Review on Photoacoustic Monitoring after Drug Delivery: From Label-Free Biomarkers to Pharmacokinetics Agents
Abstract
:1. Introduction
2. Background and Principle
2.1. Principle of Photoacoustic Imaging
2.2. Contrast of Photoacoustic Imaging
2.3. Multispectral Photoacoustic Imaging
2.4. Photoacoustic Imaging Systems
3. Label-Free Photoacoustic Monitoring of Responses to Drug Delivery
4. Photoacoustic Monitoring of Pharmacokinetics and Biodistribution of Exogenous Agents
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Lee, D.-E.; Koo, H.; Sun, I.-C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional Nanoparticles for Multimodal Imaging and Theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, G.; Sanmugam, A.; Palem, V.V.; Sevanan, M.; Sairam, A.B.; Nachiappan, N.; Youn, B.; Lee, J.S.; Nallal, M.; Park, K.H. Nanomaterials for detection of biomolecules and delivering therapeutic agents in theragnosis: A review. Int. J. Biol. Macromol. 2024, 254, 127904. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Lee, S.; Son, S.; Kim, S.H.; Leary, J.F.; Choi, K.; Kwon, I.C. Theranostic nanoparticles for future personalized medicine. J. Control. Release 2014, 190, 477–484. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2. [Google Scholar] [CrossRef]
- Kang, M.S.; Lee, S.Y.; Kim, K.S.; Han, D.-W. State of the Art Biocompatible Gold Nanoparticles for Cancer Theragnosis. Pharmaceutics 2020, 12, 701. [Google Scholar] [CrossRef]
- Ryu, J.H.; Koo, H.; Sun, I.-C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. Tumor-Targeting Multi-Functional Nanoparticles for Theragnosis: New Paradigm for Cancer Therapy. Adv. Drug Deliv. Rev. 2012, 64, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.H.; Park, H.; Kim, Y.-S.; Park, K.; Nam, H.; Lee, S.; Park, J.H.; Park, R.-W.; Kim, I.-S. Tumor-Homing Multifunctional Nanoparticles for Cancer Theragnosis: Simultaneous Diagnosis, Drug Delivery, and Therapeutic Monitoring. J. Control. Release 2010, 146, 219–227. [Google Scholar] [CrossRef]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef]
- Oh, D.; Lee, D.; Heo, J.; Kweon, J.; Yong, U.; Jang, J.; Ahn, Y.J.; Kim, C. Contrast agent-free 3D Renal ultrafast doppler imaging reveals vascular dysfunction in acute and diabetic kidney diseases. Adv. Sci. 2023, 10, 2303966. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C. CuGeO3 Nanoparticles: An Efficient Photothermal Theragnosis Agent for CT Imaging-Guided Photothermal Therapy of Cancers. Front. Bioeng. Biotechnol. 2020, 8, 590518. [Google Scholar] [CrossRef] [PubMed]
- Curry, T.; Kopelman, R.; Shilo, M.; Popovtzer, R. Multifunctional theranostic gold nanoparticles for targeted CT imaging and photothermal therapy. Contrast Media Mol. Imaging 2014, 9, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Dreifuss, T.; Barnoy, E.; Motiei, M.; Popovtzer, R. Theranostic gold nanoparticles for CT imaging. In Design and Applications of Nanoparticles in Biomedical Imaging; Springer: Cham, Switzerland, 2017; pp. 403–427. [Google Scholar]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef]
- Anani, T.; Rahmati, S.; Sultana, N.; David, A.E. MRI-traceable theranostic nanoparticles for targeted cancer treatment. Theranostics 2021, 11, 579. [Google Scholar] [CrossRef]
- Yoo, D.; Lee, J.-H.; Shin, T.-H.; Cheon, J. Theranostic magnetic nanoparticles. Acc. Chem. Res. 2011, 44, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Eo, J.S.; Paeng, J.C.; Lee, D.S. Nuclear Imaging for Functional Evaluation and Theragnosis in Liver Malignancy and Transplantation. World J. Gastroenterol. 2014, 20, 5375. [Google Scholar] [CrossRef]
- Polyak, A.; Ross, T.L. Nanoparticles for SPECT and PET imaging: Towards personalized medicine and theranostics. Curr. Med. Chem. 2018, 25, 4328–4353. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Gnanasammandhan, M.K.; Zhang, Y. Optical imaging-guided cancer therapy with fluorescent nanoparticles. J. R. Soc. Interface 2010, 7, 3–18. [Google Scholar] [CrossRef]
- Pekkanen, A.M.; DeWitt, M.R.; Rylander, M.N. Nanoparticle enhanced optical imaging and phototherapy of cancer. J. Biomed. Nanotechnol. 2014, 10, 1677–1712. [Google Scholar] [CrossRef]
- Murar, M.; Albertazzi, L.; Pujals, S. Advanced optical imaging-guided nanotheranostics towards personalized cancer drug delivery. Nanomaterials 2022, 12, 399. [Google Scholar] [CrossRef]
- Juvekar, V.; Lee, D.J.; Park, T.G.; Samanta, R.; Kasar, P.; Kim, C.; Rotermund, F.; Kim, H.M. Two-photon excitation photosensitizers for photodynamic therapy: From small-molecules to nano-complex systems. Coord. Chem. Rev. 2024, 506, 215711. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Li, W.; Li, C. Real-time dual-modal photoacoustic and fluorescence small animal imaging. Photoacoustics 2024, 36, 100593. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, J.; Kim, H.-H.; Kim, C.-S.; Kim, J. Review on Optical Imaging Techniques for Multispectral Analysis of Nanomaterials. Nanotheranostics 2022, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Pirovano, G.; Roberts, S.; Kossatz, S.; Reiner, T. Optical Imaging Modalities: Principles and Applications in Preclinical Research and Clinical Settings. J. Nucl. Med. 2020, 61, 1419–1427. [Google Scholar] [CrossRef]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.V. Photoacoustic microscopy. Laser Photonics Rev. 2013, 7, 758–778. [Google Scholar] [CrossRef]
- Bell, A.G. The Photophone. Science 1880, 1, 130–134. [Google Scholar] [CrossRef]
- Park, B.; Lee, K.M.; Park, S.; Yun, M.; Choi, H.-J.; Kim, J.; Lee, C.; Kim, H.; Kim, C. Deep tissue photoacoustic imaging of nickel (II) dithiolene-containing polymeric nanoparticles in the second near-infrared window. Theranostics 2020, 10, 2509. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Han, M.; Park, J.; Kim, T.; Ryu, H.; Seo, Y.; Kim, W.J.; Kim, H.H.; Kim, C. A photoacoustic finder fully integrated with a solid-state dye laser and transparent ultrasound transducer. Photoacoustics 2021, 23, 100290. [Google Scholar] [CrossRef]
- Cho, S.; Kim, M.; Ahn, J.; Kim, Y.; Lim, J.; Park, J.; Kim, H.H.; Kim, W.J.; Kim, C. An ultrasensitive and broadband transparent ultrasound transducer for ultrasound and photoacoustic imaging in-vivo. Nat. Commun. 2024, 15, 1444. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Kim, C.; Favazza, C.; Wang, L.V. In vivo photoacoustic tomography of chemicals: High-resolution functional and molecular optical imaging at new depths. Chem. Rev. 2010, 110, 2756–2782. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, S.M.; Park, J.; Cho, S.-W.; Han, S.; Ahn, J.; Cho, S.; Kim, C.; Kim, C.-S.; Kim, J. Transportable Multispectral Optical-Resolution Photoacoustic Microscopy using Stimulated Raman Scattering Spectrum. IEEE Trans. Instrum. Meas. 2024, 73, 4502309. [Google Scholar] [CrossRef]
- Kye, H.; Song, Y.; Ninjbadgar, T.; Kim, C.; Kim, J. Whole-Body Photoacoustic Imaging Techniques for Preclinical Small Animal Studies. Sensors 2022, 22, 5130. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Oh, D.; Kim, J.; Kim, C. Functional photoacoustic imaging: From nano-and micro-to macro-scale. Nano Converg. 2023, 10, 29. [Google Scholar] [CrossRef]
- Cho, S.-W.; Park, S.M.; Park, B.; Lee, T.G.; Kim, B.-M.; Kim, C.; Kim, J.; Lee, S.-W.; Kim, C.-S. High-speed photoacoustic microscopy: A review dedicated on light sources. Photoacoustics 2021, 24, 100291. [Google Scholar] [CrossRef]
- Xia, Q.; Lv, S.; Xu, H.; Wang, X.; Xie, Z.; Lin, R.; Zhang, J.; Shu, C.; Chen, Z.; Gong, X. Non-invasive evaluation of endometrial microvessels via in vivo intrauterine photoacoustic endoscopy. Photoacoustics 2024, 36, 100589. [Google Scholar] [CrossRef]
- Xu, Z.; Pan, Y.; Chen, N.; Zeng, S.; Liu, L.; Gao, R.; Zhang, J.; Fang, C.; Song, L.; Liu, C. Visualizing tumor angiogenesis and boundary with polygon-scanning multiscale photoacoustic microscopy. Photoacoustics 2022, 26, 100342. [Google Scholar] [CrossRef]
- Gao, R.; Chen, T.; Ren, Y.; Liu, L.; Chen, N.; Wong, K.K.; Song, L.; Ma, X.; Liu, C. Restoring the imaging quality of circular transducer array-based PACT using synthetic aperture focusing technique integrated with 2nd-derivative-based back projection scheme. Photoacoustics 2023, 32, 100537. [Google Scholar] [CrossRef]
- Chen, N.; Yu, J.; Liu, L.; Xu, Z.; Gao, R.; Chen, T.; Song, L.; Zheng, W.; Liu, C. Video-rate high-resolution single-pixel nonscanning photoacoustic microscopy. Biomed. Opt. Express 2022, 13, 3823–3835. [Google Scholar] [CrossRef]
- Gao, R.; Liu, F.; Liu, W.; Zeng, S.; Chen, J.; Gao, R.; Wang, L.; Fang, C.; Song, L.; Sedgwick, A.C. Background-suppressed tumor-targeted photoacoustic imaging using bacterial carriers. Proc. Natl. Acad. Sci. USA 2022, 119, e2121982119. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Xu, Z.; Ren, Y.; Song, L.; Liu, C. Nonlinear mechanisms in photoacoustics—Powerful tools in photoacoustic imaging. Photoacoustics 2021, 22, 100243. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Xu, Z.; Song, L.; Liu, C. Breaking acoustic limit of optical focusing using photoacoustic-guided wavefront shaping. Laser Photonics Rev. 2021, 15, 2000594. [Google Scholar] [CrossRef]
- Olefir, I.; Tzoumas, S.; Restivo, C.; Mohajerani, P.; Xing, L.; Ntziachristos, V. Deep Learning-Based Spectral Unmixing for Optoacoustic Imaging of Tissue Oxygen Saturation. IEEE Trans. Med. Imaging 2020, 39, 3643–3654. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, Y.; Yao, J. Photoacoustic Tomography of Blood Oxygenation: A Mini Review. Photoacoustics 2018, 10, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Kennedy, M.J.; Dixon, A.J.; Sun, N.; Cao, R.; Soetikno, B.T.; Chen, R.; Zhou, Q.; Shung, K.K.; Hossack, J.A. Simultaneous Photoacoustic Microscopy of Microvascular Anatomy, Oxygen Saturation, and Blood Flow. Opt. Lett. 2015, 40, 910–913. [Google Scholar] [CrossRef]
- Barulin, A.; Park, H.; Park, B.; Kim, I. Dual-wavelength UV-visible metalens for multispectral photoacoustic microscopy: A simulation study. Photoacoustics 2023, 32, 100545. [Google Scholar] [CrossRef]
- John, S.; Hester, S.; Basij, M.; Paul, A.; Xavierselvan, M.; Mehrmohammadi, M.; Mallidi, S. Niche preclinical and clinical applications of photoacoustic imaging with endogenous contrast. Photoacoustics 2023, 32, 100533. [Google Scholar] [CrossRef]
- Li, X.; Yew, Y.W.; Ram, K.V.; Oon, H.H.; Thng, S.T.G.; Dinish, U.; Olivo, M. Structural and functional imaging of psoriasis for severity assessment and quantitative monitoring of treatment response using high-resolution optoacoustic imaging. Photoacoustics 2024, 38, 100611. [Google Scholar] [CrossRef]
- Lee, H.; Han, S.; Park, S.; Cho, S.; Yoo, J.; Kim, C.; Kim, J. Ultrasound-Guided Breath-Compensation in Single-Element Photoacoustic Imaging for Three-Dimensional Whole-Body Images of Mice. Front. Phys. 2022, 10, 457. [Google Scholar] [CrossRef]
- Menger, M.M.; Körbel, C.; Bauer, D.; Bleimehl, M.; Tobias, A.L.; Braun, B.J.; Herath, S.C.; Rollmann, M.F.; Laschke, M.W.; Menger, M.D. Photoacoustic imaging for the study of oxygen saturation and total hemoglobin in bone healing and non-union formation. Photoacoustics 2022, 28, 100409. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Y.; He, L.; Liang, Y.; Wang, L. Wide-field polygon-scanning photoacoustic microscopy of oxygen saturation at 1-MHz A-line rate. Photoacoustics 2020, 20, 100195. [Google Scholar] [CrossRef] [PubMed]
- Nemirova, S.; Orlova, A.; Kurnikov, A.; Litvinova, Y.; Kazakov, V.; Ayvazyan, I.; Liu, Y.-H.; Razansky, D.; Subochev, P. Scanning optoacoustic angiography for assessing structural and functional alterations in superficial vasculature of patients with post-thrombotic syndrome: A pilot study. Photoacoustics 2024, 38, 100616. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.; Jung, Y.; Chang, S.; Park, J.; Zhang, Y.; Lovell, J.F.; Kim, C. Programmable Real-time Clinical Photoacoustic and Ultrasound Imaging System. Sci. Rep. 2016, 6, 35137. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, B.; Ha, J.; Steinberg, I.; Hooper, S.M.; Jeong, C.; Park, E.-Y.; Choi, W.; Liang, T.; Bae, J.-S.; et al. Multiparametric Photoacoustic Analysis of Human Thyroid Cancers In Vivo. Cancer Res. 2021, 81, 4849–4860. [Google Scholar] [CrossRef]
- Schoustra, S.M.; Piras, D.; Huijink, R.; Op’t Root, T.J.; Alink, L.; Kobold, W.M.F.; Steenbergen, W.; Manohar, S. Twente Photoacoustic Mammoscope 2: System Overview and Three-Dimensional Vascular Network Images in Healthy Breasts. J. Biomed. Opt. 2019, 24, 121909. [Google Scholar] [CrossRef]
- Neuschler, E.I.; Butler, R.; Young, C.A.; Barke, L.D.; Bertrand, M.L.; Böhm-Vélez, M.; Destounis, S.; Donlan, P.; Grobmyer, S.R.; Katzen, J. A Pivotal Study of Optoacoustic Imaging to Diagnose Benign and Malignant Breast Masses: A New Evaluation Tool for Radiologists. Radiology 2017, 287, 398–412. [Google Scholar] [CrossRef]
- Diot, G.; Metz, S.; Noske, A.; Liapis, E.; Schroeder, B.; Ovsepian, S.V.; Meier, R.; Rummeny, E.; Ntziachristos, V. Multispectral Optoacoustic Tomography (MSOT) of Human Breast Cancer. Clin. Cancer Res. 2017, 23, 6912–6922. [Google Scholar] [CrossRef]
- Park, E.-Y.; Lee, H.; Han, S.; Kim, C.; Kim, J. Photoacoustic Imaging Systems Based on Clinical Ultrasound Platform. Exp. Biol. Med. 2022, 247, 551–560. [Google Scholar] [CrossRef]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, H.E.; Tummers, W.S.; Gambhir, S.S. Photoacoustic Clinical Imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, D.; Mo, S.; Hong, X.; Xie, J.; Chen, Y.; Liu, L.; Song, D.; Tang, S.; Wu, H. Multimodal PA/US imaging in Rheumatoid Arthritis: Enhanced correlation with clinical scores. Photoacoustics 2024, 38, 100615. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Lee, C.; Kim, J.Y.; Kim, C. Organic Nanostructures for Photoacoustic Imaging. ChemNanoMat 2015, 2, 156–166. [Google Scholar] [CrossRef]
- Choi, W.; Park, B.; Choi, S.; Oh, D.; Kim, J.; Kim, C. Recent advances in contrast-enhanced photoacoustic imaging: Overcoming the physical and practical challenges. Chem. Rev. 2023, 123, 7379–7419. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lee, D.; Kim, S.; Kim, H.-H.; Jeong, S.; Kim, J. Contrast Agents for Photoacoustic Imaging: A Review Focusing on the Wavelength Range. Biosensors 2022, 12, 594. [Google Scholar] [CrossRef]
- Jiang, Z.; Ding, Y.; Lovell, J.F.; Zhang, Y. Design and Application of Organic Contrast Agents for Molecular Imaging in the Second Near Infrared (NIR-II) Window. Photoacoustics 2022, 28, 100426. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhu, R.; Song, J.; Yang, H.; Chen, X. Photoacoustic imaging: Contrast agents and their biomedical applications. Adv. Mater. 2019, 31, 1805875. [Google Scholar] [CrossRef]
- Xu, W.; Leskinen, J.; Sahlström, T.; Happonen, E.; Tarvainen, T.; Lehto, V.-P. Assembly of fluorophore J-aggregates with nanospacer onto mesoporous nanoparticles for enhanced photoacoustic imaging. Photoacoustics 2023, 33, 100552. [Google Scholar] [CrossRef] [PubMed]
- Choe, A.; Qin, D.; Anthony, M.Y.; Chung, E.; Jhunjhunwala, A.; Rose, J.A.; Emelianov, S.Y. pH-responsive ratiometric photoacoustic imaging of polyaniline nanoparticle-coated needle for targeted cancer biopsy. Photoacoustics 2023, 31, 100500. [Google Scholar] [CrossRef]
- Park, B.; Park, S.; Kim, J.; Kim, C. Listening to Drug Delivery and Responses via Photoacoustic Imaging. Adv. Drug Deliv. Rev. 2022, 184, 114235. [Google Scholar] [CrossRef]
- Guo, T.; Tang, Q.; Guo, Y.; Qiu, H.; Dai, J.; Xing, C.; Zhuang, S.; Huang, G. Boron Quantum Dots for Photoacoustic Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 13, 306–311. [Google Scholar] [CrossRef]
- Miao, Z.-H.; Wang, H.; Yang, H.; Li, Z.; Zhen, L.; Xu, C.-Y. Glucose-Derived Carbonaceous Nanospheres for Photoacoustic Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2016, 8, 15904–15910. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591. [Google Scholar] [CrossRef] [PubMed]
- Kwon, N.; Kim, K.H.; Park, S.; Cho, Y.; Park, E.-Y.; Lim, J.; Çetindere, S.; Tümay, S.O.; Kim, W.J.; Li, X. Hexa-BODIPY-cyclotriphosphazene based nanoparticle for NIR fluorescence/photoacoustic dual-modal imaging and photothermal cancer therapy. Biosens. Bioelectron. 2022, 216, 114612. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, C.; Cheng, Y.; Cheng, Q. Photostability enhancement of silica-coated gold nanostars for photoacoustic imaging guided photothermal therapy. Photoacoustics 2021, 23, 100284. [Google Scholar] [CrossRef]
- Han, S.; Ninjbadgar, T.; Kang, M.; Kim, C.; Kim, J. Recent Advances in Photoacoustic Agents for Theranostic Applications. Nanomaterials 2023, 13, 695. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kang, M.S.; Lee, H.; Lee, J.H.; Kim, J.; Han, D.-W.; Kim, K.S. Recent Trends in Photoacoustic Imaging Techniques for 2D Nanomaterial-Based Phototherapy. Biomedicines 2021, 9, 80. [Google Scholar] [CrossRef]
- Ding, Y.; Park, B.; Ye, J.; Wang, X.; Liu, G.; Yang, X.; Jiang, Z.; Han, M.; Fan, Y.; Song, J. Surfactant-stripped semiconducting polymer micelles for tumor theranostics and deep tissue imaging in the NIR-II window. Small 2022, 18, 2104132. [Google Scholar] [CrossRef]
- Lee, H.; Park, B.; Lee, J.; Kang, Y.; Han, M.; Lee, J.; Kim, C.; Kim, W.J. Transcytosis-Inducing Multifunctional Albumin Nanomedicines with Deep Penetration Ability for Image-Guided Solid Tumor Treatment. Small 2023, 19, 2303668. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Yang, J.; Lee, S.Y.; Kim, J.; Lee, J.; Kim, W.J.; Lee, S.; Kim, C. Deep Learning Enhances Multiparametric Dynamic Volumetric Photoacoustic Computed Tomography In Vivo (DL-PACT). Adv. Sci. 2023, 10, 2202089. [Google Scholar] [CrossRef]
- Kim, D.; Ahn, J.; Park, E.; Kim, J.Y.; Kim, C. In vivo quantitative photoacoustic monitoring of corticosteroid-induced vasoconstriction. J. Biomed. Opt. 2023, 28, 082805. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, Q.; DiSpirito, A.; Vu, T.; Rong, Q.; Peng, X.; Sheng, H.; Shen, X.; Zhou, Q.; Jiang, L. Real-time whole-brain imaging of hemodynamics and oxygenation at micro-vessel resolution with ultrafast wide-field photoacoustic microscopy. Light Sci. Appl. 2022, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Neuschmelting, V.; Kim, K.; Malekzadeh-Najafabadi, J.; Jebiwott, S.; Prakash, J.; Scherz, A.; Coleman, J.A.; Kircher, M.F.; Ntziachristos, V. WST11 vascular targeted photodynamic therapy effect monitoring by multispectral optoacoustic tomography (MSOT) in mice. Theranostics 2018, 8, 723. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, Q.; Jiang, L.; Nguyen, V.-T.; Vu, T.; Devlin, G.; Shaima, J.; Wang, X.; Chen, Y.; Ma, L. Longitudinal intravital imaging of mouse placenta. Sci. Adv. 2024, 10, eadk1278. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Z.; Chang, C.-C.; Chen, Y.-C.; Zhang, Q.; Zhang, X.-D.; Andreou, C.; Pang, J.; Liu, Z.-X.; Wang, D.-Y. Near-infrared phototheranostic iron pyrite nanocrystals simultaneously induce dual cell death pathways via enhanced Fenton reactions in triple-negative breast cancer. ACS Nano 2023, 17, 4261–4278. [Google Scholar] [CrossRef]
- Song, J.; Kang, X.; Wang, L.; Ding, D.; Kong, D.; Li, W.; Qi, J. Near-infrared-II photoacoustic imaging and photo-triggered synergistic treatment of thrombosis via fibrin-specific homopolymer nanoparticles. Nat. Commun. 2023, 14, 6881. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ke, Z.; Yang, F.; Li, K.; Chen, N.; Song, L.; Zheng, C.; Liang, D.; Liu, C. Deep learning enables superior photoacoustic imaging at ultralow laser dosages. Adv. Sci. 2021, 8, 2003097. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, L.; Ma, X.; Zhang, Y.; Liu, H.; Zheng, R.; Ren, J.; Zhou, H.; Ren, Y.; Gao, R. Dedicated photoacoustic imaging instrument for human periphery blood vessels: A new paradigm for understanding the vascular health. IEEE Trans. Biomed. Eng. 2021, 69, 1093–1100. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, N.; Li, T.; Zhang, J.; Lin, R.; Gong, X.; Song, L.; Liu, Z.; Liu, C. Motion correction in optical resolution photoacoustic microscopy. IEEE Trans. Med. Imaging 2019, 38, 2139–2150. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, G.; Lin, R.; Gong, X.; Song, L.; Li, T.; Wang, W.; Zhang, K.; Qian, X.; Zhang, H. Three-dimensional Hessian matrix-based quantitative vascular imaging of rat iris with optical-resolution photoacoustic microscopy in vivo. J. Biomed. Opt. 2018, 23, 046006. [Google Scholar] [CrossRef]
- Kim, D.; Park, E.; Park, J.; Perleberg, B.; Jeon, S.; Ahn, J.; Ha, M.; Kim, H.H.; Kim, J.Y.; Jung, C.K. An ultraviolet-transparent ultrasound transducer enables high-resolution label-free photoacoustic histopathology. Laser Photonics Rev. 2024, 18, 2300652. [Google Scholar] [CrossRef]
- Baik, J.W.; Kim, H.; Son, M.; Choi, J.; Kim, K.G.; Baek, J.H.; Park, Y.H.; An, J.; Choi, H.Y.; Ryu, S.Y. Intraoperative label-free photoacoustic histopathology of clinical specimens. Laser Photonics Rev. 2021, 15, 2100124. [Google Scholar] [CrossRef]
- Park, J.; Park, B.; Kim, T.Y.; Jung, S.; Choi, W.J.; Ahn, J.; Yoon, D.H.; Kim, J.; Jeon, S.; Lee, D. Quadruple ultrasound, photoacoustic, optical coherence, and fluorescence fusion imaging with a transparent ultrasound transducer. Proc. Natl. Acad. Sci. USA 2021, 118, e1920879118. [Google Scholar] [CrossRef]
- Park, B.; Bang, C.; Lee, C.; Han, J.; Choi, W.; Kim, J.; Park, G.; Rhie, J.; Lee, J.; Kim, C. 3D wide-field multispectral photoacoustic imaging of human melanomas in vivo: A pilot study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Tzoumas, S.; Ntziachristos, V. Spectral unmixing techniques for optoacoustic imaging of tissue pathophysiology. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20170262. [Google Scholar] [CrossRef]
- Yoon, C.; Park, E.; Misra, S.; Kim, J.Y.; Baik, J.W.; Kim, K.G.; Jung, C.K.; Kim, C. Deep learning-based virtual staining, segmentation, and classification in label-free photoacoustic histology of human specimens. Light Sci. Appl. 2024, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Kim, J.; Lee, D.; Baik, J.W.; Kim, C. Review on practical photoacoustic microscopy. Photoacoustics 2019, 15, 100141. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.Y.; Jeon, S.; Baik, J.W.; Cho, S.H.; Kim, C. Super-resolution localization photoacoustic microscopy using intrinsic red blood cells as contrast absorbers. Light Sci. Appl. 2019, 8, 156–166. [Google Scholar] [CrossRef]
- Cao, R.; Zhao, J.; Li, L.; Du, L.; Zhang, Y.; Luo, Y.; Jiang, L.; Davis, S.; Zhou, Q.; de la Zerda, A.; et al. Optical-resolution photoacoustic microscopy with a needle-shaped beam. Nat. Photonics 2023, 17, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jin, H.; Zheng, Z.; Sharma, A.; Wang, L.; Pramanik, M.; Zheng, Y. Deep and Domain Transfer Learning Aided Photoacoustic Microscopy: Acoustic Resolution to Optical Resolution. IEEE Trans. Med. Imaging 2022, 41, 3636–3648. [Google Scholar] [CrossRef]
- Moothanchery, M.; Dev, K.; Balasundaram, G.; Bi, R.; Olivo, M. Acoustic resolution photoacoustic microscopy based on microelectromechanical systems scanner. J. Biophotonics 2020, 13, e201960127. [Google Scholar] [CrossRef]
- Choi, W.; Oh, D.; Kim, C. Practical photoacoustic tomography: Realistic limitations and technical solutions. J. Appl. Phys. 2020, 127, 230903. [Google Scholar] [CrossRef]
- Lin, L.; Hu, P.; Tong, X.; Na, S.; Cao, R.; Yuan, X.; Garrett, D.C.; Shi, J.; Maslov, K.; Wang, L.V. High-speed three-dimensional photoacoustic computed tomography for preclinical research and clinical translation. Nat. Commun. 2021, 12, 882. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cho, E.C.; Chen, J.; Song, K.H.; Au, L.; Favazza, C.; Zhang, Q.; Cobley, C.M.; Gao, F.; Xia, Y. In vivo molecular photoacoustic tomography of melanomas targeted by bioconjugated gold nanocages. ACS Nano 2010, 4, 4559–4564. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Baik, J.W.; Kim, D.; Choi, K.; Lee, S.; Park, S.-M.; Kim, J.Y.; Nam, S.H.; Kim, C. In vivo photoacoustic monitoring of vasoconstriction induced by acute hyperglycemia. Photoacoustics 2023, 30, 100485. [Google Scholar] [CrossRef]
- Huda, K.; Lawrence, D.J.; Thompson, W.; Lindsey, S.H.; Bayer, C.L. In vivo noninvasive systemic myography of acute systemic vasoactivity in female pregnant mice. Nat. Commun. 2023, 14, 6286. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, M.A.; Sanaie, S.; Kuchaki Rafsanjani, M.; Hosseini, M.-S. Role of imaging in early diagnosis of acute ischemic stroke: A literature review. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 175. [Google Scholar] [CrossRef]
- Chiu, F.-Y.; Yen, Y. Imaging biomarkers for clinical applications in neuro-oncology: Current status and future perspectives. Biomark. Res. 2023, 11, 35. [Google Scholar] [CrossRef]
- Zhang, D.; Li, R.; Chen, M.; Vu, T.; Sheng, H.; Yang, W.; Hoffmann, U.; Luo, J.; Yao, J. Photoacoustic imaging of in vivo hemodynamic responses to sodium nitroprusside. J. Biophotonics 2021, 14, e202000478. [Google Scholar] [CrossRef]
- Shan, T.; Zhao, Y.; Jiang, S.; Jiang, H. In-vivo hemodynamic imaging of acute prenatal ethanol exposure in fetal brain by photoacoustic tomography. J. Biophotonics 2020, 13, e201960161. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, W.; Zhu, X.; Li, R.; Liu, W.; Chen, M.; Vu, T.; Jiang, L.; Zhou, Q.; Evans, C.L. Epinephrine-induced effects on cerebral microcirculation and oxygenation dynamics using multimodal monitoring and functional photoacoustic microscopy. Anesthesiology 2023, 139, 173–185. [Google Scholar] [CrossRef]
- Johnson, S.P.; Ogunlade, O.; Lythgoe, M.F.; Beard, P.; Pedley, R.B. Longitudinal photoacoustic imaging of the pharmacodynamic effect of vascular targeted therapy on tumors. Clin. Cancer Res. 2019, 25, 7436–7447. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, B.; Lim, H.G. Advances in photoacoustic imaging aided by nano contrast agents: Special focus on role of lymphatic system imaging for cancer theranostics. J. Nanobiotechnol. 2023, 21, 437. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, G.; Li, Q.; Liao, C.; Huang, L.; Ke, T.; Jiang, H.; Han, D. Photoacoustic imaging for the evaluation of early tumor response to antivascular treatment. Quant. Imaging Med. Surg. 2019, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Fadhel, M.N.; Appak Baskoy, S.; Wang, Y.; Hysi, E.; Kolios, M.C. Use of photoacoustic imaging for monitoring vascular disrupting cancer treatments. J. Biophotonics 2023, 16, e202000209. [Google Scholar] [CrossRef]
- Sun, N.; Zheng, S.; Rosin, D.L.; Poudel, N.; Yao, J.; Perry, H.M.; Cao, R.; Okusa, M.D.; Hu, S. Development of a photoacoustic microscopy technique to assess peritubular capillary function and oxygen metabolism in the mouse kidney. Kidney Int. 2021, 100, 613–620. [Google Scholar] [CrossRef]
- Bunke, J.; Merdasa, A.; Sheikh, R.; Albinsson, J.; Erlöv, T.; Gesslein, B.; Cinthio, M.; Reistad, N.; Malmsjö, M. Photoacoustic imaging for the monitoring of local changes in oxygen saturation following an adrenaline injection in human forearm skin. Biomed. Opt. Express 2021, 12, 4084–4096. [Google Scholar] [CrossRef]
- Petri, M.; Stoffels, I.; Jose, J.; Leyh, J.; Schulz, A.; Dissemond, J.; Schadendorf, D.; Klode, J. Photoacoustic imaging of real-time oxygen changes in chronic leg ulcers after topical application of a haemoglobin spray: A pilot study. J. Wound Care 2016, 25, 87–91. [Google Scholar] [CrossRef]
- Capozza, M.; Blasi, F.; Valbusa, G.; Oliva, P.; Cabella, C.; Buonsanti, F.; Cordaro, A.; Pizzuto, L.; Maiocchi, A.; Poggi, L. Photoacoustic imaging of integrin-overexpressing tumors using a novel ICG-based contrast agent in mice. Photoacoustics 2018, 11, 36–45. [Google Scholar] [CrossRef]
- Wood, C.A.; Han, S.; Kim, C.S.; Wen, Y.; Sampaio, D.R.; Harris, J.T.; Homan, K.A.; Swain, J.L.; Emelianov, S.Y.; Sood, A.K. Clinically translatable quantitative molecular photoacoustic imaging with liposome-encapsulated ICG J-aggregates. Nat. Commun. 2021, 12, 5410. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Choi, S.; Lee, H.; Yang, J.; Jeon, H.; Sung, M.; Kim, W.J.; Kim, C. 3D Multiparametric Photoacoustic Computed Tomography of Primary and Metastatic Tumors in Living Mice. ACS Nano 2024, 18, 18176–18190. [Google Scholar] [CrossRef]
- Singh, S.; Giammanco, G.; Hu, C.-H.; Bush, J.; Cordova, L.S.; Lawrence, D.J.; Moran, J.L.; Chitnis, P.V.; Veneziano, R. Size-tunable ICG-based contrast agent platform for targeted near-infrared photoacoustic imaging. Photoacoustics 2023, 29, 100437. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Hu, Z.; Yu, P.; He, Z.; Chen, Y.; Chen, J.; Sun, H.; Wang, S.; Zhang, F. Ultra-photostable small-molecule dyes facilitate near-infrared biophotonics. Nat. Commun. 2024, 15, 2593. [Google Scholar] [CrossRef] [PubMed]
- Rathnamalala, C.S.; Hernandez, S.; Lucero, M.Y.; Swartchick, C.B.; Kalam Shaik, A.; Hammer, N.I.; East, A.K.; Gwaltney, S.R.; Chan, J.; Scott, C.N. Xanthene-Based Nitric Oxide-Responsive Nanosensor for Photoacoustic Imaging in the SWIR Window. Angew. Chem. Int. Ed. 2023, 62, e202214855. [Google Scholar] [CrossRef] [PubMed]
- Wi, J.-S.; Kim, J.; Kim, M.Y.; Choi, S.; Jung, H.J.; Kim, C.; Na, H.-K. Theoretical and experimental comparison of the performance of gold, titanium, and platinum nanodiscs as contrast agents for photoacoustic imaging. RSC Adv. 2023, 13, 9441–9447. [Google Scholar] [CrossRef]
- Li, W.; Chen, X. Gold nanoparticles for photoacoustic imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef]
- Sun, I.-C.; Dumani, D.S.; Emelianov, S.Y. Applications of the Photocatalytic and Photoacoustic Properties of Gold Nanorods in Contrast-Enhanced Ultrasound and Photoacoustic Imaging. ACS Nano 2024, 18, 3575–3582. [Google Scholar] [CrossRef]
- Cao, Z.; Feng, L.; Zhang, G.; Wang, J.; Shen, S.; Li, D.; Yang, X. Semiconducting polymer-based nanoparticles with strong absorbance in NIR-II window for in vivo photothermal therapy and photoacoustic imaging. Biomaterials 2018, 155, 103–111. [Google Scholar] [CrossRef]
- St Lorenz, A.; Moses, A.S.; Mamnoon, B.; Demessie, A.A.; Park, Y.; Singh, P.; Taratula, O.; Taratula, O.R. A Photoacoustic Contrast Nanoagent with a Distinct Spectral Signature for Ovarian Cancer Management. Adv. Healthc. Mater. 2023, 12, 2202946. [Google Scholar] [CrossRef]
- Chen, Z.; Gezginer, I.; Augath, M.-A.; Ren, W.; Liu, Y.-H.; Ni, R.; Deán-Ben, X.L.; Razansky, D. Hybrid magnetic resonance and optoacoustic tomography (MROT) for preclinical neuroimaging. Light Sci. Appl. 2022, 11, 332. [Google Scholar] [CrossRef]
- Choi, W.; Park, E.-Y.; Jeon, S.; Yang, Y.; Park, B.; Ahn, J.; Cho, S.; Lee, C.; Seo, D.-K.; Cho, J.-H. Three-dimensional multistructural quantitative photoacoustic and US imaging of human feet in vivo. Radiology 2022, 303, 467–473. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, H.; Miao, Y.; Weng, J.; Huang, Z.; Fu, J.; Zhang, Y.; Lin, J.; Ye, D. Tailoring a near-infrared macrocyclization scaffold allows the control of in situ self-assembly for photoacoustic/PET bimodal imaging. Angew. Chem. Int. Ed. 2022, 61, e202200369. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Choi, S.; Kim, J.; Park, B.; Kim, C. Recent advances in deep-learning-enhanced photoacoustic imaging. Adv. Photonics Nexus 2023, 2, 054001. [Google Scholar] [CrossRef]
- Lan, H.; Jiang, D.; Yang, C.; Gao, F.; Gao, F. Y-Net: Hybrid deep learning image reconstruction for photoacoustic tomography in vivo. Photoacoustics 2020, 20, 100197. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, G.; Li, L.; Zhang, P.; Kim, J.Y.; Kim, Y.; Kim, H.H.; Wang, L.V.; Lee, S.; Kim, C. Deep learning acceleration of multiscale superresolution localization photoacoustic imaging. Light Sci. Appl. 2022, 11, 131. [Google Scholar] [CrossRef]
- Jeon, S.; Choi, W.; Park, B.; Kim, C. A deep learning-based model that reduces speed of sound aberrations for improved in vivo photoacoustic imaging. IEEE Trans. Image Process. 2021, 30, 8773–8784. [Google Scholar] [CrossRef]
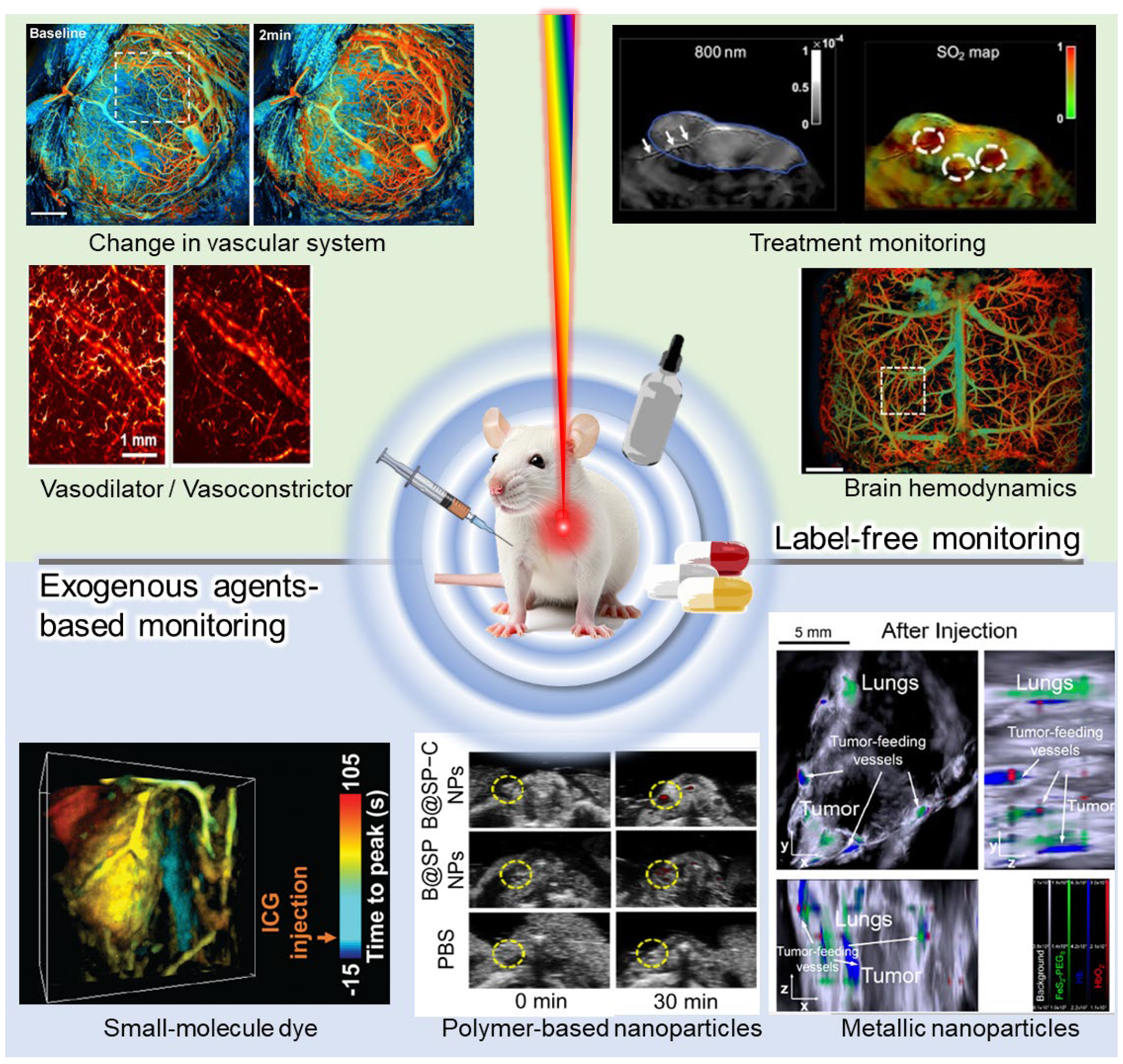
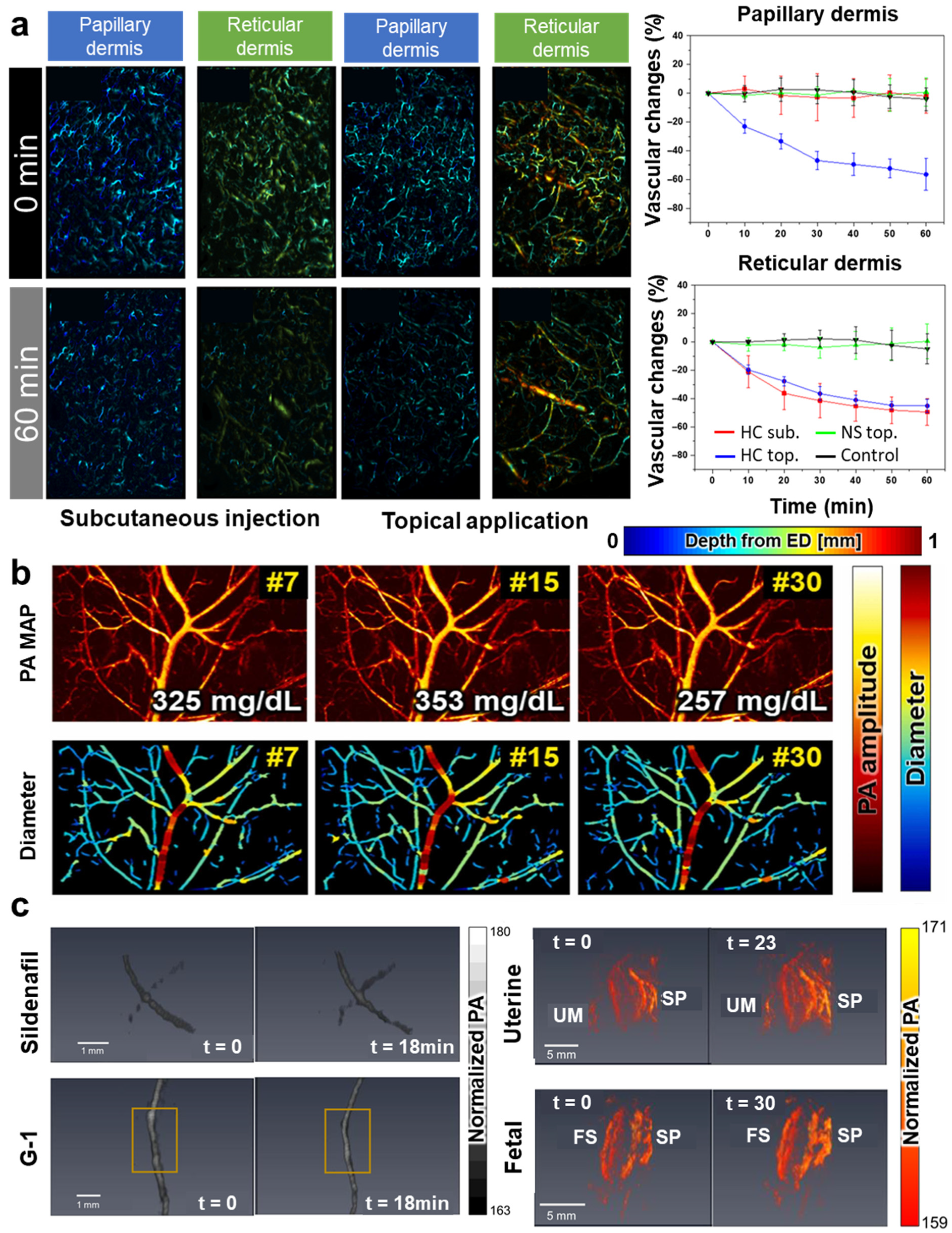
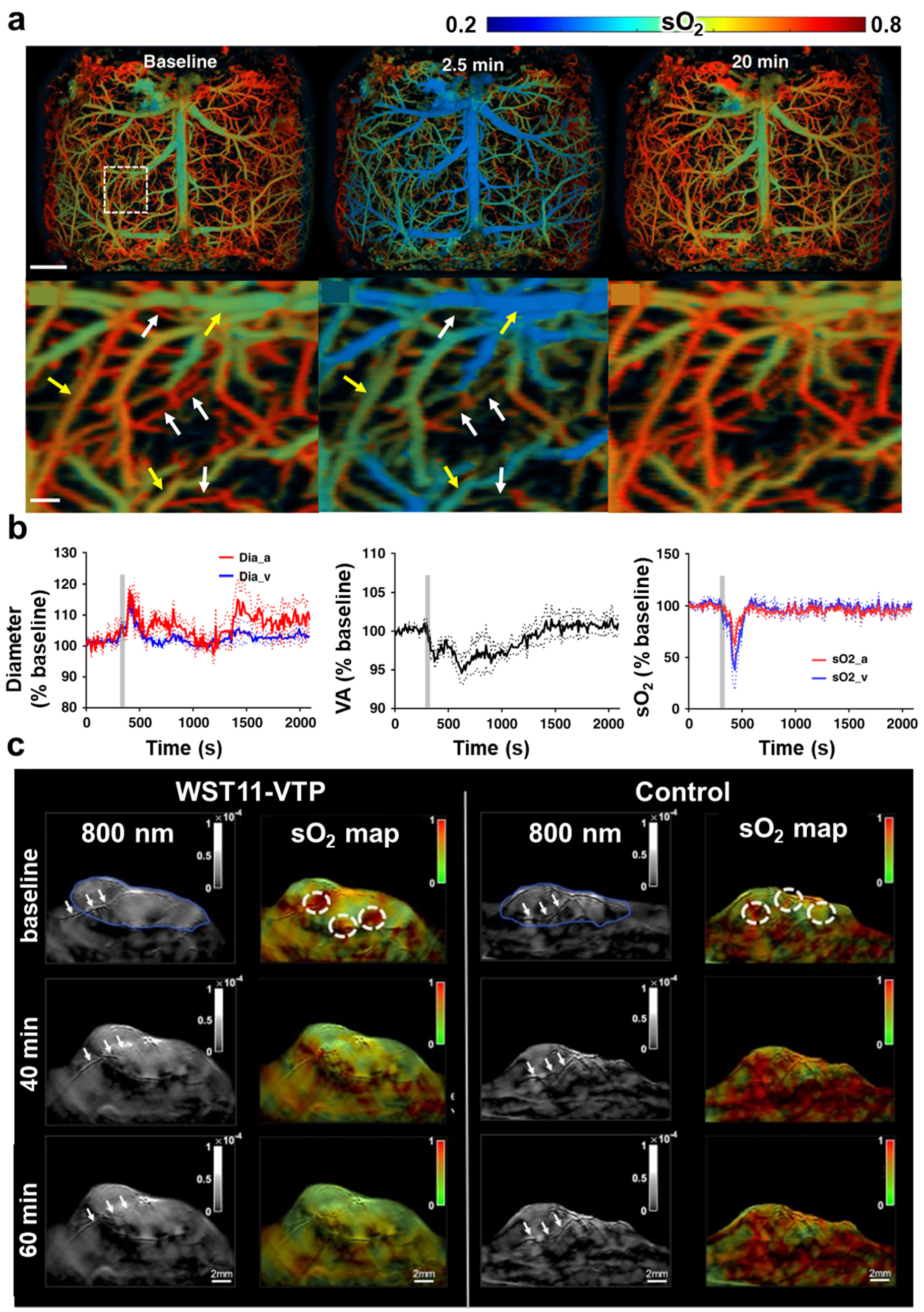
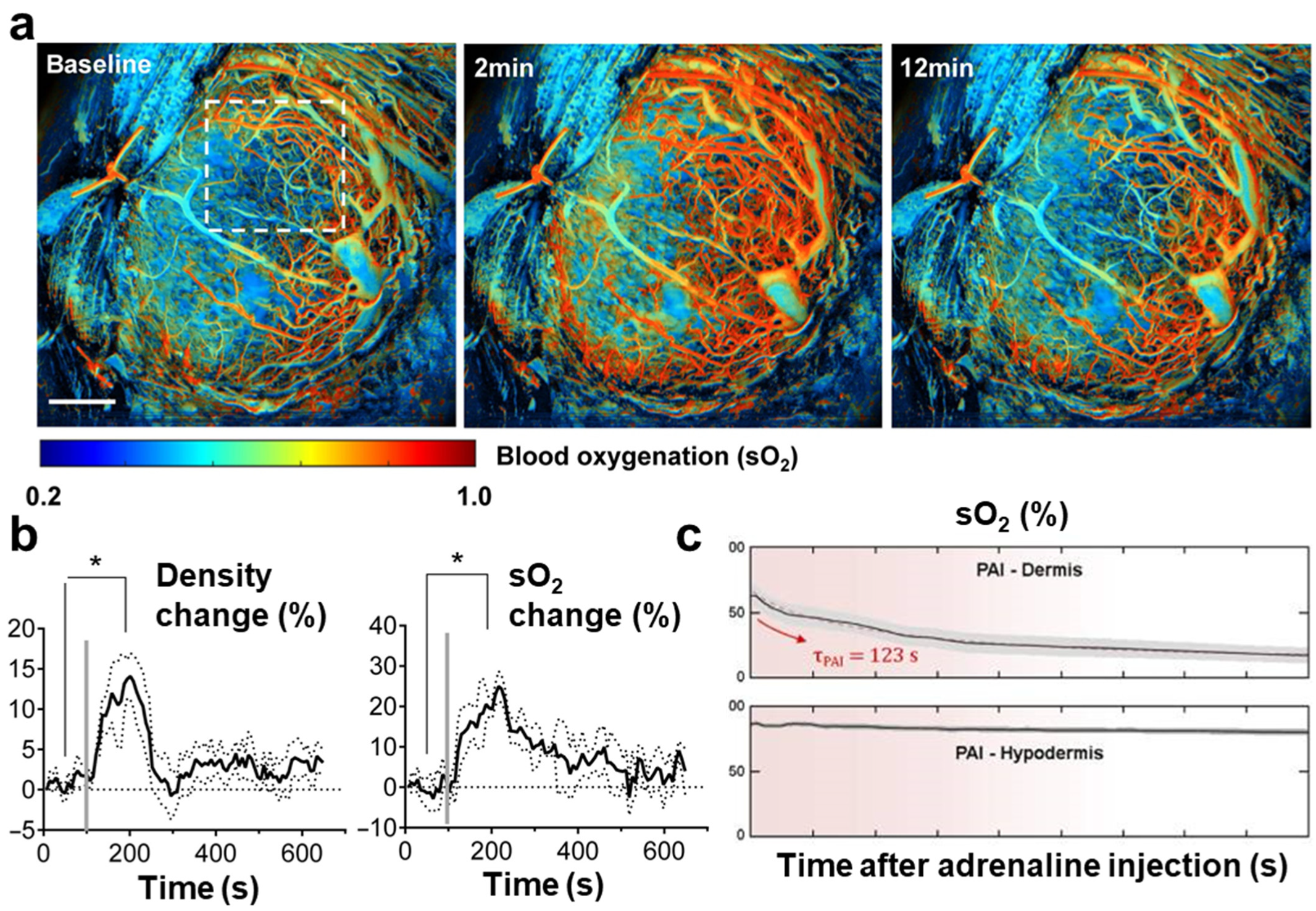
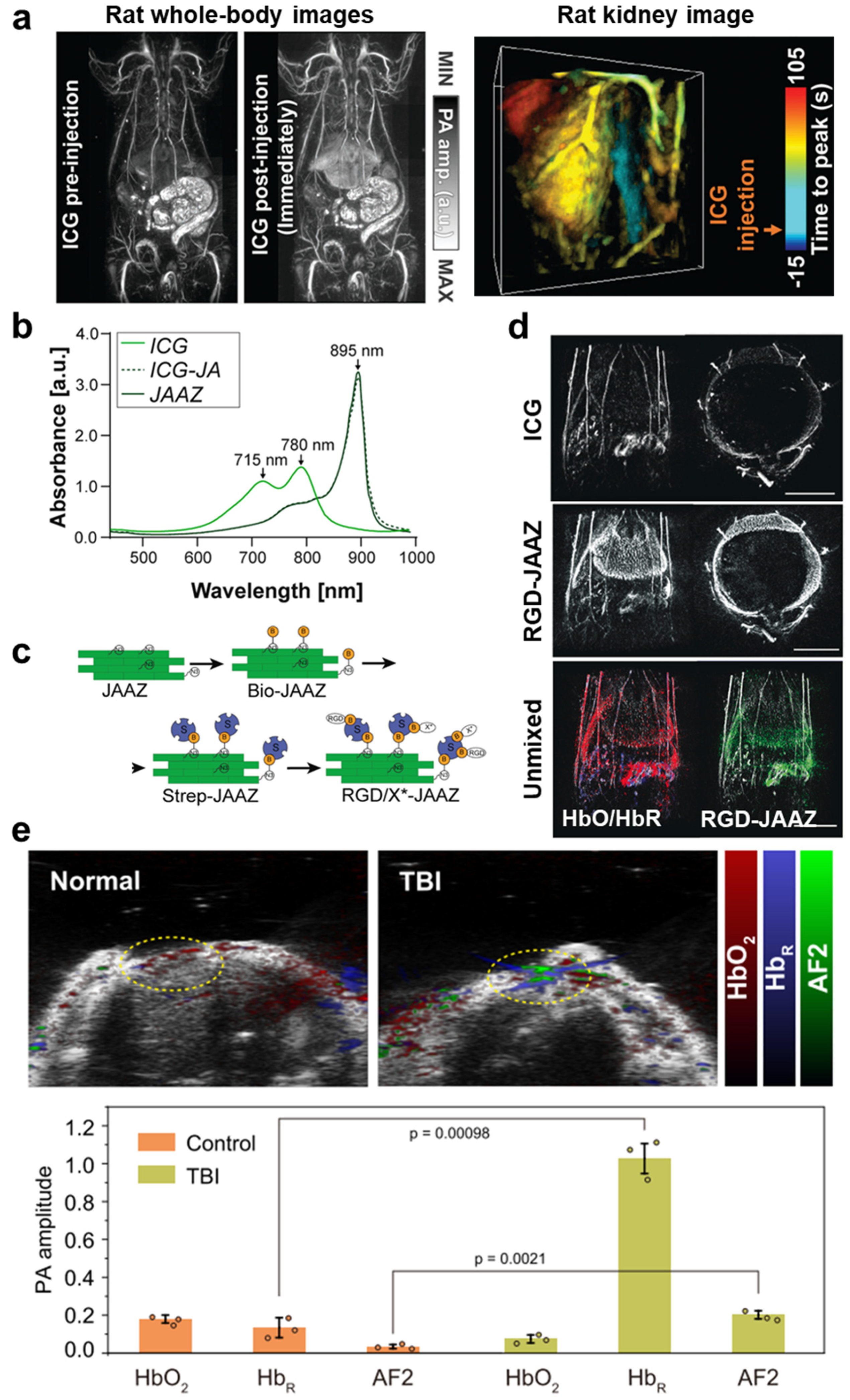
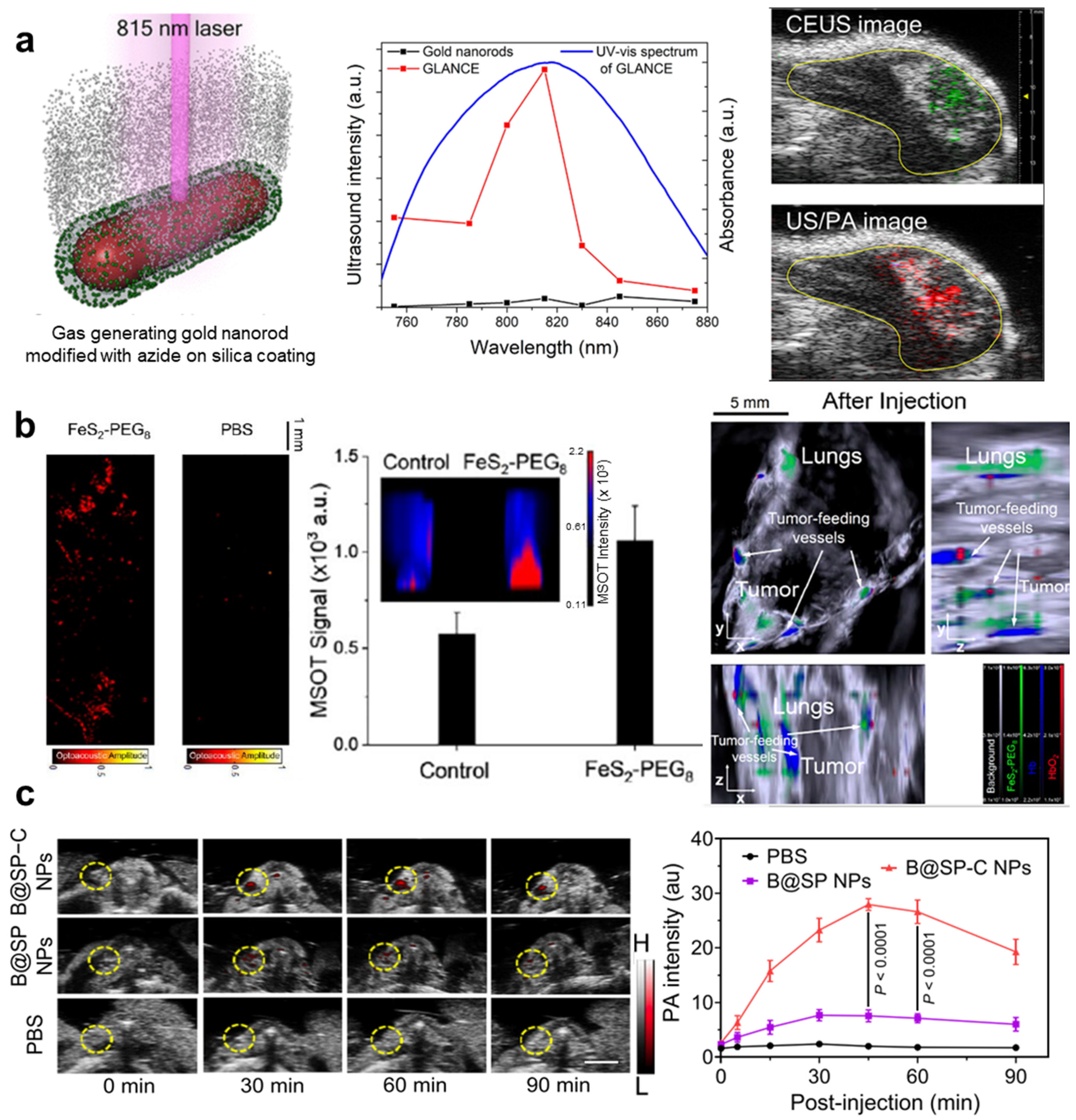
| Abbreviation | Explanation |
|---|---|
| HbO | Oxy-hemoglobin |
| HbR | Deoxy-hemoglobin |
| HbT | Total hemoglobin |
| sO2 | Oxygen saturation |
| MAP | Maximum amplitude projection |
| SNP | Sodium nitroprusside |
| DMXAA | 5,6-Dimethylxanthenone-4-acetic acid |
| VTP | Vascular-targeted photodynamic therapy |
| ICG | Indocyanine green |
| JA | J-aggregates |
| SWIR | Shortwave infrared |
| NO | Nitric oxide |
| ROS | Reactive oxygen species |
| NIR | Near infrared |
| System | Detector Specification (Center Frequency) | Lateral Resolution | Imaging Depth | Approximate Imaging Area (Target) | Ref. |
|---|---|---|---|---|---|
| OR-PAM | Single element (50 MHz) | 0.4–0.7 µm | 0.76 mm | 15 × 10 mm2 (Mouse ear) | [98] |
| Single element (n/a) | 1.2 µm | 1 mm | 6 × 10 mm2 (Mouse brain) | [99] | |
| AR-PAM | Single element (50 MHz) | 52.5 µm | 3–6 mm | 30 × 35 mm2 (Mouse ear) | [100] |
| Single element (50/75 MHz) | 84/54 µm | 2.7/1.8 mm | 9 × 10 mm2 (Mouse ear) | [101] | |
| PACT | 1024 elements Hemispherical (2 MHz) | 380 µm | 10 mm | 65 × 85 mm2 (Mouse whole body) | [80] |
| 1024 elements Arc (2.25 MHz) | 370–390 µm | 40 mm | 75 × 85 mm2 (Human breast) | [103] |
| Target Tissue | Image Modality | Detector Spec. (Center F) | Imaging Performance | Detection | Drug or Contrast Agents | Ref. |
|---|---|---|---|---|---|---|
| Skin | OR-PAM | Single (50 MHz) | LRes: 5 µm ARes: 30 µm ID: 1 mm | Vasoconstriction | Corticosteroid | [81] |
| Ear | PAM | Single (50 MHz) | LRes: 5 µm IDes: 1 mm | Vasoconstriction | Glucose | [105] |
| Placenta | PACT | 96 el. Arc (6 MHz) | LRes: 390 µm ARes: 370 µm | Vasodilation | Sildenafil and G protein-coupled receptor G-1 | [106] |
| UFF-PAM | Single (40 MHz) | LRes: 10 µm | Vasodilation Vascular structure Oxygenation | Alcohol | [84] | |
| Brain | UFF-PAM | Single (40 MHz) | LRes: 10 µm IDes: 1.5 mm | Vasoconstriction Vasodilation Oxygenation | SNP | [82] |
| OR-PAM | Single (30 MHz) | LRes: 3 µm ARes: 25 µm | Vasodilation Oxygenation | SNP | [109] | |
| PACT | 256 el. Cylinder (4 MHz) | n/a | Vascular structure Oxygenation | Alcohol | [110] | |
| PAM | n/a | ID: several millimeters | Vasoconstriction Oxygenation | Epinephrine | [111] | |
| PACT | 256 el. Linear (n/a) | n/a | Agent’s biodistribution Oxygenation | AF-based dye | [123] | |
| Tumor | All-optical PA | Fabry–Perot sensor | LRes: 50–150 µm ARes: 50–150 µm ID: 10 mm | Vascular structure | OXi4503 | [112] |
| AR-PAM | Single (25 MHz) | LRes: 130 µm ARes: 60 µm | Vascular structure Oxygenation | Bevacizumab | [114] | |
| PACT | Linear (15 MHz) | n/a | Oxygenation | DMXAA | [115] | |
| PACT | 256 el. Arc (4 MHz) | Res: 200 µm | Vascular structure Oxygenation | WST11 | [83] | |
| PACT | Array (40 MHz) | n/a | Agent’s biodistribution | Gas-generating laser-activatable nanorods | [127] | |
| PACT | 256 el. Array (5 MHz) | n/a | Agent’s biodistribution | FeS₂ nanocrystals | [85] | |
| PACT | Array (40 MHz) | ARes: 40 µm | Agent’s biodistribution | Polymeric nanoparticle | [129] | |
| Human forearm skin | PACT | Array (30 MHz) | LRes: 50 µm ARes: 110 µm IDes: 20 µm | Vasoconstriction Oxygenation | Adrenaline | [117] |
| Ceritubular capillary | PAM | Single (35 MHz) | IDes: 200 µm | Vascular structure Oxygenation | Lipopolysaccharide | [116] |
| Human leg | PACT | Linear array (21 MHz) | n/a | Oxygenation | Hemoglobin spray | [118] |
| Whole body | PACT | 1024 el. Hemispherical (2 MHz) | Isotropic R: 380 µm | Agent’s biodistribution | ICG | [80] |
| Liver and spleen | PACT | Single (5, 35 MHz) | ID: 5 mm | Agent’s biodistribution | JAAZs | [122] |
| PACT | 128 el. Array (5 MHz) | n/a | Agent’s biodistribution | Xanthene-based NO-responsive nanosensors | [124] | |
| Carotid artery | PACT | Array (n/a) | n/a | Agent’s biodistribution | Semiconducting homopolymer nanoplatform | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Choi, S.; Kim, C.; Kim, J.; Park, B. Review on Photoacoustic Monitoring after Drug Delivery: From Label-Free Biomarkers to Pharmacokinetics Agents. Pharmaceutics 2024, 16, 1240. https://doi.org/10.3390/pharmaceutics16101240
Kim J, Choi S, Kim C, Kim J, Park B. Review on Photoacoustic Monitoring after Drug Delivery: From Label-Free Biomarkers to Pharmacokinetics Agents. Pharmaceutics. 2024; 16(10):1240. https://doi.org/10.3390/pharmaceutics16101240
Chicago/Turabian StyleKim, Jiwoong, Seongwook Choi, Chulhong Kim, Jeesu Kim, and Byullee Park. 2024. "Review on Photoacoustic Monitoring after Drug Delivery: From Label-Free Biomarkers to Pharmacokinetics Agents" Pharmaceutics 16, no. 10: 1240. https://doi.org/10.3390/pharmaceutics16101240
APA StyleKim, J., Choi, S., Kim, C., Kim, J., & Park, B. (2024). Review on Photoacoustic Monitoring after Drug Delivery: From Label-Free Biomarkers to Pharmacokinetics Agents. Pharmaceutics, 16(10), 1240. https://doi.org/10.3390/pharmaceutics16101240










