Inhalable Combination Powder Formulations for Treating Latent and Multidrug-Resistant Tuberculosis: Formulation and In Vitro Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Spray-Dried Powder Formulations and Estimation of Percentage Yield
2.3. Drug Content Estimation
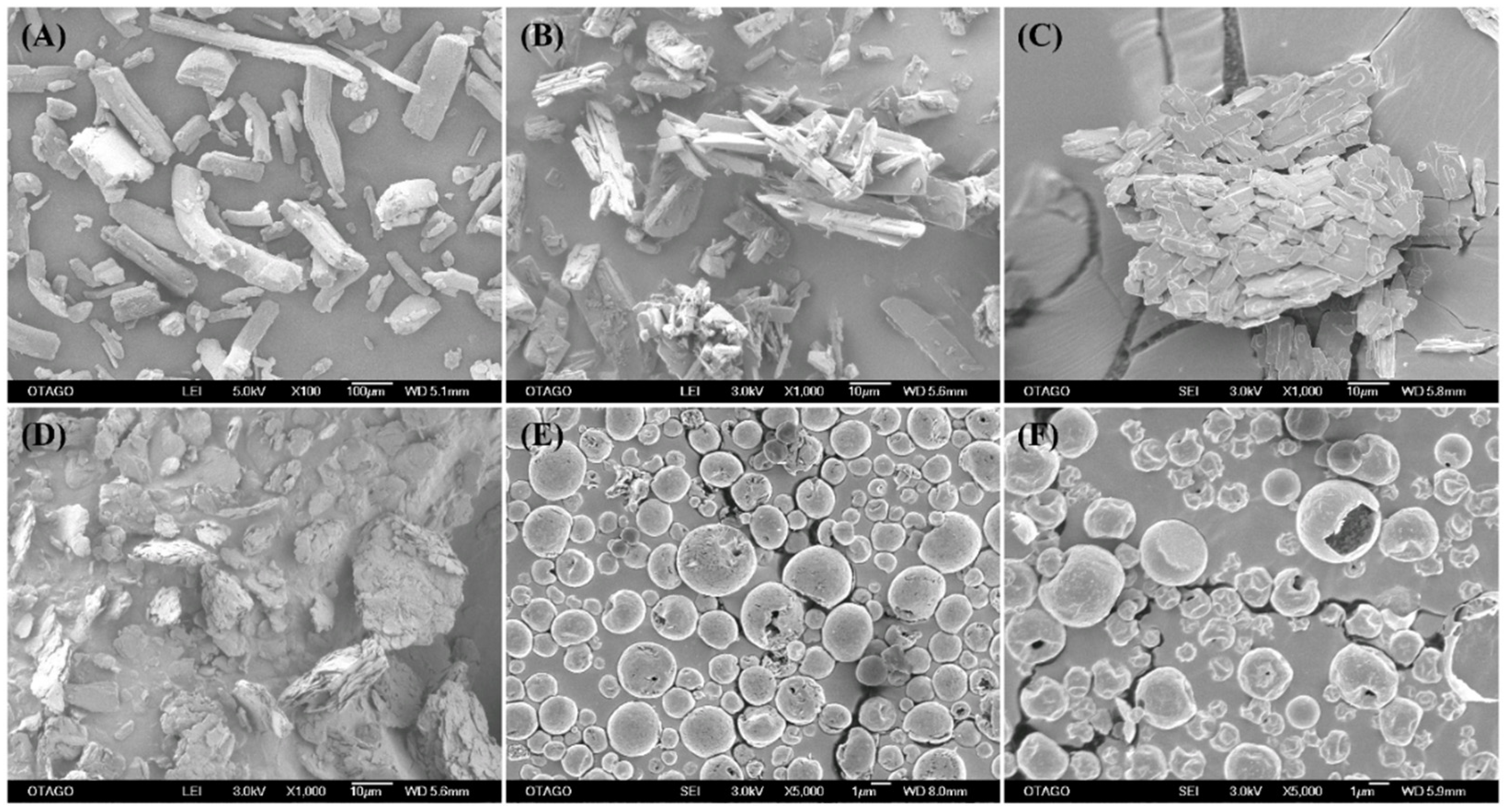
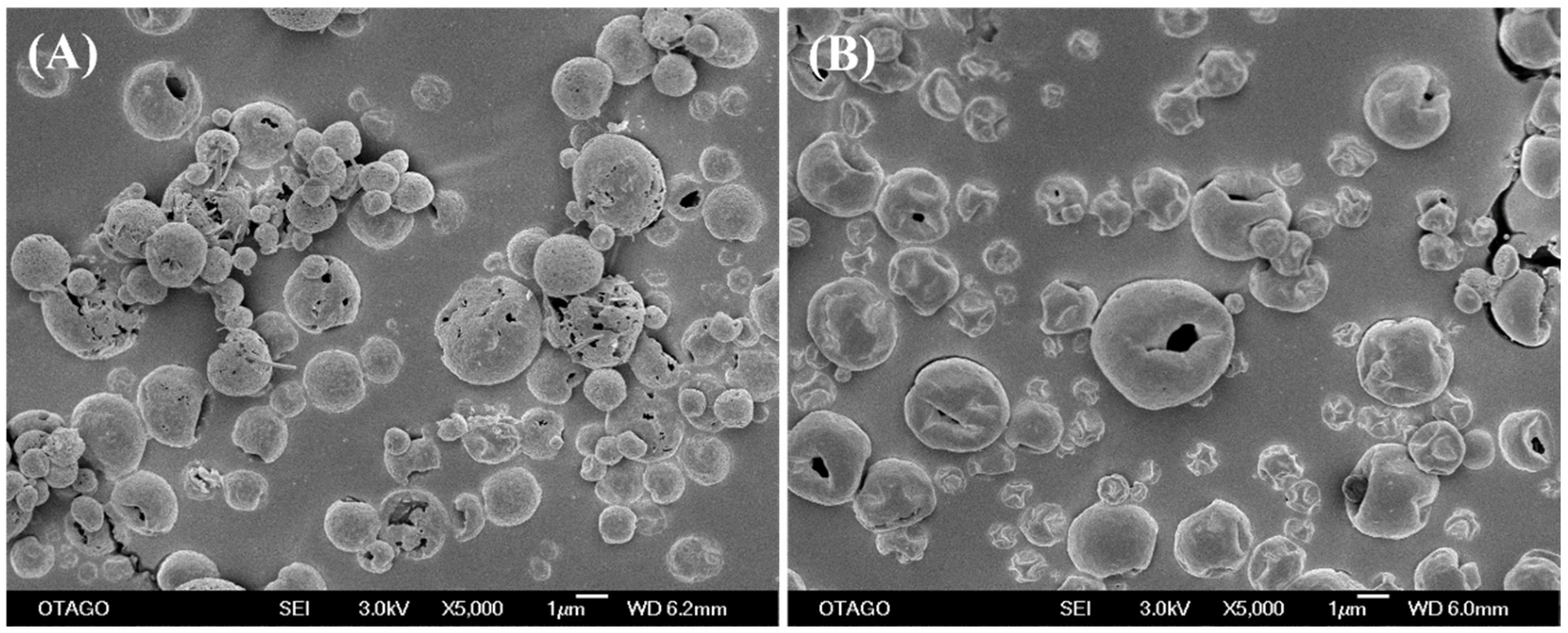
2.4. Surface Morphology by Scanning Electron Microscopy (SEM)
2.5. Particle Size Determination from SEM Images
2.6. Differential Scanning Calorimetry (DSC)
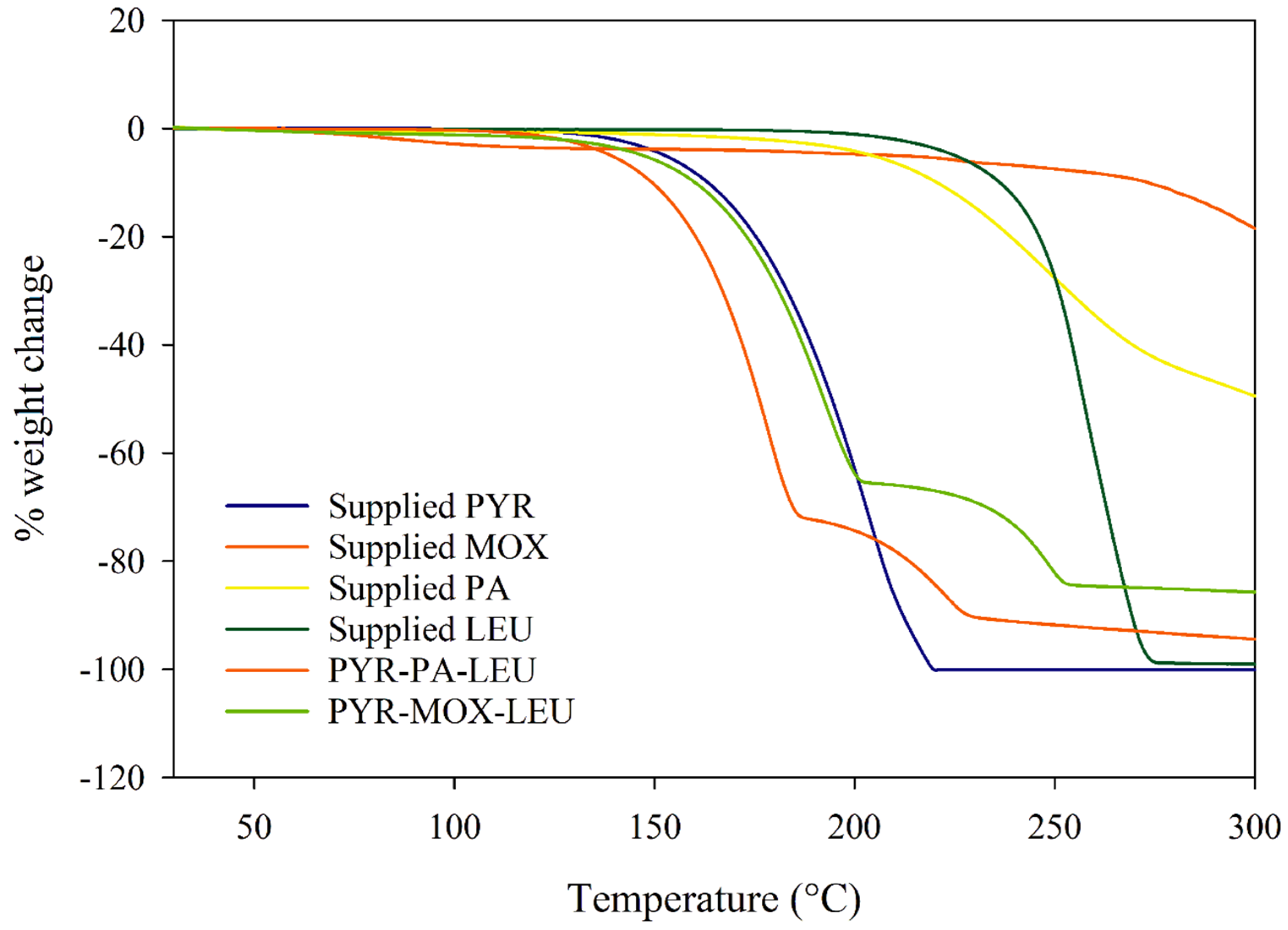
2.7. Thermogravimetric Analysis (TGA)
2.8. Hot-stage Microscopy
2.9. X-ray Powder Diffraction (XRPD) Analysis
2.10. Attenuated Total Reflectance—Fourier-Transform Infrared (ATR-FTIR) Spectroscopy
2.11. In Vitro Aerosol Dispersion Performance Using Next Generation Impactor (NGI)
2.12. HPLC Analysis
2.13. In Vitro Dissolution Study of Spray-Dried Powder Particles
2.14. Stability Study
2.15. Statistical Analysis
3. Results and Discussions
3.1. Preparation of Spray-Dried Powder Formulations and Estimation of Percentage Yield and Drug Content
3.2. Surface Morphology by SEM
3.3. Particle Size of Spray-Dried Powder Formulations
3.4. Differential Scanning Calorimetry (DSC)
3.5. Thermogravimetric Analysis (TGA)
3.6. Hot-Stage Microscopy
3.7. X-ray Powder Diffraction (XRPD) Analysis
3.8. Attenuated Total Reflectance—Fourier-Transform Infrared (ATR-FTIR) Spectroscopy
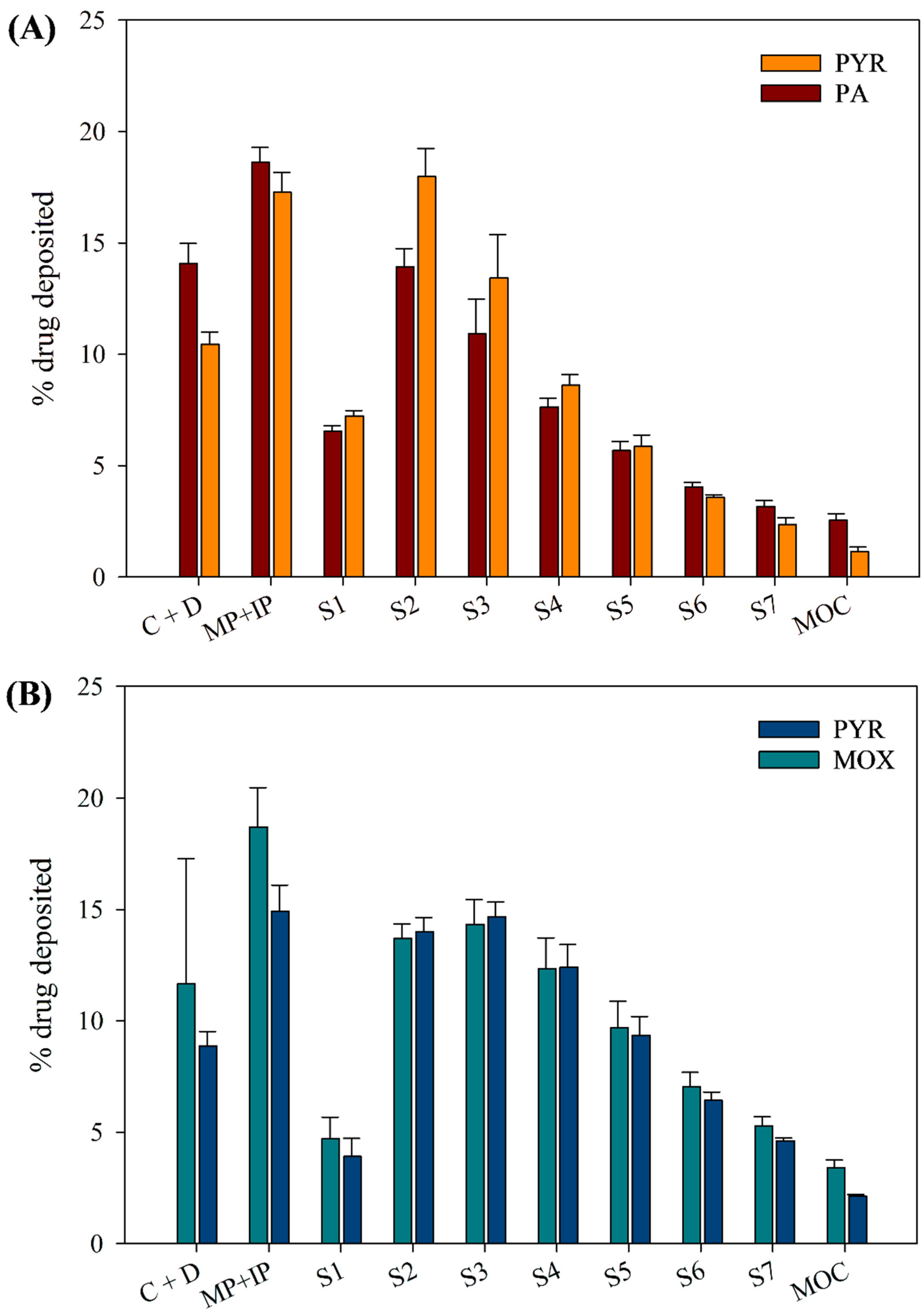
3.9. In Vitro Aerosol Dispersion Performance
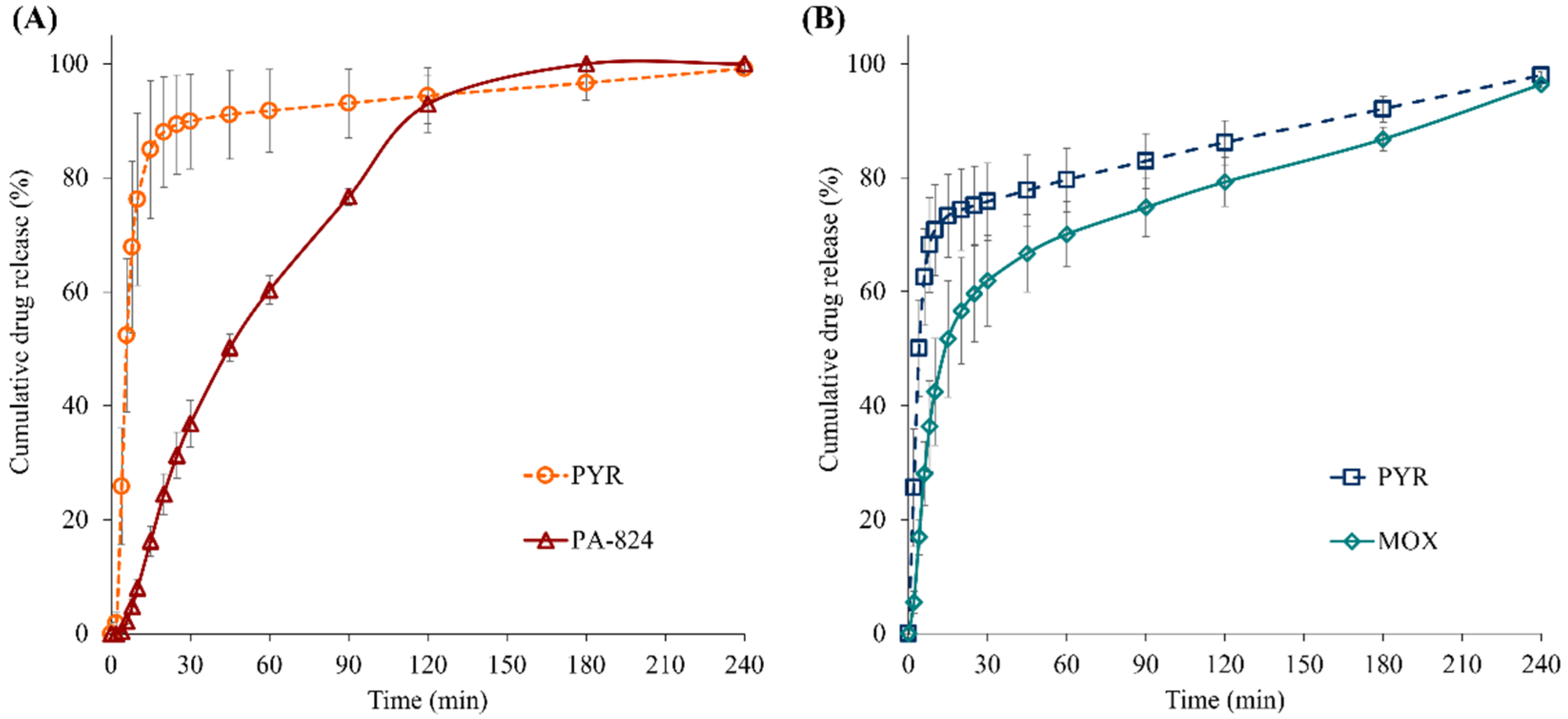
3.10. In Vitro Dissolution Study of Spray-Dried Powder Particles
3.11. Stability Study
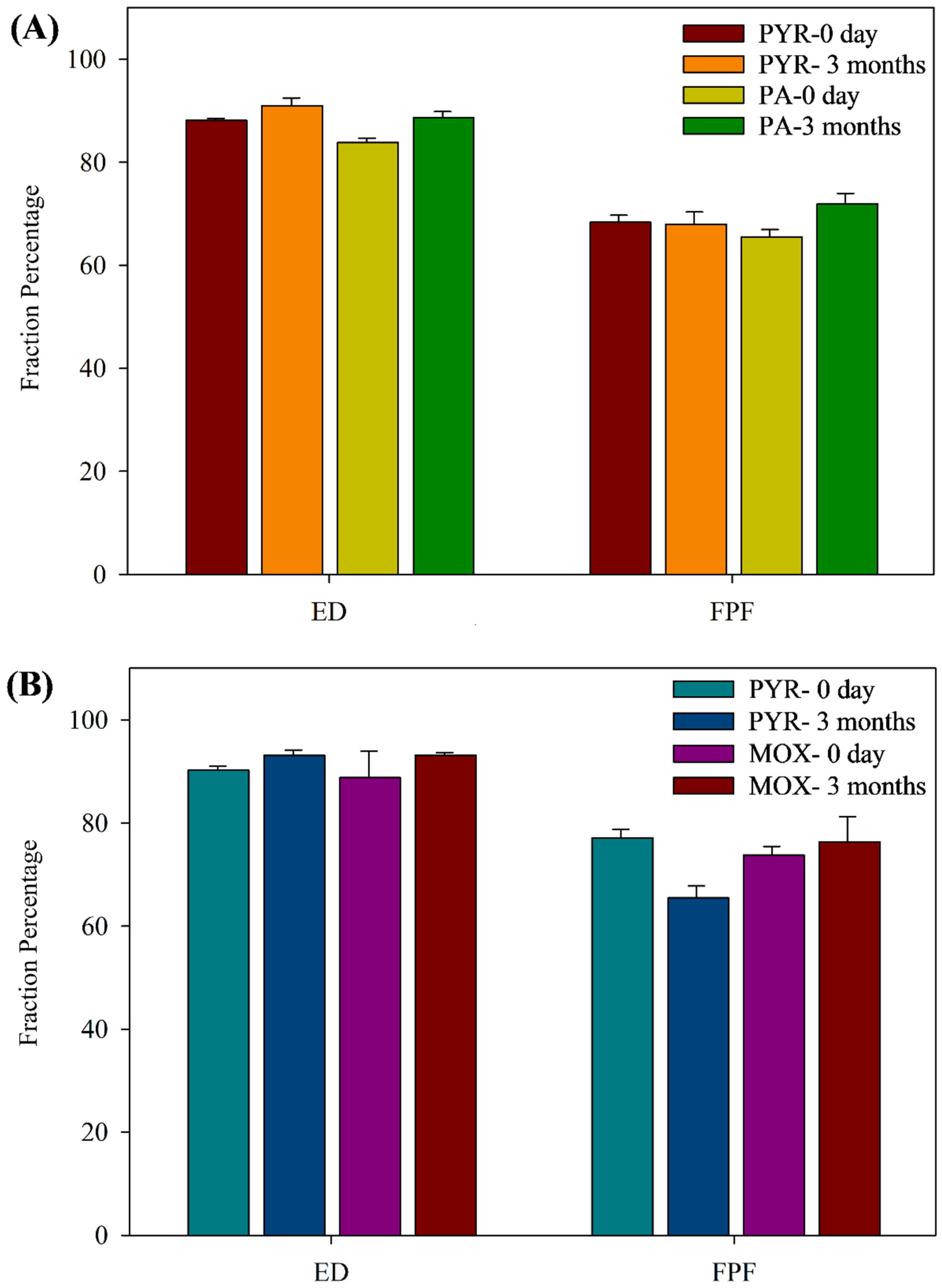
3.11.1. Drug Content and In Vitro Aerosol Dispersion Performance upon Storage
3.11.2. Morphological Changes during Storage
3.11.3. Crystallinity Changes upon Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2022; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2022.
- Bagcchi, S. WHO’s global tuberculosis report 2022. Lancet Microbe 2023, 4, e20. [Google Scholar] [CrossRef]
- Caws, M.; Thwaites, G.; Dunstan, S.; Hawn, T.R.; Lan, N.T.N.; Thuong, N.T.T.; Stepniewska, K.; Huyen, M.N.T.; Bang, N.D.; Loc, T.H.; et al. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog. 2008, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Seeberg, J. An epidemic of drug resistance: Tuberculosis in the twenty-first century. Pathogens 2023, 12, 652. [Google Scholar] [CrossRef] [PubMed]
- Pagaduan, J.V.; Altawallbeh, G. Advances in TB testing. Adv. Clin. Chem. 2023, 115, 33–62. [Google Scholar] [PubMed]
- Development, GAfTD. Handbook of Anti-Tuberculosis Agents. Tuberculosis 2008, 88, 85–170. [Google Scholar]
- WHO. WHO Consolidated Guidelines on Tuberculosis: Module 4: Treatment—Drug-Susceptible Tuberculosis Treatment; World Health Organization: Geneva, Switzerland, 2022.
- Koul, A.; Arnoult, E.; Lounis, N.; Guillemont, J.; Andries, K. The challenge of new drug discovery for tuberculosis. Nature 2011, 469, 483–490. [Google Scholar] [CrossRef]
- Somasundaram, S.; Anand, R.S.; Venkatesan, P.; Paramasivan, C. Bactericidal activity of PA-824 against mycobacterium tuberculosis under anaerobic conditions and computational analysis of its novel analogues against mutant Ddn receptor. BMC Microbiol. 2013, 13, 218. [Google Scholar] [CrossRef]
- Zumla, A.; Raviglione, M.; Hafner, R.; Fordham von Reyn, C. Tuberculosis. NEJM 2013, 368, 745–755. [Google Scholar] [CrossRef]
- Albanna, A.S.; Smith, B.M.; Cowan, D.; Menzies, D. Fixed-dose combination antituberculosis therapy: A systematic review and meta-analysis. Eur. Respir. J. 2013, 42, 721–732. [Google Scholar] [CrossRef]
- Rosenthal, I.M.; Williams, K.; Tyagi, S.; Peloquin, C.A.; Vernon, A.A.; Bishai, W.R.; Grosset, J.H.; Nuermberger, E.L. Potent twice-weekly rifapentine-containing regimens in murine tuberculosis. Am. J. Respir. Crit. Care Med. 2006, 174, 94–101. [Google Scholar] [CrossRef]
- Li, X.; Manjunatha, U.H.; Goodwin, M.B.; Knox, J.E.; Lipinski, C.A.; Keller, T.H.; Barry, C.E., 3rd; Dowd, C.S. Synthesis and antitubercular activity of 7-(R)- and 7-(S)-methyl-2-nitro-6-(S)-(4-(trifluoromethoxy)benzyloxy)-6,7-dihydro-5H-imidazo [2,1-b][1,3]oxazines, analogues of PA-824. Bioorg. Med. Chem. Lett. 2008, 18, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Lenaerts, A.J.; Gruppo, V.; Marietta, K.S.; Johnson, C.M.; Driscoll, D.K.; Tompkins, N.M.; Rose, J.D.; Reynolds, R.C.; Orme, I.M. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob. Agents Chemother. 2005, 49, 2294–2301. [Google Scholar] [CrossRef] [PubMed]
- Diacon, A.H.; Dawson, R.; Hanekom, M.; Narunsky, K.; Maritz, S.J.; Venter, A.; Donald, P.R.; van Niekerk, C.; Whitney, K.; Rouse, D.J.; et al. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob. Agents Chemother. 2010, 54, 3402–3407. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Nuermberger, E.; Yoshimatsu, T.; Williams, K.; Rosenthal, I.; Lounis, N.; Bishai, W.; Grosset, J. Bactericidal activity of the nitroimidazopyran PA-824 in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2005, 49, 2289–2293. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.; Diacon, A. PA-824, moxifloxacin and pyrazinamide combination therapy for tuberculosis. Expert Opin. Investig. Drugs 2013, 22, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Tucker, I.; Stewart, P. Inhaled dry powder formulations for treating tuberculosis. Curr. Drug Deliv. 2015, 12, 26–39. [Google Scholar] [CrossRef]
- Sung, J.C.; Garcia-Contreras, L.; Verberkmoes, J.L.; Peloquin, C.A.; Elbert, K.J.; Hickey, A.J.; Edwards, D.A. Dry powder nitroimidazopyran antibiotic PA-824 aerosol for inhalation. Antimicrob. Agents Chemother. 2009, 53, 1338–1343. [Google Scholar] [CrossRef]
- Pilcer, G.; Vanderbist, F.; Amighi, K. Spray-dried carrier-free dry powder tobramycin formulations with improved dispersion properties. J. Pharm. Sci. 2009, 98, 1463–1475. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Tang, X. Characterization of a new inhalable thymopentin formulation. Int. J. Pharm. 2009, 375, 1–7. [Google Scholar] [CrossRef]
- Dawson, R.; Diacon, A.H.; Everitt, D.; van Niekerk, C.; Donald, P.R.; Burger, D.A.; Schall, R.; Spigelman, M.; Conradie, A.; Eisenach, K.; et al. Efficiency and safety of the combination of moxifloxacin, pretomanid (PA-824), and pyrazinamide during the first 8 weeks of antituberculosis treatment: A phase 2b, open-label, partly randomised trial in patients with drug-susceptible or drug-resistant pulmonary tuberculosis. Lancet 2015, 385, 1738–1747. [Google Scholar] [CrossRef]
- Eedara, B.B.; Tucker, I.G.; Das, S.C. Phospholipid-based pyrazinamide spray-dried inhalable powders for treating tuberculosis. Int. J. Pharm. 2016, 506, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Momin, M.A.M.; Thien, S.J.; Krittaphol, W.; Das, S.C. Simultaneous HPLC assay for pretomanid (PA-824), moxifloxacin and pyrazinamide in an inhaler formulation for drug-resistant tuberculosis. J. Pharm. Biomed. Anal. 2017, 135, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Eedara, B.B.; Tucker, I.G.; Das, S.C. In vitro dissolution testing of respirable size anti-tubercular drug particles using a small volume dissolution apparatus. Int. J. Pharm. 2019, 559, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Eedara, B.B.; Tucker, I.G.; Das, S.C. A STELLA simulation model for in vitro dissolution testing of respirable size particles. Sci. Rep. 2019, 9, 18522. [Google Scholar] [CrossRef]
- Molina, C.; Kaialy, W.; Nokhodchi, A. The crucial role of leucine concentration on spray dried mannitol-leucine as a single carrier to enhance the aerosolization performance of albuterol sulfate. J. Drug Deliv. Sci. Technol. 2019, 49, 97–106. [Google Scholar] [CrossRef]
- Xu, Y.; Harinck, L.; Lokras, A.G.; Gerde, P.; Selg, E.; Sjöberg, C.O.; Franzyk, H.; Thakur, A.; Foged, C. Leucine improves the aerosol performance of dry powder inhaler formulations of siRNA-loaded nanoparticles. Int. J. Pharm. 2022, 621, 121758. [Google Scholar] [CrossRef]
- Raula, J.; Lahde, A.; Kauppinen, E.I. Aerosolization behavior of carrier-free L-leucine coated salbutamol sulphate powders. Int. J. Pharm. 2009, 365, 18–25. [Google Scholar] [CrossRef]
- Newman, S.P.; Anderson, P. Respiratory Drug Delivery: Essential Theory and Practice. Respiratory Drug Delivery Online. 2009.
- Adi, S.; Adi, H.; Chan, H.-K.; Tong, Z.; Yang, R.; Yu, A. Effects of mechanical impaction on aerosol performance of particles with different surface roughness. Powder Technol. 2013, 236, 164–170. [Google Scholar] [CrossRef]
- Eedara, B.B.; Rangnekar, B.; Doyle, C.; Cavallaro, A.; Das, S.C. The influence of surface active L-leucine and 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC) in the improvement of aerosolization of pyrazinamide and moxifloxacin co-spray dried powders. Int. J. Pharm. 2018, 542, 72–81. [Google Scholar] [CrossRef]
- Eedara, B.B.; Rangnekar, B.; Sinha, S.; Doyle, C.; Cavallaro, A.; Das, S.C. Development and characterization of high payload combination dry powders of anti-tubercular drugs for treating pulmonary tuberculosis. Eur. J. Pharm. Sci. 2018, 118, 216–226. [Google Scholar] [CrossRef]
- Cherukuvada, S.; Thakuria, R.; Nangia, A. Pyrazinamide polymorphs: Relative stability and vibrational spectroscopy. Cryst. Growth Des. 2010, 10, 3931–3941. [Google Scholar] [CrossRef]
- Castro, R.A.E.; Maria, T.M.R.; Évora, A.n.O.L.; Feiteira, J.C.; Silva, M.R.; Beja, A.M.; Canotilho, J.o.; Eusébio, M.E.S. A new insight into pyrazinamide polymorphic forms and their thermodynamic relationships. Cryst. Growth Des. 2010, 10, 274–282. [Google Scholar] [CrossRef]
- Pham, D.D.; Grégoire, N.; Couet, W.; Gueutin, C.; Fattal, E.; Tsapis, N. Pulmonary delivery of pyrazinamide-loaded large porous particles. Eur. J. Pharm. Biopharm. 2015, 94, 241–250. [Google Scholar] [CrossRef] [PubMed]




| Parameters | PYR-PA-LEU | PYR-MOX-LEU | ||
|---|---|---|---|---|
| PYR | PA | PYR | MOX | |
| Drug content (%) | 68.31 ± 0.02 | 10.12 ± 0.01 | 57.07 ± 0.03 | 15.18 ± 0.01 |
| Process yield (%) | 46.0 | 60.2 | ||
| Mean particle size (µm) | 1.9 ± 0.6 | 2.4 ± 0.7 | ||
| Particle size range (µm) | 0.6–4.1 | 1.0–5.3 | ||
| Parameters | PYR-PA-LEU | PYR-MOX-LEU | ||
|---|---|---|---|---|
| PYR | PA | PYR | MOX | |
| % Recovery | 87.93 ± 3.41 | 87.1 4 ± 2.38 | 91.31 ± 1.13 | 100.87 ± 11.29 |
| % Emitted Dose | 88.12 ± 0.38 | 83.84 ± 0.86 | 90.29 ± 0.80 | 88.84 ± 5.09 |
| % FPF | 68.34 ± 1.39 | 65.55 ± 1.42 | 77.15 ± 1.65 | 73.80 ± 1.70 |
| MMAD (µm) | 2.90 ± 0.09 | 2.58 ± 0.11 | 2.09 ± 0.10 | 1.99 ± 0.11 |
| GSD | 1.90 ± 0.08 | 2.13 ± 0.12 | 1.84 ± 0.48 | 2.21 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eedara, B.B.; Fan, C.; Sinha, S.; Khadka, P.; Das, S.C. Inhalable Combination Powder Formulations for Treating Latent and Multidrug-Resistant Tuberculosis: Formulation and In Vitro Characterization. Pharmaceutics 2023, 15, 2354. https://doi.org/10.3390/pharmaceutics15092354
Eedara BB, Fan C, Sinha S, Khadka P, Das SC. Inhalable Combination Powder Formulations for Treating Latent and Multidrug-Resistant Tuberculosis: Formulation and In Vitro Characterization. Pharmaceutics. 2023; 15(9):2354. https://doi.org/10.3390/pharmaceutics15092354
Chicago/Turabian StyleEedara, Basanth Babu, Claire Fan, Shubhra Sinha, Prakash Khadka, and Shyamal C. Das. 2023. "Inhalable Combination Powder Formulations for Treating Latent and Multidrug-Resistant Tuberculosis: Formulation and In Vitro Characterization" Pharmaceutics 15, no. 9: 2354. https://doi.org/10.3390/pharmaceutics15092354
APA StyleEedara, B. B., Fan, C., Sinha, S., Khadka, P., & Das, S. C. (2023). Inhalable Combination Powder Formulations for Treating Latent and Multidrug-Resistant Tuberculosis: Formulation and In Vitro Characterization. Pharmaceutics, 15(9), 2354. https://doi.org/10.3390/pharmaceutics15092354







