Advancements in Ocular Therapy: A Review of Emerging Drug Delivery Approaches and Pharmaceutical Technologies
Abstract
1. Introduction
2. Barriers to Ocular Drug Delivery
2.1. Static Barriers
2.2. Dynamic Barriers
3. Common Eye Diseases and Treatment
3.1. Glaucoma
3.2. Dry Eye Disease/Dry Eye Syndrome
3.3. Diabetic Retinopathy and Diabetic Macular Edema
3.4. Retinal Vein Occlusion
3.5. Uveitis
3.6. Age-Related Macular Degeneration
4. Emerging Ocular Drug Delivery Systems
4.1. Ocular Implants
4.2. Liposomes
4.3. Nanoparticles
4.4. Nanomicelles
4.5. Microparticles
4.6. Iontophoresis
4.7. In Situ Gels
4.8. Contact Lenses
4.9. Microneedles
4.10. Hydrogels
4.11. Bispecific Antibodies
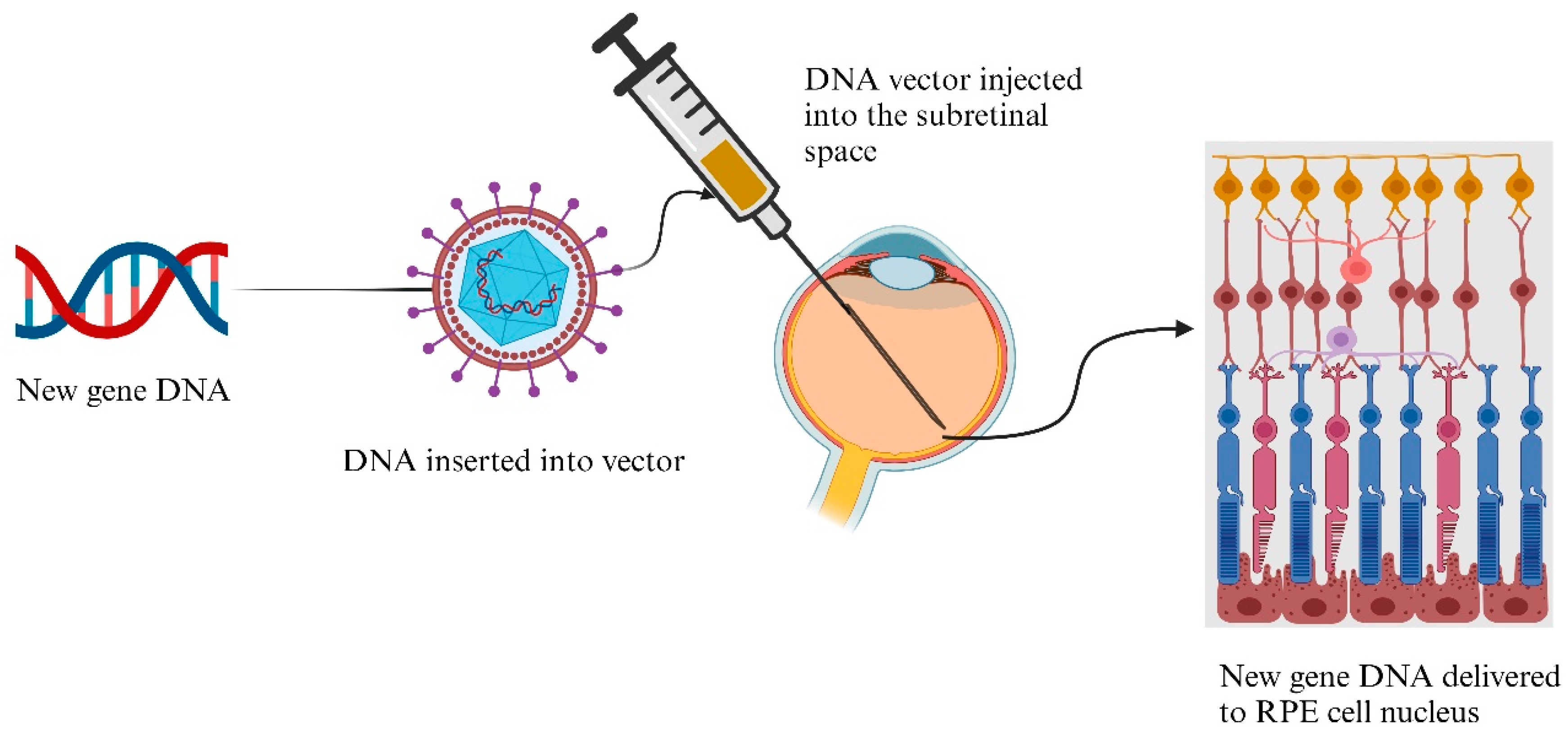
4.12. Gene Delivery
5. Pharmaceutical Technologies for Ocular Applications
5.1. Three-Dimensional Printing Technologies
5.1.1. Fused Deposition Modeling
5.1.2. Semi-Solid Extrusion
5.1.3. Vat Photopolymerization
5.2. Hot-Melt Extrusion
5.3. Injection Molding
6. Role of Digital Healthcare, AI, and ML
7. Industry and Regulatory Perspectives
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization—Blindness and Vision Impairment. Available online: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment (accessed on 24 August 2024).
- U.S. Centers for Disease Control and Prevention-Looking Ahead: Improving Our Vision for the Future|Vision and Eye Health. Available online: https://www.cdc.gov/vision-health/data-research/vision-loss-facts/improving-vision-for-future.html (accessed on 24 August 2024).
- Zhao, J.; Xu, X.; Ellwein, L.B.; Guan, H.; He, M.; Liu, P.; Lv, J.; Sheng, X.; Yang, P.; Yi, J.; et al. Causes of Visual Impairment and Blindness in the 2006 and 2014 Nine-Province Surveys in Rural China. Am. J. Ophthalmol. 2019, 197, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.; et al. Global Causes of Blindness and Distance Vision Impairment 1990–2020: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Amin, M.M.; Sayed, S. Ocular Drug Delivery: A Comprehensive Review. AAPS PharmSciTech 2023, 24, 66. [Google Scholar] [CrossRef]
- Urtti, A. Challenges and Obstacles of Ocular Pharmacokinetics and Drug Delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, K.; Järvinen, T.; Urtti, A. Ocular Absorption Following Topical Delivery. Adv. Drug Deliv. Rev. 1995, 16, 3–19. [Google Scholar] [CrossRef]
- Vidal, K.S.; Suemoto, C.K.; Moreno, A.B.; Viana, M.C.; Lotufo, P.A.; Benseñor, I.M.; Brunoni, A.R. Association Between Posterior Segment Eye Diseases, Common Mental Disorders, and Depression: Cross-Sectional and Longitudinal Analyses of Brazilian Longitudinal Study of Adult Health Cohort. J. Acad. Consult. Psychiatry 2021, 62, 70–78. [Google Scholar] [CrossRef]
- Forrester, J.V.; Dick, A.D.; Andrew, D.; McMenamin, P.G.; Roberts, F.; Pearlman, E. The Eye: Basic Sciences in Practice; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016; pp. 1–102. [Google Scholar] [CrossRef]
- Zhou, R.; Caspi, R.R. Ocular Immune Privilege. F1000 Biol. Rep. 2010, 2, 3. [Google Scholar] [CrossRef]
- Dosmar, E.; Walsh, J.; Doyel, M.; Bussett, K.; Oladipupo, A.; Amer, S.; Goebel, K. Targeting Ocular Drug Delivery: An Examination of Local Anatomy and Current Approaches. Bioengineering 2022, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Kels, B.D.; Grzybowski, A.; Grant-Kels, J.M. Human Ocular Anatomy. Clin. Dermatol. 2015, 33, 140–146. [Google Scholar] [CrossRef]
- Camburu, G.; Zemba, M.; Tătaru, C.P.; Purcărea, V.L. The Measurement of Central Corneal Thickness. Rom. J. Ophthalmol. 2023, 67, 168. [Google Scholar] [CrossRef]
- Meek, K.M.; Knupp, C.; Lewis, P.N.; Morgan, S.R.; Hayes, S. Structural Control of Corneal Transparency, Refractive Power and Dynamics. Eye 2024, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Balla, A.; Auriola, S.; Grey, A.C.; Demarais, N.J.; Valtari, A.; Heikkinen, E.M.; Toropainen, E.; Urtti, A.; Vellonen, K.S.; Ruponen, M. Partitioning and Spatial Distribution of Drugs in Ocular Surface Tissues. Pharmaceutics 2021, 13, 658. [Google Scholar] [CrossRef] [PubMed]
- Schoenwald, R.D.; Ward, R.L. Relationship between Steroid Permeability across Excised Rabbit Cornea and Octanol-Water Partition Coefficients. J. Pharm. Sci. 1978, 67, 786–788. [Google Scholar] [CrossRef]
- Mofidfar, M.; Abdi, B.; Ahadian, S.; Mostafavi, E.; Desai, T.A.; Abbasi, F.; Sun, Y.; Manche, E.E.; Ta, C.N.; Flowers, C.W. Drug Delivery to the Anterior Segment of the Eye: A Review of Current and Future Treatment Strategies. Int. J. Pharm. 2021, 607, 120924. [Google Scholar] [CrossRef]
- Wilson, S.E. Bowman’s Layer in the Cornea– Structure and Function and Regeneration. Exp. Eye Res. 2020, 195, 108033. [Google Scholar] [CrossRef] [PubMed]
- Espana, E.M.; Birk, D.E. Composition, Structure and Function of the Corneal Stroma. Exp. Eye Res. 2020, 198, 108137. [Google Scholar] [CrossRef]
- Lagali, N.; Germundsson, J.; Fagerholm, P. The Role of Bowman’s Layer in Corneal Regeneration after Phototherapeutic Keratectomy: A Prospective Study Using in Vivo Confocal Microscopy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4192–4198. [Google Scholar] [CrossRef]
- Wilson, S.E.; Torricelli, A.A.M.; Marino, G.K. Corneal Epithelial Basement Membrane: Structure, Function and Regeneration. Exp. Eye Res. 2020, 194, 108002. [Google Scholar] [CrossRef] [PubMed]
- Germundsson, J.; Karanis, G.; Fagerholm, P.; Lagali, N. Age-Related Thinning of Bowman’s Layer in the Human Cornea In Vivo. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6143–6149. [Google Scholar] [CrossRef]
- Meek, K.M.; Knupp, C. Corneal Structure and Transparency. Prog. Retin. Eye Res. 2015, 49, 1. [Google Scholar] [CrossRef]
- Santana, C.P.; Matter, B.A.; Patil, M.A.; Silva-Cunha, A.; Kompella, U.B. Corneal Permeability and Uptake of Twenty-Five Drugs: Species Comparison and Quantitative Structure-Permeability Relationships. Pharmaceutics 2023, 15, 1646. [Google Scholar] [CrossRef]
- Maurice, D.M.; Mishima, S. Ocular Pharmacokinetics; Springer: Berlin/Heidelberg, Germany, 1984; pp. 19–116. [Google Scholar] [CrossRef]
- Agarwal, R.; Iezhitsa, I.; Agarwal, P.; Abdul Nasir, N.A.; Razali, N.; Alyautdin, R.; Ismail, N.M. Liposomes in Topical Ophthalmic Drug Delivery: An Update. Drug Deliv. 2016, 23, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Rupenthal, I.D. Modern Approaches to the Ocular Delivery of Cyclosporine A. Drug Discov. Today 2016, 21, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Jwala, J.; Boddu, S.H.S.; Mitra, A.K. Recent Perspectives in Ocular Drug Delivery. Pharm. Res. 2009, 26, 1197–1216. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, M.S. Anatomy of Cornea and Ocular Surface. Indian J. Ophthalmol. 2018, 66, 190. [Google Scholar] [CrossRef]
- Joyce, N.C. Proliferative Capacity of the Corneal Endothelium. Prog. Retin. Eye Res. 2003, 22, 359–389. [Google Scholar] [CrossRef]
- Bonanno, J.A. Molecular Mechanisms Underlying the Corneal Endothelial Pump. Exp. Eye Res. 2012, 95, 2–7. [Google Scholar] [CrossRef]
- Fischbarg, J.; Maurice, D.M. An Update on Corneal Hydration Control. Exp. Eye Res. 2004, 78, 537–541. [Google Scholar] [CrossRef]
- Verkman, A.S. Aquaporins in Clinical Medicine. Annu. Rev. Med. 2012, 63, 303. [Google Scholar] [CrossRef]
- Prausnitz, M.R. Permeability of Cornea, Sclera, and Conjunctiva: A Literature Analysis for Drug Delivery to the Eye. J. Pharm. Sci. 1998, 87, 1479–1488. [Google Scholar] [CrossRef]
- Freddo, T.F. A Contemporary Concept of the Blood-Aqueous Barrier. Prog. Retin. Eye Res. 2013, 32, 181. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-Retinal Barrier. Eur. J. Ophthalmol. 2011, 21 (Suppl. 6), 3–9. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, E.; Lajunen, T.; Bhattacharya, M.; Reinisalo, M.; Rilla, K.; Kidron, H.; Terasaki, T.; Urtti, A. Selective Drug Delivery to the Retinal Cells: Biological Barriers and Avenues. J. Control. Release 2023, 361, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Abelson, M.B.; Udell, I.J.; Weston, J.H. Normal Human Tear PH by Direct Measurement. Arch. Ophthalmol. 1981, 99, 301. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D.P.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Stern, M.E. Biological Functions of Tear Film. Exp. Eye Res. 2020, 197, 108115. [Google Scholar] [CrossRef]
- Long-Term Trends in Human Eye Blink Rate—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/754827/ (accessed on 29 August 2024).
- Grassiri, B.; Zambito, Y.; Bernkop-Schnürch, A. Strategies to Prolong the Residence Time of Drug Delivery Systems on Ocular Surface. Adv. Colloid Interface Sci. 2021, 288, 102342. [Google Scholar] [CrossRef]
- Ducker, L.; Rivera, R.Y. Anatomy, Head and Neck: Eye Lacrimal Duct; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735. [Google Scholar] [CrossRef]
- Ghate, D.; Edelhauser, H.F. Barriers to Glaucoma Drug Delivery. J. Glaucoma 2008, 17, 147–156. [Google Scholar] [CrossRef]
- Sahlin, S.; Laurell, C.G.; Chen, E.; Philipson, B. Lacrimal Drainage Capacity, Age and Blink Rate. Orbit 1998, 17, 155–159. [Google Scholar] [CrossRef]
- Agarwal, P.; Rupenthal, I.D. Non-Aqueous Formulations in Topical Ocular Drug Delivery—A Paradigm Shift? Adv. Drug Deliv. Rev. 2023, 198, 114867. [Google Scholar] [CrossRef] [PubMed]
- Mishima, S.; Gasset, A.; Klyce, S.D.; Baum, J.L. Determination of Tear Volume and Tear Flow. Investig. Ophthalmol. Vis. Sci. 1966, 5, 264–276. [Google Scholar]
- Farkouh, A.; Frigo, P.; Czejka, M. Systemic Side Effects of Eye Drops: A Pharmacokinetic Perspective. Clin. Ophthalmol. 2016, 10, 2433. [Google Scholar] [CrossRef]
- Mahaling, B.; Katti, D.S. Understanding the Influence of Surface Properties of Nanoparticles and Penetration Enhancers for Improving Bioavailability in Eye Tissues in Vivo. Int. J. Pharm. 2016, 501, 1–9. [Google Scholar] [CrossRef]
- Ludwig, A. The Use of Mucoadhesive Polymers in Ocular Drug Delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- Yellepeddi, V.K.; Sheshala, R.; McMillan, H.; Gujral, C.; Jones, D.; Raghu Raj Singh, T. Punctal Plug: A Medical Device to Treat Dry Eye Syndrome and for Sustained Drug Delivery to the Eye. Drug Discov. Today 2015, 20, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Anatomy, Head and Neck, Eye Conjunctiva—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30137787/ (accessed on 26 August 2024).
- Ashique, S.; Mishra, N.; Mohanto, S.; Gowda, B.H.J.; Kumar, S.; Raikar, A.S.; Masand, P.; Garg, A.; Goswami, P.; Kahwa, I. Overview of Processed Excipients in Ocular Drug Delivery: Opportunities so Far and Bottlenecks. Heliyon 2023, 10, e23810. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A Review. JAMA 2014, 311, 1901. [Google Scholar] [CrossRef]
- Abe, R.Y.; Gracitelli, C.P.B.; Diniz-Filho, A.; Tatham, A.J.; Medeiros, F.A. Lamina Cribrosa in Glaucoma: Diagnosis and Monitoring. Curr. Ophthalmol. Rep. 2015, 3, 74. [Google Scholar] [CrossRef]
- Lu, Y.; Hua, Y.; Wang, B.; Zhong, F.; Theophanous, A.; Tahir, S.; Lee, P.Y.; Sigal, I.A. The Robust Lamina Cribrosa Vasculature: Perfusion and Oxygenation Under Elevated Intraocular Pressure. Investig. Ophthalmol. Vis. Sci. 2024, 65, 1. [Google Scholar] [CrossRef]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous Humor Dynamics: A Review. Open Ophthalmol. J. 2010, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Acute Angle-Closure Glaucoma—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/28613607/ (accessed on 26 August 2024).
- Kolko, M. Suppl 1: M5: Present and New Treatment Strategies in the Management of Glaucoma. Open Ophthalmol. J. 2015, 9, 89. [Google Scholar] [CrossRef]
- Tejwani, S.; Machiraju, P.; Nair, A.P.; Ghosh, A.; Das, R.K.; Ghosh, A.; Sethu, S. Treatment of Glaucoma by Prostaglandin Agonists and Beta-blockers in Combination Directly Reduces Pro-fibrotic Gene Expression in Trabecular Meshwork. J. Cell. Mol. Med. 2020, 24, 5195. [Google Scholar] [CrossRef] [PubMed]
- Alon, S. Selective Laser Trabeculoplasty: A Clinical Review. J. Curr. Glaucoma Pract. 2013, 7, 58. [Google Scholar] [CrossRef]
- Balas, M.; Mathew, D.J. Minimally Invasive Glaucoma Surgery: A Review of the Literature. Vision 2023, 7, 54. [Google Scholar] [CrossRef]
- Pereira, I.C.F.; van de Wijdeven, R.; Wyss, H.M.; Beckers, H.J.M.; den Toonder, J.M.J. Conventional Glaucoma Implants and the New MIGS Devices: A Comprehensive Review of Current Options and Future Directions. Eye 2021, 35, 3202–3221. [Google Scholar] [CrossRef]
- Dana, R.; Meunier, J.; Markowitz, J.T.; Joseph, C.; Siffel, C. Patient-Reported Burden of Dry Eye Disease in the United States: Results of an Online Cross-Sectional Survey. Am. J. Ophthalmol. 2020, 216, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Golden, M.I.; Meyer, J.J.; Zeppieri, M.; Patel, B.C. Dry Eye Syndrome; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Potvin, R.; Makari, S.; Rapuano, C.J. Tear Film Osmolarity and Dry Eye Disease: A Review of the Literature. Clin. Ophthalmol. 2015, 9, 2039. [Google Scholar] [CrossRef]
- Baudouin, C.; Aragona, P.; Messmer, E.M.; Tomlinson, A.; Calonge, M.; Boboridis, K.G.; Akova, Y.A.; Geerling, G.; Labetoulle, M.; Rolando, M. Role of Hyperosmolarity in the Pathogenesis and Management of Dry Eye Disease: Proceedings of the OCEAN Group Meeting. Ocul. Surf. 2013, 11, 246–258. [Google Scholar] [CrossRef]
- Wei, Y.; Asbell, P.A. The Core Mechanism of Dry Eye Disease (DED) Is Inflammation. Eye Contact Lens 2014, 40, 248. [Google Scholar] [CrossRef]
- Sheppard, J.; Shen Lee, B.; Periman, L.M. Dry Eye Disease: Identification and Therapeutic Strategies for Primary Care Clinicians and Clinical Specialists. Ann. Med. 2023, 55, 241. [Google Scholar] [CrossRef] [PubMed]
- Ames, P.; Galor, A. Cyclosporine Ophthalmic Emulsions for the Treatment of Dry Eye: A Review of the Clinical Evidence. Clin. Investig. 2015, 5, 267. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Liu, Z.; Zhang, Z.; Ao, M.; Li, X.; Wang, W. Punctal Plugs versus Artificial Tears for Treating Dry Eye: A Comparative Observation of Their Effects on Contrast Sensitivity. J. Ocul. Biol. Dis. Inform. 2012, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Zhang, C.; Zhang, J.; Gu, L.; Luo, D.; Qiu, Q. Diabetic Macular Edema: Current Understanding, Molecular Mechanisms and Therapeutic Implications. Cells 2022, 11, 3362. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Chan, P.S. Oxidative Stress and Diabetic Retinopathy. Exp. Diabetes Res. 2007, 2007, 12. [Google Scholar] [CrossRef]
- Saxena, S.; Mishra, A.; Saxena, A.; Natu, S.M. Advanced Glycation End Products and Diabetic Retinopathy. J. Ocul. Biol. Dis. Inform. 2012, 5, 63. [Google Scholar] [CrossRef]
- Hammes, H.P. Diabetic Retinopathy: Hyperglycaemia, Oxidative Stress and Beyond. Diabetologia 2018, 61, 29–38. [Google Scholar] [CrossRef]
- Lechner, J.; O’Leary, O.E.; Stitt, A.W. The Pathology Associated with Diabetic Retinopathy. Vis. Res. 2017, 139, 7–14. [Google Scholar] [CrossRef]
- Roy, S.; Ha, J.; Trudeau, K.; Beglova, E. Vascular Basement Membrane Thickening in Diabetic Retinopathy. Curr. Eye Res. 2010, 35, 1045–1056. [Google Scholar] [CrossRef]
- Penn, J.S.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.W.; Hartnett, M.E. Vascular Endothelial Growth Factor in Eye Disease. Prog. Retin. Eye Res. 2008, 27, 331. [Google Scholar] [CrossRef]
- Stewart, M.W.; Browning, D.J.; Landers, M.B. Current Management of Diabetic Tractional Retinal Detachments. Indian J. Ophthalmol. 2018, 66, 1751–1762. [Google Scholar] [CrossRef]
- Stewart, M.W. A Review of Ranibizumab for the Treatment of Diabetic Retinopathy. Ophthalmol. Ther. 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Wykoff, C.C.; Boyer, D.; Heier, J.S.; Clark, W.L.; Emanuelli, A.; Higgins, P.M.; Singer, M.; Weinreich, D.M.; Yancopoulos, G.D.; et al. Evaluation of Intravitreal Aflibercept for the Treatment of Severe Nonproliferative Diabetic Retinopathy: Results From the PANORAMA Randomized Clinical Trial. JAMA Ophthalmol. 2021, 139, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, J.F.; Fromow-Guerra, J.; Quiroz-Mercado, H.; Sanchez, J.G.; Wu, L.; Maia, M.; Berrocal, M.H.; Solis-Vivanco, A.; Farah, M.E. Primary Intravitreal Bevacizumab (Avastin) for Diabetic Macular Edema. Results from the Pan-American Collaborative Retina Study Group at 6-Month Follow-Up. Ophthalmology 2007, 114, 743–750. [Google Scholar] [CrossRef]
- Romano, F.; Lamanna, F.; Gabrielle, P.H.; Teo, K.Y.C.; Battaglia Parodi, M.; Iacono, P.; Fraser-Bell, S.; Cornish, E.E.; Nassisi, M.; Viola, F.; et al. Update on Retinal Vein Occlusion. Asia-Pac. J. Ophthalmol. 2023, 12, 196–210. [Google Scholar] [CrossRef]
- Haller, J.A.; Tomaiuolo, M.; Lucas, M.M.; Yang, C.C.; Hyman, L.; Lee, A.Y.; Lee, C.S.; Van Gelder, R.; Lorch, A.; Miller, J.W.; et al. Disparities in Retinal Vein Occlusion Presentation and Initiation of Anti-VEGF Therapy: An Academy IRIS® Registry Analysis. Ophthalmol. Retin. 2024, 8, 657–665. [Google Scholar] [CrossRef]
- Browning, D.J. Retinal Vein Occlusions: Evidence-Based Management; Springer Science & Business Media: Berlin, Germany, 2012; pp. 1–387. [Google Scholar] [CrossRef]
- Labay-Tejado, S.; Menendez-Acebal, C.; Bernal-Morales, C.; Alforja, S.; Zarranz-Ventura, J. Central Retinal Vein Occlusion. In Retinal and Choroidal Vascular Diseases of the Eye; Academic Press: Cambridge, MA, USA, 2023; pp. 165–177. [Google Scholar] [CrossRef]
- Hang, A.; Feldman, S.; Amin, A.P.; Ochoa, J.A.R.; Park, S.S. Intravitreal Anti-Vascular Endothelial Growth Factor Therapies for Retinal Disorders. Pharmaceuticals 2023, 16, 1140. [Google Scholar] [CrossRef] [PubMed]
- Uveitis—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/31082037/ (accessed on 26 August 2024).
- González, M.M.; Solano, M.M.; Porco, T.C.; Oldenburg, C.E.; Acharya, N.R.; Lin, S.C.; Chan, M.F. Epidemiology of Uveitis in a US Population-Based Study. J. Ophthalmic Inflamm. Infect. 2018, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; Kuffova, L.; Dick, A.D. Autoimmunity, Autoinflammation, and Infection in Uveitis. Am. J. Ophthalmol. 2018, 189, 77–85. [Google Scholar] [CrossRef]
- Eskandarpour, M.; Nunn, M.A.; Weston-Davies, W.; Calder, V.L. Immune-Mediated Retinal Vasculitis in Posterior Uveitis and Experimental Models: The Leukotriene (LT)B4-VEGF Axis. Cells 2021, 10, 396. [Google Scholar] [CrossRef]
- Tomkins-Netzer, O.; Niederer, R.; Greenwood, J.; Fabian, I.D.; Serlin, Y.; Friedman, A.; Lightman, S. Mechanisms of Blood-Retinal Barrier Disruption Related to Intraocular Inflammation and Malignancy. Prog. Retin. Eye Res. 2024, 99, 101245. [Google Scholar] [CrossRef] [PubMed]
- Relationship between Aqueous Humor Protein Level and Outflow Facility in Patients with Uveitis|IOVS|ARVO Journals. Available online: https://iovs.arvojournals.org/article.aspx?articleid=2200090 (accessed on 26 August 2024).
- McHarg, M.; Young, L.A.; Kesav, N.; Yakin, M.; Sen, H.N.; Kodati, S. Practice Patterns Regarding Regional Corticosteroid Treatment in Noninfectious Uveitis: A Survey Study. J. Ophthalmic Inflamm. Infect. 2022, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tao, M.; Zhu, L.; Zhang, T.; Zhang, M. Pathogenesis and Current Therapies for Non-Infectious Uveitis. Clin. Exp. Med. 2023, 23, 1089. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.B.; Wittenborn, J.S.; Burke-Conte, Z.; Gulia, R.; Robalik, T.; Ehrlich, J.R.; Lundeen, E.A.; Flaxman, A.D. Prevalence of Age-Related Macular Degeneration in the US in 2019. JAMA Ophthalmol. 2022, 140, 1202–1208. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-Related Macular Degeneration. Nat. Rev. Dis. Prim. 2021, 7, 31. [Google Scholar] [CrossRef]
- Somasundaran, S.; Constable, I.J.; Mellough, C.B.; Carvalho, L.S. Retinal Pigment Epithelium and Age-related Macular Degeneration: A Review of Major Disease Mechanisms. Clin. Experiment. Ophthalmol. 2020, 48, 1043. [Google Scholar] [CrossRef]
- Heesterbeek, T.J.; Lorés-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.E.; den Hollander, A.I. Risk Factors for Progression of Age-related Macular Degeneration. Ophthalmic Physiol. Opt. 2020, 40, 140. [Google Scholar] [CrossRef]
- Wong, J.H.C.; Ma, J.Y.W.; Jobling, A.I.; Brandli, A.; Greferath, U.; Fletcher, E.L.; Vessey, K.A. Exploring the Pathogenesis of Age-Related Macular Degeneration: A Review of the Interplay between Retinal Pigment Epithelium Dysfunction and the Innate Immune System. Front. Neurosci. 2022, 16, 1009599. [Google Scholar] [CrossRef]
- Wet Age-Related Macular Degeneration (AMD)—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/34283513/ (accessed on 26 August 2024).
- Galindo-Camacho, R.M.; Blanco-Llamero, C.; da Ana, R.; Fuertes, M.A.; Señoráns, F.J.; Silva, A.M.; García, M.L.; Souto, E.B. Therapeutic Approaches for Age-Related Macular Degeneration. Int. J. Mol. Sci. 2022, 23, 11769. [Google Scholar] [CrossRef]
- Sato-Akushichi, M.; Ono, S.; Taneda, T.; Klose, G.; Sasamori, A.; Song, Y. One-Year Outcome of Combination Therapy with Full or Reduced Photodynamic Therapy and One Anti-Vascular Endothelial Growth Factor in Pachychoroid Neovasculopathy. Pharmaceuticals 2022, 15, 483. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in Drug Delivery Systems, Challenges and Future Directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- Al Fatease, M.; Alany, A.; Abdelkader, R.G.; Recent, H.; Otero-Espinar, J.; Arango-Gonzalez, B.; Mostafa, M.; Al Fatease, A.; Alany, R.G.; Abdelkader, H. Recent Advances of Ocular Drug Delivery Systems: Prominence of Ocular Implants for Chronic Eye Diseases. Pharmaceutics 2023, 15, 1746. [Google Scholar] [CrossRef] [PubMed]
- Short, B.G. Safety Evaluation of Ocular Drug Delivery Formulations: Techniques and Practical Considerations. Toxicol. Pathol. 2008, 36, 49–62. [Google Scholar] [CrossRef] [PubMed]
- US FDA Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Available online: https://www.fda.gov/drugs/development-approval-process-drugs/orange-book-preface (accessed on 6 October 2024).
- Mohan, S.; Ratra, D. Intravitreal Implants; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Smith, T.J.; Pearson, P.A.; Blandford, D.L.; Brown, J.D.; Goins, K.A.; Hollins, J.L.; Schmeisser, E.T.; Glavinos, P.; Baldwin, L.B.; Ashton, P. Intravitreal Sustained-Release Ganciclovir. Arch. Ophthalmol. 1992, 110, 255–258. [Google Scholar] [CrossRef]
- Yasukawa, T.; Ogura, Y.; Kimura, H.; Sakurai, E.; Tabata, Y. Drug Delivery from Ocular Implants. Expert Opin. Drug Deliv. 2006, 3, 261–273. [Google Scholar] [CrossRef]
- Tao, W. Application of Encapsulated Cell Technology for Retinal Degenerative Diseases. Expert Opin. Biol. Ther. 2006, 6, 717–726. [Google Scholar] [CrossRef]
- Zlomke, C.; Barth, M.; Mäder, K. Polymer Degradation Induced Drug Precipitation in PLGA Implants—Why Less Is Sometimes More. Eur. J. Pharm. Biopharm. 2019, 139, 142–152. [Google Scholar] [CrossRef]
- Lee, S.S.; Hughes, P.; Ross, A.D.; Robinson, M.R. Biodegradable Implants for Sustained Drug Release in the Eye. Pharm. Res. 2010, 27, 2043–2053. [Google Scholar] [CrossRef]
- Abd, A.J.; Kanwar, R.K.; Kanwar, J.R. Aged Macular Degeneration: Current Therapeutics for Management and Promising New Drug Candidates. Drug Discov. Today 2017, 22, 1671–1679. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Mishra, G.P.; Bagui, M.; Tamboli, V.; Mitra, A.K. Recent Applications of Liposomes in Ophthalmic Drug Delivery. J. Drug Deliv. 2011, 2011, 863734. [Google Scholar] [CrossRef] [PubMed]
- Mehrandish, S.; Mirzaeei, S. A Review on Ocular Novel Drug Delivery Systems of Antifungal Drugs: Functional Evaluation and Comparison of Conventional and Novel Dosage Forms. Adv. Pharm. Bull. 2021, 11, 28. [Google Scholar] [CrossRef]
- Honda, M.; Asai, T.; Oku, N.; Araki, Y.; Tanaka, M.; Ebihara, N. Liposomes and Nanotechnology in Drug Development: Focus on Ocular Targets. Int. J. Nanomed. 2013, 8, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Torkashvand, A.; Izadian, A.; Hajrasouliha, A. Advances in Ophthalmic Therapeutic Delivery: A Comprehensive Overview of Present and Future Directions. Surv. Ophthalmol. 2024, 69, 967–983. [Google Scholar] [CrossRef]
- Sanap, S.N.; Bisen, A.C.; Mishra, A.; Biswas, A.; Agrawal, S.; Yadav, K.S.; Krishna, A.; Chopra, S.; Mugale, M.N.; Bhatta, R.S. QbD Based Antifungal Drug-Loaded Ophthalmic Liposomal Formulation for the Management of Fungal Keratitis: In Vitro, Ex Vivo and in Vivo Pharmacokinetic Studies. J. Drug Deliv. Sci. Technol. 2022, 74, 103517. [Google Scholar] [CrossRef]
- Gómez-Ballesteros, M.; López-Cano, J.J.; Bravo-Osuna, I.; Herrero-Vanrell, R.; Molina-Martínez, I.T. Osmoprotectants in Hybrid Liposome/HPMC Systems as Potential Glaucoma Treatment. Polymers 2019, 11, 929. [Google Scholar] [CrossRef] [PubMed]
- Onugwu, A.L.; Nwagwu, C.S.; Onugwu, O.S.; Echezona, A.C.; Agbo, C.P.; Ihim, S.A.; Emeh, P.; Nnamani, P.O.; Attama, A.A.; Khutoryanskiy, V.V. Nanotechnology Based Drug Delivery Systems for the Treatment of Anterior Segment Eye Diseases. J. Control. Release 2023, 354, 465–488. [Google Scholar] [CrossRef]
- Tavakoli, S.; Peynshaert, K.; Lajunen, T.; Devoldere, J.; del Amo, E.M.; Ruponen, M.; De Smedt, S.C.; Remaut, K.; Urtti, A. Ocular Barriers to Retinal Delivery of Intravitreal Liposomes: Impact of Vitreoretinal Interface. J. Control. Release 2020, 328, 952–961. [Google Scholar] [CrossRef]
- Keam, S.J.; Scott, L.J.; Curran, M.P. Verteporfin: A Review of Its Use in the Management of Subfoveal Choroidal Neovascularisation. Drugs 2003, 63, 2521–2554. [Google Scholar] [CrossRef]
- Lee, S.; Dausch, S.; Maierhofer, G.; Dausch, D. A New Therapy Concept for the Treatment of Dry Eye--The Usefulness of Phospholipid Liposomes. Klin. Monatsblatter Augenheilkd. 2004, 221, 825–836. [Google Scholar] [CrossRef]
- Dausch Dieter, A.; Suwan, L.; Sabine, D.; Jae Chan, K.; Gregor, S.; Wanda, M.; Augenheilkunde, A.; St Marien, K. Comparative Study of Treatment of the Dry Eye Syndrome Due to Disturbances of the Tear Film Lipid Layer with Lipid-Containing Tear Substitutes Efficacy of Lipid. Klin. Monatsblatter Augenheilkd. 2006, 223, 974–983. [Google Scholar]
- Vaneev, A.; Tikhomirova, V.; Chesnokova, N.; Popova, E.; Beznos, O.; Kost, O.; Klyachko, N. Nanotechnology for Topical Drug Delivery to the Anterior Segment of the Eye. Int. J. Mol. Sci. 2021, 22, 12368. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.C.; Chen, Y.H.; Lu, D.W. Overview of Recent Advances in Nano-Based Ocular Drug Delivery. Int. J. Mol. Sci. 2023, 24, 15352. [Google Scholar] [CrossRef]
- Akhter, S.; Anwar, M.; Siddiqui, M.A.; Ahmad, I.; Ahmad, J.; Ahmad, M.Z.; Bhatnagar, A.; Ahmad, F.J. Improving the Topical Ocular Pharmacokinetics of an Immunosuppressant Agent with Mucoadhesive Nanoemulsions: Formulation Development, in-Vitro and in-Vivo Studies. Colloids Surf. B Biointerfaces 2016, 148, 19–29. [Google Scholar] [CrossRef]
- Pinto Reis, C.; Neufeld, R.J.; Ribeiro, A.J.; Veiga, F.; Nanoencapsulation, I. Methods for Preparation of Drug-Loaded Polymeric Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 8–21. [Google Scholar] [CrossRef]
- Khalil, I.A.; Ali, I.H.; El-Sherbiny, I.M. Noninvasive Biodegradable Nanoparticles-in-Nanofibers Single-Dose Ocular Insert: In Vitro, Ex Vivo and in Vivo Evaluation. Nanomedicine 2019, 14, 33–55. [Google Scholar] [CrossRef]
- Modi, D.; Mohammad; Warsi, M.H.; Garg, V.; Bhatia, M.; Kesharwani, P.; Jain, G.K. Formulation Development, Optimization, and in Vitro Assessment of Thermoresponsive Ophthalmic Pluronic F127-Chitosan in Situ Tacrolimus Gel. J. Biomater. Sci. Polym. Ed. 2021, 32, 1678–1702. [Google Scholar] [CrossRef]
- Mohammed, N.; Sanoj Rejinold, N.; Mangalathillam, S.; Biswas, R.; Nair, S.V.; Jayakumar, R. Fluconazole Loaded Chitin Nanogels as a Topical Ocular Drug Delivery Agent for Corneal Fungal Infections. J. Biomed. Nanotechnol. 2013, 9, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef]
- Tang, Z.; Yin, L.; Zhang, Y.; Yu, W.; Wang, Q.; Zhan, Z. Preparation and Study of Two Kinds of Ophthalmic Nano-Preparations of Everolimus. Drug Deliv. 2019, 26, 1235–1242. [Google Scholar] [CrossRef]
- Cholkar, K.; Patel, A.; Dutt Vadlapudi, A.K.; Mitra, A. Novel Nanomicellar Formulation Approaches for Anterior and Posterior Segment Ocular Drug Delivery. Recent Pat. Nanomedicinee 2012, 2, 82–95. [Google Scholar] [CrossRef]
- Bose, A.; Burman, D.R.; Sikdar, B.; Patra, P. Nanomicelles: Types, Properties and Applications in Drug Delivery. IET Nanobiotechnol. 2021, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Liang, Z.; Zhang, Z.; Yang, J.; Song, F.; Zhou, T.; Li, J.; Zhang, J. Novel Nanomicelle Butenafine Formulation for Ocular Drug Delivery against Fungal Keratitis: In Vitro and In Vivo Study. Eur. J. Pharm. Sci. 2024, 192, 106629. [Google Scholar] [CrossRef]
- Vilar, G.; Tulla-Puche, J.; Albericio, F. Polymers and Drug Delivery Systems. Curr. Drug Deliv. 2012, 9, 367–394. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Guest, J.M.; Malbin, B.; Abrams, G.; Parendo, A.; Das, S.; Okeagu, C.; Ross, B.X.; Kumar, A.; Lin, X. Accuracy of Intravitreal Injection Volume for Aflibercept Pre-Filled Syringe and BD Luer-Lok One-Milliliter Syringe. Int. J. Retin. Vitr. 2022, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Naguib, S.; DeJulius, C.R.; Backstrom, J.R.; Haider, A.A.; Ang, J.M.; Boal, A.M.; Calkins, D.J.; Duvall, C.L.; Rex, T.S. Intraocular Sustained Release of EPO-R76E Mitigates Glaucoma Pathogenesis by Activating the NRF2/ARE Pathway. Antioxidants 2023, 12, 556. [Google Scholar] [CrossRef]
- Arranz-Romera, A.; Davis, B.M.; Bravo-Osuna, I.; Esteban-Pérez, S.; Molina-Martínez, I.T.; Shamsher, E.; Ravindran, N.; Guo, L.; Cordeiro, M.F.; Herrero-Vanrell, R. Simultaneous Co-Delivery of Neuroprotective Drugs from Multi-Loaded PLGA Microspheres for the Treatment of Glaucoma. J. Control. Release 2019, 297, 26–38. [Google Scholar] [CrossRef]
- Puricelli, C.; Gigliotti, C.L.; Stoppa, I.; Sacchetti, S.; Pantham, D.; Scomparin, A.; Rolla, R.; Pizzimenti, S.; Dianzani, U.; Boggio, E.; et al. Use of Poly Lactic-Co-Glycolic Acid Nano and Micro Particles in the Delivery of Drugs Modulating Different Phases of Inflammation. Pharmaceutics 2023, 15, 1772. [Google Scholar] [CrossRef]
- Bejjani, R.A.; Andrieu, C.; Bloquel, C.; Berdugo, M.; BenEzra, D.; Behar-Cohen, F. Electrically Assisted Ocular Gene Therapy. Surv. Ophthalmol. 2007, 52, 196–208. [Google Scholar] [CrossRef]
- Myles, M.E.; Neumann, D.M.; Hill, J.M. Recent Progress in Ocular Drug Delivery for Posterior Segment Disease: Emphasis on Transscleral Iontophoresis. Adv. Drug Deliv. Rev. 2005, 57, 2063–2079. [Google Scholar] [CrossRef]
- Gratieri, T.; Santer, V.; Kalia, Y.N. Basic Principles and Current Status of Transcorneal and Transscleral Iontophoresis. Expert Opin. Drug Deliv. 2017, 14, 1091–1102. [Google Scholar] [CrossRef]
- Madni, A.; Rahem, M.A.; Tahir, N.; Sarfraz, M.; Jabar, A.; Rehman, M.; Kashif, P.M.; Badshah, S.F.; Khan, K.U.; Santos, H.A. Non-Invasive Strategies for Targeting the Posterior Segment of Eye. Int. J. Pharm. 2017, 530, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Pescina, S.; Santi, P.; Ferrari, G.; Nicoli, S. Trans-Scleral Delivery of Macromolecules. Ther. Deliv. 2011, 2, 1331–1349. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.B.; Shastri, D.H.; Shelat, P.K.; Shukla, A.K. Ophthalmic Drug Delivery System: Challenges and Approaches. Syst. Rev. Pharm. 2010, 1, 113–120. [Google Scholar] [CrossRef]
- Kim, S.N.; Min, C.H.; Kim, Y.K.; Ha, A.; Park, C.G.; Lee, S.H.; Park, K.H.; Choy, Y.B. Iontophoretic Ocular Delivery of Latanoprost-Loaded Nanoparticles via Skin-Attached Electrodes. Acta Biomater. 2022, 144, 32–41. [Google Scholar] [CrossRef]
- Davis, J.L.; Gilger, B.C.; Robinson, M.R. Novel Approaches to Ocular Drug Delivery. Curr. Opin. Mol. Ther. 2004, 6, 195–205. [Google Scholar]
- Eljarrat-Binstock, E.; Pe’er, J.; Domb, A.J. New Techniques for Drug Delivery to the Posterior Eye Segment. Pharm. Res. 2010, 27, 530–543. [Google Scholar] [CrossRef]
- Garg, A.; Agrawal, R.; Singh Chauhan, C.; Deshmukh, R. In-Situ Gel: A Smart Carrier for Drug Delivery. Int. J. Pharm. 2024, 652, 123819. [Google Scholar] [CrossRef]
- Campos, P.M.; Petrilli, R.; Lopez, R.F. The Prominence of the Dosage Form Design to Treat Ocular Diseases. Int. J. Pharm. 2020, 586, 119577. [Google Scholar] [CrossRef]
- Paul, S.; Majumdar, S.; Chakraborty, M. Revolutionizing Ocular Drug Delivery: Recent Advancements in in Situ Gel Technology. Bull. Natl. Res. Cent. 2023, 47, 1–16. [Google Scholar] [CrossRef]
- Al-Tahami, K.; Singh, J. Smart polymer based delivery systems for peptides and proteins. Recent Pat. Drug Deliv. Formul. 2007, 1, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Bonacucina, G.; Cespi, M.; Mencarelli, G.; Giorgioni, G.; Palmieri, G.F. Thermosensitive self-assembling block copolymers as drug delivery systems. Polymers 2011, 3, 779–811. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Nie, S.; Liu, H.; Ding, P.; Pan, W. Study of an Alginate/HPMC-Based in Situ Gelling Ophthalmic Delivery System for Gatifloxacin. Int. J. Pharm. 2006, 315, 12–17. [Google Scholar] [CrossRef]
- Cardoso, C.O.; Ferreira-Nunes, R.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. In Situ Gelling Microemulsion for Topical Ocular Delivery of Moxifloxacin and Betamethasone. J. Mol. Liq. 2022, 360, 119559. [Google Scholar] [CrossRef]
- Shivam, U.U.; Siddhi, K.C.; Devarshi, U.G.; Umeshkumar, M.U.; Jayvadan, K.P. Nanoparticles Laden In Situ Gel for Sustained Drug Release after Topical Ocular Administration. J. Drug Deliv. Sci. Technol. 2020, 57, 101736. [Google Scholar] [CrossRef]
- Jimenez, J.; Washington, M.A.; Resnick, J.L.; Nischal, K.K.; Fedorchak, M.V. A Sustained Release Cysteamine Microsphere/Thermoresponsive Gel Eyedrop for Corneal Cystinosis Improves Drug Stability. Drug Deliv. Transl. Res. 2021, 11, 2224–2238. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Li, X.; Kebebe, D.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research Progress of In-Situ Gelling Ophthalmic Drug Delivery System. Asian J. Pharm. Sci. 2019, 14, 1–15. [Google Scholar] [CrossRef]
- Rykowska, I.; Nowak, I.; Nowak, R. Soft Contact Lenses as Drug Delivery Systems: A Review. Molecules 2021, 26, 5577. [Google Scholar] [CrossRef]
- Toffoletto, N.; Saramago, B.; Serro, A.P. Therapeutic ophthalmic lenses: A review. Pharmaceutics 2020, 13, 36. [Google Scholar] [CrossRef]
- Zhao, L.; Song, J.; Du, Y.; Ren, C.; Guo, B.; Bi, H. Therapeutic Applications of Contact Lens-Based Drug Delivery Systems in Ophthalmic Diseases. Drug Deliv. 2023, 30, 2219419. [Google Scholar] [CrossRef] [PubMed]
- Hui, A. Contact Lenses for Ophthalmic Drug Delivery. Clin. Exp. Optom. 2017, 100, 494–512. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.M.; Subbaraman, L.; Jones, L. Contact Lenses for Antifungal Ocular Drug Delivery: A Review. Expert Opin. Drug Deliv. 2014, 11, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Desai, D.T.; Shetty, K.H.; Shah, D.O.; Willcox, M.D.P. Advances and Challenges in the Nanoparticles-Laden Contact Lenses for Ocular Drug Delivery. Int. J. Pharm. 2021, 608, 121090. [Google Scholar] [CrossRef] [PubMed]
- Toffoletto, N.; Salema-Oom, M.; Nicoli, S.; Pescina, S.; González-Fernández, F.M.; Pinto, C.A.; Saraiva, J.A.; Alves de Matos, A.P.; Vivero-Lopez, M.; Huete-Toral, F.; et al. Dexamethasone Phosphate and Penetratin Co-Eluting Contact Lenses: A Strategy to Enhance Ocular Drug Permeability. Int. J. Pharm. 2024, 650, 123685. [Google Scholar] [CrossRef]
- Bengani, L.C.; Hsu, K.H.; Gause, S.; Chauhan, A. Contact Lenses as a Platform for Ocular Drug Delivery. Expert Opin. Drug Deliv. 2013, 10, 1483–1496. [Google Scholar] [CrossRef]
- Berdy, G.J.; Abelson, M.B.; Smith, L.M.; George, M.A. Preservative-Free Artificial Tear Preparations: Assessment of Corneal Epithelial Toxic Effects. Arch. Ophthalmol. 1992, 110, 528–532. [Google Scholar] [CrossRef]
- Wu, C.; Or, P.W.; Chong, J.I.T.; Pathirage Don, I.K.K.; Lee, C.H.C.; Wu, K.; Yu, M.; Lam, D.C.C.; Yang, Y. Extended Delivery of Pirfenidone with Novel, Soft Contact Lenses In Vitro and In Vivo. J. Ocul. Pharmacol. Ther. 2021, 37, 75–83. [Google Scholar] [CrossRef]
- Wu, C.; Or, P.W.; Chong, J.I.T.; Isuru, I.K.; Lee, C.H.C.; Wu, K.; Yu, M.; Lam, D.C.C.; Yang, Y. Controllable Release of Pirfenidone by Polyvinyl Alcohol Film Embedded Soft Contact Lenses in Vitro and in Vivo. Drug Deliv. 2021, 28, 634–641. [Google Scholar] [CrossRef]
- Ding, X.; Ben-Shlomo, G.; Que, L. Soft Contact Lens with Embedded Microtubes for Sustained and Self-Adaptive Drug Delivery for Glaucoma Treatment. ACS Appl. Mater. Interfaces 2020, 12, 45789–45795. [Google Scholar] [CrossRef]
- Xu, J.; Ge, Y.; Bu, R.; Zhang, A.; Feng, S.; Wang, J.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; et al. Co-Delivery of Latanoprost and Timolol from Micelles-Laden Contact Lenses for the Treatment of Glaucoma. J. Control. Release 2019, 305, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Huo, Q.; Lin, X.; Chu, X.; Deng, Z.; Guo, H.; Peng, Y.; Lu, S.; Zhou, X.; Wang, X. Drug-Free Contact Lens Based on Quaternized Chitosan and Tannic Acid for Bacterial Keratitis Therapy and Corneal Repair. Carbohydr. Polym. 2022, 286, 119314. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Goudie, M.J.; Tebon, P.; Sun, W.; Luo, Z.; Lee, J.; Zhang, S.; Fetah, K.; Kim, H.J.; Xue, Y.; et al. Non-Transdermal Microneedles for Advanced Drug Delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 41–59. [Google Scholar] [CrossRef]
- Jakka, D.; Matadh, A.V.; Shankar, V.K.; Shivakumar, H.N.; Narasimha Murthy, S. Polymer Coated Polymeric (PCP) Microneedles for Controlled Delivery of Drugs (Dermal and Intravitreal). J. Pharm. Sci. 2022, 111, 2867–2878. [Google Scholar] [CrossRef]
- Özkiriş, A.; Erkiliç, K. Complications of Intravitreal Injection of Triamcinolone Acetonide. Can. J. Ophthalmol. 2005, 40, 63–68. [Google Scholar] [CrossRef]
- Matadh, A.V.; Jakka, D.; Pragathi, S.G.; Poornima, K.; Shivakumar, H.N.; Murthy, R.N.; Rangappa, S.; Shivanna, M.; Murthy, S.N. Polymer Coated Polymeric Microneedles for Intravitreal Delivery of Dexamethasone. Exp. Eye Res. 2023, 231, 109467. [Google Scholar] [CrossRef]
- Matadh, A.V.; Jakka, D.; Pragathi, S.G.; Rangappa, S.; Shivakumar, H.N.; Maibach, H.; Reena, N.M.; Murthy, S.N. Polymer-Coated Polymeric (PCP) Microneedles for Controlled Dermal Delivery of 5-Fluorouracil. AAPS PharmSciTech 2023, 24, 1–6. [Google Scholar] [CrossRef]
- Matadh, A.V.; Jakka, D.; Kumar, A.; Shivakumar, H.N.; Murthy, R.N.; Murthy, S.N. Polymer-Coated Polymeric (PCP) Microneedles for Controlled Regional Drug Delivery. In Design and Applications of Microneedles in Drug Delivery and Therapeutics; Academic Press: Cambridge, MA, USA, 2024; pp. 175–187. [Google Scholar] [CrossRef]
- Chao, Z.; Dong, C.; Fang, H. Current Perspective on Microneedles for Ocular Drug Delivery. Saudi J. Med. Pharm. Sci. 2017, 3, 772–776. [Google Scholar] [CrossRef]
- Jiang, J.; Gill, H.S.; Ghate, D.; McCarey, B.E.; Patel, S.R.; Edelhauser, H.F.; Prausnitz, M.R. Coated Microneedles for Drug Delivery to the Eye. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4038–4043. [Google Scholar] [CrossRef]
- Gupta, P.; Yadav, K.S. Applications of Microneedles in Delivering Drugs for Various Ocular Diseases. Life Sci. 2019, 237, 116907. [Google Scholar] [CrossRef]
- Gadziński, P.; Froelich, A.; Wojtyłko, M.; Białek, A.; Krysztofiak, J.; Osmałek, T. Microneedle-Based Ocular Drug Delivery Systems–Recent Advances and Challenges. Beilstein J. Nanotechnol. 2022, 13, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, J.; Wang, Y.; Zhu, Y.; Lin, D.; Lei, L.; Vakal, S.; Wang, J.; Li, X. A Rapid Corneal Healing Microneedle for Efficient Ocular Drug Delivery. Small 2022, 18, 2104657. [Google Scholar] [CrossRef]
- Kim, J.H.; Song, H.B.; Lee, K.J.; Seo, I.H.; Lee, J.Y.; Lee, S.M.; Kim, J.H.; Ryu, W. Impact Insertion of Transfer-Molded Microneedle for Localized and Minimally Invasive Ocular Drug Delivery. J. Control. Release 2015, 209, 272–279. [Google Scholar] [CrossRef]
- Wu, Y.; Vora, L.K.; Donnelly, R.F.; Singh, T.R.R. Rapidly Dissolving Bilayer Microneedles Enabling Minimally Invasive and Efficient Protein Delivery to the Posterior Segment of the Eye. Drug Deliv. Transl. Res. 2023, 13, 2142–2158. [Google Scholar] [CrossRef]
- Dugam, S.; Tade, R.; Dhole, R.; Nangare, S. Emerging Era of Microneedle Array for Pharmaceutical and Biomedical Applications: Recent Advances and Toxicological Perspectives. Future J. Pharm. Sci. 2021, 7, 1–26. [Google Scholar] [CrossRef]
- Rojekar, S.; Parit, S.; Gholap, A.D.; Manchare, A.; Nangare, S.N.; Hatvate, N.T.; Sugandhi, V.V.; Paudel, K.R.; Ingle, R.G. Revolutionizing Eye Care: Exploring the Potential of Microneedle Drug Delivery. Preprints 2024. [Google Scholar] [CrossRef]
- Fang, G.; Yang, X.; Wang, Q.; Zhang, A.; Tang, B. Hydrogels-Based Ophthalmic Drug Delivery Systems for Treatment of Ocular Diseases. Mater. Sci. Eng. C 2021, 127, 112212. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Espina, M.; Doktorovova, S.; Souto, E.B.; García, M.L. Lipid Nanoparticles (SLN, NLC): Overcoming the Anatomical and Physiological Barriers of the Eye—Part I—Barriers and Determining Factors in Ocular Delivery. Eur. J. Pharm. Biopharm. 2017, 110, 70–75. [Google Scholar] [CrossRef]
- Alami-Milani, M.; Zakeri-Milani, P.; Valizadeh, H.; Salehi, R.; Salatin, S.; Naderinia, A.; Jelvehgari, M. Novel Pentablock Copolymers as Thermosensitive Self-Assembling Micelles for Ocular Drug Delivery. Adv. Pharm. Bull. 2017, 7, 11–20. [Google Scholar] [CrossRef]
- Díaz-Tomé, V.; Luaces-Rodríguez, A.; Silva-Rodríguez, J.; Blanco-Dorado, S.; García-Quintanilla, L.; Llovo-Taboada, J.; Blanco-Méndez, J.; García-Otero, X.; Varela-Fernández, R.; Herranz, M.; et al. Ophthalmic Econazole Hydrogels for the Treatment of Fungal Keratitis. J. Pharm. Sci. 2018, 107, 1342–1351. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Y.; Bao, Z.; Lin, D.; Liu, H.; Yu, A.; Lei, L.; Li, X.; Xu, X. Thermosensitive Glycol Chitosan-Based Hydrogel as a Topical Ocular Drug Delivery System for Enhanced Ocular Bioavailability. Int. J. Pharm. 2019, 570, 118688. [Google Scholar] [CrossRef] [PubMed]
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P.W.H.I. Bispecific Antibodies: A Mechanistic Review of the Pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608. [Google Scholar] [CrossRef]
- Husain, B.; Ellerman, D. Expanding the Boundaries of Biotherapeutics with Bispecific Antibodies. Biodrugs 2018, 32, 441. [Google Scholar] [CrossRef] [PubMed]
- Kontermann, R.E. Recombinant Bispecific Antibodies for Cancer Therapy. Acta Pharmacol Sin. 2005, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kontermann, R.E.; Brinkmann, U. Bispecific Antibodies. Drug. Discov. Today 2015, 20, 838–847. [Google Scholar] [CrossRef]
- Garber, K. Bispecific Antibodies Rise Again. Nat. Rev. Drug Discov. 2014, 13, 799–801. [Google Scholar] [CrossRef]
- Brinkmann, U.; Kontermann, R.E. The Making of Bispecific Antibodies. MAbs 2017, 9, 182. [Google Scholar] [CrossRef]
- Goebeler, M.E.; Stuhler, G.; Bargou, R. Bispecific and Multispecific Antibodies in Oncology: Opportunities and Challenges. Nat. Rev. Clin. Oncol. 2024, 21, 539–560. [Google Scholar] [CrossRef]
- Klein, C.; Brinkmann, U.; Reichert, J.M.; Kontermann, R.E. The Present and Future of Bispecific Antibodies for Cancer Therapy. Nat. Rev. Drug Discov. 2024, 23, 301–319. [Google Scholar] [CrossRef]
- Lim, K.S.; Zhu, X.; Zhou, D.; Ren, S.; Phipps, A. Clinical Pharmacology Strategies for Bispecific Antibody Development: Learnings from FDA-Approved Bispecific Antibodies in Oncology. Clin. Pharmacol. Ther. 2024, 116, 315–327. [Google Scholar] [CrossRef]
- Przepiorka, D.; Ko, C.W.; Deisseroth, A.; Yancey, C.L.; Candau-Chacon, R.; Chiu, H.J.; Gehrke, B.J.; Gomez-Broughton, C.; Kane, R.C.; Kirshner, S.; et al. FDA Approval: Blinatumomab. Clin. Cancer Res. 2015, 21, 4035–4039. [Google Scholar] [CrossRef] [PubMed]
- Panos, G.D.; Lakshmanan, A.; Dadoukis, P.; Ripa, M.; Motta, L.; Amoaku, W.M. Faricimab: Transforming the Future of Macular Diseases Treatment—A Comprehensive Review of Clinical Studies. Drug Des. Devel. Ther. 2023, 17, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Vabysmo|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/vabysmo (accessed on 4 August 2024).
- Sulak, R.; Liu, X.; Smedowski, A. The Concept of Gene Therapy for Glaucoma. Neural Regen. Res. 2024, 19, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Shreya, A.B.; Raychaudhuri, R.; Pandey, A.; Lewis, S.A.; Hazarika, M.; Bhandary, S.V.; Rao, B.S.S.; Mutalik, S. Small Interfering RNAs (SiRNAs) Based Gene Silencing Strategies for the Treatment of Glaucoma: Recent Advancements and Future Perspectives. Life Sci. 2021, 264, 118712. [Google Scholar] [CrossRef] [PubMed]
- Brar, A.S.; Parameswarappa, D.C.; Takkar, B.; Narayanan, R.; Jalali, S.; Mandal, S.; Fujinami, K.; Padhy, S.K. Gene Therapy for Inherited Retinal Diseases: From Laboratory Bench to Patient Bedside and Beyond. Ophthalmol. Ther. 2024, 13, 21–50. [Google Scholar] [CrossRef]
- Ma, C.C.; Wang, Z.L.; Xu, T.; He, Z.Y.; Wei, Y.Q. The Approved Gene Therapy Drugs Worldwide: From 1998 to 2019. Biotechnol. Adv. 2020, 40, 107502. [Google Scholar] [CrossRef]
- Kiser, P.D. Retinal Pigment Epithelium 65 KDa Protein (RPE65): An Update. Prog. Retin. Eye Res. 2022, 88, 101013. [Google Scholar] [CrossRef]
- Girach, A.; Audo, I.; Birch, D.G.; Huckfeldt, R.M.; Lam, B.L.; Leroy, B.P.; Michaelides, M.; Russell, S.R.; Sallum, J.M.F.; Stingl, K.; et al. RNA-Based Therapies in Inherited Retinal Diseases. Ther. Adv. Ophthalmol. 2022, 14, 25158414221134602. [Google Scholar] [CrossRef]
- Kansara, V.S.; Hancock, S.E.; Muya, L.W.; Ciulla, T.A. Suprachoroidal Delivery Enables Targeting, Localization and Durability of Small Molecule Suspensions. J. Control. Release 2022, 349, 1045–1051. [Google Scholar] [CrossRef]
- Martínez, T.; González, M.V.; Roehl, I.; Wright, N.; Pañeda, C.; Jiménez, A.I. In Vitro and in Vivo Efficacy of SYL040012, a Novel SiRNA Compound for Treatment of Glaucoma. Mol. Ther. 2014, 22, 81–91. [Google Scholar] [CrossRef]
- Yin, D.; Ling, S.; Wang, D.; Dai, Y.; Jiang, H.; Zhou, X.; Paludan, S.R.; Hong, J.; Cai, Y. Targeting Herpes Simplex Virus with CRISPR–Cas9 Cures Herpetic Stromal Keratitis in Mice. Nat. Biotechnol. 2021, 39, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Zode, G.; Kasetti, R.B.; Ran, F.A.; Yan, W.; Sharma, T.P.; Bugge, K.; Searby, C.C.; Fingert, J.H.; Zhang, F.; et al. CRISPR-Cas9–Based Treatment of Myocilin-Associated Glaucoma. Proc. Natl. Acad. Sci. USA 2017, 114, 11199–11204. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.K.; Symons, R.C.A.; Shah, S.M.; Quinlan, E.J.; Tabandeh, H.; Do, D.V.; Reisen, G.; Lockridge, J.A.; Short, B.; Guerciolini, R.; et al. RNAi-Based Treatment for Neovascular Age-Related Macular Degeneration by Sirna-027. Am. J. Ophthalmol. 2010, 150, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Samul, R.; Silva, R.L.; Akiyama, H.; Liu, H.; Saishin, Y.; Hackett, S.F.; Zinnen, S.; Kossen, K.; Fosnaugh, K.; et al. Suppression of Ocular Neovascularization with SiRNA Targeting VEGF Receptor 1. Gene Ther. 2006, 13, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Controversies in Treatment Approaches: Gene Therapy,...—Google Scholar. Available online: https://scholar.google.com/scholar?q=ControversiesinTreatmentApproaches:GeneTherapy,IVF,StemCells,andPharmacogenomics%7C (accessed on 22 June 2024).
- Mehta, N.; Robbins, D.A.; Yiu, G. Ocular Inflammation and Treatment Emergent Adverse Events in Retinal Gene Therapy. Int. Ophthalmol. Clin. 2021, 61, 151–177. [Google Scholar] [CrossRef]
- Zaiss, A.-K.; Liu, Q.; Bowen, G.P.; Wong, N.C.W.; Bartlett, J.S.; Muruve, D.A. Differential Activation of Innate Immune Responses by Adenovirus and Adeno-Associated Virus Vectors. J. Virol. 2002, 76, 4580–4590. [Google Scholar] [CrossRef]
- Walters, L. The Ethics of Human Gene Therapy. Nature 1986, 320, 225–227. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular Drug Delivery. AAPS J. 2010, 12, 348. [Google Scholar] [CrossRef]
- Giri, B.R.; Maniruzzaman, M. Fabrication of Sustained-Release Dosages Using Powder-Based Three-Dimensional (3D) Printing Technology. AAPS PharmSciTech 2022, 24, 4. [Google Scholar] [CrossRef]
- Giri, B.R.; Poudel, S.; Kim, D.W. Cellulose and Its Derivatives for Application in 3D Printing of Pharmaceuticals. J. Pharm. Investig. 2020, 51, 1–22. [Google Scholar] [CrossRef]
- Alomari, M.; Mohamed, F.H.; Basit, A.W.; Gaisford, S. Personalised Dosing: Printing a Dose of One’s Own Medicine. Int. J. Pharm. 2015, 494, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Trenfield, S.J.; Pollard, T.D.; Ong, J.J.; Elbadawi, M.; McCoubrey, L.E.; Goyanes, A.; Gaisford, S.; Basit, A.W. Connected Healthcare: Improving Patient Care Using Digital Health Technologies. Adv. Drug Deliv. Rev. 2021, 178, 113958. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Viaño, I.; Trenfield, S.J.; Basit, A.W.; Goyanes, A. Translating 3D Printed Pharmaceuticals: From Hype to Real-World Clinical Applications. Adv. Drug Deliv. Rev. 2021, 174, 553–575. [Google Scholar] [CrossRef]
- Giri, B.R.; Song, E.S.; Kwon, J.; Lee, J.H.; Park, J.B.; Kim, D.W. Fabrication of Intragastric Floating, Controlled Release 3D Printed Theophylline Tablets Using Hot-Melt Extrusion and Fused Deposition Modeling. Pharmaceutics 2020, 12, 77. [Google Scholar] [CrossRef]
- Kulkarni, V.R.; Saha, T.; Raj Giri, B.; Lu, A.; Das, S.C.; Maniruzzaman, M. Recent Advancements in Pharmaceutical 3D Printing Industry. J. Drug Deliv. Sci. Technol. 2024, 100, 106072. [Google Scholar] [CrossRef]
- Cerda, J.R.; Arifi, T.; Ayyoubi, S.; Knief, P.; Paloma Ballesteros, M.; Keeble, W.; Barbu, E.; Marie Healy, A.; Lalatsa, A.; Serrano, D.R. Personalised 3D Printed Medicines: Optimising Material Properties for Successful Passive Diffusion Loading of Filaments for Fused Deposition Modelling of Solid Dosage Forms. Pharmaceutics 2020, 12, 345. [Google Scholar] [CrossRef]
- Mohamdeen, Y.M.G.; Tabriz, A.G.; Tighsazzadeh, M.; Nandi, U.; Khalaj, R.; Andreadis, I.; Boateng, J.S.; Douroumis, D. Development of 3D Printed Drug-Eluting Contact Lenses. J. Pharm. Pharmacol. 2022, 74, 1467–1476. [Google Scholar] [CrossRef]
- Wu, L.; Park, J.; Kamaki, Y.; Kim, B. Optimization of the Fused Deposition Modeling-Based Fabrication Process for Polylactic Acid Microneedles. Microsystems Nanoeng. 2021, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Viaño, I.; Januskaite, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A. Semi-Solid Extrusion 3D Printing in Drug Delivery and Biomedicine: Personalised Solutions for Healthcare Challenges. J. Control. Release 2021, 332, 367–389. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent Trends in Bioinks for 3D Printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef]
- Tagami, T.; Goto, E.; Kida, R.; Hirose, K.; Noda, T.; Ozeki, T. Lyophilized Ophthalmologic Patches as Novel Corneal Drug Formulations Using a Semi-Solid Extrusion 3D Printer. Int. J. Pharm. 2022, 617, 121448. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, N.; Luo, J.; Qin, M.; Di Luca, M.; Mathew, E.; Tagalakis, A.D.; Lamprou, D.A.; Yu-Wai-Man, C. 3D-Printed Long-Acting 5-Fluorouracil Implant to Prevent Conjunctival Fibrosis in Glaucoma. J. Pharm. Pharmacol. 2023, 75, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Paleel, F.; Qin, M.; Tagalakis, A.D.; Yu-Wai-Man, C.; Lamprou, D.A. Manufacturing and Characterisation of 3D-Printed Sustained-Release Timolol Implants for Glaucoma Treatment. Drug Deliv. Transl. Res. 2024, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Deshmane, S.; Kendre, P.; Mahajan, H.; Jain, S. Stereolithography 3D Printing Technology in Pharmaceuticals: A Review. Drug Dev. Ind. Pharm. 2021, 47, 1362–1372. [Google Scholar] [CrossRef]
- Fitaihi, R.; Abukhamees, S.; Chung, S.H.; Craig, D.Q.M. Optimization of Stereolithography 3D Printing of Microneedle Micro-Molds for Ocular Drug Delivery. Int. J. Pharm. 2024, 658, 124195. [Google Scholar] [CrossRef]
- Chanabodeechalermrung, B.; Chaiwarit, T.; Udomsom, S.; Rachtanapun, P.; Piboon, P.; Jantrawut, P. Determination of Vat-Photopolymerization Parameters for Microneedles Fabrication and Characterization of HPMC/PVP K90 Dissolving Microneedles Utilizing 3D-Printed Mold. Sci. Rep. 2024, 14, 16174. [Google Scholar] [CrossRef]
- Xu, X.; Awwad, S.; Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Brocchini, S.; Gaisford, S.; Goyanes, A.; Basit, A.W. 3D Printed Punctal Plugs for Controlled Ocular Drug Delivery. Pharmaceutics 2021, 13, 1421. [Google Scholar] [CrossRef]
- Alam, F.; Elsherif, M.; Alqattan, B.; Salih, A.; Lee, S.M.; Yetisen, A.K.; Park, S.; Butt, H. 3D Printed Contact Lenses. ACS Biomater. Sci. Eng. 2021, 7, 794–803. [Google Scholar] [CrossRef]
- Antonara, L.; Dallas, P.P.; Rekkas, D.M. A Novel 3D Printing Enabled Method for Fast and Reliable Construction of Polymeric Microneedles Using Experimental Design. J. Drug Deliv. Sci. Technol. 2022, 68, 102888. [Google Scholar] [CrossRef]
- Khoshnood, N.; Frampton, J.P.; Badri, A.; Zamanian, A. 3D Bioprinting of Betamethasone-Loaded Gellan Gum–Polyethyleneimine Composite Hydrogels for Ocular Drug Delivery. Int. J. Bioprint. 2024, 10, 3440. [Google Scholar] [CrossRef]
- Goto, E.; Tagami, T.; Ogawa, K.; Ozeki, T. Fabrication of 3D-Printed Contact Lens Composed of Polyethylene Glycol Diacrylate for Controlled Release of Azithromycin. Biol. Pharm. Bull. 2023, 46, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Mathew, E.; Pitzanti, G.; Gomes Dos Santos, A.L.; Lamprou, D.A. Optimization of Printing Parameters for Digital Light Processing 3d Printing of Hollow Microneedle Arrays. Pharmaceutics 2021, 13, 1837. [Google Scholar] [CrossRef] [PubMed]
- Koutsamanis, I.; Roblegg, E.; Spoerk, M. Controlled Delivery via Hot-Melt Extrusion: A Focus on Non-Biodegradable Carriers for Non-Oral Applications. J. Drug Deliv. Sci. Technol. 2023, 81, 104289. [Google Scholar] [CrossRef]
- Ahmed, J.; Giri, B.R.; Thomas, L.; Al-Attar, H.; Maniruzzaman, M. Continuous Manufacturing of Vitamin D3 and Iron Enriched Granules by Means of a Novel Twin-Screw Dry Granulation Process. Powder Technol. 2022, 412, 117975. [Google Scholar] [CrossRef]
- Karnik, I.; Youssef, A.A.A.; Joshi, P.; Munnangi, S.R.; Narala, S.; Varner, C.; Vemula, S.K.; Majumdar, S.; Repka, M. Formulation Development and Characterization of Dual Drug Loaded Hot-Melt Extruded Inserts for Better Ocular Therapeutic Outcomes: Sulfacetamide/Prednisolone. J. Drug Deliv. Sci. Technol. 2023, 84, 104558. [Google Scholar] [CrossRef]
- Shadambikar, G.; Marathe, S.; Patil, A.; Joshi, R.; Bandari, S.; Majumdar, S.; Repka, M. Novel Application of Hot Melt Extrusion Technology for Preparation and Evaluation of Valacyclovir Hydrochloride Ocular Inserts. AAPS PharmSciTech 2021, 22, 1–7. [Google Scholar] [CrossRef]
- Tambe, S.; Jain, D.; Rawat, R.; Mali, S.; Pagano, M.A.; Brunati, A.M.; Amin, P. MeltSerts Technology (Brinzolamide Ocular Inserts via Hot-Melt Extrusion): QbD-Steered Development, Molecular Dynamics, in Vitro, Ex Vivo and in Vivo Studies. Int. J. Pharm. 2023, 648, 123579. [Google Scholar] [CrossRef]
- Costello, M.A.; Liu, J.; Chen, B.; Wang, Y.; Qin, B.; Xu, X.; Li, Q.; Lynd, N.A.; Zhang, F. Drug Release Mechanisms of High-Drug-Load, Melt-Extruded Dexamethasone Intravitreal Implants. Eur. J. Pharm. Biopharm. 2023, 187, 46–56. [Google Scholar] [CrossRef]
- Ghebremeskel, A.N.; Robinson, M.R. Prostamide-Containing Intraocular Implants and Methods of Use Thereof. U.S. Patent 9,492,316, 15 November 2016. [Google Scholar]
- Deng, X.; Gould, M.; Ali, M.A. Fabrication and Characterisation of Melt-Extruded Chitosan/Keratin/PCL/PEG Drug-Eluting Sutures Designed for Wound Healing. Mater. Sci. Eng. C. Mater. Biol. Appl. 2021, 120, 111696. [Google Scholar] [CrossRef]
- Nagy, Z.K.; Balogh, A.; Drávavölgyi, G.; Ferguson, J.; Pataki, H.; Vajna, B.; Marosi, G. Solvent-Free Melt Electrospinning for Preparation of Fast Dissolving Drug Delivery System and Comparison with Solvent-Based Electrospun and Melt Extruded Systems. J. Pharm. Sci. 2013, 102, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Czepiel, M.; Bańkosz, M.; Sobczak-Kupiec, A. Advanced Injection Molding Methods: Review. Materials 2023, 16, 5802. [Google Scholar] [CrossRef]
- Zema, L.; Loreti, G.; Melocchi, A.; Maroni, A.; Gazzaniga, A. Injection Molding and Its Application to Drug Delivery. J. Control. Release 2012, 159, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Tsung, T.H.; Tsai, Y.C.; Lee, H.P.; Chen, Y.H.; Lu, D.W. Biodegradable Polymer-Based Drug-Delivery Systems for Ocular Diseases. Int. J. Mol. Sci. 2023, 24, 12976. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377. [Google Scholar] [CrossRef]
- Allyn, M.M.; Luo, R.H.; Hellwarth, E.B.; Swindle-Reilly, K.E. Considerations for Polymers Used in Ocular Drug Delivery. Front. Med. 2022, 8, 787644. [Google Scholar] [CrossRef]
- Akhter, M.H.; Ahmad, I.; Alshahrani, M.Y.; Al-Harbi, A.I.; Khalilullah, H.; Afzal, O.; Altamimi, A.S.A.; Najib Ullah, S.N.M.; Ojha, A.; Karim, S. Drug Delivery Challenges and Current Progress in Nanocarrier-Based Ocular Therapeutic System. Gels 2022, 8, 82. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Wu, X.; Jiang, J.; Qiang, W.; Xie, H.; Zhou, H.; Wu, S.; Shao, Y.; Chen, W. Artificial Intelligence in Ophthalmology: The Path to the Real-World Clinic. Cell Rep. Med. 2023, 4, 101095. [Google Scholar] [CrossRef]
- DeepMind’s AI Can Detect over 50 Eye Diseases as Accurately as a Doctor—The Verge. Available online: https://www.theverge.com/2018/8/13/17670156/deepmind-ai-eye-disease-doctor-moorfields (accessed on 30 July 2024).
- Yelne, S.; Chaudhary, M.; Dod, K.; Sayyad, A.; Sharma, R. Harnessing the Power of AI: A Comprehensive Review of Its Impact and Challenges in Nursing Science and Healthcare. Cureus 2023, 15, e49252. [Google Scholar] [CrossRef]
- Cicinelli, M.; Marmamula, S.; Khanna, R. Comprehensive Eye Care—Issues, Challenges, and Way Forward. Indian J. Ophthalmol. 2020, 68, 316. [Google Scholar] [CrossRef]
- Artificial Intelligence to Manage the AMD Burden|Retinal Physician. Available online: https://www.retinalphysician.com/issues/2024/januaryfebruary/artificial-intelligence-to-manage-the-amd-burden/ (accessed on 30 July 2024).
- Kazanskiy, N.L.; Khonina, S.N.; Butt, M.A. Smart Contact Lenses—A Step towards Non-Invasive Continuous Eye Health Monitoring. Biosensors 2023, 13, 933. [Google Scholar] [CrossRef] [PubMed]
- Blanco-González, A.; Cabezón, A.; Seco-González, A.; Conde-Torres, D.; Antelo-Riveiro, P.; Piñeiro, Á.; Garcia-Fandino, R. The Role of AI in Drug Discovery: Challenges, Opportunities, and Strategies. Pharmaceuticals 2023, 16, 891. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.N.; Ceulemans, H.; Boyd, J.D.; Carpenter, A.E. Image-Based Profiling for Drug Discovery: Due for a Machine-Learning Upgrade? Nat. Rev. Drug Discov. 2021, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Pushkaran, A.C.; Arabi, A.A. From Understanding Diseases to Drug Design: Can Artificial Intelligence Bridge the Gap? Artif. Intell. Rev. 2024, 57, 1–39. [Google Scholar] [CrossRef]
- Kamya, P.; Ozerov, I.V.; Pun, F.W.; Tretina, K.; Fokina, T.; Chen, S.; Naumov, V.; Long, X.; Lin, S.; Korzinkin, M.; et al. PandaOmics: An AI-Driven Platform for Therapeutic Target and Biomarker Discovery. J. Chem. Inf. Model. 2024, 64, 3961–3969. [Google Scholar] [CrossRef]
- Mondal, H.; Kim, H.J.; Mohanto, N.; Jee, J.P. A Review on Dry Eye Disease Treatment: Recent Progress, Diagnostics, and Future Perspectives. Pharmaceutics 2023, 15, 990. [Google Scholar] [CrossRef]
- FDA Approves First-of-Its-Kind Glaucoma Treatment|BrightFocus Foundation. Available online: https://www.brightfocus.org/glaucoma/news/fda-approves-first-its-kind-glaucoma-treatment (accessed on 31 July 2024).
- AI Used to Advance Drug Delivery System for Glaucoma and Other Chronic Diseases|Johns Hopkins Medicine. Available online: https://www.hopkinsmedicine.org/news/newsroom/news-releases/2023/05/ai-used-to-advance-drug-delivery-system-for-glaucoma-and-other-chronic-diseases (accessed on 31 July 2024).
- Hsueh, H.T.; Chou, R.T.; Rai, U.; Liyanage, W.; Kim, Y.C.; Appell, M.B.; Pejavar, J.; Leo, K.T.; Davison, C.; Kolodziejski, P.; et al. Machine Learning-Driven Multifunctional Peptide Engineering for Sustained Ocular Drug Delivery. Nat. Commun. 2023, 14, 2509. [Google Scholar] [CrossRef]
- US FDA GDUFA Commitment Letter. Available online: https://www.fda.gov/media/153631/download?attachment (accessed on 27 August 2024).
- FY2016 Regulatory Science Report: Ophthalmic Products|FDA. Available online: https://www.fda.gov/industry/generic-drug-user-fee-amendments/fy2016-regulatory-science-report-ophthalmic-products (accessed on 27 August 2024).
- Office of Generic Drugs FY 2018 GDUFA Science and Research Report|FDA. Available online: https://www.fda.gov/drugs/generic-drugs/office-generic-drugs-fy-2018-gdufa-science-and-research-report (accessed on 7 October 2024).
- Quality Considerations for Topical Ophthalmic Drug Products|FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/quality-considerations-topical-ophthalmic-drug-products (accessed on 24 August 2024).
- US FDA Guidance for Industry Extended Release Oral Dosage Forms: Development, Evaluation, and Application of In Vitro/In Vivo Correlations. Available online: https://www.fda.gov/media/70939/download (accessed on 27 August 2024).







| Active Ingredient | Proprietary Name | Strength | Indication | Route of Administration | Efficacy Duration | Rate Controlling Systems | Year of Approval |
|---|---|---|---|---|---|---|---|
| Ganciclovir | Vitrasert | 4.5 mg | Cytomegalovirus | Implantation | 5–8 months | PVA and EVA | 1996 |
| Fluocinolone acetonide | Retisert | 0.59 mg | Uveitis | Intravitreal | 30 months | PVA | 2005 |
| Dexamethasone | Ozurdex | 0.7 mg | DME, RVO, and uveitis | Intravitreal | Up to 6 months Depends on the conditions | PLGA | 2009 |
| Fluocinolone acetonide | Iluvien | 0.19 mg | DME | Intravitreal | 36 months | Polyimide tube, PVA, and silicone adhesive | 2014 |
| Fluocinolone acetonide | Yutiq | 0.18 mg | Uveitis | Intravitreal | 36 months | Polyimide tube, PVA, and silicone adhesive | 2018 |
| Bimatoprost | Durysta | 10 mcg | Reducing IOP | Ophthalmic/intracameral | Several months | PLGA, PDLA, PDLLA and PEG3350 | 2020 |
| Ranibizumab | Suvismo | 2 mg | Wet AMD | Intravitreal | Up to 6 months | Port delivery system | 2021 |
| Travoprost | iDose TR | 75 mcg | Reducing IOP | Intracameral | 3 months | Titanium reservoir coated with semipermeable membrane | 2023 |
| Device/Dosage Forms | APIs (Drug Load % w/w) | Excipients | 3D Printing Technologies | Findings/Applications | Ref. |
|---|---|---|---|---|---|
| Contact lenses | Timolol maleate (1%) | EVA and PLA | HME-FDM | Drug-eluting contact lenses provided an initial burst release followed by a sustained release for 3 days for the treatment of glaucoma. | [236] |
| Microneedles (MNs) | Rhodamine B (model drug) | PLA | FDM | The MN can be coated with APIs for various biomedical uses. | [237] |
| Molds for dissolving MNs | Galantamine hydrobromide | PLA filaments, PVA/PVP | FDM | FDM 3D printing was used to develop molds which were used to prepare API-loaded MNs | [249] |
| Ophthalmic patches | Levofloxacin (0.5%) | HPMC, mannitol, and xylitol | SSE | Antibacterial effect for eye infections. Most of the drug is released within 60–120 min. | [240] |
| Intracameral implants | Timolol maleate (5–10%) | PCL | SSE | The 3D-printed implants were developed to deliver sustained drug release over eight weeks for treating glaucoma. | [242] |
| Implants | 5-fluorouracil (1%) | PCL and chitosan | SSE | The implant was developed to prevent conjunctival fibrosis post-glaucoma surgery. | [241] |
| Hydrogel-based scaffold | Betamethasone sodium phosphate (2.5%) | Polyethyleneimine | SSE | The 3D-bioprinted hydrogel scaffold has the potential to manage ocular inflammation. | [250] |
| Dissolving MNs | Placebo | PVP and PVA | SLA | Placebo MNs developed for potential ocular applications. | [245] |
| Molds for dissolving MNs | Placebo | PLA | SLA | The molds were 3D-printed to produce HPMC and PVP K90 dissolving MNs. | [246] |
| Punctal plugs | Dexamethasone (10–20%) | PEGDA and PEG 400 | DLP | Plugs made with 100% PEGDA showed prolonged releases for over 21 days for treating dry eye disease. | [247] |
| Contact lenses | Azithromycin (1%) | PEGDA and PEG 400 | DLP | The lenses demonstrated antimicrobial properties with an inhibition zone diameter of 30 mm, similar to commercial eye drops. | [251] |
| Hollow MNs | Placebo | Biocompatible commercial resins | DLP | An angled-printed MN showed optimal geometries compared to a flat-printed (at 0° to the base plate) MN. | [252] |
| Dosage Form | APIs | Excipients | Key Findings | Refs. |
|---|---|---|---|---|
| Fixed-dose combination ocular inserts | Prednisolone sodium phosphate and Sulfacetamide sodium | PEO, HPC-HF, and EC | The HPC-HF- and EC-containing inserts showed sustained drug release profiles and were stable for >90 days at 25 and 40 °C. Optimum bio-adhesive strength and smooth surface finish were observed, making them suitable for topical ocular application. | [255] |
| Inserts | Valacyclovir HCL | HPC EF-HPMC K4M and PEG 400 | The ocular inserts were fabricated to treat corneal keratitis. The inserts showed a sustained drug release profile, dissolving completely in 8 h, and enhanced permeation. | [256] |
| Inserts | Brinzolamide (BRZ) | HPMC and Poloxamer 407 | The solubility and residence duration of BRZ in the polymer matrix were influenced by various interactions, including ionic, Van der Waals, H-bonding, and electrostatic forces. The inserts showed a sustained-release profile for 24 h and better IOP control and remained stable at ambient temperature and 4 °C for 6 months. Drug release was governed by swelling, polymer chain relaxation, and diffusion phenomena. | [257] |
| Biodegradable implant | Dexamethasone | PLGA | The implant showed an irregular surface with 6% internal porosity and a triphasic drug release profile. Physicochemical characterizations revealed limited interaction between the drug and the polymer, resulting in a two-phase system of dexamethasone crystals embedded within a PLGA matrix. The reverse-engineered implant and Ozurdex showed similar compositions and structural similarities, allowing for an equivalent in vitro release profile. | [258] |
| Biodegradable intracameral implant | Prostamide | PEG 3350 and PLGA | The implant is rod-shaped and formed by a hot-melt extrusion process, with the implant being 150 to 300 μm in diameter or width, 0.50 to 2.5 mm in length, and 30 to 100 μg in total weight. It effectively reduces IOP for at >2 months after placement in the anterior chamber of the eye. | [259] |
| Monofilamnt (500–700 μm) | Diclofenac potassium | PEG–PCL–chitosan–keratin blend | Amorphous and miscible solid dispersions were created. Rapid and sustained drug release rates were achieved with the PEG/PCL/chitosan/keratin blends at various combinations. Presence of hydrophilic and phobic polymers improved the solubility of the diclofenac potassium with a tunable release rate. | [260] |
| Monofilamnt (20 μm) | Carvdilol | Eudragit® E | Up to 20% of carvedilol was loaded. Fast release of carvedilol, which has poor water solubility. Comparable drug loading and drug release with suture fibers with similar compositions produced by solvent-free melt electrospinning and solvent-based electrospinning. | [261] |
| Pharmaceutical Technologies | Advantages | Limitations |
|---|---|---|
| 3D printing |
|
|
| Hot-melt extrusion |
|
|
| Injection molding |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giri, B.R.; Jakka, D.; Sandoval, M.A.; Kulkarni, V.R.; Bao, Q. Advancements in Ocular Therapy: A Review of Emerging Drug Delivery Approaches and Pharmaceutical Technologies. Pharmaceutics 2024, 16, 1325. https://doi.org/10.3390/pharmaceutics16101325
Giri BR, Jakka D, Sandoval MA, Kulkarni VR, Bao Q. Advancements in Ocular Therapy: A Review of Emerging Drug Delivery Approaches and Pharmaceutical Technologies. Pharmaceutics. 2024; 16(10):1325. https://doi.org/10.3390/pharmaceutics16101325
Chicago/Turabian StyleGiri, Bhupendra Raj, Deeksha Jakka, Michael A. Sandoval, Vineet R. Kulkarni, and Quanying Bao. 2024. "Advancements in Ocular Therapy: A Review of Emerging Drug Delivery Approaches and Pharmaceutical Technologies" Pharmaceutics 16, no. 10: 1325. https://doi.org/10.3390/pharmaceutics16101325
APA StyleGiri, B. R., Jakka, D., Sandoval, M. A., Kulkarni, V. R., & Bao, Q. (2024). Advancements in Ocular Therapy: A Review of Emerging Drug Delivery Approaches and Pharmaceutical Technologies. Pharmaceutics, 16(10), 1325. https://doi.org/10.3390/pharmaceutics16101325






