Lipid-Based Nanoformulations for Drug Delivery: An Ongoing Perspective
Abstract
1. Introduction
2. Micro- and Nanoemulsions
2.1. Microemulsions
2.1.1. Method of Preparation

2.1.2. Applications
2.2. Nanoemulsions
2.2.1. Methods of Preparation
2.2.2. Applications
3. Self-Emulsifying Drug Delivery Systems (SEDDS)
3.1. Classic SEDDS
3.2. Self-Micro Emulsifying Drug Delivery System (SMEDDS)
3.3. Self-Nano-Emulsifying Drug Delivery Systems (SNEDDS)
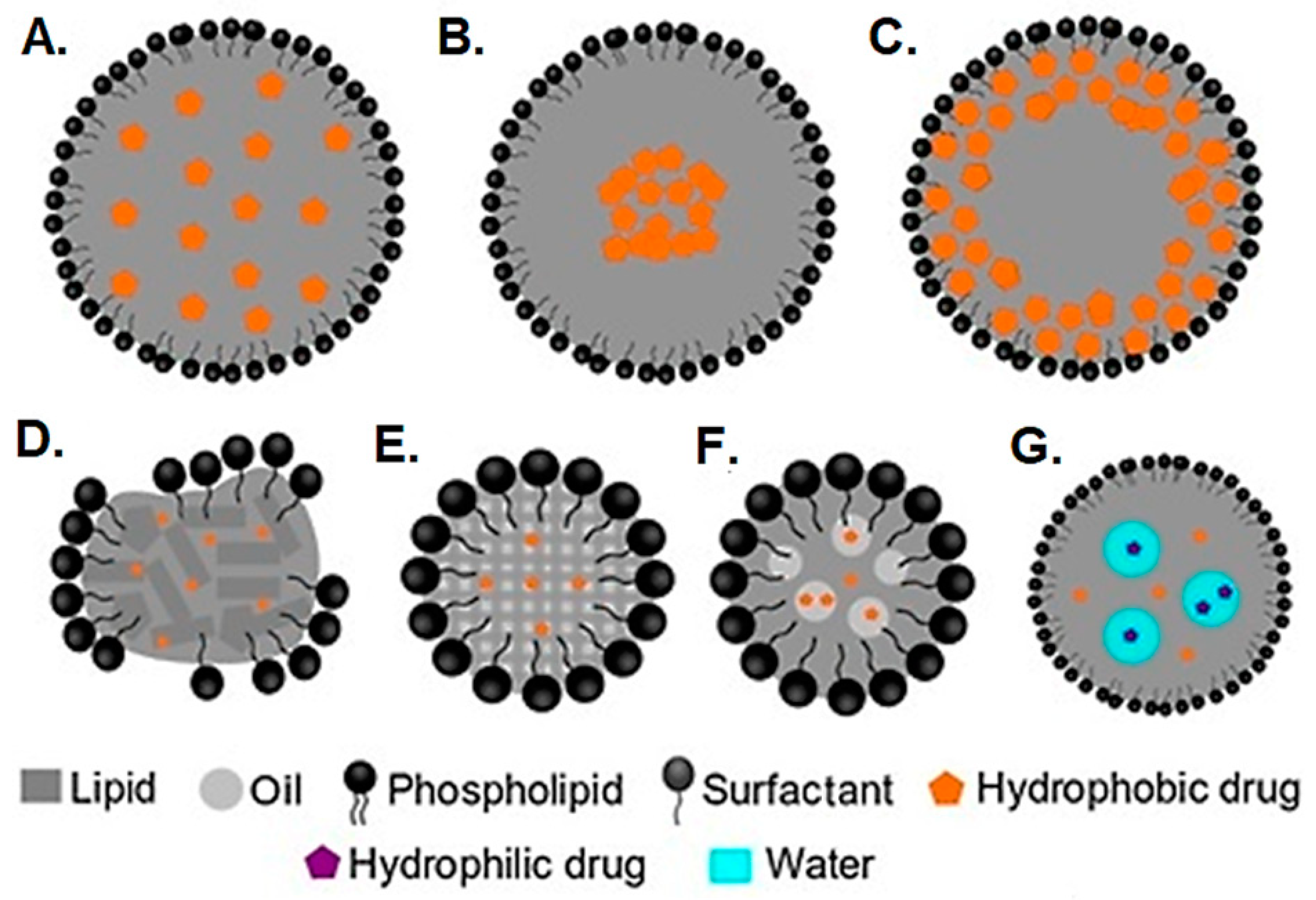
4. Solid-Phase Lipid Nanoparticles: Solid Lipid Nanoparticles and Nanostructured Lipid Nanoparticles
4.1. Applications
4.1.1. Controlled Release of Drugs
4.1.2. Intravenous Delivery of Drugs
4.1.3. Targeted Delivery to the Brain

4.1.4. Topical Delivery of Drugs and Cosmetics
5. Vesicular Drug Delivery Systems: Liposomes and Niosomes
5.1. Formulation and Preparation Methods
5.2. Applications
5.2.1. Gene Delivery
5.2.2. Vaccine Delivery
5.2.3. Anticancer Drug Delivery
5.2.4. Topical Drug Delivery
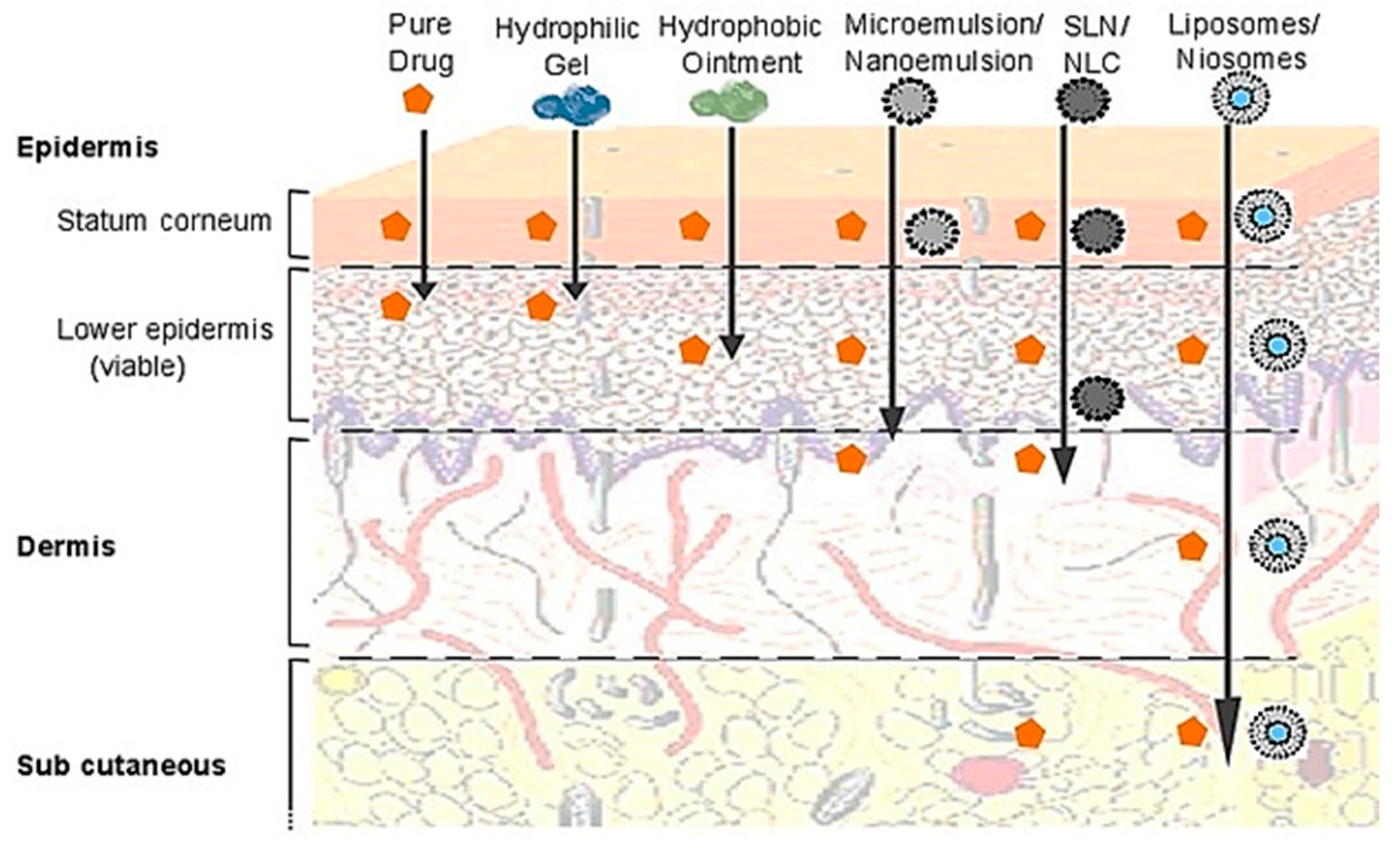
5.2.5. Oral Drug Delivery
5.2.6. Diagnostic and Theragnostic Agents
5.2.7. Stimuli-Responsive Liposomes for Targeted Drug Delivery
6. Lipid Nanoparticles (LNPs)
6.1. Formulation
6.2. Mechanisms for Nucleic Acid Delivery
6.2.1. Cellular Uptake and Intracellular Trafficking
6.2.2. Endosomal Escape and Cytoplasmic Release
6.2.3. Mechanisms of Gene Expression: Transcription, Translation, and Antigen Presentation
6.3. Clinical Development of LNP-Based Nucleic Acid Vaccines
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lou, J.; Duan, H.; Qin, Q.; Teng, Z.; Gan, F.; Zhou, X.; Zhou, X. Advances in oral drug delivery systems: Challenges and opportunities. Pharmaceutics 2023, 15, 484. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.M.; Fonte, P.; Oliveira, A.; Madureira, A.R.; Sarmento, B.; Pintado, M.E. Optimization of two biopolymer-based oral films for the delivery of bioactive molecules. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Mehta, C.H.; Nayak, U.Y. Multiple approaches for achieving drug solubility: An in silico perspective. Drug Discov. Today 2020, 25, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.; Fonte, P.; Costa, A.; Reis, C.C.; Nunes, R.; Almeida, A.; Ferreira, D.; Oliva, M.; Sarmento, B. Pharmacological and toxicological assessment of innovative self-assembled polymeric micelles as powders for insulin pulmonary delivery. Nanomedicine 2016, 11, 2305–2317. [Google Scholar] [CrossRef]
- Augsburger, L.L.; Hoag, S.W. Pharmaceutical Dosage Forms-Tablets; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Tung, N.-T.; Tran, C.-S.; Tran, T.-C.; Nguyen, K.-T.; Pham, T.-A.; Ngo, T.-N. Synergistic effect of miscible cellulose-based microparticles and pH modulators on the bioavailability of a weakly basic drug and its metabolites. Int. J. Biol. Macromol. 2023, 233, 123555. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, Z.; Yin, B.; Goh, H.M.; Toh, D.W.K.; Kim, J.E. Effects of dietary fat type and emulsification on carotenoid absorption: A randomized crossover trial. Am. J. Clin. Nutr. 2023, 117, 1017–1025. [Google Scholar] [CrossRef]
- Rajput, T.; Chauhan, M.K. Bilosome: A bile salt based novel carrier system gaining interest in pharmaceutical research. J. Drug Deliv. Ther. 2017, 7, 4–16. [Google Scholar] [CrossRef]
- Bolhassani, A. Lipid-based delivery systems in development of genetic and subunit vaccines. Mol. Biotechnol. 2023, 65, 669–698. [Google Scholar] [CrossRef]
- Rampado, R.; Peer, D. Design of experiments in the optimization of nanoparticle-based drug delivery systems. J. Control. Release 2023, 358, 398–419. [Google Scholar] [CrossRef]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and nanoemulsions in skin drug delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef]
- Ascenso, A.; Simões, S.; Marto, J.; Ribeiro, H.M.; Almeida, A.J. Colloidal Disperse Systems: Microemulsions and Nanoemulsions. Nanocarriers Drug Deliv. Concepts Appl. 2021, 73–81. [Google Scholar]
- Guo, Y.; Zhang, X.; Wang, X.; Zhang, L.; Xu, Z.; Sun, D. Nanoemulsions Stable against Ostwald Ripening. Langmuir 2024, 40, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, R.; Jaiswal, P.; Patel, D.K.; Yadav, P.K. Lipid-based drug delivery system (LBDDS): An emerging paradigm to enhance oral bioavailability of poorly soluble drugs. Biomed. Mater. Devices 2023, 1, 648–663. [Google Scholar] [CrossRef]
- Ait-Touchente, Z.; Zine, N.; Jaffrezic-Renault, N.; Errachid, A.; Lebaz, N.; Fessi, H.; Elaissari, A. Exploring the versatility of microemulsions in cutaneous drug delivery: Opportunities and challenges. Nanomaterials 2023, 13, 1688. [Google Scholar] [CrossRef] [PubMed]
- Tartaro, G.; Mateos, H.; Schirone, D.; Angelico, R.; Palazzo, G. Microemulsion microstructure(s): A tutorial review. Nanomaterials 2020, 10, 1657. [Google Scholar] [CrossRef]
- Leung, R.; Hou, M.J.; Shah, D.O. Microemulsions: Formation, structure, properties, and novel applications. In Surfactants in Chemical/Process Engineering; Routledge: London, UK, 2017; pp. 315–368. [Google Scholar]
- Gunarto, C.; Ju, Y.-H.; Putro, J.N.; Tran-Nguyen, P.L.; Soetaredjo, F.E.; Santoso, S.P.; Ayucitra, A.; Angkawijaya, A.E.; Ismadji, S. Effect of a Nonionic Surfactant on the Pseudoternary Phase Diagram and Stability of Microemulsion. J. Chem. Eng. Data 2020, 65, 4024–4033. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsion-based oral delivery systems for lipophilic bioactive components: Nutraceuticals and pharmaceuticals. Ther. Deliv. 2013, 4, 841–857. [Google Scholar] [CrossRef]
- Ameta, R.K.; Soni, K.; Bhattarai, A. Recent advances in improving the bioavailability of hydrophobic/lipophilic drugs and their delivery via self-emulsifying formulations. Colloids Interfaces 2023, 7, 16. [Google Scholar] [CrossRef]
- Zaeim, D.; Mulet-Cabero, A.-I.; Read, S.A.; Liu, W.; Han, J.; Wilde, P.J. Effect of oil droplet size on the gastric digestion of milk protein emulsions using a semi-dynamic gastric model. Food Hydrocoll. 2022, 124, 107278. [Google Scholar] [CrossRef]
- Suhail, N.; Alzahrani, A.K.; Basha, W.J.; Kizilbash, N.; Zaidi, A.; Ambreen, J.; Khachfe, H.M. Microemulsions: Unique properties, pharmacological applications, and targeted drug delivery. Front. Nanotechnol. 2021, 3, 754889. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, N.; Liu, C.; Zhuo, Y.; Liang, L.; Gan, Y.; Yu, M. Oral delivery of nucleic acid therapeutics: Challenges, strategies, and opportunities. Drug Discov. Today 2023, 28, 103507. [Google Scholar] [CrossRef] [PubMed]
- Wellert, S.; Engelskirchen, S.; Hellweg, T.; Holderer, O. Where Does an Enzyme Reside in a Bicontinuous Structure? EPJ Web Conf. 2023, 286, 04001. [Google Scholar] [CrossRef]
- Chavda, V.P.; Gogoi, N.; Vaghela, D.A.; Balar, P.C.; Daware, S.; Dave, D.J. Parenteral microemulsion for drug delivery: Advances and update. J. Drug Deliv. Sci. Technol. 2023, 89, 104991. [Google Scholar] [CrossRef]
- Das, B.; Kumar, B.; Begum, W.; Bhattarai, A.; Mondal, M.H.; Saha, B. Comprehensive review on applications of surfactants in vaccine formulation, therapeutic and cosmetic pharmacy and prevention of pulmonary failure due to COVID-19. Chem. Afr. 2022, 5, 459–480. [Google Scholar] [CrossRef]
- Dong, J.; Zhu, X.-m.; Wu, F.-y.; Yang, B.-q.; Feng, H.; Dong, Y.-f.; Gu, W.; Chen, J. Development of galangal essential oil-based microemulsion gel for transdermal delivery of flurbiprofen: Simultaneous permeability evaluation of flurbiprofen and 1, 8-cineole. Drug Dev. Ind. Pharm. 2020, 46, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Kajbafvala, A.; Salabat, A. Microemulsion and microemulsion gel formulation for transdermal delivery of rutin: Optimization, in-vitro/ex-vivo evaluation and SPF determination. J. Dispers. Sci. Technol. 2022, 43, 1848–1857. [Google Scholar] [CrossRef]
- Çağlar, E.Ş.; Okur, M.E.; Aksu, B.; Üstündağ Okur, N. Transdermal delivery of acemetacin loaded microemulsions: Preparation, characterization, in vitro–ex vivo evaluation and in vivo analgesic and anti-inflammatory efficacy. J. Dispers. Sci. Technol. 2024, 45, 662–672. [Google Scholar] [CrossRef]
- Patel, P.; Pol, A.; Kalaria, D.; Date, A.A.; Kalia, Y.; Patravale, V. Microemulsion-based gel for the transdermal delivery of rasagiline mesylate: In vitro and in vivo assessment for Parkinson’s therapy. Eur. J. Pharm. Biopharm. 2021, 165, 66–74. [Google Scholar] [CrossRef]
- Froelich, A.; Osmałek, T.; Jadach, B.; Puri, V.; Michniak-Kohn, B. Microemulsion-based media in nose-to-brain drug delivery. Pharmaceutics 2021, 13, 201. [Google Scholar] [CrossRef]
- Meirinho, S.; Rodrigues, M.; Santos, A.O.; Falcão, A.; Alves, G. Intranasal Microemulsion as an Innovative and Promising Alternative to the Oral Route in Improving Stiripentol Brain Targeting. Pharmaceutics 2023, 15, 1641. [Google Scholar] [CrossRef]
- Alaayedi, M.H.; Maraie, N.K. Lomustine’s nanoemulsion as nose-to-brain drug delivery system for CNS tumor treatment. Saudi Pharm. J. 2023, 31, 101692. [Google Scholar] [CrossRef] [PubMed]
- Shah, B. Microemulsion as a promising carrier for nose to brain delivery: Journey since last decade. J. Pharm. Investig. 2021, 51, 611–634. [Google Scholar] [CrossRef]
- Meng, W.; Sun, H.; Mu, T.; Garcia-Vaquero, M. Chitosan-based Pickering emulsion: A comprehensive review on their stabilizers, bioavailability, applications and regulations. Carbohydr. Polym. 2023, 304, 120491. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Ahmadi, S.H.; Babak, P.; Bryant, S.L.; Kantzas, A. On the Stability of Pickering and Classical Nanoemulsions: Theory and Experiments. Langmuir 2023, 39, 6975–6991. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho-Guimarães, F.B.; Correa, K.L.; de Souza, T.P. A Review of Pickering Emulsions: Perspectives and Applications. Pharmaceuticals 2022, 15, 1413. [Google Scholar] [CrossRef]
- Jug, M.; Yoon, B.K.; Jackman, J.A. Cyclodextrin-based Pickering emulsions: Functional properties and drug delivery applications. J. Incl. Phenom. Macrocycl. Chem. 2021, 101, 31–50. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, B.; Tang, X.; Che, Z.; Hu, F.; Shen, C.; Wu, W.; Shen, B.; Yuan, H. Fabrication and in vitro/vivo evaluation of drug nanocrystals self-stabilized Pickering emulsion for oral delivery of quercetin. Pharmaceutics 2022, 14, 897. [Google Scholar] [CrossRef]
- Aulton, M.E.; Taylor, K. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Sneha, K.; Kumar, A. Nanoemulsions: Techniques for the preparation and the recent advances in their food applications. Innov. Food Sci. Emerg. Technol. 2022, 76, 102914. [Google Scholar]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Jadhav, K.; Singh, R.; Ray, E.; Verma, R.K. Application of nanoemulsion in pharmaceuticals industry. In Industrial Applications of Nanoemulsion; Elsevier: Amsterdam, The Netherlands, 2024; pp. 107–156. [Google Scholar]
- Vinchhi, P.; Patel, J.K.; Patel, M.M. High-Pressure Homogenization Techniques for Nanoparticles. In Emerging Technologies for Nanoparticle Manufacturing; Springer: Berlin/Heidelberg, Germany, 2021; pp. 263–285. [Google Scholar]
- Martin-Piñero, M.J.; Muñoz, J.; Alfaro-Rodriguez, M.-C. Improvement of the rheological properties of rosemary oil nanoemulsions prepared by microfluidization and vacuum evaporation. J. Ind. Eng. Chem. 2020, 91, 340–346. [Google Scholar] [CrossRef]
- Alhasso, B.; Ghori, M.U.; Conway, B.R. Development of Nanoemulsions for Topical Application of Mupirocin. Pharmaceutics 2023, 15, 378. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Huang, W.; Wang, Q.; Yang, G. Green synthesis of garlic oil nanoemulsion using ultrasonication technique and its mechanism of antifungal action against Penicillium italicum. Ultrason. Sonochem. 2020, 64, 104970. [Google Scholar] [CrossRef] [PubMed]
- Pillai, P.; Saw, R.K.; Mandal, A. Formulation and characterization of ionic liquid-based nanoemulsion for enhanced oil recovery applications. J. Mol. Liq. 2024, 397, 124189. [Google Scholar] [CrossRef]
- Pathak, K.; Pattnaik, S.; Swain, K. Application of nanoemulsions in drug delivery. In Nanoemulsions; Elsevier: Amsterdam, The Netherlands, 2018; pp. 415–433. [Google Scholar]
- Mohammed, A.N.; Ishwarya, S.P.; Nisha, P. Nanoemulsion versus microemulsion systems for the encapsulation of beetroot extract: Comparison of physicochemical characteristics and betalain stability. Food Bioprocess Technol. 2021, 14, 133–150. [Google Scholar] [CrossRef]
- Pandey, P.; Gulati, N.; Makhija, M.; Purohit, D.; Dureja, H. Nanoemulsion: A novel drug delivery approach for enhancement of bioavailability. Recent Pat. Nanotechnol. 2020, 14, 276–293. [Google Scholar] [CrossRef]
- Kaya, E.C.; Oztop, M.H.; Alpas, H. Effect of high-pressure processing (HPP) on production and characterization of chia seed oil nanoemulsions. LWT 2021, 141, 110872. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Egea, M.B.; Salas-Mellado, M.d.l.M.; Segura-Campos, M.R. Chia Oil and Mucilage Nanoemulsion: Potential Strategy to Protect a Functional Ingredient. Int. J. Mol. Sci. 2023, 24, 7384. [Google Scholar] [CrossRef]
- Ozogul, Y.; Karsli, G.T.; Durmuş, M.; Yazgan, H.; Oztop, H.M.; McClements, D.J.; Ozogul, F. Recent developments in industrial applications of nanoemulsions. Adv. Colloid Interface Sci. 2022, 304, 102685. [Google Scholar] [CrossRef]
- Moghassemi, S.; Dadashzadeh, A.; Azevedo, R.B.; Amorim, C.A. Nanoemulsion applications in photodynamic therapy. J. Control. Release 2022, 351, 164–173. [Google Scholar] [CrossRef]
- Gursoy, R.N.; Benita, S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharmacother. 2004, 58, 173–182. [Google Scholar] [CrossRef]
- Salawi, A. Self-emulsifying drug delivery systems: A novel approach to deliver drugs. Drug Deliv. 2022, 29, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, L.; Zhu, W.; Guo, R.; Sun, H.; Chen, X.; Deng, N. Barriers and strategies of cationic liposomes for cancer gene therapy. Mol. Ther.-Methods Clin. Dev. 2020, 18, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ye, J.; Zhang, Q. Self-emulsifying drug delivery system improve oral bioavailability: Role of excipients and physico-chemical characterization. Pharm. Nanotechnol. 2020, 8, 290–301. [Google Scholar] [CrossRef]
- Dhaval, M.; Vaghela, P.; Patel, K.; Sojitra, K.; Patel, M.; Patel, S.; Dudhat, K.; Shah, S.; Manek, R.; Parmar, R. Lipid-based emulsion drug delivery systems—A comprehensive review. Drug Deliv. Transl. Res. 2022, 12, 1616–1639. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, C.H.; Xu, Z.P. Self-nanoemulsifying drug-delivery system and solidified self-nanoemulsifying drug-delivery system. In Nanocarriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 421–449. [Google Scholar]
- Nardin, I.; Köllner, S. Successful development of oral SEDDS: Screening of excipients from the industrial point of view. Adv. Drug Deliv. Rev. 2019, 142, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications. Adv. Colloid Interface Sci. 2020, 275, 102061. [Google Scholar] [CrossRef]
- Jörgensen, A.M.; Friedl, J.D.; Wibel, R.; Chamieh, J.; Cottet, H.; Bernkop-Schnürch, A. Cosolvents in self-emulsifying drug delivery systems (SEDDS): Do they really solve our solubility problems? Mol. Pharm. 2020, 17, 3236–3245. [Google Scholar] [CrossRef]
- Rehman, F.U.; Farid, A.; Shah, S.U.; Dar, M.J.; Rehman, A.U.; Ahmed, N.; Rashid, S.A.; Shaukat, I.; Shah, M.; Albadrani, G.M. Self-emulsifying drug delivery systems (SEDDS): Measuring energy dynamics to determine thermodynamic and kinetic stability. Pharmaceuticals 2022, 15, 1064. [Google Scholar] [CrossRef]
- Andrade, F.; Fonte, P.; Oliva, M.; Videira, M.; Ferreira, D.; Sarmento, B. Solid state formulations composed by amphiphilic polymers for delivery of proteins: Characterization and stability. Int. J. Pharm. 2015, 486, 195–206. [Google Scholar] [CrossRef]
- Bashir, M.A.; Khan, A.; Shah, S.I.; Ullah, M.; Khuda, F.; Abbas, M.; Goh, K.W.; Ming, L.C. Development and Evaluation of Self-Emulsifying Drug-Delivery System–Based Tablets for Simvastatin, a BCS Class II Drug. Drug Des. Dev. Ther. 2023, 17, 261–272. [Google Scholar] [CrossRef]
- Yang, X.; Gao, P.; Jiang, Z.; Luo, Q.; Mu, C.; Cui, M. Preparation and evaluation of self-emulsifying drug delivery system (SEDDS) of cepharanthine. AAPS PharmSciTech 2021, 22, 245. [Google Scholar]
- Goel, H.; Siddiqui, L.; Mahtab, A.; Talegaonkar, S. Fabrication design, process technologies, and convolutions in the scale-up of nanotherapeutic delivery systems. In Nanoparticle Therapeutics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 47–131. [Google Scholar]
- Dholakiya, A.; Dudhat, K.; Patel, J.; Mori, D. An integrated QbD based approach of SMEDDS and liquisolid compacts to simultaneously improve the solubility and processability of hydrochlorthiazide. J. Drug Deliv. Sci. Technol. 2021, 61, 102162. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Omari-Siaw, E.; Adu-Frimpong, M.; Liu, J.; Xu, X.; Yu, J. Enhanced oral bioavailability of Bisdemethoxycurcumin-loaded self-microemulsifying drug delivery system: Formulation design, in vitro and in vivo evaluation. Int. J. Pharm. 2020, 590, 119887. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.M.; Yang, T.L.; Putri, A.D.; Chen, C.T. Application of design of experiments in the development of self-microemulsifying drug delivery systems. Pharmaceuticals 2023, 16, 283. [Google Scholar] [CrossRef] [PubMed]
- Mandić, J.; Pirnat, V.; Luštrik, M.; Ilić, I.G.; Vrečer, F.; Gašperlin, M.; Pobirk, A.Z. Solidification of SMEDDS by fluid bed granulation and manufacturing of fast drug release tablets. Int. J. Pharm. 2020, 583, 119377. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.; Ye, J.; Dong, W.; Zhang, X.; Xu, Y.; Hu, J.; Wang, R.; Xia, X.; Yang, Y. Improved safety and anti-glioblastoma efficacy of cat3-encapsulated smedds through metabolism modification. Molecules 2021, 26, 484. [Google Scholar] [CrossRef]
- Jain, S.K.; Panchal, N.; Singh, A.; Thakur, S.; Shahtaghi, N.R.; Sharma, S.; Guleria, A. Novel self-micro emulsifying drug delivery system for safe intramuscular delivery with improved pharmacodynamics and pharmacokinetics. Curr. Drug Deliv. 2021, 18, 1533–1549. [Google Scholar] [CrossRef]
- Sapiun, Z.; Imran, A.K.; Dewi, S.T.R.; Pade, D.; Ibrahim, W.; Tungadi, R.; Abdulkadir, W.S.; Banne, Y.; Rifai, Y.; Sartini, S. Formulation and Characterization of Self Nano-Emulsifying Drug Delivery System (SNEDDS) Fraction of N-Hexane: Ethyl Acetate from Sesewanua Leaf (Clerodendrum Fragrans Wild.). Int. J. Appl. Pharm. 2023, 15, 72–77. [Google Scholar] [CrossRef]
- Mahmood, A.; Bernkop-Schnürch, A. SEDDS: A game changing approach for the oral administration of hydrophilic macromolecular drugs. Adv. Drug Deliv. Rev. 2019, 142, 91–101. [Google Scholar] [CrossRef]
- Griesser, J.; Hetényi, G.; Kadas, H.; Demarne, F.; Jannin, V.; Bernkop-Schnürch, A. Self-emulsifying peptide drug delivery systems: How to make them highly mucus permeating. Int. J. Pharm. 2018, 538, 159–166. [Google Scholar] [CrossRef]
- Abd-Elhakeem, E.; Teaima, M.H.; Abdelbary, G.A.; El Mahrouk, G.M. Bioavailability enhanced clopidogrel-loaded solid SNEDDS: Development and in-vitro/in-vivo characterization. J. Drug Deliv. Sci. Technol. 2019, 49, 603–614. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, Y.J.; Woo, M.R.; Cheon, S.; Ji, S.H.; Im, D.; Ud Din, F.; Kim, J.O.; Youn, Y.S.; Oh, K.T.; et al. New potential application of hydroxypropyl-β-cyclodextrin in solid self-nanoemulsifying drug delivery system and solid dispersion. Carbohydr. Polym. 2021, 271, 118433. [Google Scholar] [CrossRef] [PubMed]
- Bahiraei, M.; Derakhshandeh, K.; Mahjub, R. Hydrophobic ion pairing with cationic derivatives of α-, ß-, and γ-cyclodextrin as a novel approach for development of a self-nano-emulsifying drug delivery system (SNEDDS) for oral delivery of heparin. Drug Dev. Ind. Pharm. 2021, 47, 1809–1823. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Khan, M.Z.; Tayyab, M.; Madni, A.; Khalid, Q. Self-Nanoemulsification of Healthy Oils to Enhance the Solubility of Lipophilic Drugs. JoVE (J. Vis. Exp.) 2022, 185, e63995. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A. Bioactive SNEDDS Containing Curcumin for the Treatment of Inflammation; Quaid-i-Azam University: Islamabad, Pakistan, 2023. [Google Scholar]
- Kazi, M.; Khan, M.F.; Nasr, F.A.; Ahmed, M.Z.; Alqahtani, A.S.; Ali, M.M.; Aldughaim, M.S. Development of Curcumin and Piperine-Loaded Bio-Active Self-Nanoemulsifying Drugs and Investigation of Their Bioactivity in Zebrafish Embryos and Human Hematological Cancer Cell Lines. Int. J. Nanomed. 2023, 18, 1793–1808. [Google Scholar] [CrossRef]
- Sherif, A.Y.; Shahba, A.A.-W. Development of a Multifunctional Oral Dosage Form via Integration of Solid Dispersion Technology with a Black Seed Oil-Based Self-Nanoemulsifying Drug Delivery System. Biomedicines 2023, 11, 2733. [Google Scholar] [CrossRef]
- Park, H.; Ha, E.-S.; Kim, M.-S. Current status of supersaturable self-emulsifying drug delivery systems. Pharmaceutics 2020, 12, 365. [Google Scholar] [CrossRef]
- Muller, R.H.; Shegokar, R.; M Keck, C. 20 years of lipid nanoparticles (SLN & NLC): Present state of development & industrial applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar]
- Barroso, L.C.; Viegas, C.; Vieira, J.; Ferreira-Pêgo, C.; Costa, J.; Fonte, P. Lipid-based carriers for food ingredients delivery. J. Food Eng. 2021, 295, 110451. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Kumar, N.S.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Munir, M.; Zaman, M.; Waqar, M.A.; Khan, M.A.; Alvi, M.N. Solid lipid nanoparticles: A versatile approach for controlled release and targeted drug delivery. J. Liposome Res. 2023, 34, 335–348. [Google Scholar] [CrossRef]
- Fonte, P.; Andrade, F.; Azevedo, C.; Pinto, J.; Seabra, V.; van de Weert, M.; Reis, S.; Sarmento, B. Effect of the Freezing Step in the Stability and Bioactivity of Protein-Loaded PLGA Nanoparticles Upon Lyophilization. Pharm. Res. 2016, 33, 2777–2793. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.I.; Pinho, C.; Fonte, P.; Sarmento, B.; Dias, A.C.P. Development, characterization, antioxidant and hepatoprotective properties of poly(Ɛ-caprolactone) nanoparticles loaded with a neuroprotective fraction of Hypericum perforatum. Int. J. Biol. Macromol. 2018, 110, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Pucek-Kaczmarek, A.; Celary, D.; Bazylińska, U. Natural-Origin Betaine Surfactants as Promising Components for the Stabilization of Lipid Carriers. Int. J. Mol. Sci. 2024, 25, 955. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H.; Chen, H.-L.; Dong, J.-R. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) as food-grade nanovehicles for hydrophobic nutraceuticals or bioactives. Appl. Sci. 2023, 13, 1726. [Google Scholar] [CrossRef]
- Souto, E.; Wissing, S.; Barbosa, C.; Müller, R. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int. J. Pharm. 2004, 278, 71–77. [Google Scholar] [CrossRef]
- Borges, A.; Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rehman, M.; Ihsan, A.; Madni, A.; Bajwa, S.Z.; Shi, D.; Webster, T.J.; Khan, W.S. Solid lipid nanoparticles for thermoresponsive targeting: Evidence from spectrophotometry, electrochemical, and cytotoxicity studies. Int. J. Nanomed. 2017, 12, 8325–8336. [Google Scholar] [CrossRef]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef]
- Eroğlu, C.; Sinani, G.; Ülker, Z. Current state of lipid nanoparticles (SLN and NLC) for skin applications. Curr. Pharm. Des. 2023, 29, 1632–1644. [Google Scholar] [CrossRef]
- Ahmad, J. Lipid Nanoparticles Based Cosmetics with Potential Application in Alleviating Skin Disorders. Cosmetics 2021, 8, 84. [Google Scholar] [CrossRef]
- Gupta, V.; Mohapatra, S. Nanotechnology in Cosmetics and Cosmeceuticals—A Review of Latest Advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Assali, M.; Zaid, A.N. Features, applications, and sustainability of lipid nanoparticles in cosmeceuticals. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2022, 30, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Makoni, P.A.; Wa Kasongo, K.; Walker, R.B. Short term stability testing of efavirenz-loaded solid lipid nanoparticle (SLN) and nanostructured lipid carrier (NLC) dispersions. Pharmaceutics 2019, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- López, K.L.; Ravasio, A.; González-Aramundiz, J.V.; Zacconi, F.C. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) Prepared by Microwave and Ultrasound-Assisted Synthesis: Promising Green Strategies for the Nanoworld. Pharmaceutics 2023, 15, 1333. [Google Scholar] [CrossRef]
- Jahangirian, H.; Kalantari, K.; Izadiyan, Z.; Rafiee-Moghaddam, R.; Shameli, K.; Webster, T.J. A review of small molecules and drug delivery applications using gold and iron nanoparticles. Int. J. Nanomed. 2019, 14, 1633–1657. [Google Scholar] [CrossRef]
- Smith, T.; Affram, K.; Nottingham, E.L.; Han, B.; Amissah, F.; Krishnan, S.; Trevino, J.; Agyare, E. Application of smart solid lipid nanoparticles to enhance the efficacy of 5-fluorouracil in the treatment of colorectal cancer. Sci. Rep. 2020, 10, 16989. [Google Scholar] [CrossRef]
- Fonte, P.; Nogueira, T.; Gehm, C.; Ferreira, D.; Sarmento, B. Chitosan-coated solid lipid nanoparticles enhance the oral absorption of insulin. Drug Deliv. Transl. Res. 2011, 1, 299–308. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Maeng, H.J. Pharmacokinetics and Pharmacodynamics of Intranasal Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Nose-to-Brain Delivery. Pharmaceutics 2022, 14, 572. [Google Scholar] [CrossRef]
- Soares, S.; Fonte, P.; Costa, A.; Andrade, J.; Seabra, V.; Ferreira, D.; Reis, S.; Sarmento, B. Effect of freeze-drying, cryoprotectants and storage conditions on the stability of secondary structure of insulin-loaded solid lipid nanoparticles. Int. J. Pharm. 2013, 456, 370–381. [Google Scholar] [CrossRef]
- Araújo, F.; Shrestha, N.; Shahbazi, M.A.; Fonte, P.; Mäkilä, E.M.; Salonen, J.J.; Hirvonen, J.T.; Granja, P.L.; Santos, H.A.; Sarmento, B. The impact of nanoparticles on the mucosal translocation and transport of GLP-1 across the intestinal epithelium. Biomaterials 2014, 35, 9199–9207. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Enhanced bioaccessibility and stability of iron through W/O/W double emulsion-based solid lipid nanoparticles and coating with water-soluble chitosan. Int. J. Biol. Macromol. 2022, 209, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Tootoonchi, M.H.; Fraker, C.A.; Walls, J.D. Reverse-dialysis can be misleading for drug release studies in emulsions as demonstrated by NMR dilution experiments. Int. J. Pharm. 2021, 608, 121093. [Google Scholar] [CrossRef] [PubMed]
- Dawson, K.A.; Yan, Y. Current understanding of biological identity at the nanoscale and future prospects. Nat. Nanotechnol. 2021, 16, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.-y.; Wang, M.-t.; Chen, F.; Gong, T.; Jian, Y.-l.; Zhang, Z.-r.; Huang, Y. Lung-targeting delivery of dexamethasone acetate loaded solid lipid nanoparticles. Arch. Pharmacal Res. 2007, 30, 519–525. [Google Scholar] [CrossRef]
- Tucak-Smajić, A.; Ruseska, I.; Letofsky-Papst, I.; Vranić, E.; Zimmer, A. Development and Characterization of Cationic Nanostructured Lipid Carriers as Drug Delivery Systems for miRNA-27a. Pharmaceuticals 2023, 16, 1007. [Google Scholar] [CrossRef]
- Yuan, H.; Miao, J.; Du, Y.-Z.; You, J.; Hu, F.-Q.; Zeng, S. Cellular uptake of solid lipid nanoparticles and cytotoxicity of encapsulated paclitaxel in A549 cancer cells. Int. J. Pharm. 2008, 348, 137–145. [Google Scholar] [CrossRef]
- Aghabagherzadeh, M.; Karimi, E.; Zareian, M. Folic Acid-Conjugated Chitosan-Coated Solid Lipid Nanoparticles: Precision Targeting of Artemisia vulgaris Essential Oils for Anticancer Therapy. Chem. Biodivers. 2024, 21, e202300187. [Google Scholar] [CrossRef]
- Fathy Abd-Ellatef, G.-E.; Gazzano, E.; Chirio, D.; Ragab Hamed, A.; Belisario, D.C.; Zuddas, C.; Peira, E.; Rolando, B.; Kopecka, J.; Assem Said Marie, M. Curcumin-loaded solid lipid nanoparticles bypass p-glycoprotein mediated doxorubicin resistance in triple negative breast cancer cells. Pharmaceutics 2020, 12, 96. [Google Scholar] [CrossRef]
- Seo, Y.; Lim, H.; Park, H.; Yu, J.; An, J.; Yoo, H.Y.; Lee, T. Recent Progress of lipid nanoparticles-based lipophilic drug delivery: Focus on surface modifications. Pharmaceutics 2023, 15, 772. [Google Scholar] [CrossRef]
- Terribile, G.; Di Girolamo, S.; Donzelli, E.; Re, F.; Gasco, P.; Sancini, G. The fantastic voyage of solid lipid nanoparticles from the lung to the brain: Non-invasive tomographic imaging as a feasible refinement process. Biomed. Sci. Eng. 2023, 4. [Google Scholar] [CrossRef]
- Athalye, M.; Teli, D.; Chorawala, M.; Sharma, A.; Patel, R.; Dua, K.; Singh, S.K.; Gupta, G.; Patel, M. Apolipoprotein E3 functionalized lipid-drug conjugated nanoparticles of Levetiracetam for enhanced delivery to the brain: In-vitro cell line studies and in-vivo study. Int. J. Biol. Macromol. 2024, 254, 127799. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Verma, A.; Saharan, V.A. Lipid drug carriers for cancer therapeutics: An insight into lymphatic targeting, P-gp, CYP3A4 modulation and bioavailability enhancement. Adv. Pharm. Bull. 2020, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Madni, A.; Ihsan, A.; Khan, W.S.; Khan, M.I.; Mahmood, M.A.; Ashfaq, M.; Bajwa, S.Z.; Shakir, I. Solid and liquid lipid-based binary solid lipid nanoparticles of diacerein: In vitro evaluation of sustained release, simultaneous loading of gold nanoparticles, and potential thermoresponsive behavior. Int. J. Nanomed. 2015, 10, 2805–2814. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Madni, A.; Shi, D.; Ihsan, A.; Tahir, N.; Chang, K.; Javed, I.; Webster, T. Enhanced blood brain barrier permeability and glioblastoma cell targeting via thermoresponsive lipid nanoparticles. Nanoscale 2017, 9, 15434–15440. [Google Scholar] [CrossRef]
- Pinheiro, R.; Granja, A.; Loureiro, J.A.; Pereira, M.; Pinheiro, M.; Neves, A.; Reis, S. RVG29-functionalized lipid nanoparticles for quercetin brain delivery and Alzheimer’s disease. Pharm. Res. 2020, 37, 139. [Google Scholar] [CrossRef]
- Müller, J.; Bauer, K.N.; Prozeller, D.; Simon, J.; Mailänder, V.; Wurm, F.R.; Winzen, S.; Landfester, K. Coating nanoparticles with tunable surfactants facilitates control over the protein corona. Biomaterials 2017, 115, 1–8. [Google Scholar] [CrossRef]
- Chen, T.; Pan, F.; Luo, G.; Jiang, K.; Wang, H.; Ding, T.; Li, W.; Zhan, C.; Wei, C. Morphology-driven protein corona manipulation for preferential delivery of lipid nanodiscs. Nano Today 2022, 46, 101609. [Google Scholar] [CrossRef]
- Zhang, Z.; Guan, J.; Jiang, Z.; Yang, Y.; Liu, J.; Hua, W.; Mao, Y.; Li, C.; Lu, W.; Qian, J.; et al. Brain-targeted drug delivery by manipulating protein corona functions. Nat. Commun. 2019, 10, 3561. [Google Scholar] [CrossRef]
- Kržič, M.; Šentjurc, M.; Kristl, J. Improved skin oxygenation after benzyl nicotinate application in different carriers as measured by EPR oximetry in vivo. J. Control. Release 2001, 70, 203–211. [Google Scholar] [CrossRef]
- Jain, S.; Addan, R.; Kushwah, V.; Harde, H.; Mahajan, R.R. Comparative assessment of efficacy and safety potential of multifarious lipid based Tacrolimus loaded nanoformulations. Int. J. Pharm. 2019, 562, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef]
- Wang, F.; Chen, J.; Dai, W.; He, Z.; Zhai, D.; Chen, W. Pharmacokinetic studies and anticancer activity of curcumin-loaded nanostructured lipid carriers. Acta Pharm. 2017, 67, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Pashizeh, F.; Moeinabadi-Bidgoli, K.; Eyvazi, Y.; Akbari, T.; Moghaddam, Z.S.; Eskandarisani, M.; Farahmand, F.; Hafezi, Y.; Jevinani, H.N. Co-encapsulation of hydrophilic and hydrophobic drugs into niosomal nanocarrier for enhanced breast cancer therapy: In silico and in vitro studies. Environ. Res. 2023, 239, 117292. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, K.; Pani, A.; Chowdhury, T.; Kundu, A.; Thomas, S. Multi-vesicular Liposome and its Applications: A Novel Chemically Modified Approach for Drug Delivery Application. Mini Rev. Med. Chem. 2024, 24, 26–38. [Google Scholar] [CrossRef]
- Viegas, C.; Seck, F.; Fonte, P. An insight on lipid nanoparticles for therapeutic proteins delivery. J. Drug Deliv. Sci. Technol. 2022, 77, 103839. [Google Scholar] [CrossRef]
- Crommelin, D.J.; van Hoogevest, P.; Storm, G. The role of liposomes in clinical nanomedicine development. What now? Now what? J. Control. Release 2020, 318, 256–263. [Google Scholar] [CrossRef]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef]
- Kheilnezhad, B.; Hadjizadeh, A. Factors affecting the penetration of niosome into the skin, their laboratory measurements and dependency to the niosome composition: A review. Curr. Drug Deliv. 2021, 18, 555–569. [Google Scholar] [CrossRef]
- Patel, P.; Parashar, A.K.; Kaurav, M.; Yadav, K.; Singh, D.; Gupta, G.; Kurmi, B.D. Niosome: A Vesicular Drug Delivery Tool. In Nanoparticles and Nanocarriers Based Pharmaceutical Formulations; Bentham Science Publishers: Sharjah, United Arab Emirates, 2022; pp. 333–364. [Google Scholar]
- Khan, D.H.; Bashir, S.; Khan, M.I.; Figueiredo, P.; Santos, H.A.; Peltonen, L. Formulation optimization and in vitro characterization of rifampicin and ceftriaxone dual drug loaded niosomes with high energy probe sonication technique. J. Drug Deliv. Sci. Technol. 2020, 58, 101763. [Google Scholar] [CrossRef]
- Havlikova, M.; Szabova, J.; Mravcova, L.; Venerova, T.; Chang, C.-H.; Pekar, M.; Jugl, A.; Mravec, F. Cholesterol Effect on Membrane Properties of Cationic Ion Pair Amphiphile Vesicles at Different Temperatures. Langmuir 2021, 37, 2436–2444. [Google Scholar] [CrossRef] [PubMed]
- Moammeri, A.; Chegeni, M.M.; Sahrayi, H.; Ghafelehbashi, R.; Memarzadeh, F.; Mansouri, A.; Akbarzadeh, I.; Hejabi, F.; Abtahi, M.S.; Ren, Q. Current advances in niosomes applications for drug delivery and cancer treatment. Mater. Today Bio 2023, 23, 100837. [Google Scholar] [CrossRef] [PubMed]
- Nigro, F.; Cerqueira Pinto, C.d.S.; dos Santos, E.P.; Mansur, C.R.E. Niosome-based hydrogel as a potential drug delivery system for topical and transdermal applications. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 444–461. [Google Scholar] [CrossRef]
- Yasamineh, S.; Yasamineh, P.; Kalajahi, H.G.; Gholizadeh, O.; Yekanipour, Z.; Afkhami, H.; Eslami, M.; Kheirkhah, A.H.; Taghizadeh, M.; Yazdani, Y. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int. J. Pharm. 2022, 624, 121878. [Google Scholar] [CrossRef] [PubMed]
- Akbari, J.; Saeedi, M.; Morteza-Semnani, K.; Hashemi, S.M.H.; Babaei, A.; Eghbali, M.; Mohammadi, M.; Rostamkalaei, S.S.; Asare-Addo, K.; Nokhodchi, A. Innovative topical niosomal gel formulation containing diclofenac sodium (niofenac). J. Drug Target. 2022, 30, 108–117. [Google Scholar] [CrossRef]
- Khan, M.I.; Madni, A.; Hirvonen, J.; Peltonen, L. Ultrasonic processing technique as a green preparation approach for diacerein-loaded niosomes. AAPS PharmSciTech 2017, 18, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, M.G.; He, Y.; Yeo, Y. Recent trends in the delivery of RNA drugs: Beyond the liver, more than vaccine. Eur. J. Pharm. Biopharm. 2024, 197, 114203. [Google Scholar] [CrossRef]
- Bishani, A.; Makarova, D.M.; Shmendel, E.V.; Maslov, M.A.; Sen ‘kova, A.V.; Savin, I.A.; Gladkikh, D.V.; Zenkova, M.A.; Chernolovskaya, E.L. Influence of the Composition of Cationic Liposomes on the Performance of Cargo Immunostimulatory RNA. Pharmaceutics 2023, 15, 2184. [Google Scholar] [CrossRef]
- Attia, N.; Mashal, M.; Grijalvo, S.; Eritja, R.; Zárate, J.; Puras, G.; Pedraz, J.L. Stem cell-based gene delivery mediated by cationic niosomes for bone regeneration. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 521–531. [Google Scholar] [CrossRef]
- Al Qtaish, N.; Gallego, I.; Villate-Beitia, I.; Sainz-Ramos, M.; López-Méndez, T.B.; Grijalvo, S.; Eritja, R.; Soto-Sánchez, C.; Martínez-Navarrete, G.; Fernández, E. Niosome-based approach for in situ gene delivery to retina and brain cortex as immune-privileged tissues. Pharmaceutics 2020, 12, 198. [Google Scholar] [CrossRef]
- Eda Sutova, H.; Kutlu, O.; Cetinel, S. Niosomal Drug Delivery Systems for Ocular Disease-Recent Advances and Future Prospects. Nanomaterials 2020, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, E.; Puras, G.; Agirre, M.; Zárate, J.; Grijalvo, S.; Pons, R.; Eritja, R.; Martinez-Navarrete, G.; Soto-Sánchez, C.; Fernández, E. Niosomes based on synthetic cationic lipids for gene delivery: The influence of polar head-groups on the transfection efficiency in HEK-293, ARPE-19 and MSC-D1 cells. Org. Biomol. Chem. 2015, 13, 1068–1081. [Google Scholar] [CrossRef] [PubMed]
- Awashra, M.; Młynarz, P. The toxicity of nanoparticles and their interaction with cells: An in vitro metabolomic perspective. Nanoscale Adv. 2023, 5, 2674–2723. [Google Scholar] [CrossRef] [PubMed]
- Fuller, D.H.; Berglund, P. Amplifying RNA Vaccine Development. N. Engl. J. Med. 2020, 382, 2469–2471. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, B.; Gupta, R.; Patel, P.; Salave, S.; Sharma, A.; Desai, D.; Benival, D.; Kommineni, N. Emerging Trends in Lipid-Based Vaccine Delivery: A Special Focus on Developmental Strategies, Fabrication Methods, and Applications. Vaccines 2023, 11, 661. [Google Scholar] [CrossRef]
- Ali, A.; Waris, A.; Khan, M.A.; Asim, M.; Khan, A.U.; Khan, S.; Zeb, J. Recent advancement, immune responses, and mechanism of action of various vaccines against intracellular bacterial infections. Life Sci. 2023, 314, 121332. [Google Scholar] [CrossRef]
- Liu, M.A. A Comparison of Plasmid DNA and mRNA as Vaccine Technologies. Vaccines 2019, 7, 37. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Wuebben, C.; Bartok, E.; Hartmann, G. Innate sensing of mRNA vaccines. Curr. Opin. Immunol. 2022, 79, 102249. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, X.-Y.; Lu, A.; Wang, X.-Y.; Jiang, L.-X.; Wang, J.-C. Non-viral vectors for RNA delivery. J. Control. Release 2022, 342, 241–279. [Google Scholar] [CrossRef]
- Swetha, K.; Kotla, N.G.; Tunki, L.; Jayaraj, A.; Bhargava, S.K.; Hu, H.; Bonam, S.R.; Kurapati, R. Recent advances in the lipid nanoparticle-mediated delivery of mRNA vaccines. Vaccines 2023, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Stolk, D.A.; Gabrowska, J.; Twilhaar, M.N.; Ambrosini, M.; Storm, G.; Van Der Vliet, H.J.; De Gruijl, T.D.; Van Kooyk, Y.; Den Haan, J.M. Liposomal nanovaccine containing A-galactosylceramide and ganglioside GM3 stimulates robust CD8+ T cell responses via CD169+ macrophages and CDC1. VU Res. Portal 2022, 9, 56. [Google Scholar] [CrossRef]
- Lian, T.; Ho, R.J. Trends and developments in liposome drug delivery systems. J. Pharm. Sci. 2001, 90, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.N.; Mishra, V.; Rawat, A.; Dubey, P.; Mahor, S.; Jain, S.; Chatterji, D.; Vyas, S.P. Non-invasive vaccine delivery in transfersomes, niosomes and liposomes: A comparative study. Int. J. Pharm. 2005, 293, 73–82. [Google Scholar] [CrossRef]
- Beg, S.; Alharbi, K.S.; Alruwaili, N.K.; Alotaibi, N.H.; Almalki, W.H.; Alenezi, S.K.; Altowayan, W.M.; Alshammari, M.S.; Rahman, M. Nanotherapeutic systems for delivering cancer vaccines: Recent advances. Nanomedicine 2020, 15, 1527–1537. [Google Scholar] [CrossRef]
- Di, J.; Xie, F.; Xu, Y. When liposomes met antibodies: Drug delivery and beyond. Adv. Drug Deliv. Rev. 2020, 154, 151–162. [Google Scholar] [CrossRef]
- Moutabian, H.; Ghahramani-Asl, R.; Mortezazadeh, T.; Laripour, R.; Narmani, A.; Zamani, H.; Ataei, G.; Bagheri, H.; Farhood, B.; Sathyapalan, T. The cardioprotective effects of nano-curcumin against doxorubicin-induced cardiotoxicity: A systematic review. Biofactors 2022, 48, 597–610. [Google Scholar] [CrossRef]
- Dorostkar, H.; Haghiralsadat, B.F.; Hemati, M.; Safari, F.; Hassanpour, A.; Naghib, S.M.; Roozbahani, M.H.; Mozafari, M.; Moradi, A. Reduction of doxorubicin-induced cardiotoxicity by Co-administration of smart liposomal doxorubicin and free quercetin: In vitro and in vivo studies. Pharmaceutics 2023, 15, 1920. [Google Scholar] [CrossRef]
- Davarpanah, F.; Khalili Yazdi, A.; Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M. Magnetic delivery of antitumor carboplatin by using PEGylated-Niosomes. DARU J. Pharm. Sci. 2018, 26, 57–64. [Google Scholar] [CrossRef]
- Luiz, M.T.; Dutra, J.A.P.; de Cássia Ribeiro, T.; Carvalho, G.C.; Sábio, R.M.; Marchetti, J.M.; Chorilli, M. Folic acid-modified curcumin-loaded liposomes for breast cancer therapy. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128935. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Liu, B. Formulation strategies for folate-targeted liposomes and their biomedical applications. Pharmaceutics 2019, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Gottesmann, M.; Goycoolea, F.M.; Steinbacher, T.; Menogni, T.; Hensel, A. Smart drug delivery against Helicobacter pylori: Pectin-coated, mucoadhesive liposomes with antiadhesive activity and antibiotic cargo. Appl. Microbiol. Biotechnol. 2020, 104, 5943–5957. [Google Scholar] [CrossRef] [PubMed]
- Dasa, S.S.K.; Diakova, G.; Suzuki, R.; Mills, A.M.; Gutknecht, M.F.; Klibanov, A.L.; Slack-Davis, J.K.; Kelly, K.A. Plectin-targeted liposomes enhance the therapeutic efficacy of a PARP inhibitor in the treatment of ovarian cancer. Theranostics 2018, 8, 2782. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Guo, R.; Zhang, L.; Liu, W.; Kong, L.; Liu, Y.; Yu, Y.; Zang, J.; Chen, W.; Li, X. Mannose-modified celastrol liposomes targeted activated macrophages for rheumatoid arthritis treatment in vitro and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2024, 91, 105185. [Google Scholar] [CrossRef]
- Hagimori, M.; Chinda, Y.; Suga, T.; Yamanami, K.; Kato, N.; Inamine, T.; Fuchigami, Y.; Kawakami, S. Synthesis of high functionality and quality mannose-grafted lipids to produce macrophage-targeted liposomes. Eur. J. Pharm. Sci. 2018, 123, 153–161. [Google Scholar] [CrossRef]
- Demir, B.; Moulahoum, H.; Ghorbanizamani, F.; Barlas, F.B.; Yesiltepe, O.; Gumus, Z.P.; Meral, K.; Demirkol, D.O.; Timur, S. Carbon dots and curcumin-loaded CD44-Targeted liposomes for imaging and tracking cancer chemotherapy: A multi-purpose tool for theranostics. J. Drug Deliv. Sci. Technol. 2021, 62, 102363. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Loureiro, J.A.; Coelho, M.A.; Pereira, M.C. Transferrin receptor-targeted nanocarriers: Overcoming barriers to treat glioblastoma. Pharmaceutics 2022, 14, 279. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Bak, M.; Melander, F.; Thomsen, M.S.; Burkhart, A.; Kempen, P.J.; Andresen, T.L.; Moos, T. Modulating the antibody density changes the uptake and transport at the blood-brain barrier of both transferrin receptor-targeted gold nanoparticles and liposomal cargo. J. Control. Release 2019, 295, 237–249. [Google Scholar] [CrossRef]
- Moreira, T.d.S.; Silva, A.D.O.; Vasconcelos, B.R.F.; Santos, E.d.S.; de Sousa, A.C.C.; de Freitas, J.V.B.; de Oliveira, Y.S.; Vidal, L.M.T.; Ribeiro, F.d.O.S.; de Araújo, A.R. DOPE/CHEMS-Based EGFR-Targeted Immunoliposomes for Docetaxel Delivery: Formulation Development, Physicochemical Characterization and Biological Evaluation on Prostate Cancer Cells. Pharmaceutics 2023, 15, 915. [Google Scholar] [CrossRef]
- Canato, E.; Grigoletto, A.; Zanotto, I.; Tedeschini, T.; Campara, B.; Quaglio, G.; Toffoli, G.; Mandracchia, D.; Dinarello, A.; Tiso, N. Anti-HER2 Super Stealth Immunoliposomes for Targeted-Chemotherapy. Adv. Healthc. Mater. 2023, 12, 2301650. [Google Scholar] [CrossRef]
- Thumrongsiri, N.; Dana, P.; Bawab, R.; Tanyapanyachon, P.; Treetidnipa, C.; Saengkrit, N.; Sathornsumetee, S. Assessment of therapeutic effect of CD20-targeted immunoliposome in primary central nervous system lymphoma. Biomed. Pharmacother. 2022, 150, 112979. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Fu, Y.-X.; Peng, H. Targeting tumor cells with antibodies enhances anti-tumor immunity. Biophys. Rep. 2018, 4, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Lakkadwala, S.; dos Santos Rodrigues, B.; Sun, C.; Singh, J. Biodistribution of TAT or QLPVM coupled to receptor targeted liposomes for delivery of anticancer therapeutics to brain in vitro and in vivo. Nanomed. Nanotechnol. Biol. Med. 2020, 23, 102112. [Google Scholar] [CrossRef] [PubMed]
- Matei, A.-M.; Caruntu, C.; Tampa, M.; Georgescu, S.R.; Matei, C.; Constantin, M.M.; Constantin, T.V.; Calina, D.; Ciubotaru, D.A.; Badarau, I.A.; et al. Applications of Nanosized-Lipid-Based Drug Delivery Systems in Wound Care. Appl. Sci. 2021, 11, 4915. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Froiio, F.; Mancuso, A.; Iannone, M.; Fresta, M.; Fiorito, S.; Celia, C.; Paolino, D. In vitro and in vivo trans-epidermal water loss evaluation following topical drug delivery systems application for pharmaceutical analysis. J. Pharm. Biomed. Anal. 2020, 186, 113295. [Google Scholar] [CrossRef]
- Téllez, J.; Echeverry, M.C.; Romero, I.; Guatibonza, A.; Santos Ramos, G.; Borges De Oliveira, A.C.; Frézard, F.; Demicheli, C. Use of liposomal nanoformulations in antileishmania therapy: Challenges and perspectives. J. Liposome Res. 2021, 31, 169–176. [Google Scholar] [CrossRef]
- Chaud, M.V.; Amaral, V.A.; Batain, F.; Crescencio, K.M.M.; dos Santos, C.A.; Rebelo, M.A.; Soeiro, V.S. Nanobiotechnological Strategies for Treatment of Tegumentary and Visceral Leishmaniasis Including Resistance Strains. In Nanotechnology in Skin, Soft Tissues and Bone Infections; Springer: Cham, Switzerland, 2020; pp. 183–204. [Google Scholar]
- Guo, J.; Ping, Q.; Chen, Y. Pharmacokinetic behavior of cyclosporin A in rabbits by oral administration of lecithin vesicle and Sandimmun Neoral. Int. J. Pharm. 2001, 216, 17–21. [Google Scholar] [CrossRef]
- Jadon, P.S.; Gajbhiye, V.; Jadon, R.S.; Gajbhiye, K.R.; Ganesh, N. Enhanced oral bioavailability of griseofulvin via niosomes. AAPS PharmSciTech 2009, 10, 1186–1192. [Google Scholar] [CrossRef]
- Khan, M.I.; Madni, A.; Peltonen, L. Development and in-vitro characterization of sorbitan monolaurate and poloxamer 184 based niosomes for oral delivery of diacerein. Eur. J. Pharm. Sci. 2016, 95, 88–95. [Google Scholar] [CrossRef]
- Sgorla, D.; Bunhak, É.J.; Cavalcanti, O.A.; Fonte, P.; Sarmento, B. Exploitation of lipid-polymeric matrices at nanoscale for drug delivery applications. Expert Opin. Drug Deliv. 2016, 13, 1301–1309. [Google Scholar] [CrossRef]
- Yazdian-Robati, R.; Ahmadi, S.M.; Mavandadnejad, F.; Ebrahimnejad, P.; Amirkhanloo, S.; Shad, A. Liposome-and niosome-based drug delivery for pancreatic cancer. In Recent Advances in Nanocarriers for Pancreatic Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2024; pp. 95–114. [Google Scholar]
- Madni, A.; Sarfraz, M.; Rehman, M.; Ahmad, M.; Akhtar, N.; Ahmad, S.; Tahir, N.; Ijaz, S.; Al-Kassas, R.; Löbenberg, R. Liposomal drug delivery: A versatile platform for challenging clinical applications. J. Pharm. Pharm. Sci. 2014, 17, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, M.; Shiraishi, T.; Nielsen, P.E. Enzyme-triggered release of the antisense octaarginine-pna conjugate from phospholipase A2 sensitive liposomes. ACS Appl. Bio Mater. 2020, 3, 1018–1025. [Google Scholar] [CrossRef]
- Wang, X.; Tong, J.; He, Z.; Yang, X.; Meng, F.; Liang, H.; Zhang, X.; Luo, L. Paclitaxel-potentiated photodynamic theranostics for synergistic tumor ablation and precise anticancer efficacy monitoring. ACS Appl. Mater. Interfaces 2020, 12, 5476–5487. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Zhang, J.; Jiao, J.; Qin, W.; Yang, X. Photodynamic therapy for prostate cancer: Recent advances, challenges and opportunities. Front. Oncol. 2022, 12, 980239. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhou, S.; Zhang, X.; Li, C.; Ji, S.; Mao, H. Mitochondrion-specific dendritic lipopeptide liposomes for targeted sub-cellular delivery. Nat. Commun. 2021, 12, 2390. [Google Scholar] [CrossRef] [PubMed]
- Kommineni, N.; Chaudhari, R.; Conde, J.; Tamburaci, S.; Cecen, B.; Chandra, P.; Prasad, R. Engineered Liposomes in Interventional Theranostics of Solid Tumors. ACS Biomater. Sci. Eng. 2023, 9, 4527–4557. [Google Scholar] [CrossRef]
- Xiang, J.; Tong, X.; Shi, F.; Yan, Q.; Yu, B.; Zhao, Y. Near-infrared light-triggered drug release from UV-responsive diblock copolymer-coated upconversion nanoparticles with high monodispersity. J. Mater. Chem. B 2018, 6, 3531–3540. [Google Scholar] [CrossRef]
- Huang, X.; Chen, L.; Zhang, Y.; Zhou, S.; Cai, H.-H.; Li, T.; Jin, H.; Cai, J.; Zhou, H.; Pi, J. GE11 peptide conjugated liposomes for EGFR-targeted and chemophotothermal combined anticancer therapy. Bioinorg. Chem. Appl. 2021, 2021. [Google Scholar] [CrossRef]
- Sebeke, L.C.; Gómez, J.D.C.; Heijman, E.; Rademann, P.; Simon, A.C.; Ekdawi, S.; Vlachakis, S.; Toker, D.; Mink, B.L.; Schubert-Quecke, C. Hyperthermia-induced doxorubicin delivery from thermosensitive liposomes via MR-HIFU in a pig model. J. Control. Release 2022, 343, 798–812. [Google Scholar] [CrossRef]
- Lee, S.; Han, H.; Koo, H.; Na, J.H.; Yoon, H.Y.; Lee, K.E.; Lee, H.; Kim, H.; Kwon, I.C.; Kim, K. Extracellular matrix remodeling in vivo for enhancing tumor-targeting efficiency of nanoparticle drug carriers using the pulsed high intensity focused ultrasound. J. Control. Release Off. J. Control. Release Soc. 2017, 263, 68–78. [Google Scholar] [CrossRef]
- Souri, M.; Soltani, M.; Kashkooli, F.M.; Shahvandi, M.K. Engineered strategies to enhance tumor penetration of drug-loaded nanoparticles. J. Control. Release 2022, 341, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, K.; Barzegar-Fallah, A.; Banstola, A.; Rizwan, S.B.; Reynolds, J.N. Ultrasound-Mediated Blood–Brain Barrier Disruption for Drug Delivery: A Systematic Review of Protocols, Efficacy, and Safety Outcomes from Preclinical and Clinical Studies. Pharmaceutics 2022, 14, 833. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-J.; Hsu, H.-L.; Lan, Y.-H.; Chen, J.-P. Thermosensitive Cationic Magnetic Liposomes for Thermoresponsive Delivery of CPT-11 and SLP2 shRNA in Glioblastoma Treatment. Pharmaceutics 2023, 15, 1169. [Google Scholar] [CrossRef] [PubMed]
- Pham, S.H.; Choi, Y.; Choi, J. Stimuli-responsive nanomaterials for application in antitumor therapy and drug delivery. Pharmaceutics 2020, 12, 630. [Google Scholar] [CrossRef]
- Liu, C.; Chen, H.; Zhou, H.; Yu, S.; Zhao, Y.; Wang, N.; Yao, W.; Lu, A.-H.; Qiao, W. MRI-FI-guided superimposed stimulus-responsive co-assembled liposomes for optimizing transmembrane drug delivery pathways and improving cancer efficacy. Appl. Mater. Today 2022, 26, 101368. [Google Scholar] [CrossRef]
- Yao, W.; Liu, C.; Wang, N.; Zhou, H.; Chen, H.; Qiao, W. An MRI-guided targeting dual-responsive drug delivery system for liver cancer therapy. J. Colloid Interface Sci. 2021, 603, 783–798. [Google Scholar] [CrossRef]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.D.F.R.E.; Blasi, M. The use of viral vectors in vaccine development. npj Vaccines 2022, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Kanvinde, S.; Kulkarni, T.; Deodhar, S.; Bhattacharya, D.; Dasgupta, A. Non-Viral Vectors for Delivery of Nucleic Acid Therapies for Cancer. BioTech 2022, 11, 6. [Google Scholar] [CrossRef]
- Broderick, K.E.; Humeau, L.M. Electroporation-enhanced delivery of nucleic acid vaccines. Expert Rev. Vaccines 2015, 14, 195–204. [Google Scholar] [CrossRef]
- Lopes, C.; Cristóvão, J.; Silvério, V.; Lino, P.R. Microfluidic production of mRNA-loaded lipid nanoparticles for vaccine applications. Expert Opin. Drug Deliv. 2022, 19, 1381–1395. [Google Scholar] [CrossRef]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Samaridou, E.; Heyes, J.; Lutwyche, P. Lipid nanoparticles for nucleic acid delivery: Current perspectives. Adv. Drug Deliv. Rev. 2020, 154–155, 37–63. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.P. Review of COVID-19 mRNA Vaccines: BNT162b2 and mRNA-1273. J. Pharm. Pract. 2022, 35, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Ndwandwe, D.; Wiysonge, C.S. COVID-19 vaccines. Curr. Opin. Immunol. 2021, 71, 111–116. [Google Scholar] [CrossRef]
- Pérez-Betancourt, Y.; Araujo, P.M.; Távora, B.D.C.L.F.; Pereira, D.R.; Faquim-Mauro, E.L.; Carmona-Ribeiro, A.M. Cationic and Biocompatible Polymer/Lipid Nanoparticles as Immunoadjuvants. Pharmaceutics 2021, 13, 1859. [Google Scholar] [CrossRef]
- Silva, A.; Martins-Gomes, C.; Coutinho, T.; Fangueiro, J.; Sanchez-Lopez, E.; Pashirova, T.; Andreani, T.; Souto, E. Soft Cationic Nanoparticles for Drug Delivery: Production and Cytotoxicity of Solid Lipid Nanoparticles (SLNs). Appl. Sci. 2019, 9, 4438. [Google Scholar] [CrossRef]
- Paliwal, R.; Paliwal, S.R.; Kenwat, R.; Kurmi, B.D.; Sahu, M.K. Solid lipid nanoparticles: A review on recent perspectives and patents. Expert Opin. Ther. Pat. 2020, 30, 179–194. [Google Scholar] [CrossRef]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, J.; Shen, R.; Lin, J.; Li, S.; Lu, X.; Stelzel, J.L. Screening for lipid nanoparticles that modulate the immune activity of helper T cells towards enhanced antitumour activity. Nat. Biomed. Eng. 2023, 8, 544–560. [Google Scholar] [CrossRef]
- Patel, S.; Ashwanikumar, N.; Robinson, E.; Xia, Y.; Mihai, C.; Griffith III, J.P.; Hou, S.; Esposito, A.A.; Ketova, T.; Welsher, K. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat. Commun. 2020, 11, 983. [Google Scholar] [PubMed]
- Kim, J.; Jozic, A.; Lin, Y.; Eygeris, Y.; Bloom, E.; Tan, X.; Acosta, C.; MacDonald, K.D.; Welsher, K.D.; Sahay, G. Engineering lipid nanoparticles for enhanced intracellular delivery of mRNA through Inhalation. ACS Nano 2022, 16, 14792–14806. [Google Scholar] [PubMed]
- Patel, S.K.; Billingsley, M.M.; Mukalel, A.J.; Thatte, A.S.; Hamilton, A.G.; Gong, N.; El-Mayta, R.; Safford, H.C.; Merolle, M.; Mitchell, M.J. Bile acid-containing lipid nanoparticles enhance extrahepatic mRNA delivery. Theranostics 2024, 14, 1. [Google Scholar] [PubMed]
- Cheng, M.H.Y.; Leung, J.; Zhang, Y.; Strong, C.; Basha, G.; Momeni, A.; Chen, Y.; Jan, E.; Abdolahzadeh, A.; Wang, X. Induction of Bleb Structures in Lipid Nanoparticle Formulations of mRNA Leads to Improved Transfection Potency. Adv. Mater. 2023, 35, 2303370. [Google Scholar]
- Neves, A.R.; Queiroz, J.F.; Costa Lima, S.A.; Figueiredo, F.; Fernandes, R.; Reis, S. Cellular uptake and transcytosis of lipid-based nanoparticles across the intestinal barrier: Relevance for oral drug delivery. J. Colloid Interface Sci. 2016, 463, 258–265. [Google Scholar] [CrossRef]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef]
- Herrera, M.; Kim, J.; Eygeris, Y.; Jozic, A.; Sahay, G. Illuminating endosomal escape of polymorphic lipid nanoparticles that boost mRNA delivery. Biomater. Sci. 2021, 9, 4289–4300. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, J.M.; Haque, S. Strategies in the design of endosomolytic agents for facilitating endosomal escape in nanoparticles. Biochimie 2019, 160, 61–75. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Cullis, P.R.; Van Der Meel, R. Lipid Nanoparticles Enabling Gene Therapies: From Concepts to Clinical Utility. Nucleic Acid Ther. 2018, 28, 146–157. [Google Scholar] [CrossRef]
- del Pozo-Rodríguez, A.; Solinís, M.Á.; Rodríguez-Gascón, A. Applications of lipid nanoparticles in gene therapy. Eur. J. Pharm. Biopharm. 2016, 109, 184–193. [Google Scholar] [CrossRef]
- Jung, H.N.; Lee, S.-Y.; Lee, S.; Youn, H.; Im, H.-J. Lipid nanoparticles for delivery of RNA therapeutics: Current status and the role of in vivo imaging. Theranostics 2022, 12, 7509–7531. [Google Scholar] [CrossRef] [PubMed]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Granados-Riveron, J.T.; Aquino-Jarquin, G. Engineering of the current nucleoside-modified mRNA-LNP vaccines against SARS-CoV-2. Biomed. Pharmacother. 2021, 142, 111953. [Google Scholar] [CrossRef] [PubMed]
- Bettini, E.; Locci, M. SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond. Vaccines 2021, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Kraynyak, K.A.; Blackwood, E.; Agnes, J.; Tebas, P.; Giffear, M.; Amante, D.; Reuschel, E.L.; Purwar, M.; Christensen-Quick, A.; Liu, N.; et al. SARS-CoV-2 DNA Vaccine INO-4800 Induces Durable Immune Responses Capable of Being Boosted in a Phase 1 Open-Label Trial. J. Infect. Dis. 2022, 225, 1923–1932. [Google Scholar] [CrossRef]
- Bhuyan, P.K.; Dallas, M.; Kraynyak, K.; Herring, T.; Morrow, M.; Boyer, J.; Duff, S.; Kim, J.; Weiner, D.B. Durability of response to VGX-3100 treatment of HPV16/18 positive cervical HSIL. Hum. Vaccines Immunother. 2021, 17, 1288–1293. [Google Scholar] [CrossRef]
- Wagner, R.; Meißner, J.; Grabski, E.; Sun, Y.; Vieths, S.; Hildt, E. Regulatory concepts to guide and promote the accelerated but safe clinical development and licensure of COVID-19 vaccines in Europe. Allergy 2022, 77, 72–82. [Google Scholar] [CrossRef]
- Billington, J.; Deschamps, I.; Erck, S.C.; Gerberding, J.L.; Hanon, E.; Ivol, S.; Shiver, J.W.; Spencer, J.A.; Hoof, J.V. Developing Vaccines for SARS-CoV-2 and Future Epidemics and Pandemics: Applying Lessons from Past Outbreaks. Health Secur. 2020, 18, 241–249. [Google Scholar] [CrossRef]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in mRNA Vaccines for Infectious Diseases. Front. Immunol. 2019, 10, 594. [Google Scholar] [CrossRef]
- Tan, L.; Sun, X. Recent advances in mRNA vaccine delivery. Nano Res. 2018, 11, 5338–5354. [Google Scholar] [CrossRef]
- Poovi, G.; Damodharan, N. Lipid nanoparticles: A challenging approach for oral delivery of BCS Class-II drugs. Future J. Pharm. Sci. 2018, 4, 191–205. [Google Scholar] [CrossRef]
- Oberli, M.A.; Reichmuth, A.M.; Dorkin, J.R.; Mitchell, M.J.; Fenton, O.S.; Jaklenec, A.; Anderson, D.G.; Langer, R.; Blankschtein, D. Lipid Nanoparticle Assisted mRNA Delivery for Potent Cancer Immunotherapy. Nano Lett. 2017, 17, 1326–1335. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, M.; Tahir, N.; Sohail, M.F.; Qadri, M.U.; Duarte, S.O.D.; Brandão, P.; Esteves, T.; Javed, I.; Fonte, P. Lipid-Based Nanoformulations for Drug Delivery: An Ongoing Perspective. Pharmaceutics 2024, 16, 1376. https://doi.org/10.3390/pharmaceutics16111376
Rehman M, Tahir N, Sohail MF, Qadri MU, Duarte SOD, Brandão P, Esteves T, Javed I, Fonte P. Lipid-Based Nanoformulations for Drug Delivery: An Ongoing Perspective. Pharmaceutics. 2024; 16(11):1376. https://doi.org/10.3390/pharmaceutics16111376
Chicago/Turabian StyleRehman, Mubashar, Nayab Tahir, Muhammad Farhan Sohail, Muhammad Usman Qadri, Sofia O. D. Duarte, Pedro Brandão, Teresa Esteves, Ibrahim Javed, and Pedro Fonte. 2024. "Lipid-Based Nanoformulations for Drug Delivery: An Ongoing Perspective" Pharmaceutics 16, no. 11: 1376. https://doi.org/10.3390/pharmaceutics16111376
APA StyleRehman, M., Tahir, N., Sohail, M. F., Qadri, M. U., Duarte, S. O. D., Brandão, P., Esteves, T., Javed, I., & Fonte, P. (2024). Lipid-Based Nanoformulations for Drug Delivery: An Ongoing Perspective. Pharmaceutics, 16(11), 1376. https://doi.org/10.3390/pharmaceutics16111376










