A New Paclitaxel Formulation Based on Secretome Isolated from Mesenchymal Stem Cells Shows a Significant Cytotoxic Effect on Osteosarcoma Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bone Marrow Mesenchymal Stem Cells Secretome Production
2.2. Determination of PTX Sensitivity in MSCs and OS Cell Lines
2.3. Secretome Cytotoxicity Activities
2.4. Secretome Characterization by Nanoparticle Tracking Analysis
2.5. PTX Determination in Secretome Preparation via HPLC-MS/MS Analysis
3. Results
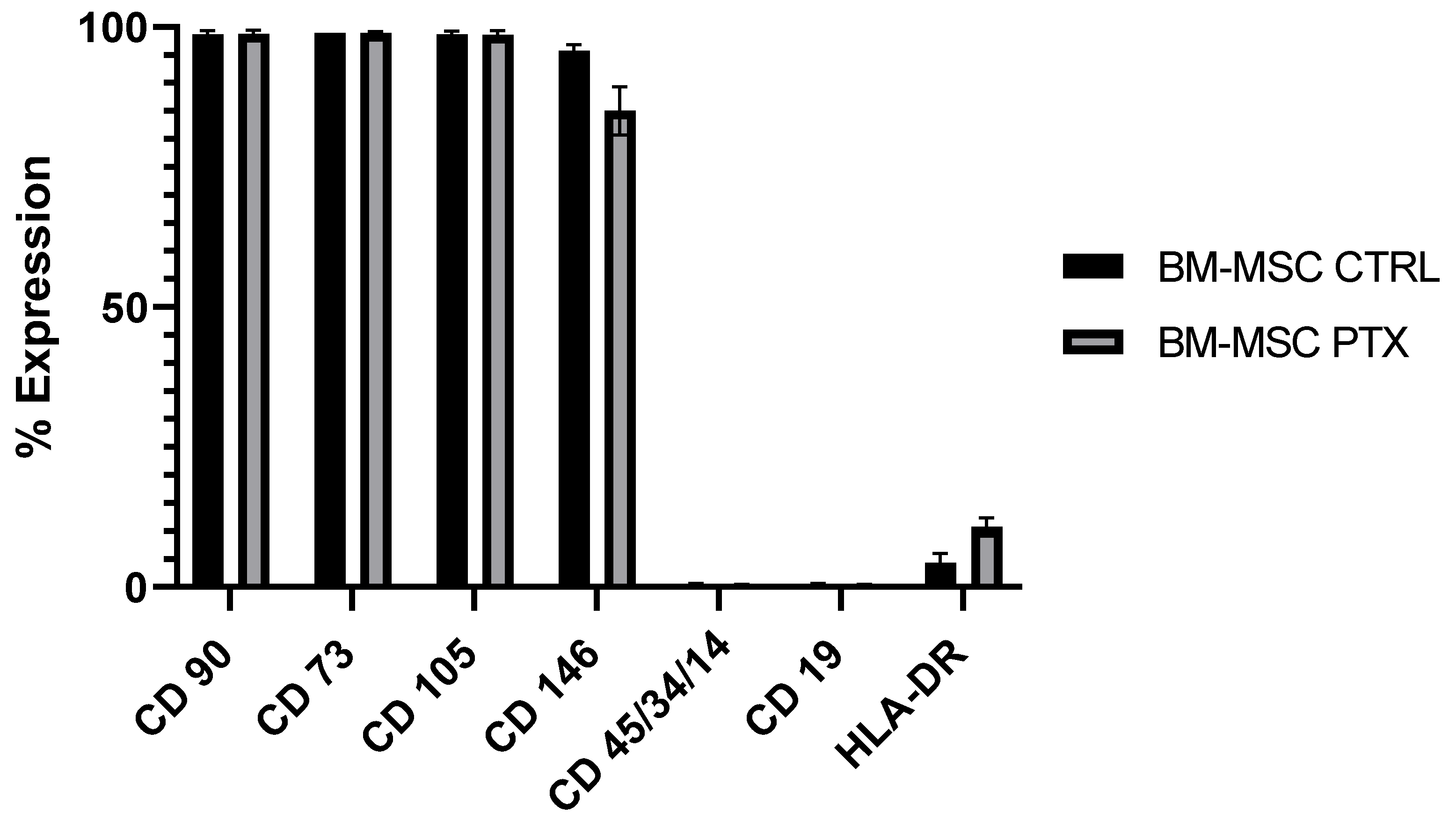
3.1. Characterization of BM-MSCs and Sensitivity to PTX
| ID | SEX | AGE | ID SECRETOME |
|---|---|---|---|
| BM-MSC-1 | Male | 6 years | SECR-CTRL-1; SECR-PTX-1 |
| BM-MSC-2 | Male | 9 years | SECR-CTRL-2; SECR-PTX-2 |
| BM-MSC-3 | Female | 4 years | SECR-CTRL-3; SECR-PTX-3 |
3.2. Secretome Cytotoxicity Effect on OS Cell Lines
3.3. Characterization of the Secretome by Nanoparticle Tracking Analysis (NTA)
3.4. High-Performance Liquid Chromatography (HPLC-MS/MS) Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hingorani, P.; Janeway, K.; Crompton, B.D.; Kadoch, C.; Mackall, C.L.; Khan, J.; Shern, J.F.; Schiffman, J.; Mirabello, L.; Savage, S.A.; et al. Current State of Pediatric Sarcoma Biology and Opportunities for Future Discovery: A Report from the Sarcoma Translational Research Workshop. Cancer Genet. 2016, 209, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E. Update on Survival in Osteosarcoma. Orthop. Clin. N. Am. 2016, 47, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Ingley, K.M.; Maleddu, A.; Grange, F.L.; Gerrand, C.; Bleyer, A.; Yasmin, E.; Whelan, J.; Strauss, S.J. Current Approaches to Management of Bone Sarcoma in Adolescent and Young Adult Patients. Pediatr. Blood Cancer 2022, 69, e29442. [Google Scholar] [CrossRef]
- Lenna, S.; Bellotti, C.; Duchi, S.; Martella, E.; Columbaro, M.; Dozza, B.; Ballestri, M.; Guerrini, A.; Sotgiu, G.; Frisoni, T.; et al. Mesenchymal Stromal Cells Mediated Delivery of Photoactive Nanoparticles Inhibits Osteosarcoma Growth in Vitro and in a Murine in Vivo Ectopic Model. J. Exp. Clin. Cancer Res. 2020, 39, 40. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Moon, Y.J.; Chun, H.J.; Yang, D.H. Doxorubicin·Hydrochloride/Cisplatin-Loaded Hydrogel/Nanosized (2-Hydroxypropyl)-Beta-Cyclodextrin Local Drug-Delivery System for Osteosarcoma Treatment In Vivo. Nanomaterials 2019, 9, E1652. [Google Scholar] [CrossRef] [PubMed]
- Loebinger, M.R.; Janes, S.M. Stem Cells as Vectors for Antitumour Therapy. Thorax 2010, 65, 362–369. [Google Scholar] [CrossRef]
- Pessina, A.; Bonomi, A.; Coccè, V.; Invernici, G.; Navone, S.; Cavicchini, L.; Sisto, F.; Ferrari, M.; Viganò, L.; Locatelli, A.; et al. Mesenchymal Stromal Cells Primed with Paclitaxel Provide a New Approach for Cancer Therapy. PLoS ONE 2011, 6, e28321. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef]
- Babajani, A.; Soltani, P.; Jamshidi, E.; Farjoo, M.H.; Niknejad, H. Recent Advances on Drug-Loaded Mesenchymal Stem Cells with Anti-neoplastic Agents for Targeted Treatment of Cancer. Front. Bioeng. Biotechnol. 2020, 8, 748. [Google Scholar] [CrossRef]
- Scioli, M.G.; Artuso, S.; D’Angelo, C.; Porru, M.; D’Amico, F.; Bielli, A.; Gentile, P.; Cervelli, V.; Leonetti, C.; Orlandi, A. Adipose-Derived Stem Cell-Mediated Paclitaxel Delivery Inhibits Breast Cancer Growth. PLoS ONE 2018, 13, e0203426. [Google Scholar] [CrossRef]
- Jafarinia, M.; Alsahebfosoul, F.; Salehi, H.; Eskandari, N.; Ganjalikhani-Hakemi, M. Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Novel Cell-Free Therapy. Immunol. Investig. 2020, 49, 758–780. [Google Scholar] [CrossRef] [PubMed]
- Mareschi, K.; Marini, E.; Niclot, A.G.S.B.; Barone, M.; Pinnetta, G.; Adamini, A.; Spadea, M.; Labanca, L.; Lucania, G.; Ferrero, I.; et al. A New Human Platelet Lysate for Mesenchymal Stem Cell Production Compliant with Good Manufacturing Practice Conditions. Int. J. Mol. Sci. 2022, 23, 3234. [Google Scholar] [CrossRef] [PubMed]
- Lisini, D.; Nava, S.; Frigerio, S.; Pogliani, S.; Maronati, G.; Marcianti, A.; Coccè, V.; Bondiolotti, G.; Cavicchini, L.; Paino, F.; et al. Automated Large-Scale Production of Paclitaxel Loaded Mesenchymal Stromal Cells for Cell Therapy Applications. Pharmaceutics 2020, 12, E411. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Damaser, M.S. Stem Cells as Drug Delivery Methods: Application of Stem Cell Secretome for Regeneration. Adv. Drug Deliv. Rev. 2015, 82–83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Walker, F.E. Paclitaxel (TAXOL): Side effects and patient education issues. Semin. Oncol. Nurs. 1993, 9, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, G.; Jaffe, N. The Epidemiology of Osteosarcoma. Cancer Treat. Res. 2009, 152, 3–13. [Google Scholar] [CrossRef]
- Grünewald, T.G.; Alonso, M.; Avnet, S.; Banito, A.; Burdach, S.; Cidre-Aranaz, F.; Di Pompo, G.; Distel, M.; Dorado-Garcia, H.; Garcia-Castro, J.; et al. Sarcoma Treatment in the Era of Molecular Medicine. EMBO Mol. Med. 2020, 12, e11131. [Google Scholar] [CrossRef]
- Wei, H.; Chen, J.; Wang, S.; Fu, F.; Zhu, X.; Wu, C.; Liu, Z.; Zhong, G.; Lin, J. A Nanodrug Consisting of Doxorubicin and Exosome Derived from Mesenchymal Stem Cells for Osteosarcoma Treatment In Vitro. Int. J. Nanomed. 2019, 14, 8603–8610. [Google Scholar] [CrossRef]
- Khan, M.; Adil, S.E.; Olson, A.L. The role of mesenchymal stem cells in oncology and regenerative medicine. Future Oncol. 2017, 13, 821–831. [Google Scholar] [CrossRef]
- Sevelda, P.; Mayerhofer, K.; Obermair, A.; Stolzlechner, J.; Kurz, C. Thrombosis with paclitaxel. Lancet 1994, 343, 727. [Google Scholar] [CrossRef]
- Bari, E.; Perteghella, S.; Di Silvestre, D.; Sorlini, M.; Catenacci, L.; Sorrenti, M.; Marrubini, G.; Rossi, R.; Tripodo, G.; Mauri, P.; et al. Pilot Production of Mesenchymal Stem/Stromal Freeze-Dried Secretome for Cell-Free Regenerative Nanomedicine: A Validated GMP-Compliant Process. Cells 2018, 7, E190. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Perteghella, S.; Catenacci, L.; Sorlini, M.; Croce, S.; Mantelli, M.; Avanzini, M.A.; Sorrenti, M.; Torre, M.L. Freeze-Dried and GMP-Compliant Pharmaceuticals Containing Exosomes for Acellular Mesenchymal Stromal Cell Immunomodulant Therapy. Nanomedicine 2019, 14, 753–765. [Google Scholar] [CrossRef] [PubMed]




| ID Sample | PTX-IC50 after 24 h of Treatment | PTX-IC50 after 5 Days of Treatment |
|---|---|---|
| SJSA | 22.8 µg/mL | 86.2 pg/mL |
| MG63 | 22.6 µg/mL | 52.1 pg/mL |
| HOS | 18.6 µg/mL | No detection |
| ID Sample | Particle Concentration (NPs /mL) | Particle Z Potential (mV) |
|---|---|---|
| SECR-CTRL-1 | 2.70 × 109 ± 0.09 × 109 | −12.4 ± 3.9 |
| SECR-CTRL-2 | 2.40 × 109 ± 0.05 × 109 | −36.4 ± 2.6 |
| SECR-CTRL-3 | 2.50 × 109 ± 0.09 × 109 | −15.5 ± 3.7 |
| SECR-PTX-1 | 0.51 × 109 ± 0.02 × 109 | −13.6 ± 2.8 |
| SECR-PTX-2 | 0.74 × 109 ± 0.02 × 109 | −17.5 ± 8.7 |
| SECR-PTX-3 | 0.49 × 109 ± 0.01 × 109 | −26.3 ± 5.3 |
| ID SECRETOME | PTX Concentration pg/mL |
|---|---|
| SECR-CTRL-1 | no detection |
| SECR-CTRL-2 | no detection |
| SECR-CTRL-3 | no detection |
| SECR-PTX-1 | 86.05 |
| SECR-PTX-2 | 31.03 |
| SECR-PTX-3 | 52.38 |
| ID Sample | No. of Cells (Tot) | % MSC Viability | No. of Cells/SECR-mL | No. of EVs (Tot) | No. of EVs/mL | No. of EVs/Cells | Content of PTX (pg/mL) | Content of PTX/Cell (pg/cell) | Content of PTX/EVs (pg/EVs) |
|---|---|---|---|---|---|---|---|---|---|
| SECR-PTX-1 | 1.92 × 106 | 85.7% | 9.60 × 104 | 1.01 × 1010 | 5.07 × 108 | 5277.8 | 86.5 | 9.01 × 10−4 | 1.71 × 10−7 |
| SECR-PTX-2 | 9.07 × 105 | 94.6% | 4.53 × 104 | 1.47 × 1010 | 7.37 × 108 | 16,250.0 | 31.0 | 6.84 × 10−4 | 4.21 × 10−8 |
| SECR-PTX-3 | 1.86 × 106 | 94.9% | 9.30 × 104 | 9.73 × 109 | 4.87 × 108 | 5233.0 | 52.4 | 5.63 × 10−4 | 1.08 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banche Niclot, A.G.S.; Marini, E.; Ferrero, I.; Barbero, F.; Rosso, E.; Fenoglio, I.; Barge, A.; Pessina, A.; Coccè, V.; Paino, F.; et al. A New Paclitaxel Formulation Based on Secretome Isolated from Mesenchymal Stem Cells Shows a Significant Cytotoxic Effect on Osteosarcoma Cell Lines. Pharmaceutics 2023, 15, 2340. https://doi.org/10.3390/pharmaceutics15092340
Banche Niclot AGS, Marini E, Ferrero I, Barbero F, Rosso E, Fenoglio I, Barge A, Pessina A, Coccè V, Paino F, et al. A New Paclitaxel Formulation Based on Secretome Isolated from Mesenchymal Stem Cells Shows a Significant Cytotoxic Effect on Osteosarcoma Cell Lines. Pharmaceutics. 2023; 15(9):2340. https://doi.org/10.3390/pharmaceutics15092340
Chicago/Turabian StyleBanche Niclot, Alessia Giovanna Santa, Elena Marini, Ivana Ferrero, Francesco Barbero, Elena Rosso, Ivana Fenoglio, Alessandro Barge, Augusto Pessina, Valentina Coccè, Francesca Paino, and et al. 2023. "A New Paclitaxel Formulation Based on Secretome Isolated from Mesenchymal Stem Cells Shows a Significant Cytotoxic Effect on Osteosarcoma Cell Lines" Pharmaceutics 15, no. 9: 2340. https://doi.org/10.3390/pharmaceutics15092340
APA StyleBanche Niclot, A. G. S., Marini, E., Ferrero, I., Barbero, F., Rosso, E., Fenoglio, I., Barge, A., Pessina, A., Coccè, V., Paino, F., Mareschi, K., & Fagioli, F. (2023). A New Paclitaxel Formulation Based on Secretome Isolated from Mesenchymal Stem Cells Shows a Significant Cytotoxic Effect on Osteosarcoma Cell Lines. Pharmaceutics, 15(9), 2340. https://doi.org/10.3390/pharmaceutics15092340










