, 36–40. [Google Scholar] [CrossRef]
- Chaturvedi, M.; Kaczmarek, L. Mmp-9 inhibition: A therapeutic strategy in ischemic stroke. Mol. Neurobiol. 2014, 49, 563–573. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, G.; Kushwah, A.S.; Surampalli, G.; Singh, T.G.; Gupta, S. Arbutin protects brain against middle cerebral artery occlusion-reperfusion (MCAo/R) injury. Biochem. Biophys. Res. Commun. 2021, 577, 52–57. [Google Scholar] [CrossRef]
- Bhat, J.A.; Gupta, S.; Kumar, M. Neuroprotective effects of theobromine in transient global cerebral ischemia-reperfusion rat model. Biochem. Biophys. Res. Commun. 2021, 571, 74–80. [Google Scholar] [CrossRef]
- Blixt, J.; Song, Y.; Wanecek, M.; Gunnarson, E. EPO has multiple positive effects on astrocytes in an experimental model of ischemia. Brain Res. 2023, 1802, 148207. [Google Scholar] [CrossRef] [PubMed]
- Garzón, F.; Coimbra, D.; Parcerisas, A.; Rodriguez, Y.; García, J.C.; Soriano, E.; Rama, R. NeuroEPO Preserves Neurons from Glutamate-Induced Excitotoxicity. J. Alzheimer’s Dis. JAD 2018, 65, 1469–1483. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kang, S.H.; Kim, D.K.; Lee, N.S.; Jeong, Y.G.; Han, S.Y. Protective Effect of Cholic Acid-Coated Poly Lactic-Co-Glycolic Acid (PLGA) Nanoparticles Loaded with Erythropoietin on Experimental Stroke. J. Nanosci. Nanotechnol. 2019, 19, 6524–6533. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, K.; Jain, S.K.; Krishnamurthy, S. Pharmacokinetic and Pharmacodynamic Properties of Indole-3-carbinol in Experimental Focal Ischemic Injury. Eur. J. Drug Metab. Pharmacokinet. 2022, 47, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Eftimiadi, G.; Soligo, M.; Manni, L.; Di Giuda, D.; Calcagni, M.L.; Chiaretti, A. Topical delivery of nerve growth factor for treatment of ocular and brain disorders. Neural Regen. Res. 2021, 16, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Huo, D.; Zeng, L.T.; Fan, G.Q.; Shen, T.; Zhang, T.M.; Cai, J.P.; Cui, J. Mesencephalic astrocyte-derived neurotrophic factor (MANF): Structure, functions and therapeutic potential. Ageing Res. Rev. 2022, 82, 101763. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Zhang, Z.; Zhao, Y.; Liu, J.; Qiu, J.; Gong, Y.; Fan, W.; Guo, Y.; Guo, Y.; Xu, Z.; et al. The impact of acupuncture on neuroplasticity after ischemic stroke: A literature review and perspectives. Front. Cell. Neurosci. 2022, 16, 817732. [Google Scholar] [CrossRef]
- Elia, A.; Fossati, S. Autonomic nervous system and cardiac neuro-signaling pathway modulation in cardiovascular disorders and Alzheimer’s disease. Front. Physiol. 2023, 14, 1060666. [Google Scholar] [CrossRef]
- Alfonsetti, M.; d’Angelo, M.; Castelli, V. Neurotrophic factor-based pharmacological approaches in neurological disorders. Neural Regen. Res. 2023, 18, 1220–1228. [Google Scholar] [CrossRef]
- Dou, S.H.; Cui, Y.; Huang, S.M.; Zhang, B. The Role of Brain-Derived Neurotrophic Factor Signaling in Central Nervous System Disease Pathogenesis. Front. Hum. Neurosci. 2022, 16, 924155. [Google Scholar] [CrossRef]
- Karantali, E.; Kazis, D.; Papavasileiou, V.; Prevezianou, A.; Chatzikonstantinou, S.; Petridis, F.; McKenna, J.; Luca, A.C.; Trus, C.; Ciobica, A.; et al. Serum BDNF Levels in Acute Stroke: A Systematic Review and Meta-Analysis. Medicina 2021, 57, 297. [Google Scholar] [CrossRef]
- Sims, S.K.; Wilken-Resman, B.; Smith, C.J.; Mitchell, A.; McGonegal, L.; Sims-Robinson, C. Brain-Derived Neurotrophic Factor and Nerve Growth Factor Therapeutics for Brain Injury: The Current Translational Challenges in Preclinical and Clinical Research. Neural Plast. 2022, 2022, 3889300. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; O’Connor, M.; Wang, G.; Han, F. Brain-Derived Neurotrophic Factor and Its Potential Therapeutic Role in Stroke Comorbidities. Neural Plast. 2020, 2020, 1969482. [Google Scholar] [CrossRef]
- Nistor-Cseppentö, D.C.; Jurcău, M.C.; Jurcău, A.; Andronie-Cioară, F.L.; Marcu, F. Stem Cell- and Cell-Based Therapies for Ischemic Stroke. Bioengineering 2022, 9, 717. [Google Scholar] [CrossRef]
- Zhao, T.; Zhu, T.; Xie, L.; Li, Y.; Xie, R.; Xu, F.; Tang, H.; Zhu, J. Neural Stem Cells Therapy for Ischemic Stroke: Progress and Challenges. Transl. Stroke Res. 2022, 13, 665–675. [Google Scholar] [CrossRef]
- Tang, H.; Li, Y.; Tang, W.; Zhu, J.; Parker, G.C.; Zhang, J.H. Endogenous Neural Stem Cell-induced Neurogenesis after Ischemic Stroke: Processes for Brain Repair and Perspectives. Transl. Stroke Res. 2022, 14, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Pan, S.; Ma, Y.; Kong, W.; Qi, Z.; Yang, X. Effect of electrical stimulation combined with graphene-oxide-based membranes on neural stem cell proliferation and differentiation. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Guo, W.; Zheng, S.; Fu, C.; Ma, Y.; Pan, S.; Liu, Q.; Yang, X. Enhancement of neural stem cell survival, proliferation and differentiation by IGF-1 delivery in graphene oxide-incorporated PLGA electrospun nanofibrous mats. RSC Adv. 2019, 9, 8315–8325. [Google Scholar] [CrossRef]
- Patel, M.; Min, J.H.; Hong, M.H.; Lee, H.J.; Kang, S.; Yi, S.; Koh, W.G. Culture of neural stem cells on conductive and microgrooved polymeric scaffolds fabricated via electrospun fiber-template lithography. Biomed. Mater. 2020, 15, 045007. [Google Scholar] [CrossRef] [PubMed]
- Shabani, Z.; Rahbarghazi, R.; Karimipour, M.; Ghadiri, T.; Salehi, R.; Sadigh-Eteghad, S.; Farhoudi, M. Transplantation of bioengineered Reelin-loaded PLGA/PEG micelles can accelerate neural tissue regeneration in photothrombotic stroke model of mouse. Bioeng. Transl. Med. 2022, 7, e10264. [Google Scholar] [CrossRef]
- Asgari Taei, A.; Dargahi, L.; Khodabakhsh, P.; Kadivar, M.; Farahmandfar, M. Hippocampal neuroprotection mediated by secretome of human mesenchymal stem cells against experimental stroke. CNS Neurosci. Ther. 2022, 28, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.; Lim, J.; Choi, H.; Kang, H.; Li Jeon, N.; Son, Y. Human bone marrow-derived mesenchymal stem cells play a role as a vascular pericyte in the reconstruction of human BBB on the angiogenesis microfluidic chip. Biomaterials 2021, 279, 121210. [Google Scholar] [CrossRef]
- Tan, N.; Xin, W.; Huang, M.; Mao, Y. Mesenchymal stem cell therapy for ischemic stroke: Novel insight into the crosstalk with immune cells. Front. Neurol. 2022, 13, 1048113. [Google Scholar] [CrossRef]
- Tang, W.; Lv, X.; Huang, J.; Wang, B.; Lin, L.; Shen, Y.; Yao, Y. Neuroprotective Effect of Stroke Pretreated Mesenchymal Stem Cells Against Cerebral Ischemia/Reperfusion Injury in Rats. World Neurosurg. 2022, 165, E1–E11. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, M.; Preynat-Seauve, O.; De Bruin, K.; Pepper, M.S. Stem cell therapy for neurological disorders. S. Afr. Med. J. 2019, 109, 70–77. [Google Scholar] [CrossRef]
- Zhou, L.; Tu, J.; Fang, G.; Deng, L.; Gao, X.; Guo, K.; Kong, J.; Lv, J.; Guan, W.; Yang, C. Combining PLGA Scaffold and MSCs for Brain Tissue Engineering: A Potential Tool for Treatment of Brain Injury. Stem Cells Int. 2018, 2018, 5024175. [Google Scholar] [CrossRef]
- Mohammadalizadeh, M.; Dabirian, S.; Akrami, M.; Hesari, Z. SPION based magnetic PLGA nanofibers for neural differentiation of mesenchymal stem cells. Nanotechnology 2022, 33, 375101. [Google Scholar] [CrossRef]
- Kazemi, L.; Rahbarghazi, R.; Salehi, R.; Abedelahi, A.; Niari, S.A.; Karimipour, M.; Nasrabadi, H.T. Superior Synaptogenic Effect of Electrospun PLGA-PEG Nanofibers Versus PLGA Nanofibers on Human Neural SH-SY5Y Cells in a Three-Dimensional Culture System. J. Mol. Neurosci. 2020, 70, 1967–1976. [Google Scholar] [CrossRef]
- Grayston, A.; Zhang, Y.; Garcia-Gabilondo, M.; Arrúe, M.; Martin, A.; Kopcansky, P.; Timko, M.; Kovac, J.; Strbak, O.; Castellote, L.; et al. Endovascular administration of magnetized nanocarriers targeting brain delivery after stroke. J. Cereb. Blood Flow Metab. 2022, 42, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, Q.; Li, K.; Li, X.; Wang, C.; Xue, L.; Ju, C.; Zhang, C. A neutrophil-mimetic magnetic nanoprobe for molecular magnetic resonance imaging of stroke-induced neuroinflammation. Biomater. Sci. 2021, 9, 5247–5258. [Google Scholar] [CrossRef]
- Khalin, I.; Severi, C.; Heimburger, D.; Wehn, A.; Hellal, F.; Reisch, A.; Klymchenko, A.S.; Plesnila, N. Dynamic tracing using ultra-bright labeling and multi-photon microscopy identifies endothelial uptake of poloxamer 188 coated poly(lactic-co-glycolic acid) nano-carriers in vivo. Nanomedicine 2022, 40, 102511. [Google Scholar] [CrossRef]
- Yun, X.; Maximov, V.D.; Yu, J.; Zhu, H.; Vertegel, A.A.; Kindy, M.S. Nanoparticles for targeted delivery of antioxidant enzymes to the brain after cerebral ischemia and reperfusion injury. J. Cereb. Blood Flow Metab. 2013, 33, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Ghuman, H.; Hitchens, T.K.; Modo, M. A systematic optimization of (19)F MR image acquisition to detect macrophage invasion into an ECM hydrogel implanted in the stroke-damaged brain. NeuroImage 2019, 202, 116090. [Google Scholar] [CrossRef] [PubMed]
- Deuchar, G.A.; Brennan, D.; Holmes, W.M.; Shaw, M.; Macrae, I.M.; Santosh, C. Perfluorocarbon Enhanced Glasgow Oxygen Level Dependent (GOLD) Magnetic Resonance Metabolic Imaging Identifies the Penumbra Following Acute Ischemic Stroke. Theranostics 2018, 8, 1706–1722. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, E.; Swider, E.; Staal, A.H.J.; White, P.B.; van Riessen, N.K.; Glaßer, G.; Lieberwirth, I.; Musyanovych, A.; Serra, C.A.; Srinivas, M.; et al. Continuous-Flow Production of Perfluorocarbon-Loaded Polymeric Nanoparticles: From the Bench to Clinic. ACS Appl. Mater. Interfaces 2020, 12, 49335–49345. [Google Scholar] [CrossRef]
- Adel, M.; Zahmatkeshan, M.; Akbarzadeh, A.; Rabiee, N.; Ahmadi, S.; Keyhanvar, P.; Rezayat, S.M.; Seifalian, A.M. Chemotherapeutic effects of Apigenin in breast cancer: Preclinical evidence and molecular mechanisms; enhanced bioavailability by nanoparticles. Biotechnol. Rep. 2022, 34, e00730. [Google Scholar] [CrossRef]
- Creemers, J.H.A.; Pawlitzky, I.; Grosios, K.; Gileadi, U.; Middleton, M.R.; Gerritsen, W.R.; Mehra, N.; Rivoltini, L.; Walters, I.; Figdor, C.G.; et al. Assessing the safety, tolerability and efficacy of PLGA-based immunomodulatory nanoparticles in patients with advanced NY-ESO-1-positive cancers: A first-in-human phase I open-label dose-escalation study protocol. BMJ Open 2021, 11, e050725. [Google Scholar] [CrossRef]
- Saito, H.; Couso-Queiruga, E.; Shiau, H.J.; Stuhr, S.; Prasad, H.; Allareddy, T.V.; Reynolds, M.A.; Avila-Ortiz, G. Evaluation of poly lactic-co-glycolic acid-coated β-tricalcium phosphate for alveolar ridge preservation: A multicenter randomized controlled trial. J. Periodontol. 2021, 92, 524–535. [Google Scholar] [CrossRef]
- Lecio, G.; Ribeiro, F.V.; Pimentel, S.P.; Reis, A.A.; da Silva, R.V.C.; Nociti-Jr, F.; Moura, L.; Duek, E.; Casati, M.; Casarin, R.C.V. Novel 20% doxycycline-loaded PLGA nanospheres as adjunctive therapy in chronic periodontitis in type-2 diabetics: Randomized clinical, immune and microbiological trial. Clin. Oral Investig. 2020, 24, 1269–1279. [Google Scholar] [CrossRef]
- Raju, K.; Mani, U.M.; Vaidyanathan, A.K. Evaluating the osteogenic potential of insulin-like growth factor-1 microspheres on osteoblastic activity around dental implants in patients with type 2 diabetes mellitus using bone scintigraphy: A split-mouth randomized controlled trial. J. Prosthet. Dent. 2023, 129, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Guan, C.; Yuan, J.; Cao, X.; Qin, L.; Li, Y.; Li, Z.; Nie, S.; Hou, S.; Zhang, M.; et al. Two-year safety evaluation of a biodegradable polymer sirolimus-eluting stent with increased drug elution and polymer absorption kinetics in complex patient and lesion cohort. Catheter. Cardiovasc. Interv. 2020, 95, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Vesga, B.; Hernandez, H.; Moncada, M.; Gasior, P.; Higuera, S.; Dager, A.; Arana, C.; Delgado, J.A.; Généreux, P.; Maehara, A.; et al. Three-month evaluation of strut healing using a novel optical coherence tomography analytical method following bioresorbable polymer everolimus-eluting stent implantation in humans: The TIMELESS study. Coron. Artery Dis. 2017, 28, 126–134. [Google Scholar] [CrossRef]
- Jia, B.; Zhang, X.; Ma, N.; Mo, D.; Gao, F.; Sun, X.; Song, L.; Liu, L.; Deng, Y.; Xu, X.; et al. Comparison of Drug-Eluting Stent with Bare-Metal Stent in Patients with Symptomatic High-grade Intracranial Atherosclerotic Stenosis: A Randomized Clinical Trial. JAMA Neurol. 2022, 79, 176–184. [Google Scholar] [CrossRef] [PubMed]
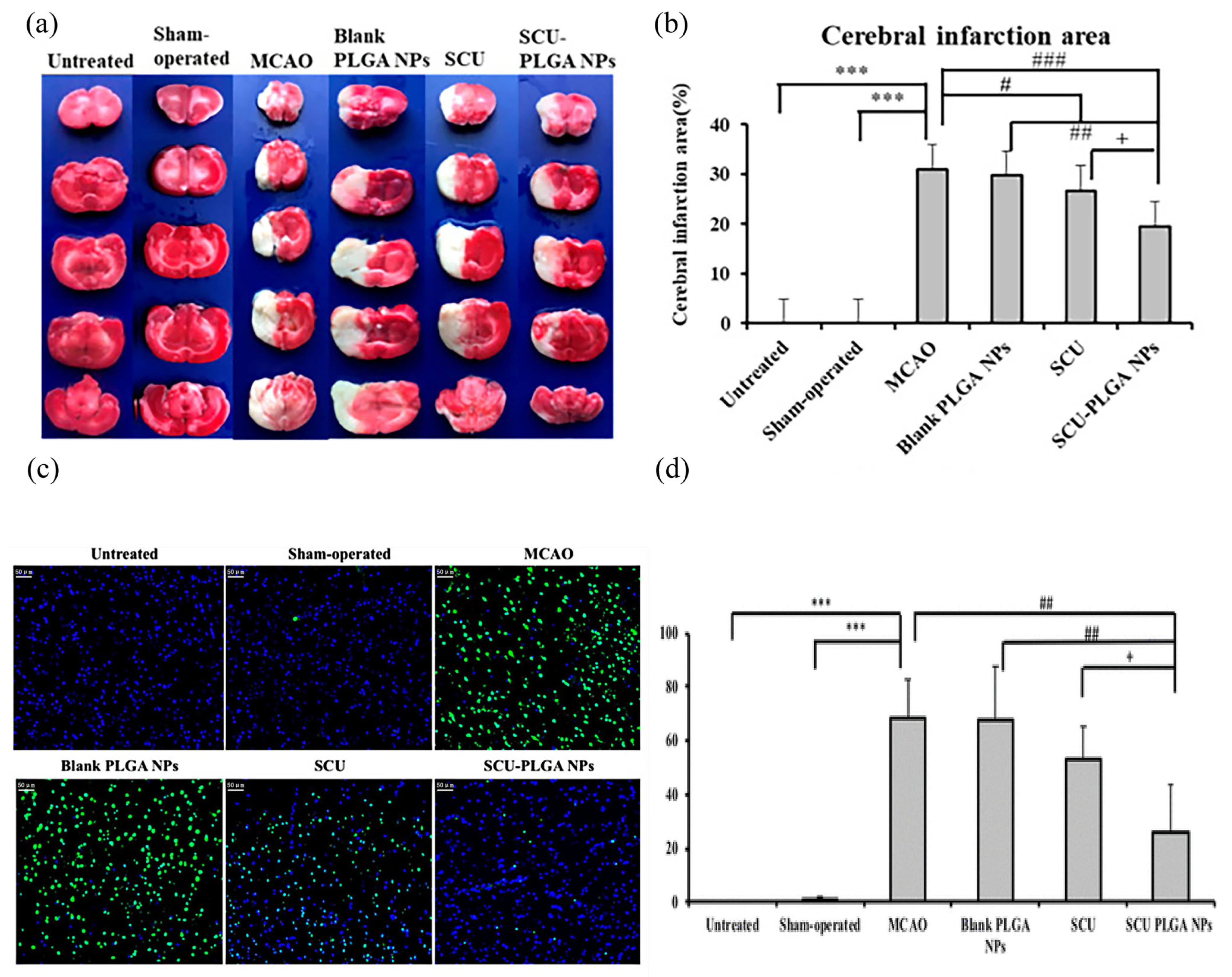
| Therapeutic Mechanism | Nanocarrier | Loaded Drug | Synthesis Method | Average Diameter (nm) | Research Type | Year of Publication | Refs. |
|---|---|---|---|---|---|---|---|
| Thrombolytic therapy | PEG-PLGA NPs | t-PA | Single-emulsion solvent diffusion/evaporation | 276.20 ± 27.58 | In vitro | 2019 | [63] |
| Peptide conjugated PLGA magnetic NPs | rtPA | Single-emulsion solvent evaporation | 321.1 ± 26.9 | In vivo and in vitro | 2020 | [64] | |
| CREKA polypeptide modified PLGA NPs | rtPA, PFH | Carbodiimide method | 178.56 ± 1.25 | In vivo and in vitro | 2022 | [65] | |
| CS-GRGD modified PLGA NPs | Indocyanine green (ICG) complex of urokinase (ICG@uPA) | Double-emulsion solvent evaporation | 68 ± 2 | In vivo and in vitro | 2022 | [66] | |
| Anti-oxidative stress and anti-apoptosis | mPEG-bPLGA NPs | Edaravone | Nanoprecipitation | 155 ± 2.5 | In vitro | 2022 | [67] |
| PLGA NPs | Scutellarin | Nanoprecipitation | 187.89 ± 3.42 | In vivo and in vitro | 2022 | [68] | |
| PLGA NPs | Tanshinone IIA | Nanoprecipitation | 91.34 ± 1.3 | In vivo and in vitro | 2021 | [69] | |
| PLGA NPs | NL-1 | Emulsification and solvent evaporation | 123.9 ± 17.1 | In vivo and in vitro | 2022 | [70] | |
| PLGA NPs | Ex-4 | Water–oil–water (w/o/w) emulsion solvent evaporation | 68 ± 3.2 | In vivo and in vitro | 2022 | [71] | |
| PEG-PLGA NPs | Curcumin | Modified emulsion diffusion evaporation | 71 ± 9.5 | In vivo | 2019 | [72] | |
| Anti-inflammation | RVG29 peptide-modified polyethylene glycol–polylactic acid–glycolic acid copolymer NPs encapsulated in the membrane of neutrophils | Baicalin | Double emulsification | 89~130 | In vivo and in vitro | 2022 | [73] |
| α-lipoic acid modified PLGA NPs | Cannabidiol | Classic emulsion/solvent evaporation | 110.3 ± 3.6 | In vivo and in vitro | 2022 | [74] | |
| PLGA NPs | Perampanel | Emulsification/ solvent evaporation | 216.7 | In vitro and in vivo | 2022 | [75] | |
| PLGA NPs | PINK1 siRNA | emulsification/ solvent evaporation | - | In vivo and in vitro | 2022 | [76] | |
| Inhibition of neuroexcitatory toxicity | PLGA NPs | Estradiol | Emulsion diffusion | 98 ± 1.9 | In vitro | 2014 | [77] |
| PLGA NPs | TIMP-1 | Multiple emulsion and solvent evaporation | - | In vitro | 2012 | [78] | |
| PLGA NPs | rhEPO | W/o/w emulsion solvent evaporation | 42 | In vitro | 2014 | [79] | |
| PLGA NPs | Indole-3-methanol | Oil-in-water (o/w) emulsion solvent evaporation | 61 | In vitro | 2015 | [80] | |
| Supplementing with neurotrophic factor | PLGA NPs | BDNF | Nanoprecipitation | 186.6 ± 19.11 | In vivo | 2020 | [81] |
| PLGA NPs-HAMC composite | BDNF | W/o/w double-emulsion solvent evaporation | - | In vivo | 2019 | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Huang, L.; Feng, J.; Yang, X. The Recent Applications of PLGA-Based Nanostructures for Ischemic Stroke. Pharmaceutics 2023, 15, 2322. https://doi.org/10.3390/pharmaceutics15092322
Yan J, Huang L, Feng J, Yang X. The Recent Applications of PLGA-Based Nanostructures for Ischemic Stroke. Pharmaceutics. 2023; 15(9):2322. https://doi.org/10.3390/pharmaceutics15092322
Chicago/Turabian StyleYan, Jun, Lei Huang, Juan Feng, and Xue Yang. 2023. "The Recent Applications of PLGA-Based Nanostructures for Ischemic Stroke" Pharmaceutics 15, no. 9: 2322. https://doi.org/10.3390/pharmaceutics15092322
APA StyleYan, J., Huang, L., Feng, J., & Yang, X. (2023). The Recent Applications of PLGA-Based Nanostructures for Ischemic Stroke. Pharmaceutics, 15(9), 2322. https://doi.org/10.3390/pharmaceutics15092322