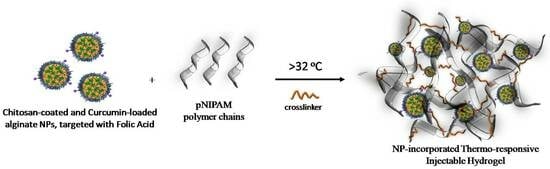
Thermo-Responsive Hydrogels Encapsulating Targeted Core–Shell Nanoparticles as Injectable Drug Delivery Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Alginate Nanoparticles (AA-NPs)
2.3. Preparation of Curcumin-Loaded Alginate Nanoparticles (AA-Cur-NPs)
2.4. Preparation of Chitosan-Coated Curcumin-Loaded Alginate Nanoparticles (CS[AA-Cur-NPs])
2.5. Conjugation of Folic Acid (FA) on the Surface of Nanoparticles (FA-CS[AA-Cur-NPs])
2.6. Characterization Methods for Prepared Nanoparticles
2.7. Determination of Drug Encapsulation Efficiency
2.8. Preparation of Nanoparticle-Embedded Hydrogel Scaffolds (HG/CS(AA-NPs))
2.9. Characterization of Nanoparticle-Embedded Hydrogel Scaffolds (HG/CS(AA-NPs))
2.10. Drug Release Studies
3. Results and Discussion
3.1. Preparation of Drug-Loaded Nanoparticles
3.2. Characterization of Nanoparticle-Embedded Hydrogel Scaffolds (HG/CS(AA-Cur NPs))
3.2.1. Morphological Characterization
3.2.2. Water Uptake Capacity
3.2.3. Mechanical Characterization
3.3. Drug Release Studies of Nanoparticles and Nanoparticle-Embedded Hydrogel Scaffolds
3.4. Thermo-Responsiveness and Injectability of Nanoparticle-Embedded Hydrogel Scaffolds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahinroosta, M.; Jomeh Farsangi, Z.; Allahverdi, A.; Shakoori, Z. Hydrogels as Intelligent Materials: A Brief Review of Synthesis, Properties and Applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- He, Y.; Tsao, H.K.; Jiang, S. Improved Mechanical Properties of Zwitterionic Hydrogels with Hydroxyl Groups. J. Phys. Chem. B 2012, 116, 5766–5770. [Google Scholar] [CrossRef]
- Summonte, S.; Racaniello, G.F.; Lopedota, A.; Denora, N.; Bernkop-Schnürch, A. Thiolated Polymeric Hydrogels for Biomedical Application: Cross-Linking Mechanisms. J. Control. Release 2021, 330, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Holt, B.D.; Wright, Z.M.; Arnold, A.M.; Moy, A.C.; Sydlik, S.A. Injectable Amine Functionalized Graphene and Chondroitin Sulfate Hydrogel with Potential for Cartilage Regeneration. J. Mater. Chem. B 2019, 7, 2442–2453. [Google Scholar] [CrossRef]
- Jain, E.; Neal, S.; Graf, H.; Tan, X.; Balasubramaniam, R.; Huebsch, N. Copper-Free Azide–Alkyne Cycloaddition for Peptide Modification of Alginate Hydrogels. ACS Appl. Bio. Mater. 2021, 4, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chai, L.; Li, W.; Xiao, L.P.; Chen, X.; Sun, R.C. Tunning the Properties of PH-Responsive Lignin-Based Hydrogels by Regulating Hydroxyl Content. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128815. [Google Scholar] [CrossRef]
- Thornton, P.D.; Mart, R.J.; Webb, S.J.; Ulijn, R.V. Enzyme-Responsive Hydrogel Particles for the Controlled Release of Proteins: Designing Peptide Actuators to Match Payload. Soft Matter 2008, 4, 821–827. [Google Scholar] [CrossRef]
- Emam, H.E.; Shaheen, T.I. Design of a Dual PH and Temperature Responsive Hydrogel Based on Esterified Cellulose Nanocrystals for Potential Drug Release. Carbohydr. Polym. 2022, 278, 118925. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Chen, C.; Cheng, Y. Magnetic-Responsive Hydrogels: From Strategic Design to Biomedical Applications. J. Control. Release 2021, 335, 541–556. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-Responsive Hydrogels: Theory, Modern Advances, and Applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef]
- Varghese, J.S.; Chellappa, N.; Fathima, N.N. Colloids and Surfaces B: Biointerfaces Gelatin—Carrageenan Hydrogels: Role of Pore Size Distribution on Drug Delivery Process. Colloids Surf. B Biointerfaces 2014, 113, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Liaw, C.; Ji, S.; Guvendiren, M. Engineering 3D Hydrogels for Personalized In Vitro Human Tissue Models. Adv. Healthc. Mater. 2018, 7, 1701165. [Google Scholar] [CrossRef]
- Markwell, S.M.; Mukherjee, S.; Brat, D.J.; Olson, C.L. Methods for in Vitro Modeling of Glioma Invasion: Choosing Tools to Meet the Need. Glia 2020, 68, 2173–2191. [Google Scholar]
- Hauck, M.; Hellmold, D.; Kubelt, C.; Synowitz, M.; Adelung, R.; Schütt, F.; Held-Feindt, J. Localized Drug Delivery Systems in High-Grade Glioma Therapy—From Construction to Application. Adv. Healthcare Mater. 2022, 5, 2200013. [Google Scholar] [CrossRef]
- Braccini, S.; Tacchini, C.; Chiellini, F.; Puppi, D. Polymeric Hydrogels for In Vitro 3D Ovarian Cancer Modeling. Int. J. Mol. Sci. 2022, 23, 3265. [Google Scholar] [CrossRef]
- Daud, H.; Ghani, A.; Najaf, D.; Ahmad, N.; Nazir, S. Preparation and Characterization of Guar Gum Based Biopolymeric Hydrogels for Controlled Release of Antihypertensive Drug. Arab. J. Chem. 2021, 14, 103111. [Google Scholar] [CrossRef]
- Aslzad, S.; Savadi, P.; Dalir, E.; Omidi, Y.; Fathi, M.; Barar, J. Chitosan/Dialdehyde Starch Hybrid in Situ Forming Hydrogel for Ocular Delivery of Betamethasone. Mater. Today Commun. 2022, 33, 104873. [Google Scholar] [CrossRef]
- Cheon, S.; Keun, I.; Park, K. Hydrogels for Delivery of Bioactive Agents: A Historical Perspective. Adv. Drug Deliv. Rev. 2013, 65, 17–20. [Google Scholar]
- Afzal, A.; Jalalah, M.; Noor, A.; Khaliq, Z.; Qadir, M.B.; Masood, R.; Nazir, A.; Ahmad, S.; Ahmad, F.; Irfan, M.; et al. Development and Characterization of Drug Loaded PVA/PCL Fibres for Wound Dressing Applications. Polymers 2023, 15, 1355. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Polymers Blending as Release Modulating Tool in Drug Delivery. Front. Mater. 2021, 8, 752813. [Google Scholar] [CrossRef]
- Raza, M.A. Irradiated Ch/GG/PVP-Based Stimuli-Responsive Hydrogels for Controlled Drug Release. J. Appl. Polym. Sci. 2020, 137, 49041. [Google Scholar] [CrossRef]
- Spizzirri, U.G. Functional Polymers for Controlled Drug Release. Pharmaceutics 2020, 12, 135. [Google Scholar] [CrossRef]
- Alexis, V.; Jorge, S.; Johanna, A.R.; Julio, C.; Investigación, G. De Use of Hydrogels as Controlled Drug Release Systems in Breast Cancer. Eur. Chem. Bull. 2023, 94, e2023100. [Google Scholar]
- Huang, G.; Gao, J.; Hu, Z.; John, J.V.S.; Ponder, B.C.; Moro, D. Controlled Drug Release from Hydrogel Nanoparticle Networks. J. Control. Release 2004, 94, 303–311. [Google Scholar] [CrossRef]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J. Nanoparticle—Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Xia, X.; Marquez, M.; Weng, H.; Tang, L. Controlled Release from and Tissue Response to Physically Bonded Hydrogel Nanoparticle Assembly. Macromol. Symp. 2005, 227, 275–284. [Google Scholar] [CrossRef]
- Mook, S.; Se, L.; Oh, H.; Hoon, H. Dual Growth Factor-Releasing Nanoparticle/Hydrogel System for Cartilage Tissue Engineering. J. Mater. Sci. Mater. Med. 2010, 21, 2593–2600. [Google Scholar]
- Jiang, Y.; Krishnan, N.; Heo, J.; Fang, R.H.; Zhang, L. Nanoparticle—Hydrogel Superstructures for Biomedical Applications. J. Control. Release 2020, 324, 505–521. [Google Scholar] [CrossRef]
- Yang, H.; Tyagi, P.; Kadam, R.S.; Holden, C.A.; Kompella, U.B. Hybrid Dendrimer Hydrogel/PLGA Nanoparticle Platform Sustains Drug Delivery for One Week and Anti-Glaucoma Effects for Four Days Following One-Time Topical Administration. ACS Nano 2012, 6, 7595–7606. [Google Scholar] [CrossRef]
- Machado, N.D.; Fernandez, M.A.; Haring, M.; Saldias, C.; Diaz, D.D. Niosomes encapsulated in biohydrogels for tunable delivery of phytoalexin resveratrol. RSC Adv. 2019, 9, 7601–7609. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Chen, M.; Gong, H.; Thamphiwatana, S.; Eckmann, L.; Gao, W.; Zhang, L. A Bioadhesive Nanoparticle—Hydrogel Hybrid System for Localized Antimicrobial Drug Delivery. ACS Appl. Mater. Interfaces. 2016, 8, 18367–18374. [Google Scholar] [CrossRef] [PubMed]
- Khoee, S.; Kardani, M. Preparation of PCL/PEG Superporous Hydrogel Containing Drug-Loaded Nanoparticles: The Effect of Hydrophobic-Hydrophilic Interface on the Physical Properties. Eur. Polym. J. 2014, 58, 180–190. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, Z.; Wang, W.; Peng, Y.Y.; Narain, R. Injectable, Self-Healing, and Multi-Responsive Hydrogels via Dynamic Covalent Bond Formation between Benzoxaborole and Hydroxyl Groups. Biomacromolecules 2019, 20, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Mellati, A.; Akhtari, J. Injectable Hydrogels: A Review of Injectability Mechanisms and Biomedical Applications. Res. Mol. Med. 2019, 6, 1–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Shen, X.; Hu, J.; Jan, J. Reduction- and PH-Sensitive Lipoic Acid-Modi Fi Ed Poly (L-Lysine) and Polypeptide/Silica Hybrid Hydrogels/Nanogels. Polymers 2016, 86, 32–41. [Google Scholar] [CrossRef]
- Zhu, J.; Li, F.; Wang, X.; Yu, J.; Wu, D. Hyaluronic Acid and Polyethylene Glycol Hybrid Hydrogel Encapsulating Nanogel with Hemostasis and Sustainable Antibacterial Property for Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 13304–13316. [Google Scholar] [CrossRef] [PubMed]
- Dadashzadeh, A.; Imani, R.; Moghassemi, S.; Omidfar, K.; Abolfathi, N. Study of Hybrid Alginate/Gelatin Hydrogel-Incorporated Niosomal Aloe Vera Capable of Sustained Release of Aloe Vera as Potential Skin Wound Dressing. Polym. Bull. 2020, 77, 387–403. [Google Scholar] [CrossRef]
- Diego, S.; Jolla, L. Nanoparticle-Hydrogel: A Hybrid Biomaterial System for Localized Drug Delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar]
- Kostarelos, K.; Prato, M.; Va, E. Nanocomposite Hydrogels: 3D Polymer À Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar]
- Hsu, X.L.; Wu, L.C.; Hsieh, J.Y.; Huang, Y.Y. Nanoparticle-Hydrogel Composite Drug Delivery System for Potential Ocular Applications. Polymers 2021, 13, 642. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Zare, M.; Thomas, V.; Kumar, T.S.S.; Ramakrishna, S. Nano-Based Drug Delivery Systems: Conventional Drug Delivery Routes, Recent Developments and Future Prospects. Med. Drug Discov. 2022, 15, 100134. [Google Scholar] [CrossRef]
- Elliott, I.; Shoichet, M.S. Acta Biomaterialia Controlled Release of Bioactive PDGF-AA from a Hydrogel/Nanoparticle Composite. Acta Biomater. 2015, 25, 35–42. [Google Scholar] [CrossRef]
- Du, W.; Zong, Q.; Guo, R.; Ling, G.; Zhang, P. Injectable Nanocomposite Hydrogels for Cancer Therapy. Macromol. Biosci. 2021, 21, 202100186. [Google Scholar] [CrossRef] [PubMed]
- Vyas, D.; Patel, M.; Wairkar, S. Strategies for Active Tumor Targeting-an Update. Eur. J. Pharmacol. 2022, 915, 174512. [Google Scholar] [CrossRef]
- Iyer, K.S. Distinction Between Active and Passive Targeting of Nanoparticles Dictate Their Overall Therapeutic Efficacy. Langmuir 2018, 34, 15343–15349. [Google Scholar]
- Wang, X.; Ye, L.; He, W.; Teng, C.; Sun, S.; Lu, H.; Li, S.; Lv, L.; Cao, X.; Yin, H.; et al. In Situ Targeting Nanoparticles-Hydrogel Hybrid System for Combined Chemo-Immunotherapy of Glioma. J. Control. Release 2022, 345, 786–797. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.L. Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems. Polymers 2021, 13, 2086. [Google Scholar] [CrossRef]
- Alexander, A.; Ajazuddin; Khan, J.; Saraf, S.; Saraf, S. Polyethylene Glycol (PEG)-Poly(N-Isopropylacrylamide) (PNIPAAm) Based Thermosensitive Injectable Hydrogels for Biomedical Applications. Eur. J. Pharm. Biopharm. 2014, 88, 575–585. [Google Scholar] [CrossRef]
- Navath, R.S.; Menjoge, A.R.; Dai, H.; Romero, R.; Kannan, S.; Kannan, R.M. Injectable PAMAM Dendrimer-PEG Hydrogels for the Treatment of Genital Infections: Formulation and in Vitro and in Vivo Evaluation. Mol. Pharm. 2011, 8, 1209–1223. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Bavel, N.V.; Lewrenz, A.; Issler, T.; Pang, L.; Anikovskiy, M.; Prenner, E.J. Synthesis of Alginate Nanoparticles Using Hydrolyzed and Enzyme-Digested Alginate Using the Ionic Gelation and Water-in-Oil Emulsion Method. Polymers 2023, 15, 1319. [Google Scholar] [CrossRef] [PubMed]
- Venkata, V.; Reddy, S.; Kuppusamy, G. International Journal of Biological Macromolecules Curcumin Loaded Chitosan Nanoparticles Impregnated into Collagen-Alginate Scaffolds for Diabetic Wound Healing. Int. J. Biol. Macromol. 2016, 93, 1519–1529. [Google Scholar]
- Access, O. Preparation and Characterization of Magnetic Nanoparticles with Chitosan Coating Preparation and Characterization of Magnetic Nanoparticles with Chitosan Coating. J. Phys. Conf. Ser. 2009, 187, 012036. [Google Scholar]
- Song, H.; Su, C.; Cui, W.; Zhu, B.; Liu, L.; Chen, Z.; Zhao, L. Folic Acid-Chitosan Conjugated Nanoparticles for Improving Tumor-Targeted Drug Delivery. BioMed Res. Int. 2013, 2013, 723158. [Google Scholar] [CrossRef] [PubMed]
- Mourdikoudis, S.; Pallares, R.M. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Li, X.; Su, J.; Kamal, Z.; Guo, P.; Wu, X.; Lu, L.; Wu, H.; Qiu, M. Odorranalectin Modified PEGPLGA/PEG—PBLG Curcumin-Loaded Nanoparticle for Intranasal Administration. Drug Dev. Ind. Pharm. 2020, 46, 899–909. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, X.; Guo, B. Multifunctional Interpenetrating Polymer Network Hydrogels Based on Methacrylated Alginate for the Delivery of Small Molecule Drugs and Sustained Release of Protein. Biomacromolecules 2014, 15, 3246–3252. [Google Scholar] [CrossRef]
- Sokker, H.H.; Ghaffar, A.M.A.; Gad, Y.H.; Aly, A.S. Synthesis and Characterization of Hydrogels Based on Grafted Chitosan for the Controlled Drug Release. Carbohydr. Polym. 2009, 75, 222–229. [Google Scholar] [CrossRef]
- Drapala, P.W.; Brey, E.M.; Mieler, W.F.; Venerus, D.C.; Derwent, J.J.K.; Pérez-luna, V.H.; Drapala, P.W.; Brey, E.M.; Mieler, W.F.; Venerus, D.C.; et al. Role of Thermo-Responsiveness and Poly (Ethylene Glycol) Diacrylate Cross-Link Density on Protein Release from Poly (N-Isopropylacrylamide) Hydrogels Diacrylate Cross-Link Density on Protein Release From. J. Biomater. Sci. Polym. Ed. 2021, 22, 59–75. [Google Scholar] [CrossRef]
- Safakas, K.; Saravanou, S.-F.; Iatridi, Z.; Tsitsilianis, C. Thermo-Responsive Injectable Hydrogels Formed by Self-Assembly of Alginate-Based Heterograft Copolymers. Gels 2023, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Qindeel, M.; Ahmed, N.; Sabir, F.; Khan, S.; Ur-Rehman, A. Development of novel pH-sensitive nanoparticles loaded hydrogel for transdermal drug delivery. Drug Dev. Ind. Pharm. 2019, 45, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S. Mechanobiology of the Female Reproductive System. Reprod. Med. Biol. 2021, 20, 371–401. [Google Scholar] [CrossRef] [PubMed]






| No * | CaCl2 (50 mM) (mL) | NaAlg (0.5%) (mL) | L-Lysine (0.1%) (mL) | SDS (0.1%) (mL) | Temp (°C) |
|---|---|---|---|---|---|
| 1 | 4.75 | 0.25 | 2 | 2 | 25 |
| 2 | 4.75 | 0.25 | 2 | 2 | 40 |
| 3 | 4.75 | 0.25 | 3 | 3 | 25 |
| 4 | 4.75 | 0.25 | 3 | 3 | 40 |
| Nanoparticle | Hydrodynamic Volume (nm) 1 | PDI 1 | Zeta Potential (mV) 2 | EE% 3 |
|---|---|---|---|---|
| AA NP | 249.2 | 0.79 | −0.4 ± 0.4 | - |
| CS (AA NP) | 261.4 | 0.64 | 1.7 ± 0.6 | - |
| AA-Cur NP | 233.1 | 0.67 | −2.1 ± 0.4 | 94 |
| CS (AA-Cur NP) | 245.2 | 0.75 | 1.9 ± 0.5 | 88 |
| FA-CS(AA-Cur NP) | 264.2 | 0.19 | 0.8 ± 0.3 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ertugral-Samgar, E.G.; Ozmen, A.M.; Gok, O. Thermo-Responsive Hydrogels Encapsulating Targeted Core–Shell Nanoparticles as Injectable Drug Delivery Systems. Pharmaceutics 2023, 15, 2358. https://doi.org/10.3390/pharmaceutics15092358
Ertugral-Samgar EG, Ozmen AM, Gok O. Thermo-Responsive Hydrogels Encapsulating Targeted Core–Shell Nanoparticles as Injectable Drug Delivery Systems. Pharmaceutics. 2023; 15(9):2358. https://doi.org/10.3390/pharmaceutics15092358
Chicago/Turabian StyleErtugral-Samgar, Elif Gulin, Ali Murad Ozmen, and Ozgul Gok. 2023. "Thermo-Responsive Hydrogels Encapsulating Targeted Core–Shell Nanoparticles as Injectable Drug Delivery Systems" Pharmaceutics 15, no. 9: 2358. https://doi.org/10.3390/pharmaceutics15092358