Development and Optimization of a Novel Lozenge Containing a Metronidazole-Peppermint Oil-Tranexamic Acid Self-Nanoemulsified Delivery System to Be Used after Dental Extraction: In Vitro Evaluation and In Vivo Appraisal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Estimation of the Required Hydrophilic-Lipophilic Balance (RHLB) for MZ-Loaded PO
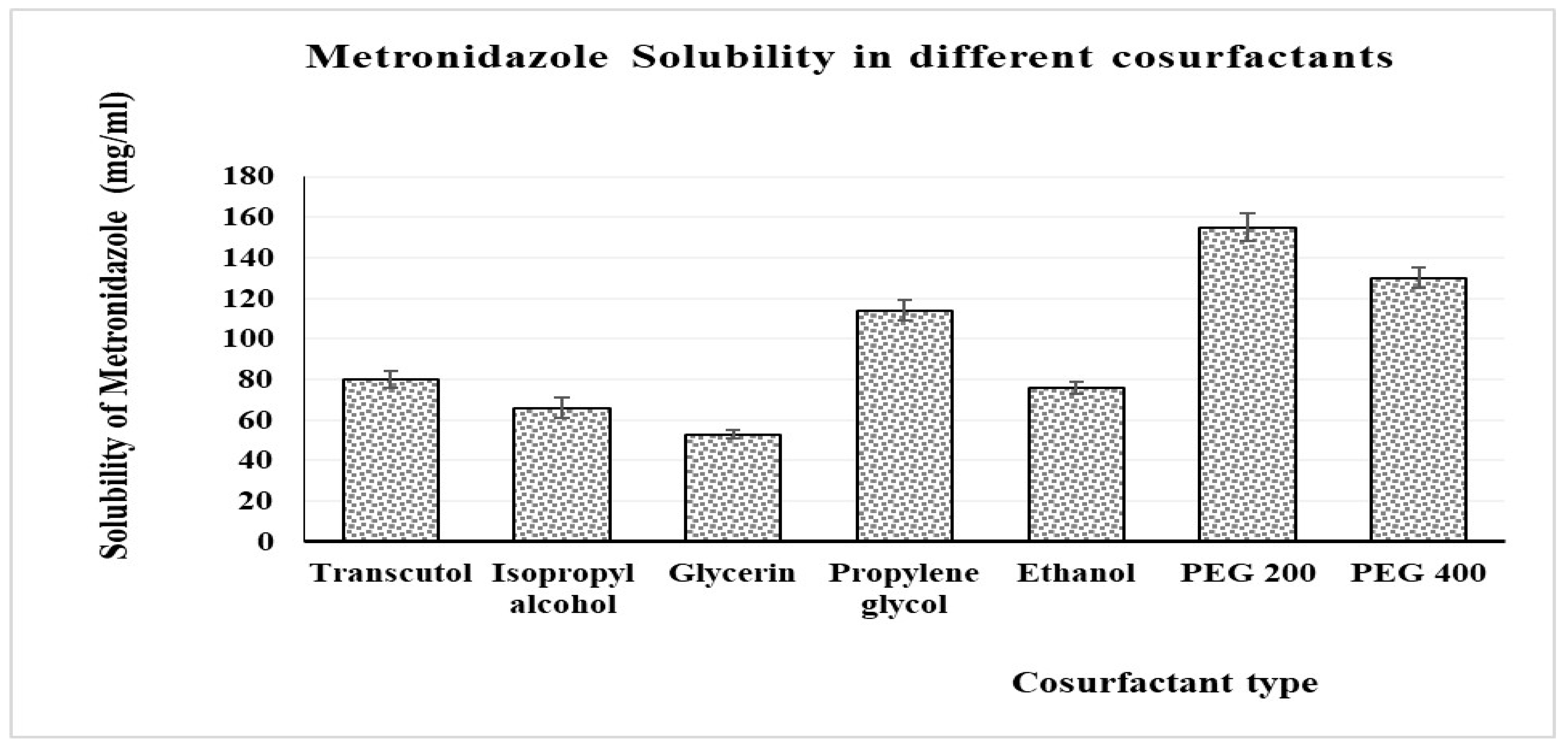
2.2.2. Solubility Studies
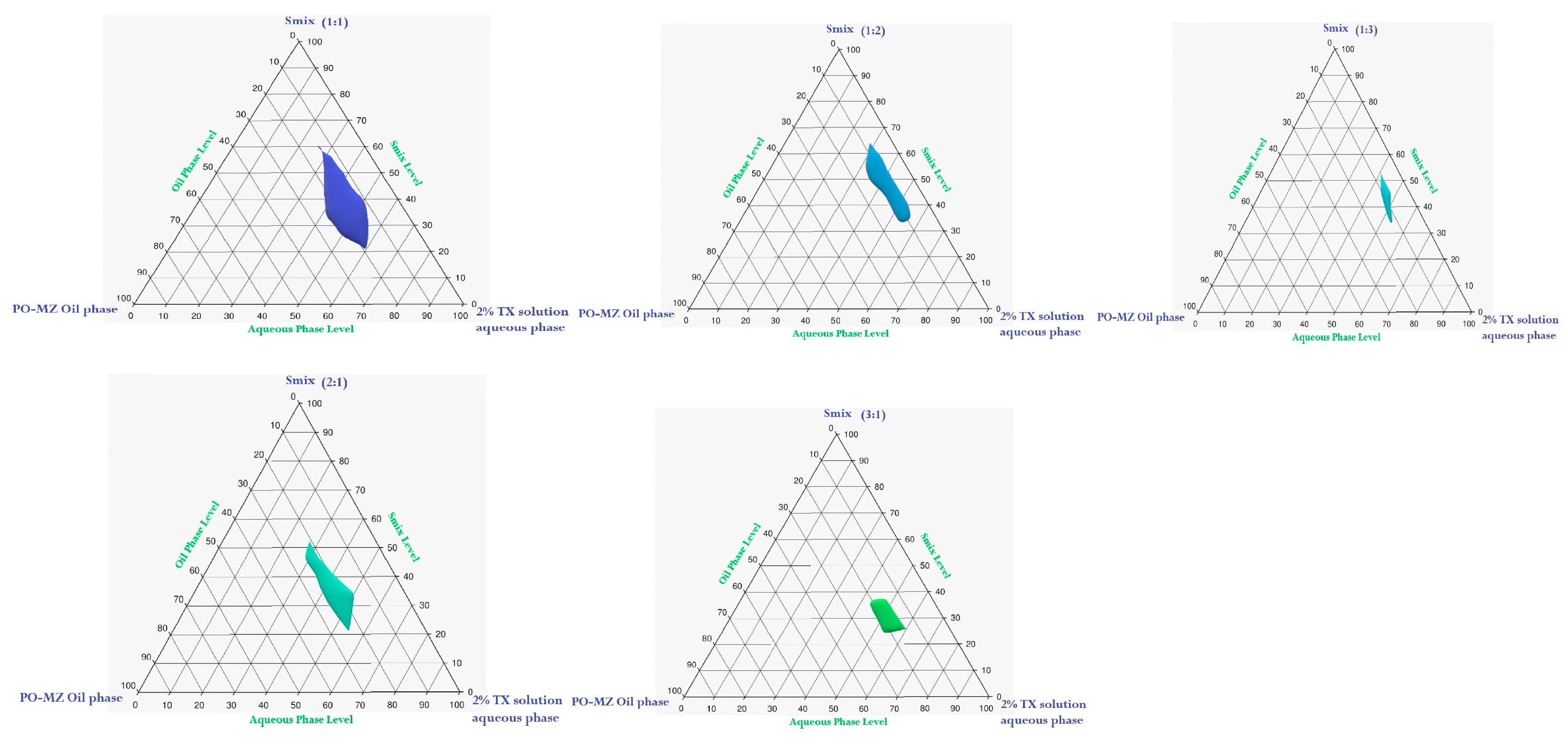
2.2.3. Construction of Pseudoternary Phase Diagrams
2.2.4. Statistical Designing of Experiments and Optimization of Self-Nanoemulsifying Drug-Delivery Systems (SNEDDS)
2.2.5. MZ-PO-TX–Loaded NEs
2.2.6. Characterization of the NEs
Droplet Size (Y1)
Blood Coagulation Time (Y2)
- Animal Handling and Care
- Estimation of Coagulation Time (Y2)
IL-6 Level (Y3)
Antibacterial Activity (Y4)
2.2.7. Optimization of the MZ-PO-TX-NE
2.2.8. Characterization of the Optimized MZ-PO-TX-NEs
Stability Index Determination
Evaluation of EE% of MZ in the Optimized MZ-PO-TX-NE
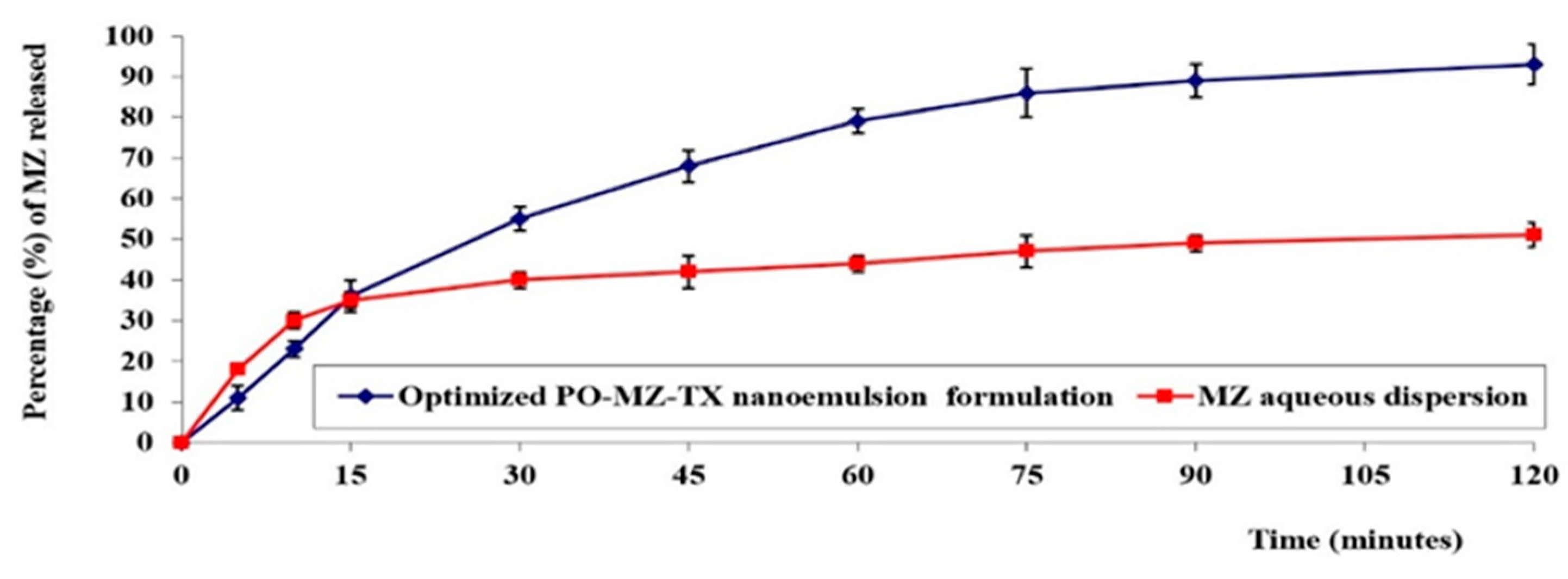
In Vitro Release Study of MZ-PO-TX-NEs
2.2.9. Formulation and Characterization of the Lozenge Loaded with Optimized MZ-PO-TX-NE
2.2.10. Characterization of Lozenges
Stability Studies
Assessment of MIC Values, IL-6 Levels, and Coagulation Times for the Optimized Lozenges Loaded with the Optimized MZ-PO-TX-NEs
3. Results and Discussion
3.1. Calculation of RHLB for PO Loaded with MZ
3.2. Pseudoternary Phase Diagram
3.3. Solubility Study
3.4. Characterization of MZ-PO-TX-NEs
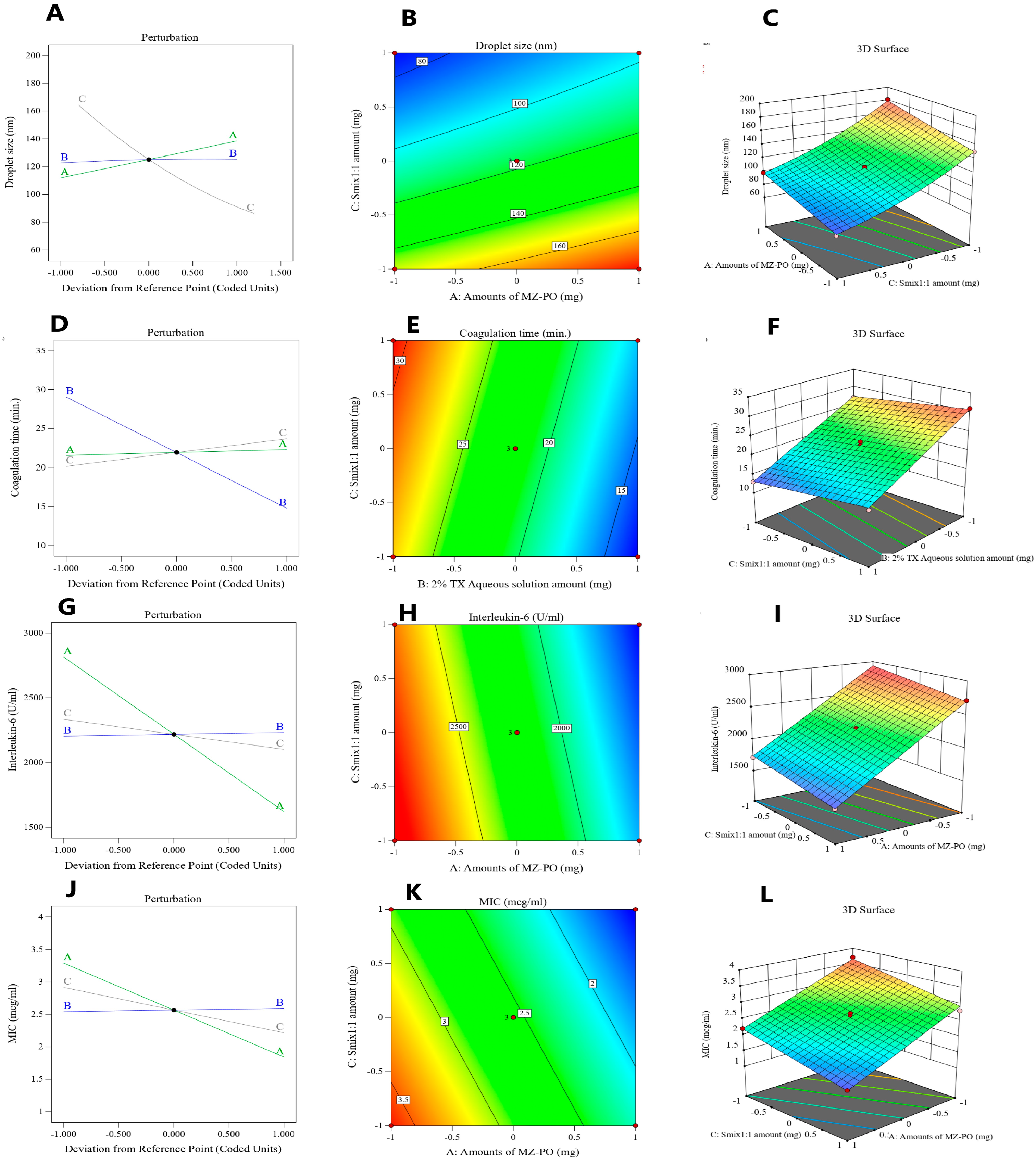
3.4.1. Droplet Size
3.4.2. Blood Coagulation Time
3.4.3. Serum IL-6 Measurements
3.4.4. Antibacterial Activity Evaluation (Y4)
3.5. Optimization of the MZ-PO-TX-NEs
3.6. Characterization of the Optimized MZ-PO-TX-NE
3.6.1. Stability Index Determination
3.6.2. Evaluation of the EE% of MZ in the Optimized MZ-PO-TX-NE
3.6.3. In Vitro Release Study of MZ-PO-TX-NE
3.7. Characterization of Lozenges
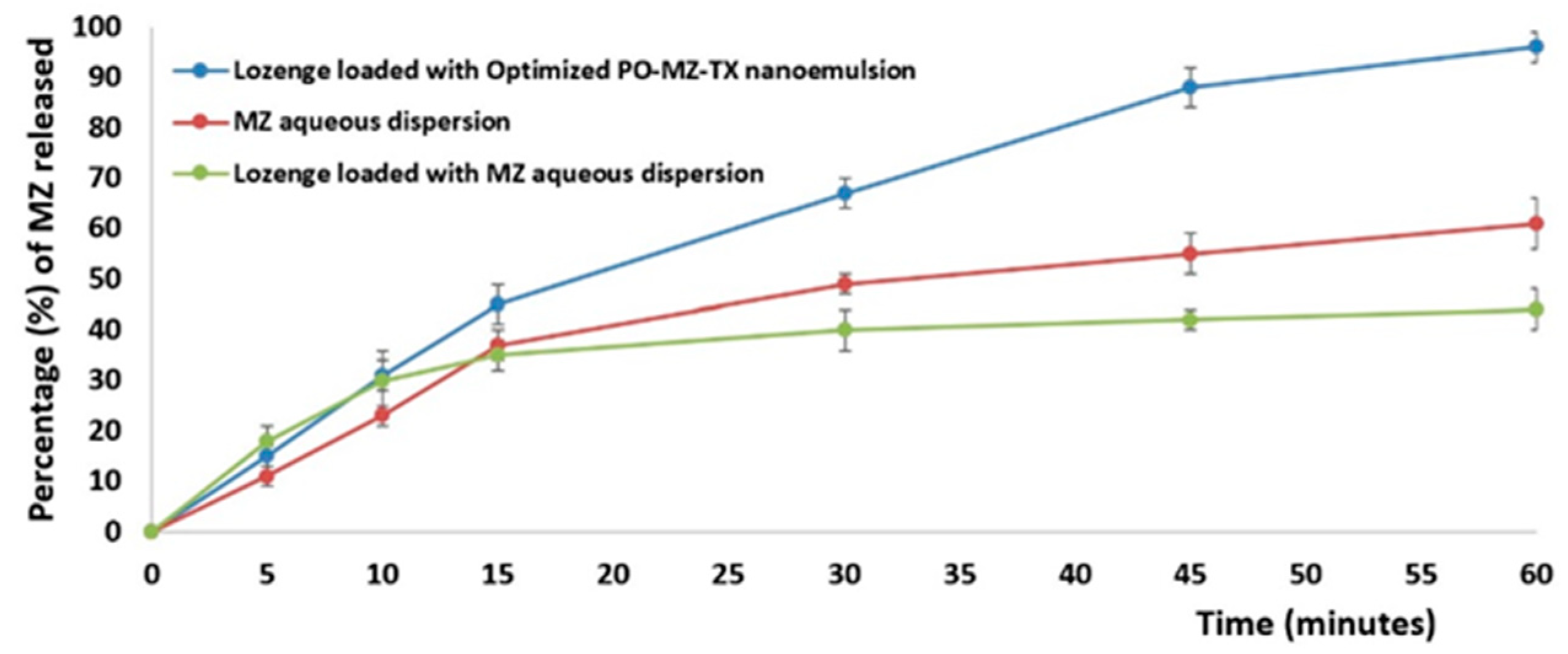
3.7.1. In Vitro Release Study
3.7.2. Stability Study
3.7.3. Assessment of MIC Values, IL-6 Levels, and Coagulation Times for the Optimal Lozenge Loaded with the Optimal MZ-PO-TX-NE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Passarelli, P.C.; Pagnoni, S.; Piccirillo, G.B.; Desantis, V.; Benegiamo, M.; Liguori, A.; Papa, R.; Papi, P.; Pompa, G.; D’Addona, A. Reasons for Tooth Extractions and Related Risk Factors in Adult Patients: A Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 2575. [Google Scholar] [CrossRef] [PubMed]
- Beuling, M.G.; Agterbos, P.C.G.; van Riet, P.C.T.; Ho, J.P.T.F.; de Vries, R.; Kober, J.; de Lange, J. Forces and movements during tooth extraction: A scoping review. Adv. Oral Maxillofacial Surg. 2023, 9, 100391. [Google Scholar] [CrossRef]
- Arteagoitia, I.; Ramos, E.; Santamaria, G.; Barbier, L.; Alvarez, J.; Santamaria, J. Amoxicillin/clavulanic acid 2000/125 mg to prevent complications due to infection following completely bone-impacted lower third molar removal: A clinical trial. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Severe Tooth Loss: A Systematic Review and Meta-analysis. J. Dent. Res. 2014, 93 (Suppl. S7), 20S–28S. [Google Scholar] [CrossRef]
- Chrysanthakopoulos, N.A. Reasons for extraction of permanent teeth in Greece: A five-year follow-up study. Int. Dent. J. 2011, 61, 19–24. [Google Scholar] [CrossRef]
- Cho, H.; Lynham, A.J.; Hsu, E. Postoperative interventions to reduce inflammatory complications after third molar surgery:review of the current evidence. Aust. Dent. J. 2017, 62, 412–419. [Google Scholar] [CrossRef]
- Alkhabuli, J.; Kokovic, V.; Emad, A. Post extraction lingual mucosal ulceration with bone necrosis. Saudi J. Dent. Res. 2016, 7, 34–37. [Google Scholar] [CrossRef]
- Akhtar, A.; Macfarlane, R.J.; Waseem, M. Pre-operative assessment and post-operative care in elective shoulder surgery. Open Orthop. J. 2013, 7, 316–322. [Google Scholar] [CrossRef]
- Lodi, G.; Azzi, L.; Varoni, E.M.; Pentenero, M.; Del Fabbro, M.; Carrassi, A.; Sardella, A.; Manfredi, M. Antibiotics to prevent complications following tooth extractions. Cochrane Database Syst. Rev. 2021, 2, CD003811. [Google Scholar]
- Hersh, E.V.; Moore, P.A.; Grosser, T.; Polomano, R.C.; Farrar, J.T.; Saraghi, M.; Juska, S.A.; Mitchell, C.H.; Theken, K.N. Nonsteroidal Anti-Inflammatory Drugs and Opioids in Postsurgical Dental Pain. J. Dent. Res. 2020, 99, 777–786. [Google Scholar] [CrossRef]
- Kulkarni, M.P.; Sharma, A.; Tanwar, S.; Vandana, P.B.; Wadhwa, S.; Singh, G.; Kumar, P.; Kumar, R. Pharmaceutical Lozenges: Recent Trends and Developments with an Update on Research and Patents. Recent Adv. Drug. Deliv. Formul. 2022, 16, 45–54. [Google Scholar]
- Phaemachud, T.; Tuntarawongsa, S. Clotrimazol Soft Lozenges Fabricated with Melting and Mold Technique. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 579–586. [Google Scholar]
- Majekodunmi, S.O. A Review on Lozenges. Am. J. Med. Med. Sci. 2015, 5, 99–104. [Google Scholar]
- Costa, F.W.G.; Rodrigues, R.R.; Sousa, L.H.T.D.; Carvalho, F.S.R.; Chaves, F.N.; Fernandes, C.P.; Pereira, K.M.A.; Soares, E.C.S. Local hemostatic measures in anticoagulated patients undergoing oral surgery: A systematized literature review. Acta Cir. Bras. 2013, 28, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Felix, J.; Chaban, P.; Ouanounou, A. Dental Management of Patients Undergoing Antithrombotic Therapy. J. Can. Dent. Assoc. 2020, 86, k17. [Google Scholar]
- Chaudhari, D.M.; Renjen, P.N.; Hasan, U.; Goyal, N.; Ahmad, K. Metronidazole-Induced Encephalopathy (MIE). Cureus 2022, 14, e33133. [Google Scholar] [CrossRef]
- Leitsch, D. A review on metronidazole: An old warhorse in antimicrobial chemotherapy. Parasitology 2019, 146, 1167–1178. [Google Scholar] [CrossRef]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. N. Am. 2009, 23, 791–815. [Google Scholar] [CrossRef]
- Löfmark, S.; Edlund, C.; Nord, C.E. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin. Infect. Dis. 2010, 50 (Suppl. S1), S16–S23. [Google Scholar] [CrossRef]
- Kolokythas, A.; Olech, E.; Miloro, M. Alveolar osteitis: A comprehensive review of concepts and controversies. Int. J. Dent. 2010, 2010, 249073. [Google Scholar] [CrossRef]
- Cardoso, C.L.; Rodrigues, M.T.V.; Júnior, O.F.; Garlet, G.P.; de Carvalho, P.S.P. Clinical Concepts of Dry Socket. J. Oral Maxillofac. Surg. 2010, 68, 1922–1932. [Google Scholar] [CrossRef] [PubMed]
- Reekie, D.; Downes, P.; Devlin, C.V.; Nixon, G.M.; Devlin, H. The prevention of ‘dry socket’ with topical metronidazole in general dental practice. Br. Dent. J. 2006, 200, 210–213; discussion 206. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef]
- Kalemba, D.; Synowiec, A. Agrobiological Interactions of Essential Oils of Two Menthol Mints: Mentha piperita and Mentha arvensis. Molecules 2020, 25, 59. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; Taha-Abdelaziz, K.; Hawwas, H.A.E.-H.; Ghosh, S.; AlKafaas, S.S.; Moawad, M.M.M.; Saied, E.M.; Kassem, I.I.; Mawad, A.M.M. Antimicrobial Resistance and Recent Alternatives to Antibiotics for the Control of Bacterial Pathogens with an Emphasis on Foodborne Pathogens. Antibiotics 2023, 12, 274. [Google Scholar] [CrossRef] [PubMed]
- Chumpitazi, B.P.; Kearns, G.L.; Shulman, R.J. Review article: The physiological effects and safety of peppermint oil and its efficacy in irritable bowel syndrome and other functional disorders. Aliment. Pharmacol. Ther. 2018, 47, 738–752. [Google Scholar] [CrossRef]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Bin Emran, T.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Thompson, A.; Meah, D.; Ahmed, N.; Conniff-Jenkins, R.; Chileshe, E.; Phillips, C.O.; Claypole, T.C.; Forman, D.W.; Row, P.E. Comparison of the antibacterial activity of essential oils and extracts of medicinal and culinary herbs to investigate potential new treatments for irritable bowel syndrome. BMC Complement. Altern. Med. 2013, 13, 338. [Google Scholar] [CrossRef]
- Ghasemi-Pirbaluti, M.; Motaghi, E.; Bozorgi, H. The effect of menthol on acute experimental colitis in rats. Eur. J. Pharmacol. 2017, 805, 101–107. [Google Scholar] [CrossRef]
- Silverman, H.A.; Chen, A.; Kravatz, N.L.; Chavan, S.S.; Chang, E.H. Involvement of Neural Transient Receptor Potential Channels in Peripheral Inflammation. Front. Immunol. 2020, 11, 590261. [Google Scholar] [CrossRef]
- Rozza, A.L.; Beserra, F.P.; Vieira, A.J.; Oliveira de Souza, E.; Hussni, C.A.; Martinez, E.R.M.; Nóbrega, R.H.; Pellizzon, C.H. The Use of Menthol in Skin Wound Healing-Anti-Inflammatory Potential, Antioxidant Defense System Stimulation and Increased Epithelialization. Pharmaceutics 2021, 13, 1902. [Google Scholar] [CrossRef]
- Ramachandran, R.; Hyun, E.; Zhao, L.; Lapointe, T.K.; Chapman, K.; Hirota, C.L.; Ghosh, S.; McKemy, D.D.; Vergnolle, N.; Beck, P.L.; et al. TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc. Natl. Acad. Sci. USA 2013, 110, 7476–7481. [Google Scholar] [CrossRef] [PubMed]
- Rizg, W.Y.; Hosny, K.M.; Elgebaly, S.S.; Alamoudi, A.J.; Felimban, R.I.; Tayeb, H.H.; Alharbi, M.; Bukhary, H.A.; Abualsunun, W.A.; Almehmady, A.M.; et al. Preparation and Optimization of Garlic Oil/Apple Cider Vinegar Nanoemulsion Loaded with Minoxidil to Treat Alopecia. Pharmaceutics 2021, 13, 2150. [Google Scholar] [CrossRef]
- Mushtaq, A.; Wani, S.M.; Malik, A.R.; Gull, A.; Ramniwas, S.; Nayik, G.A.; Ercisli, S.; Marc, R.A.; Ullah, R.; Bari, A. Recent insights into Nanoemulsions: Their preparation, properties and applications. Food Chem. X 2023, 18, 100684. [Google Scholar] [CrossRef] [PubMed]
- Azmi, N.A.N.; Elgharbawy, A.A.M.; Motlagh, S.R.; Samsudin, N.; Salleh, H.M. Nanoemulsions: Factory for Food, Pharmaceutical and Cosmetics. Processes 2019, 7, 617. [Google Scholar] [CrossRef]
- Tayeb, H.H.; Felimban, R.; Almaghrabi, S.; Hasaballah, N. Nanoemulsions: Formulation, characterization, biological fate, and potential role against COVID-19 and other viral outbreaks. Colloid Inter. Sci. Commun. 2021, 45, 100533. [Google Scholar] [CrossRef] [PubMed]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M.J. Nanoemulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Bowden, G.D.; Pichler, B.J.; Maurer, A. A Design of Experiments (DoE) Approach Accelerates the Optimization of Copper-Mediated 18F-Fluorination Reactions of Arylstannanes. Sci. Rep. 2019, 9, 11370. [Google Scholar] [CrossRef]
- Duraković, B. Design of experiments application, concepts, examples: State of the art. Perio. Eng. Natural Sci. 2017, 5, 421–439. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, R.; Ahuja, N. Optimizing drug delivery systems using systematic “design of experiments.” Part I: Fundamental aspects. Crit. Rev. Ther. Drug. Carrier. Syst. 2005, 22, 27–105. [Google Scholar] [CrossRef]
- Orafidiya, L.O.; Oladimeji, F.A. Determination of the required HLB values of some essential oils. Int. J. Pharm. 2002, 237, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Meher, J.G.; Yadav, N.P.; Sahu, J.J.; Sinha, P. Determination of required hydrophilic-lipophilic balance of citronella oil and development of stable cream formulation. Drug. Dev. Ind. Pharm. 2013, 39, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Sindi, A.M.; Rizg, W.Y.; Khan, M.K.; Alkhalidi, H.M.; Alharbi, W.S.; Sabei, F.Y.; Alfayez, E.; Alkharobi, H.; Korayem, M.; Majrashi, M.; et al. Tailoring and optimization of a honey-based nanoemulgel loaded with an itraconazole-thyme oil nanoemulsion for oral candidiasis. Drug. Deliv. 2023, 30, 2173337. [Google Scholar] [CrossRef]
- Hosny, K.M.; Sindi, A.M.; Alkhalidi, H.M.; Kurakula, M.; Hassan, A.H.; Bakhaidar, R.B.; Abualsunun, W.A.; Almehmady, A.M.; Khames, A.; Rizg, W.Y.; et al. Development of omega-3 loxoprofen-loaded nanoemulsion to limit the side effect associated with NSAIDs in treatment of tooth pain. Drug. Deliv. 2021, 28, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Brake, M.A.; Ivanciu, L.; Maroney, S.A.; Martinez, N.D.; Mast, A.E.; Westrick, R.J. Assessing Blood Clotting and Coagulation Factors in Mice. Curr. Protoc. Mouse Biol. 2019, 9, e61. [Google Scholar] [CrossRef]
- Hosny, K.M.; Alhakamy, N.A.; Sindi, A.M.; Khallaf, R.A. Coconut Oil Nanoemulsion Loaded with a Statin Hypolipidemic Drug for Management of Burns: Formulation and In Vivo Evaluation. Pharmaceutics 2020, 12, 1061. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Hosny, K.M.; Khallaf, R.A.; Asfour, H.Z.; Rizg, W.Y.; Alhakamy, N.A.; Sindi, A.M.; Alkhalidi, H.M.; Abualsunun, W.A.; Bakhaidar, R.B.; Almehmady, A.M.; et al. Development and Optimization of Cinnamon Oil Nanoemulgel for Enhancement of Solubility and Evaluation of Antibacterial, Antifungal and Analgesic Effects against Oral Microbiota. Pharmaceutics 2021, 13, 1008. [Google Scholar] [CrossRef]
- Kotamkar, H.; Burange, S.; Rathod, G.; Ghorpade, D. Preparation and Evaluation of Novel Candy Lozenges Containing Fluoxetine Hydrochloride. Int. J. Sci. Develop. Res. 2020, 5, 559–565. [Google Scholar]
- Hosny, K.; Asfour, H.; Rizg, W.; Alhakamy, N.A.; Sindi, A.; Alkhalidi, H.; Abualsunun, W.; Bakhaidar, R.; Almehmady, A.M.; Akeel, S.; et al. Formulation, Optimization, and Evaluation of Oregano Oil Nanoemulsions for the Treatment of Infections Due to Oral Microbiota. Int. J. Nanomed. 2021, 16, 5465–5478. [Google Scholar] [CrossRef]
- McCormack, P.L. Tranexamic acid: A review of its use in the treatment of hyperfibrinolysis. Drugs 2012, 72, 585–617. [Google Scholar] [CrossRef]
- Cai, J.; Ribkoff, J.; Olson, S.; Raghunathan, V.; Al-Samkari, H.; DeLoughery, T.G.; Shatzel, J.J. The many roles of tranexamic acid: An overview of the clinical indications for TXA in medical and surgical patients. Eup. J. Haemat 2020, 104, 79–87. [Google Scholar] [CrossRef]
- Findlay, R.D.; Taeusch, H.W.; David-Cu, R.; Walther, F.J. Lysis of red blood cells and alveolar epithelial toxicity by therapeutic pulmonary surfactants. Pediatr. Res. 1995, 37, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.K.; Baranowski, B.; Berry, L.; Liu, M.; Rafii, B.; Post, M.; O’Brodovich, H.; Monagle, P.; Andrew, M. Influence of mechanical stretch on thrombin regulation by fetal mixed lung cells. Am. J. Respir. Cell Mol. Biol. 1998, 19, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Shenkman, B.; Einav, Y.; Salomon, O.; Varon, D.; Savion, N. Testing agonist-induced platelet aggregation by the Impact-R [Cone and plate(let) analyzer (CPA)]. Platelets 2008, 19, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar]
- Atta, A.H.; Alkofahi, A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J. Ethnopharmacol. 1998, 60, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.L.; Li, L.H.; Weng, Y.C.; Hua, K.F.; Ju, T.C. Eucalyptus essential oils inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through reducing MAPK and NF-κB pathways. BMC Complement Med. Ther. 2020, 20, 200. [Google Scholar] [CrossRef]
- Perraud, A.L.; Knowles, H.M.; Schmitz, C. Novel aspects of signaling and ion-homeostasis regulation in immunocytes. The TRPM ion channels and their potential role in modulating the immune response. Mol. Immunol. 2004, 41, 657–673. [Google Scholar] [CrossRef]
- Kazkayası, I.; Telli, G. Evaluation of Anti-Inflammatory Activity of Metronidazole Treatment On Carrageenan Induced Paw Edema in Mice. Fabad J. Pharm. Sci. 2022, 47, 175–182. [Google Scholar]
- Del Rosso, J.Q.; Baum, E.W. Comprehensive medical management of rosacea: An interim study report and literature review. J. Clin. Aesthet. Dermatol 2008, 1, 20–25. [Google Scholar] [PubMed]
- Szentmihályi, K.; Süle, K.; Egresi, A.; Blázovics, A.; May, Z. Metronidazole does not show direct antioxidant activity in in vitro global systems. Heliyon 2021, 7, e06902. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pabón, M.C.; Ortega-Cuadros, M. Thymol, menthol and eucalyptol as agents for microbiological control in the oral cavity: A scoping review. Rev. Colomb. Cienc. Quím. Farm 2020, 49, 44–69. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; McClements, D.J. Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Sci. Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Sindi, A.M.; Mair, Y.H.; Khallaf, R.A. Oral gel loaded by ethotransfersomes of antifungal drug for oral thrush: Preparation, characterization, and assessment of antifungal activity. J. Drug. Deliv. Sci. Technol. 2021, 66, 102841. [Google Scholar] [CrossRef]
- Gomaa, E.; El Deeb, S.; Ibrahim, A.E.; Faisal, M.M. Bimodal Release Two-In-One Clonazepam Matrix Lozenge Tablets for Managing Anxiety-Related Disorders: Formulation, Optimization and In Vivo Evaluation. Sci. Pharm. 2022, 90, 43. [Google Scholar] [CrossRef]

| Studied Factors | Levels | ||
|---|---|---|---|
| 1 | 0 | −1 | |
| A = Amounts of MZ-PO (mg) | 240 | 180 | 120 |
| B = 2% TX Aqueous solution amount (mg) | 600 | 450 | 300 |
| C = Smix1:1 amount (mg) | 600 | 400 | 200 |
| Dependent variables | Constrains | ||
| Y1 = Droplet size (nm) | Minimize | ||
| Y2 = Coagulation time (minutes) | Minimize | ||
| Y3 = Interleukin-6 (U/mL) | Minimize | ||
| Y4 = minimum inhibitory concentration (MIC) against Treponema denticola (µg/mL) | Minimize | ||
| RHLB | Tween 80 Ratio | Span 80 Ratio | Droplet Size of Formed PO-MZ-TX Emulsion |
|---|---|---|---|
| 10 | 0.532 | 0.468 | 421 ± 38 nm |
| 10.5 | 0.579 | 0.421 | 348 ± 24 nm |
| 11 | 0.626 | 0.374 | 295 ± 18 nm |
| 11.5 | 0.672 | 0.328 | 370 ± 16 nm |
| 12 | 0.719 | 0.281 | 445 ± 37 nm |
| 12.5 | 0.766 | 0.234 | 477 ± 29 nm |
| 13 | 0.813 | 0.187 | 522 ± 36 nm |
| 13.5 | 0.859 | 0.141 | 568 ± 40 nm |
| 14 | 0.906 | 0.094 | 603 ± 41 nm |
| Run | A:Amounts of MZ-PO (mg) | B:2% TX Aqueous Solution Amount (mg) | C:Smix1:1 Amount (mg) | Y1:Droplet Size (nm) | Y2:Coagulation Time (min) | Y3:Interleukin-6 (U/mL) | Y4:MIC (µg/mL) | PDI |
|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 116 ±1.8 | 23 ± 0.2 | 2217 ± 50 | 2.7 ± 0.20 | 0.15 |
| 2 | 0 | 0 | 0 | 118 ± 3.1 | 22 ± 0.8 | 2241 ± 42 | 2.6 ± 0.16 | 0.12 |
| 3 | 1 | −1 | 0 | 126 ± 3.5 | 29 ± 1.0 | 1630 ± 30 | 1.7 ± 0.15 | 0.25 |
| 4 | 0 | −1 | 1 | 85 ± 1.1 | 31 ± 2.2 | 2033 ± 37 | 2.3 ± 0.22 | 0.31 |
| 5 | −1 | 0 | 1 | 73 ± 2.1 | 24 ± 1.3 | 2713 ± 51 | 2.8 ± 0.14 | 0.19 |
| 6 | 0 | 0 | 0 | 117 ± 2.2 | 22 ± 0.4 | 2228 ± 38 | 2.6 ± 0.11 | 0.27 |
| 7 | 0 | −1 | −1 | 161 ± 7.0 | 27 ± 1.1 | 2339 ± 49 | 2.9 ± 0.10 | 0.33 |
| 8 | 1 | 1 | 0 | 130 ± 1.9 | 15 ± 0.5 | 1645 ± 19 | 1.8 ± 0.23 | 0.18 |
| 9 | 1 | 0 | 1 | 98 ± 0.5 | 24 ± 1.8 | 1500 ± 24 | 1.5 ± 0.11 | 0.36 |
| 10 | −1 | −1 | 0 | 103 ± 1.4 | 28 ± 2.2 | 2844 ± 41 | 3.2 ± 0.27 | 0.22 |
| 11 | −1 | 0 | −1 | 150 ± 5.9 | 20 ± 1.7 | 2862 ± 35 | 3.7 ± 0.23 | 0.28 |
| 12 | −1 | 1 | 0 | 105 ± 2.5 | 14 ± 1.9 | 2839 ± 55 | 3.3 ± 0.18 | 0.32 |
| 13 | 0 | 1 | −1 | 164 ± 2.7 | 13 ± 0.5 | 2369 ± 62 | 2.9 ± 0.09 | 0.16 |
| 14 | 1 | 0 | −1 | 181 ± 5.6 | 21 ± 1.2 | 1711 ± 42 | 2.2 ± 0.13 | 0.29 |
| 15 | 0 | 1 | 1 | 87 ± 4.1 | 16 ± 3.1 | 2106 ± 45 | 2.3 ± 0.21 | 0.3 |
| Dependent Variables | Model | R2 | Adjusted R2 | Predicted R2 | Sequential p-Value | F-Value | Lack of Fit p-Value | Adequate Precision | Significant Terms | PRESS |
|---|---|---|---|---|---|---|---|---|---|---|
| Y1 | Quadratic | 0.9988 | 0.9967 | 0.9833 | 0.0001 | 475.03 | 0.0299 | 70.8239 | A, C, C2 | 232 |
| Y2 | linear | 0.9927 | 0.9907 | 0.9869 | 0.0001 | 497.30 | 0.6527 | 63.8954 | B, C | 5.68 |
| Y3 | linear | 0.9957 | 0.9945 | 0.9910 | 0.0976 | 847.59 | 0.0976 | 80.9488 | A, C | 26,788.49 |
| Y4 | linear | 0.9842 | 0.9799 | 0.9699 | 0.0001 | 228.36 | 0.3129 | 47.8345 | A, C | 0.1568 |
| Solution | PO-MZ Amount (mg) | Smix1:1 Amount (mg) | 2% TX Amount (mg) | Droplet Size (nm) | Blood Coagulation Time (min) | MIC (μg/mL) | IL-6 Level (IU/mL) | Desirability |
|---|---|---|---|---|---|---|---|---|
| Expected value | 240 | 600 | 600 | 98.3 | 16.9 | 1.51 | 1519 | 0.874 |
| Tentative value | 240 | 600 | 600 | 96 | 16.5 | 1.50 | 1530 | 0.874 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alissa, M.; Hjazi, A.; Abusalim, G.S.; Aloraini, G.S.; Alghamdi, S.A.; Rizg, W.Y.; Hosny, K.M.; Alblowi, J.A.; Alkharobi, H. Development and Optimization of a Novel Lozenge Containing a Metronidazole-Peppermint Oil-Tranexamic Acid Self-Nanoemulsified Delivery System to Be Used after Dental Extraction: In Vitro Evaluation and In Vivo Appraisal. Pharmaceutics 2023, 15, 2342. https://doi.org/10.3390/pharmaceutics15092342
Alissa M, Hjazi A, Abusalim GS, Aloraini GS, Alghamdi SA, Rizg WY, Hosny KM, Alblowi JA, Alkharobi H. Development and Optimization of a Novel Lozenge Containing a Metronidazole-Peppermint Oil-Tranexamic Acid Self-Nanoemulsified Delivery System to Be Used after Dental Extraction: In Vitro Evaluation and In Vivo Appraisal. Pharmaceutics. 2023; 15(9):2342. https://doi.org/10.3390/pharmaceutics15092342
Chicago/Turabian StyleAlissa, Mohammed, Ahmed Hjazi, Ghadah S. Abusalim, Ghfren S. Aloraini, Suad A. Alghamdi, Waleed Y. Rizg, Khaled M. Hosny, Jazia A. Alblowi, and Hanaa Alkharobi. 2023. "Development and Optimization of a Novel Lozenge Containing a Metronidazole-Peppermint Oil-Tranexamic Acid Self-Nanoemulsified Delivery System to Be Used after Dental Extraction: In Vitro Evaluation and In Vivo Appraisal" Pharmaceutics 15, no. 9: 2342. https://doi.org/10.3390/pharmaceutics15092342
APA StyleAlissa, M., Hjazi, A., Abusalim, G. S., Aloraini, G. S., Alghamdi, S. A., Rizg, W. Y., Hosny, K. M., Alblowi, J. A., & Alkharobi, H. (2023). Development and Optimization of a Novel Lozenge Containing a Metronidazole-Peppermint Oil-Tranexamic Acid Self-Nanoemulsified Delivery System to Be Used after Dental Extraction: In Vitro Evaluation and In Vivo Appraisal. Pharmaceutics, 15(9), 2342. https://doi.org/10.3390/pharmaceutics15092342







