Common Mitochondrial Targets of Curcumin and Cinnamic Acid, the Membrane-Active Natural Phenolic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Preparation of Rat Liver Mitochondria
2.3. Assay of Swelling of Mitochondria
2.4. Determination of Mitochondrial Membrane Potential and Calcium Retention Capacity
2.5. Assay of Oxidation of Succinate and NAD-Dependent Substrates by the Methyl Thiazolyl Tetrazolium (MTT) Assay and 2,6-Dichlorophenolindophenol (DCPIP)
2.6. Determination of the Redox State of Pyridine Nucleotides and Oxidative Phosphorylation in Mitochondria
2.7. Determination of Respiration Rates
2.8. Statistical Analysis
3. Results
3.1. Influence of Curcumin on Mitochondrial Permeability Transition Pore (mPTP) Opening
3.2. Influence of Curcumin on the Membrane Potential and Activity of Dehydrogenases
3.3. Influence of Curcumin on Mitochondrial Respiration and Oxidative Phosphorylation
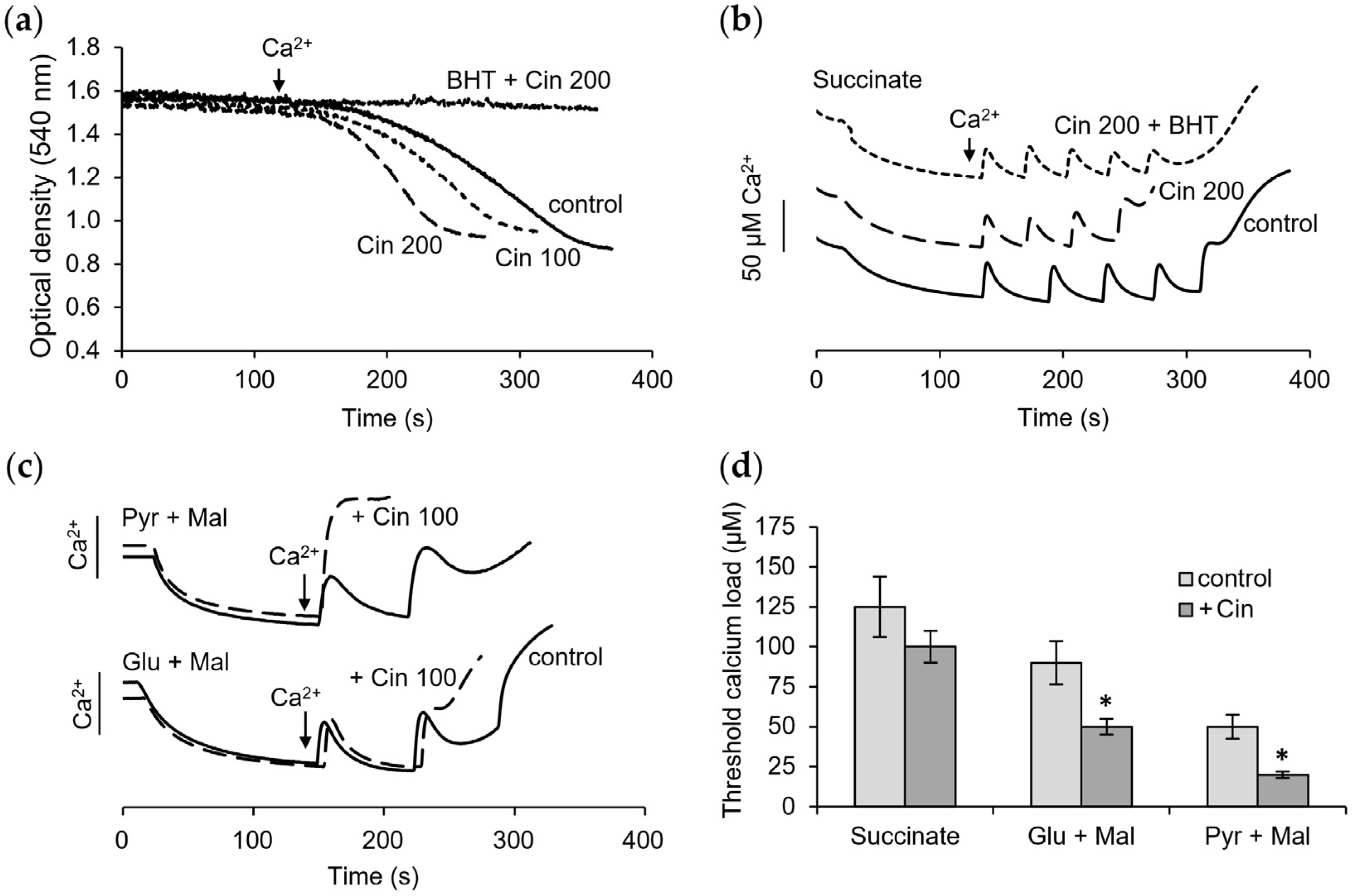
3.4. Influence of Cinnamic Acid on Mitochondrial Functions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BHT | butylhydroxytoluene |
| CRC | calcium retention capacity |
| CsA | cyclosporin A |
| DCPIP | 2,6-Dichlorophenolindophenol |
| mPTP | mitochondrial permeability transition pore |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenytetrazolium bromide |
| PMS | phenazinemethosulfate |
| SDH | succinate dehydrogenase |
| TPP+ | tetraphenylphosphonium |
References
- Zeng, Y.F.; Guo, Q.H.; Wei, X.Y.; Chen, S.Y.; Deng, S.; Liu, J.J.; Yin, N.; Liu, Y.; Zeng, W.J. Cardioprotective effect of curcumin on myocardial ischemia/reperfusion injury: A meta-analysis of preclinical animal studies. Front. Pharmacol. 2023, 14, 1184292. [Google Scholar] [CrossRef] [PubMed]
- Hanyu, F.; Zheng, H.; Jiaqi, W.; Tairan, D.; Yiyuanzi, Z.; Qiwen, Y.; Ying, L.; Hongchun, Z.; Lu, L. Protective effects and mechanism of curcumin in animal models of pulmonary fibrosis: A preclinical systematic review and meta-analysis. Front. Pharmacol. 2023, 14, 1258885. [Google Scholar] [CrossRef] [PubMed]
- Perales-Salinas, V.; Purushotham, S.S.; Buskila, Y. Curcumin as a potential therapeutic agent for treating neurodegenerative diseases. Neurochem. Int. 2024, 178, 105790. [Google Scholar] [CrossRef] [PubMed]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic Acid Derivatives and Their Biological Efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef] [PubMed]
- Panknin, T.M.; Howe, C.L.; Hauer, M.; Bucchireddigari, B.; Rossi, A.M.; Funk, J.L. Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials. Int. J. Mol. Sci. 2023, 24, 4476. [Google Scholar] [CrossRef]
- Hao, M.; Chu, Y.; Lei, J.; Yao, Z.; Wang, P.; Chen, Z.; Wang, K.; Sang, X.; Han, X.; Wang, L.; et al. Pharmacological Mechanisms and Clinical Applications of Curcumin: Update. Aging Dis. 2023, 14, 716–749. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, Q.; Chen, Y.; Ji, K.; Li, S.; Wu, Q.; Pan, Q.; Li, J. Review of the Protective Mechanism of Curcumin on Cardiovascular Disease. Drug Des. Devel. Ther. 2024, 18, 165–192. [Google Scholar] [CrossRef]
- Lambring, C.B.; Chen, L.; Nelson, C.; Stevens, A.; Bratcher, W.; Basha, R. Oxidative Stress and Cancer: Harnessing the Therapeutic Potential of Curcumin and Analogues Against Cancer. Eur. J. Biol. 2023, 82, 317–325. [Google Scholar] [CrossRef]
- Mirzaei, H.; Bagheri, H.; Ghasemi, F.; Khoi, J.M.; Pourhanifeh, M.H.; Heyden, Y.V.; Mortezapour, E.; Nikdasti, A.; Jeandet, P.; Khan, H.; et al. Anti-Cancer Activity of Curcumin on Multiple Myeloma. Anticancer. Agents Med. Chem. 2021, 21, 575–586. [Google Scholar] [CrossRef]
- Labbozzetta, M.; Poma, P.; Notarbartolo, M. Natural Inhibitors of P-glycoprotein in Acute Myeloid Leukemia. Int. J. Mol. Sci. 2023, 24, 4140. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Q.; Wu, Z.; Xu, Y.; Jiang, R. Curcumin for Treating Breast Cancer: A Review of Molecular Mechanisms, Combinations with Anticancer Drugs, and Nanosystems. Pharmaceutics 2024, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Sandur, S.K.; Krishna, M.; Priyadarsini, K.I. Curcumin mediates time and concentration dependent regulation of redox homeostasis leading to cytotoxicity in macrophage cells. Eur. J. Pharmacol. 2009, 611, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Khayatan, D.; Razavi, S.M.; Arab, Z.N.; Hosseini, Y.; Niknejad, A.; Momtaz, S.; Abdolghaffari, A.H.; Sathyapalan, T.; Jamialahmadi, T.; Kesharwani, P.; et al. Superoxide dismutase: A key target for the neuroprotective effects of curcumin. Mol. Cell Biochem. 2023, 479, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Eltayeb, W.A.; Rakshit, G.; El-Arabey, A.A.; Khan, J.; Aldosari, S.M.; Alshehri, B.; Abdalla, M. Dual synergistic inhibition of COX and LOX by potential chemicals from Indian daily spices investigated through detailed computational studies. Sci. Rep. 2023, 13, 8656. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant Potential of Curcumin-A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef]
- Miyazaki, K.; Xu, C.; Shimada, M.; Goel, A. Curcumin and Andrographis Exhibit Anti-Tumor Effects in Colorectal Cancer via Activation of Ferroptosis and Dual Suppression of Glutathione Peroxidase-4 and Ferroptosis Suppressor Protein-1. Pharmaceuticals 2023, 16, 383. [Google Scholar] [CrossRef]
- Foroutan, Z.; Butler, A.E.; Zengin, G.; Sahebkar, A. Curcumin and Ferroptosis: A Promising Target for Disease Prevention and Treatment. Cell Biochem. Biophys. 2024, 82, 343–349. [Google Scholar] [CrossRef]
- Scharstuhl, A.; Mutsaers, H.A.; Pennings, S.W.; Russel, F.G.; Wagener, F.A. Involvement of VDAC, Bax and ceramides in the efflux of AIF from mitochondria during curcumin-induced apoptosis. PLoS ONE 2009, 4, e6688. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yu, T.; Wang, W.; Pan, K.; Shi, D.; Sun, H. Curcumin-induced melanoma cell death is associated with mitochondrial permeability transition pore (mPTP) opening. Biochem. Biophys. Res. Commun. 2014, 448, 15–21. [Google Scholar] [CrossRef]
- Olivas-Aguirre, M.; Torres-López, L.; Pottosin, I.; Dobrovinskaya, O. Phenolic Compounds Cannabidiol, Curcumin and Quercetin Cause Mitochondrial Dysfunction and Suppress Acute Lymphoblastic Leukemia Cells. Int. J. Mol. Sci. 2020, 22, 204. [Google Scholar] [CrossRef]
- Izem-Meziane, M.; Djerdjouri, B.; Rimbaud, S.; Caffin, F.; Fortin, D.; Garnier, A.; Veksler, V.; Joubert, F.; Ventura-Clapier, R. Catecholamine-induced cardiac mitochondrial dysfunction and mPTP opening: Protective effect of curcumin. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H665–H674. [Google Scholar] [CrossRef] [PubMed]
- Cox, F.F.; Misiou, A.; Vierkant, A.; Ale-Agha, N.; Grandoch, M.; Haendeler, J.; Altschmied, J. Protective Effects of Curcumin in Cardiovascular Diseases-Impact on Oxidative Stress and Mitochondria. Cells 2022, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, S.; Zhou, Y.; Zhao, M.; Wang, Y.; Wang, C.; Lou, P.; Huang, R.; Ma, L.; Lu, Y.; et al. Indispensable role of mitochondria in maintaining the therapeutic potential of curcumin in acute kidney injury. J. Cell Mol. Med. 2021, 25, 9863–9877. [Google Scholar] [CrossRef] [PubMed]
- Sathyabhama, M.; Priya Dharshini, L.C.; Karthikeyan, A.; Kalaiselvi, S.; Min, T. The Credible Role of Curcumin in Oxidative Stress-Mediated Mitochondrial Dysfunction in Mammals. Biomolecules 2022, 12, 1405. [Google Scholar] [CrossRef]
- Beloborodova, N.; Bairamov, I.; Olenin, A.; Shubina, V.; Teplova, V.; Fedotcheva, N. Effect of phenolic acids of microbial origin on production of reactive oxygen species in mitochondria and neutrophils. J. Biomed. Sci. 2012, 19, 89. [Google Scholar] [CrossRef]
- Kita, T.; Imai, S.; Sawada, H.; Kumagai, H.; Seto, H. The Biosynthetic Pathway of Curcuminoid in Turmeric (Curcuma longa) as Revealed by 13C-Labeled Precursors. Biosci. Biotechnol. Biochem. 2008, 72, 1789–1798. [Google Scholar] [CrossRef]
- Xiang, L.; Moore, B.S. Inactivation, complementation and heterologous expression of encP, a novel bacterial phenylalanine ammonia-lyase gene. J. Biol. Chem. 2002, 277, 32505–32509. [Google Scholar] [CrossRef]
- Hill, A.M.; Thompson, L.B.; Harris, J.P.; Segret, R. Investigation of the early stages in soraphen A biosynthesis. Chem. Commun. 2003, 12, 1358–1359. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Moore, B.S. Biochemical characterization of a prokaryotic phenylalanine ammonia lyase. J. Bacteriol. 2005, 187, 4286–4289. [Google Scholar] [CrossRef]
- Fedotcheva, N.I.; Teplova, V.V.; Beloborodova, N.V. Participation of phenolic acids of microbial origin in the dysfunction of mitochondria in sepsis. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2010, 4, 50–55. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Sousa, E.; Vasconcelos, M.H.; Pinto, M. Curcumin: A Natural Lead for Potential New Drug Candidates. Curr. Med. Chem. 2015, 22, 4196–4232. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Guo, P.; He, Y.; Pi, C.; Wang, Y.; Feng, X.; Hou, Y.; Jiang, Q.; Zhao, L.; Wei, Y. Application of curcumin and its derivatives in tumor multidrug resistance. Phytother. Res. 2020, 34, 2438–2458. [Google Scholar] [CrossRef] [PubMed]
- Saragatsis, M.; Pontiki, E. Synthesis and Antioxidant Activities of Novel Pyrimidine Acrylamides as Inhibitors of Lipoxygenase: Molecular Modeling and In Silico Physicochemical Studies. Molecules 2024, 29, 1189. [Google Scholar] [CrossRef] [PubMed]
- Falbo, F.; Gemma, S.; Koch, A.; Mazzotta, S.; Carullo, G.; Ramunno, A.; Butini, S.; Schneider-Stock, R.; Campiani, G.; Aiello, F. Synthetic derivatives of natural cinnamic acids as potential anti-colorectal cancer agents. Chem. Biol. Drug Des. 2024, 103, e14415. [Google Scholar] [CrossRef]
- Ligeret, H.; Barthelemy, S.; Zini, R.; Tillement, J.P.; Labidalle, S.; Morin, D. Effects of curcumin and curcumin derivatives on mitochondrial permeability transition pore. Free Radic. Biol. Med. 2004, 36, 919–929. [Google Scholar] [CrossRef]
- Morin, D.; Barthélémy, S.; Zini, R.; Labidalle, S.; Tillement, J.P. Curcumin induces the mitochondrial permeability transition pore mediated by membrane protein thiol oxidation. FEBS Lett. 2001, 495, 131–136. [Google Scholar] [CrossRef]
- Stevens, J.F.; Revel, J.S.; Maier, C.S. Mitochondria-Centric Review of Polyphenol Bioactivity in Cancer Models. Antioxid. Redox Signal. 2018, 29, 1589–1611. [Google Scholar] [CrossRef]
- Miwa, S.; Brand, M.D. Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling. Biochem. Soc. Trans. 2003, 31 Pt 6, 1300–1301. [Google Scholar] [CrossRef]
- Gao, J.L.; Zhao, J.; Zhu, H.B.; Peng, X.; Zhu, J.X.; Ma, M.H.; Fu, Y.; Hu, N.; Tai, Y.; Xuan, X.C.; et al. Characterizations of mitochondrial uncoupling induced by chemical mitochondrial uncouplers in cardiomyocytes. Free Radic. Biol. Med. 2018, 124, 288–298. [Google Scholar] [CrossRef]
- Schulz, R.; Schlüter, K.D. Importance of Mitochondria in Cardiac Pathologies: Focus on Uncoupling Proteins and Monoamine Oxidases. Int. J. Mol. Sci. 2023, 24, 6459. [Google Scholar] [CrossRef]
- Fedotcheva, T.; Shimanovsky, N.; Fedotcheva, N. Specific features of mitochondrial dysfunction under conditions of ferroptosis induced by t-butylhydroperoxide and iron: Protective role of the inhibitors of lipid peroxidation and mitochondrial permeability transition pore opening. Membranes 2023, 13, 372. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Yan, J.; Hou, A.; Sui, W.; Sun, M. Curcumin Induces Ferroptosis in A549 CD133+ Cells through the GSH-GPX4 and FSP1-CoQ10-NADH Pathways. Discov. Med. 2023, 35, 251–263. [Google Scholar] [CrossRef]
- Kapur, A.; Ayuso, J.M.; Rehman, S.; Kumari, S.; Felder, M.; Stenerson, Z.; Skala, M.C.; Beebe, D.; Barroilhet, L.; Patankar, M.S. Oxidative phosphorylation inhibitors inhibit proliferation of endometriosis cells. Reproduction 2023, 165, 617–628. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, L.; Xu, S.; Cheng, X.; Wu, J.; Wang, Y.; Gao, W.; Bao, J.; Yu, H. Curcumin induces mitophagy by promoting mitochondrial succinate dehydrogenase activity and sensitizes human papillary thyroid carcinoma BCPAP cells to radioiodine treatment. Toxicol. In Vitro 2023, 93, 105669. [Google Scholar] [CrossRef]
- Fedotcheva, N.I.; Teplova, V.V.; Beloborodova, N.V. The role of thiol antioxidants in restoring mitochondrial functions modified by microbial metabolites. Biophysics 2012, 57, 634–639. [Google Scholar] [CrossRef]
- Conrad, M.; Pratt, D. A The chemical basis of ferroptosis. Nat. Chem. Biol. 2019, 15, 1137–1147. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, S.; Li, J.; Wang, R.; Xie, X.; Yu, X.; Pan, J.; Xu, Y.; Zheng, L. The effect of curcumin on the brain-gut axis in rat model of irritable bowel syndrome: Involvement of 5-HT-dependent signaling. Metab. Brain Dis. 2015, 30, 47–55. [Google Scholar] [CrossRef]
- Feng, W.; Wang, H.; Zhang, P.; Gao, C.; Tao, J.; Ge, Z.; Zhu, D.; Bi, Y. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1801–1812. [Google Scholar] [CrossRef]
- Elkharsawy, H.; Eldomany, R.A.; Mira, A.; Soliman, A.F.; Amir, M.; El-Sharkawy, S. New neuroprotective derivatives of cinnamic acid by biotransformation. Food Funct. 2024, 15, 4323–4337. [Google Scholar] [CrossRef]
- Beloborodova, N.V. Metabolism of Microbiota in Critical Illness (Review and Postulates). Gen. Reanimatol. 2019, 15, 62–79. [Google Scholar] [CrossRef]
- Beloborodova, N.V. Serum Aromatic Microbial Metabolites as Biological Markers in Intensive Care. In Biomarkers in Trauma, Injury and Critical Care. Biomarkers in Disease: Methods, Discoveries and Applications; Rajendram, R., Preedy, V.R., Patel, V.B., Eds.; Springer: Cham, Switzerland, 2023; Chapter 13; pp. 245–268. [Google Scholar]






| Mitochondrial Function, Target | Curcumin (10–50 µM) | Cinnamic Acid (100–200 µM)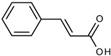 |
|---|---|---|
| Membrane potential | depolarization | no effect |
| Respiration | uncoupling effect | no effect |
| MPTP opening by Ca2+ | stimulation | stimulation |
| Resistance to calcium ion loading | decrease | decrease |
| Swelling rate | activation | activation |
| Lipid peroxidation | low activation | activation |
| SDH activity | no effect | no effect |
| GDH activity | decrease at high concentrations | decrease |
| PDH activity | decrease at high concentrations | decrease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedotcheva, T.A.; Beloborodova, N.V.; Fedotcheva, N.I. Common Mitochondrial Targets of Curcumin and Cinnamic Acid, the Membrane-Active Natural Phenolic Compounds. Pharmaceutics 2024, 16, 1272. https://doi.org/10.3390/pharmaceutics16101272
Fedotcheva TA, Beloborodova NV, Fedotcheva NI. Common Mitochondrial Targets of Curcumin and Cinnamic Acid, the Membrane-Active Natural Phenolic Compounds. Pharmaceutics. 2024; 16(10):1272. https://doi.org/10.3390/pharmaceutics16101272
Chicago/Turabian StyleFedotcheva, Tatiana A., Natalia V. Beloborodova, and Nadezhda I. Fedotcheva. 2024. "Common Mitochondrial Targets of Curcumin and Cinnamic Acid, the Membrane-Active Natural Phenolic Compounds" Pharmaceutics 16, no. 10: 1272. https://doi.org/10.3390/pharmaceutics16101272






