Investigating Strategies to Enhance the Aqueous Solubility of Ketamine HCl for Intranasal Delivery
Abstract
:1. Introduction
- 1.
- Explore the solubility profile of ketamine HCl for IN delivery by manipulating different variables including pH, co-solvent addition, and incorporation of different surfactants with the goal of achieving an effective concentration within a small volume.
- 2.
- Develop a validated method for the ketamine HCl analysis using UV-Vis spectrophotometry.
2. Material and Methods
2.1. Materials
2.2. UV-Vis Method Validation
2.2.1. Linearity
2.2.2. Selectivity
2.2.3. Precision
2.2.4. Thermal Stability
2.3. Solubility of Ketamine HCl in Various Solvents
2.4. Effect of pH on Ketamine HCl Solubility
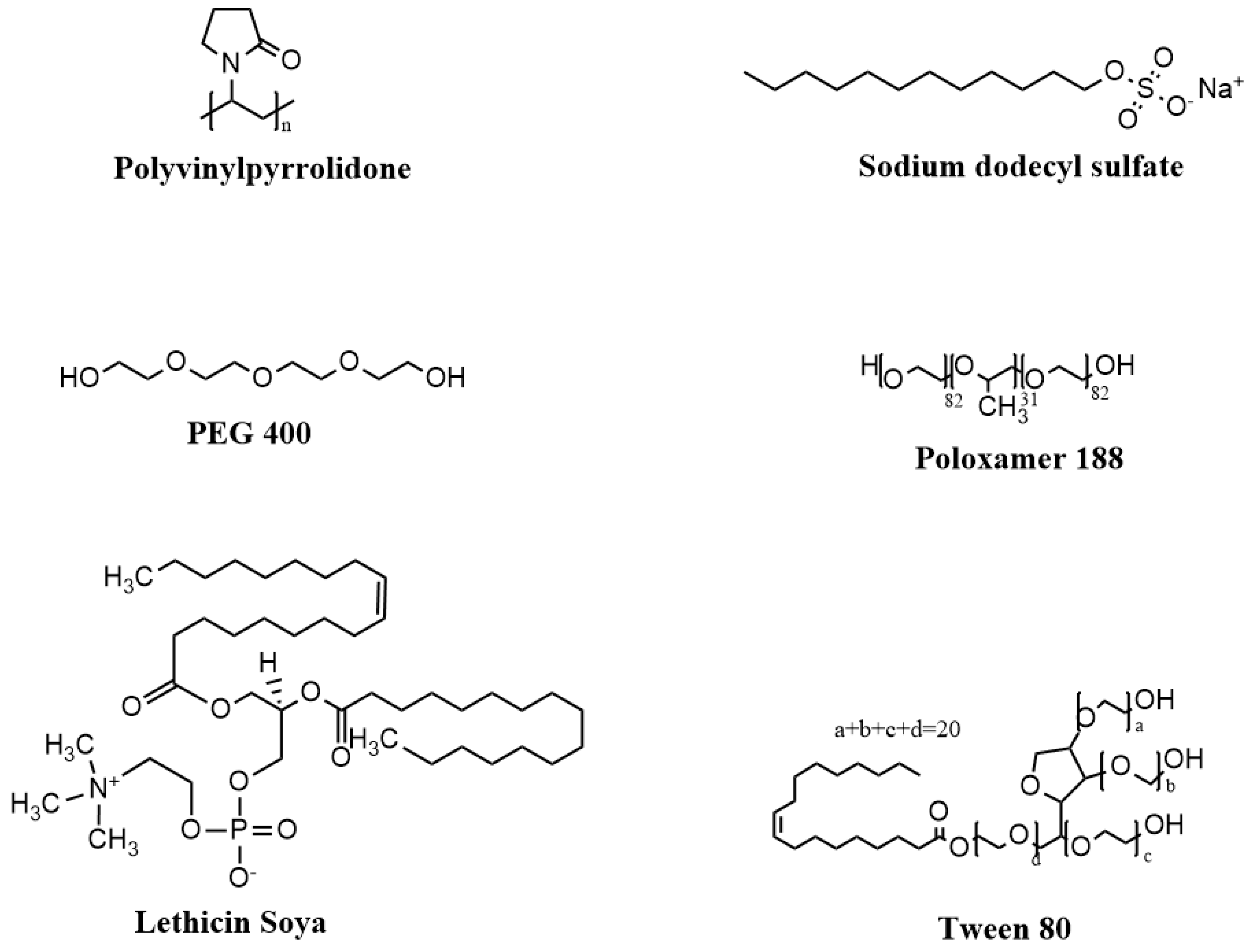
2.5. Effect of Surfactants on Ketamine HCl Solubility
2.6. Statistical Analysis
3. Results and Discussion
3.1. Method Development for Ketamine Analysis
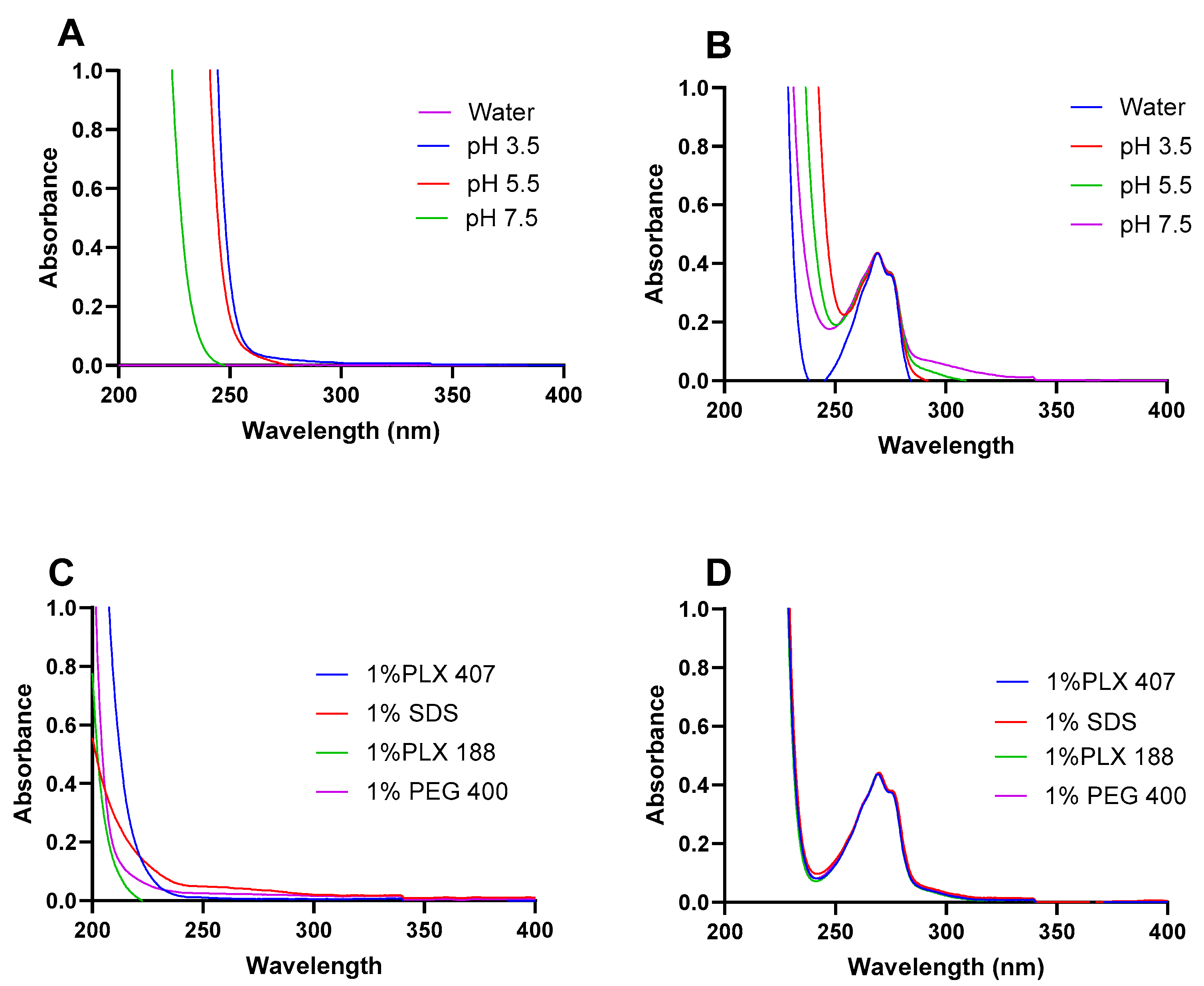
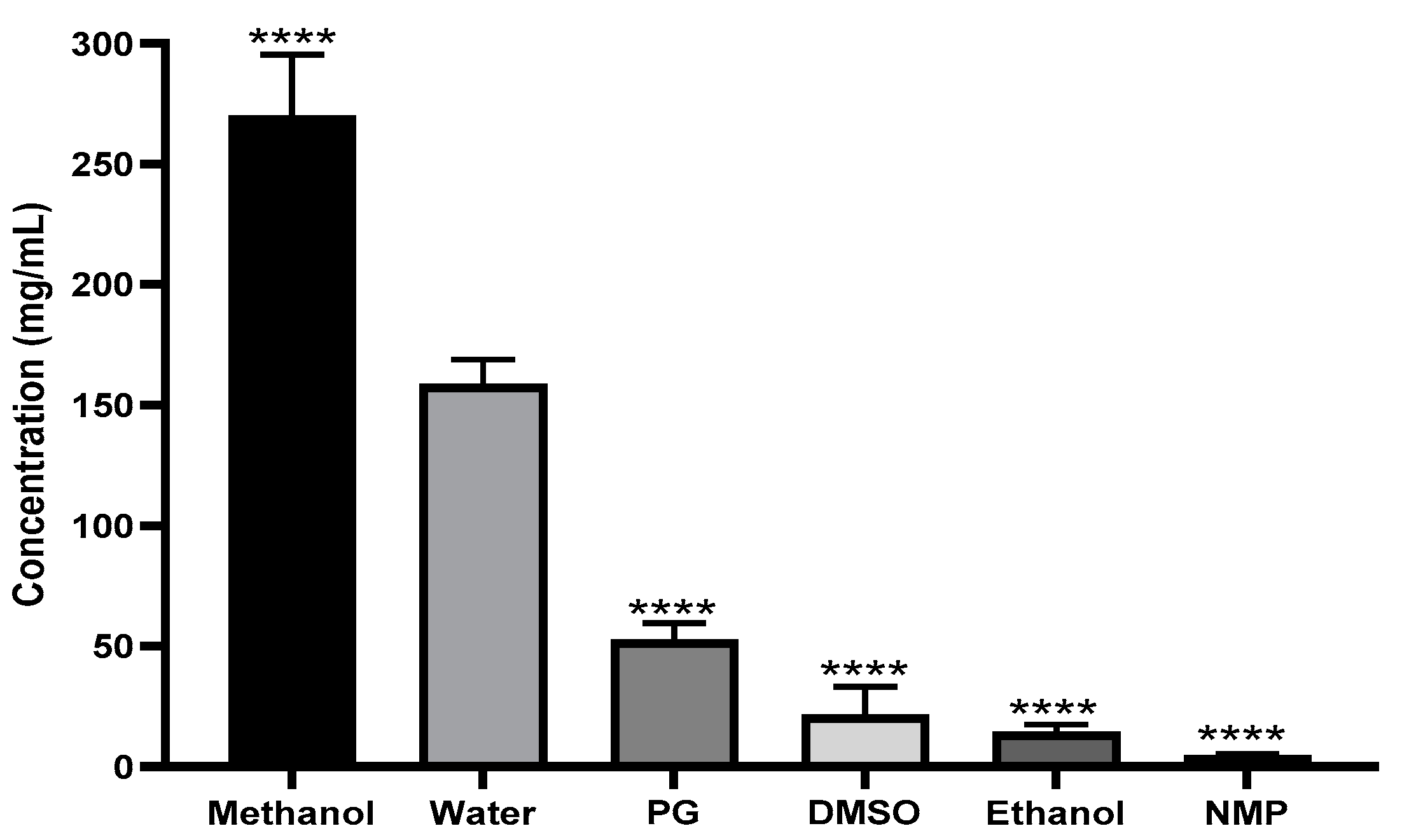
3.2. Effect of Different Solvents on Ketamine HCl Solubility
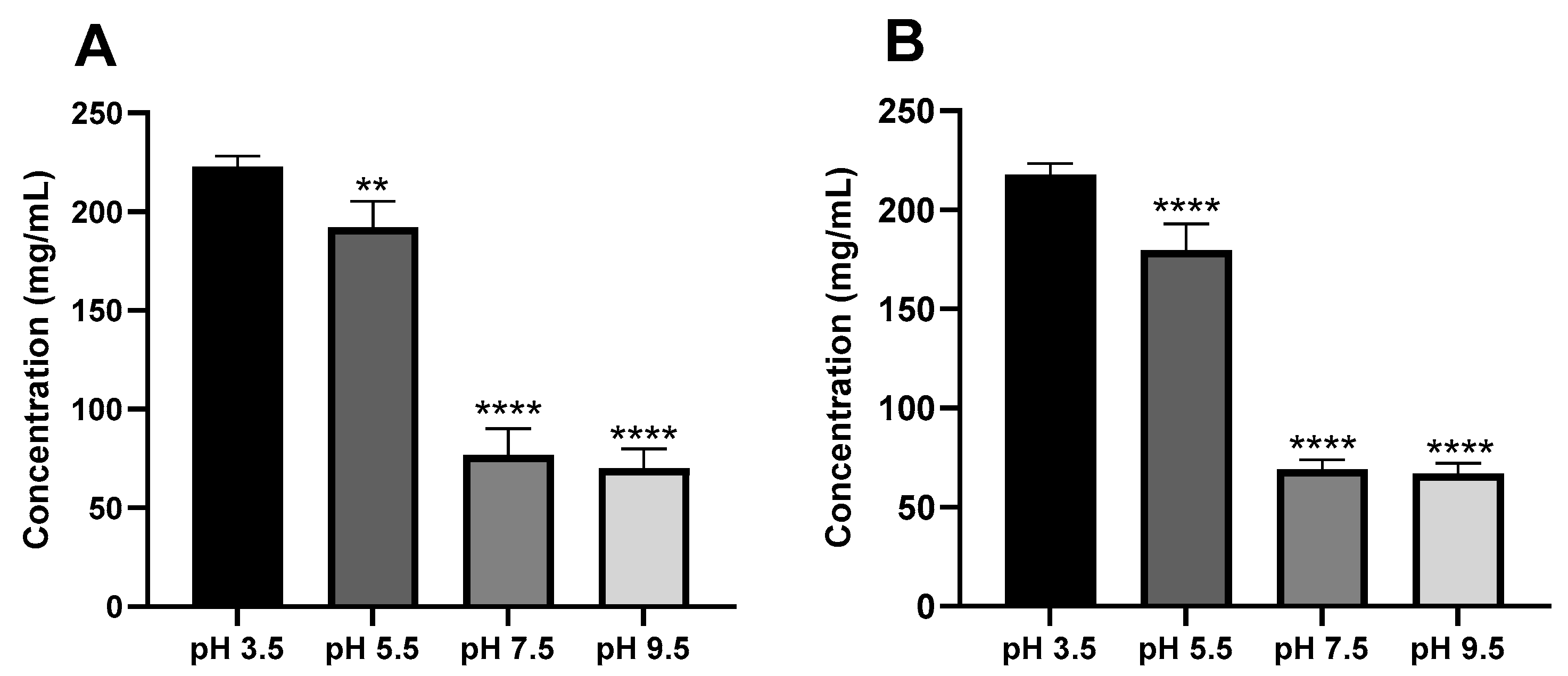
3.3. Effect of pH on Ketamine HCl Solubility
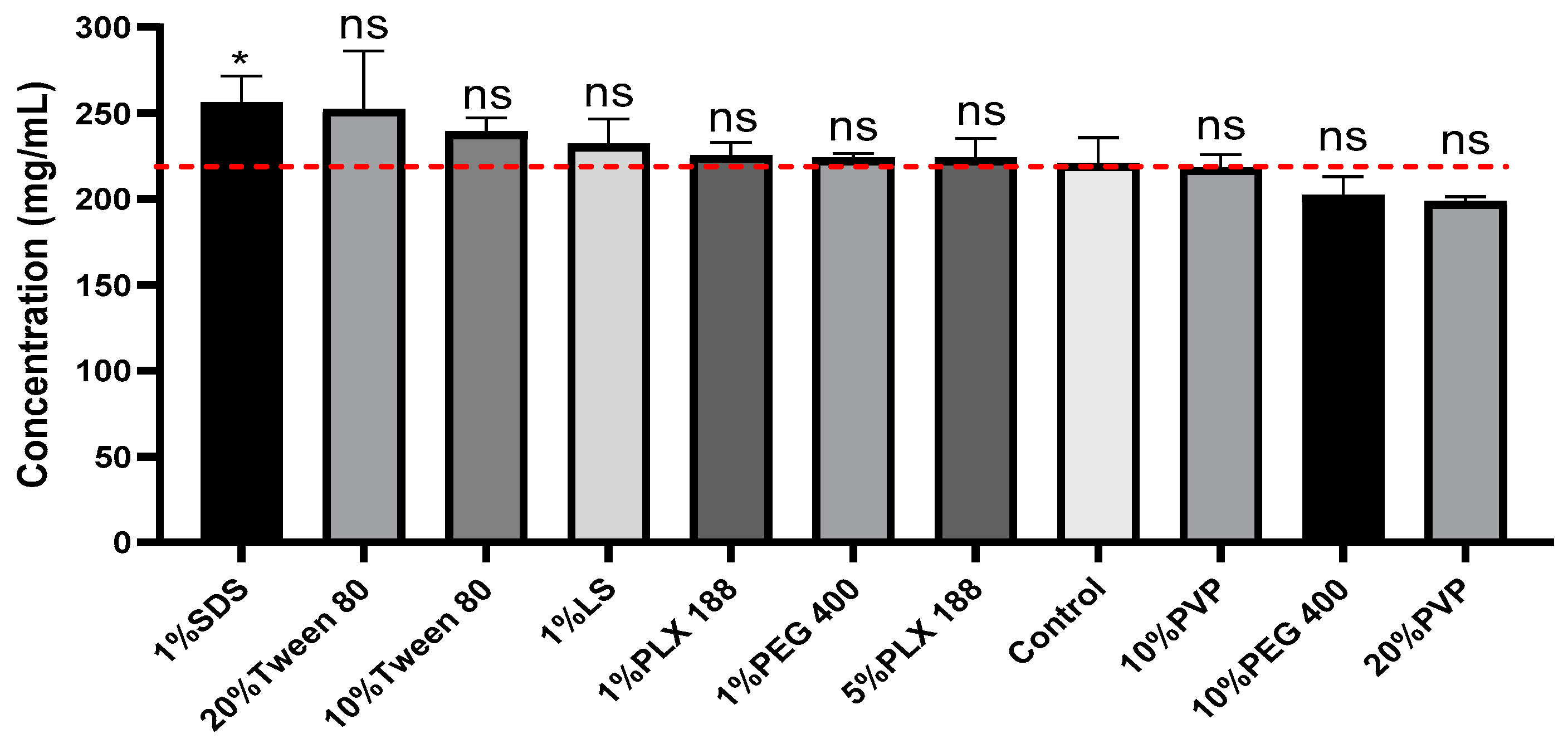
3.4. Effect of Surfactants on Ketamine HCl Solubility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peltoniemi, M.A.; Hagelberg, N.M.; Olkkola, K.T.; Saari, T.I. Ketamine: A Review of Clinical Pharmacokinetics and Pharmacodynamics in Anesthesia and Pain Therapy. Clin. Pharmacokinet. 2016, 55, 1059–1077. [Google Scholar] [CrossRef] [PubMed]
- Mion, G.; Villevieille, T. Ketamine Pharmacology: An Update (Pharmacodynamics and Molecular Aspects, Recent Findings). CNS Neurosci. Ther. 2013, 19, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Vlisides, P.E. Ketamine: 50 Years of Modulating the Mind. Front. Hum. Neurosci. 2016, 10, 612. [Google Scholar] [CrossRef] [PubMed]
- Das, J. Repurposing of Drugs-The Ketamine Story. J. Med. Chem. 2020, 63, 13514–13525. [Google Scholar] [CrossRef]
- Jelen, L.A.; Young, A.H.; Stone, J.M. Ketamine: A tale of two enantiomers. J. Psychopharmacol. 2021, 35, 109. [Google Scholar] [CrossRef]
- Nikolin, S.; Rodgers, A.; Schwaab, A.; Bahji, A.; Zarate, C.; Vazquez, G.; Loo, C. Ketamine for the treatment of major depression: A systematic review and meta-analysis. eClinicalMedicine 2023, 62, 102127. [Google Scholar] [CrossRef]
- Bahji, A.; Vazquez, G.H.; Zarate, C.A., Jr. Comparative efficacy of racemic ketamine and esketamine for depression: A systematic review and meta-analysis. J. Affect. Disord. 2021, 278, 542–555. [Google Scholar] [CrossRef]
- Trimmel, H.; Helbok, R.; Staudinger, T.; Jaksch, W.; Messerer, B.; Schöchl, H.; Likar, R. S(+)-ketamine: Current trends in emergency and intensive care medicine. Wien. Klin. Wochenschr. 2018, 130, 356. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Rosenblat, J.D.; Nemeroff, C.B.; Sanacora, G.; Murrough, J.W.; Berk, M.; Brietzke, E.; Dodd, S.; Gorwood, P.; Ho, R.; et al. Synthesizing the Evidence for Ketamine and Esketamine in Treatment-Resistant Depression: An International Expert Opinion on the Available Evidence and Implementation. Am. J. Psychiatry 2021, 178, 383–399. [Google Scholar] [CrossRef]
- Nikayin, S.; Murphy, E.; Krystal, J.H.; Wilkinson, S.T. Long-term safety of ketamine and esketamine in treatment of depression. Expert Opin. Drug Saf. 2022, 21, 777–787. [Google Scholar] [CrossRef]
- Bali, A.; Dang, A.K.; Gonzalez, D.A.; Kumar, R.; Asif, S. Clinical Uses of Ketamine in Children: A Narrative Review. Cureus 2022, 14, e27065. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, A.; Guarino, M.; Serra, S.; Spampinato, M.D.; Vanni, S.; Shiffer, D.; Voza, A.; Fabbri, A.; De Iaco, F. Narrative Review: Low-Dose Ketamine for Pain Management. J. Clin. Med. 2023, 12, 3256. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, A.; Borczyk, M. Ketamine applications beyond anesthesia—A literature review. Eur. J. Pharmacol. 2019, 860, 172547. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Riggs, L.M.; Highland, J.N.; Georgiou, P.; Pereira, E.F.R.; Albuquerque, E.X.; Thomas, C.J.; Zarate, C.A.; et al. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol. Rev. 2018, 70, 621–660. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Jang, J.H.; Lee, Y.B. Drug delivery to the brain via the nasal route of administration: Exploration of key targets and major consideration factors. J. Pharm. Investig. 2022, 53, 119–152. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2021, 12, 735–757. [Google Scholar] [CrossRef]
- Laffleur, F.; Keckeis, V. Advances in drug delivery systems: Work in progress still needed? Int. J. Pharm. 2020, 590, 119912. [Google Scholar] [CrossRef]
- Andrade, C. Ketamine for Depression, 4: In What Dose, at What Rate, by What Route, for How Long, and at What Frequency? J. Clin. Psychiatry 2017, 78, 10106. [Google Scholar] [CrossRef]
- Pires, P.C.; Rodrigues, M.; Alves, G.; Santos, A.O. Strategies to Improve Drug Strength in Nasal Preparations for Brain Delivery of Low Aqueous Solubility Drugs. Pharmaceutics 2022, 14, 588. [Google Scholar] [CrossRef]
- Chung, S.; Peters, J.M.; Detyniecki, K.; Tatum, W.; Rabinowicz, A.L.; Carrazana, E. The nose has it: Opportunities and challenges for intranasal drug administration for neurologic conditions including seizure clusters. Epilepsy Behav. Rep. 2023, 21, 100581. [Google Scholar] [CrossRef]
- Wolfe, T.R.; Braude, D.A. Intranasal Medication Delivery for Children: A Brief Review and Update. Pediatrics 2010, 126, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Del Pizzo, J.; Callahan, J.M. Intranasal medications in pediatric emergency medicine. Pediatr. Emerg. Care 2014, 30, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Abrams, R.; Morrison, J.E.; Villasenor, A.; Hencmann, D.; Da Fonseca, M.; Mueller, W. Safety and effectiveness of intranasal administration of sedative medications (ketamine, midazolam, or sufentanil) for urgent brief pediatric dental procedures. Anesth. Prog. 1993, 40, 63. [Google Scholar] [PubMed]
- Tsze, D.S.; Steele, D.W.; MacHan, J.T.; Akhlaghi, F.; Linakis, J.G. Intranasal ketamine for procedural sedation in pediatric laceration repair: A preliminary report. Pediatr. Emerg. Care 2012, 28, 767–770. [Google Scholar] [CrossRef]
- Saboo, S.; Bapat, P.; Moseson, D.E.; Kestur, U.S.; Taylor, L.S. Exploring the role of surfactants in enhancing drug release from amorphous solid dispersions at higher drug loadings. Pharmaceutics 2021, 13, 735. [Google Scholar] [CrossRef]
- Pokhrel, D.R.; Sah, M.K.; Gautam, B.; Basak, H.K.; Bhattarai, A.; Chatterjee, A. A recent overview of surfactant-drug interactions and their importance. RSC Adv. 2023, 13, 17685–17704. [Google Scholar] [CrossRef]
- Liao, Y.; Li, Z.; Zhou, Q.; Sheng, M.; Qu, Q.; Shi, Y.; Yang, J.; Lv, L.; Dai, X.; Shi, X. Saponin surfactants used in drug delivery systems: A new application for natural medicine components. Int. J. Pharm. 2021, 603, 120709. [Google Scholar] [CrossRef]
- Tahan, A.; Khojandi, M.; Salari, A.A. The theoretical investigation of solvent effects on the relative stability and 15N NMR shielding of antidepressant heterocyclic drug. Russ. J. Phys. Chem. A 2016, 90, 130–135. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Chen, G.; Farajtabar, A.; Zhao, H. Solubility modelling and solvent effect for domperidone in twelve green solvents. J. Mol. Liq. 2018, 261, 50–56. [Google Scholar] [CrossRef]
- Lomba, L.; García, C.B.; Ribate, M.P.; Giner, B.; Zuriaga, E. Applications of Deep Eutectic Solvents Related to Health, Synthesis, and Extraction of Natural Based Chemicals. Appl. Sci. 2021, 11, 10156. [Google Scholar] [CrossRef]
- Almotairy, A.; Almutairi, M.; Althobaiti, A.; Alyahya, M.; Sarabu, S.; Alzahrani, A.; Zhang, F.; Bandari, S.; Repka, M.A. Effect of pH Modifiers on the Solubility, Dissolution Rate, and Stability of Telmisartan Solid Dispersions Produced by Hot-melt Extrusion Technology. J. Drug Deliv. Sci. Technol. 2021, 65, 102674. [Google Scholar] [CrossRef] [PubMed]
- Gaohua, L.; Miao, X.; Dou, L. Crosstalk of physiological pH and chemical pKa under the umbrella of physiologically based pharmacokinetic modeling of drug absorption, distribution, metabolism, excretion, and toxicity. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Al Ktash, M.; Stefanakis, M.; Boldrini, B.; Ostertag, E.; Brecht, M. Characterization of Pharmaceutical Tablets Using UV Hyperspectral Imaging as a Rapid In-Line Analysis Tool. Sensors 2021, 21, 4436. [Google Scholar] [CrossRef] [PubMed]
- Brands, R.; Tebart, N.; Thommes, M.; Bartsch, J. UV/Vis spectroscopy as an in-line monitoring tool for tablet content uniformity. J. Pharm. Biomed. Anal. 2023, 236, 115721. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, A.; Gavat, C.-C. Ultraviolet (UV) Spectrophotometric Analysis of Ketoprofen in Tablets–Statistical Validation of Proposed Method. Mater. Proc. 2023, 14, 60. [Google Scholar] [CrossRef]
- Rambla-Alegre, M.; Esteve-Romero, J.; Carda-Broch, S. Is it really necessary to validate an analytical method or not? That is the question|Enhanced Reader. J. Chromatogr. A 2012, 1232, 101–109. [Google Scholar] [CrossRef]
- Peris-Vicente, J.; Esteve-Romero, J.; Carda-Broch, S. Validation of Analytical Methods Based on Chromatographic Techniques: An Overview. Anal. Sep. Sci. 2015, 5, 1757–1808. [Google Scholar] [CrossRef]
- Beiler, B.; Barraud, D.; Vigneron, J.; Demoré, B. Physicochemical stability of an admixture of lidocaine and ketamine in polypropylene syringe used in opioid-free anaesthesia. Eur. J. Hosp. Pharm. 2020, 27, e79–e83. [Google Scholar] [CrossRef]
- Espinoza, S.M.; Guadalupe, R.; Pimentel, C.; San, E.; Martinez, M.; Casa~ Nas Pimentel, R.G. UV–Vis spectroscopic quantification of residual acetone during the development of nanoparticulate drug delivery systems. Pharm. Dev. Technol. 2019, 24, 751–760. [Google Scholar] [CrossRef]
- Saraf, A.; Sharma, S.; Sachar, S. Evaluation of surfactants as solubilizing medium for levofloxacin. J. Mol. Liq. 2020, 319, 114060. [Google Scholar] [CrossRef]
- Kamberi, M.; Tran, T.N. UV–visible spectroscopy as an alternative to liquid chromatography for determination of everolimus in surfactant-containing dissolution media: A useful approach based on solid-phase extraction. J. Pharm. Biomed. Anal. 2012, 70, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Rapalli, V.K.; Kaul, V.; Gorantla, S.; Waghule, T.; Dubey, S.K.; Pandey, M.M.; Singhvi, G. UV Spectrophotometric method for characterization of curcumin loaded nanostructured lipid nanocarriers in simulated conditions: Method development, in-vitro and ex-vivo applications in topical delivery. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117392. [Google Scholar] [CrossRef] [PubMed]
- Boddu, S.H.S.; Kumari, S. A Short Review on the Intranasal Delivery of Diazepam for Treating Acute Repetitive Seizures. Pharmaceutics 2020, 12, 1167. [Google Scholar] [CrossRef] [PubMed]
- Duereh, A.; Guo, H.; Honma, T.; Hiraga, Y.; Sato, Y.; Lee Smith, R.; Inomata, H. Solvent Polarity of Cyclic Ketone (Cyclopentanone, Cyclohexanone): Alcohol (Methanol, Ethanol) Renewable Mixed-Solvent Systems for Applications in Pharmaceutical and Chemical Processing. Ind. Eng. Chem. Res. 2018, 57, 7331–7344. [Google Scholar] [CrossRef]
- Hoerr, C.W.; Harwood, H.J.; Ralston, A.W. Solubilities of high molecular weight symmetrical normal aliphatic secondary amines. J. Org. Chem. 1944, 9, 201–210. [Google Scholar] [CrossRef]
- Spencer, J.N.; Wolbach, W.S.; Hovick, J.W.; Ansel, L.; Modarress, K.J. Hydrogen bonding by alcohols and amines. J. Solution Chem. 1985, 14, 805–814. [Google Scholar] [CrossRef]
- Abuhelwa, A.Y.; Williams, D.B.; Upton, R.N.; Foster, D.J.R. Food, gastrointestinal pH, and models of oral drug absorption. Eur. J. Pharm. Biopharm. 2017, 112, 234–248. [Google Scholar] [CrossRef]
- Shoghi, E.; Fuguet, E.; Bosch, E.; Ràfols, C. Solubility–pH profiles of some acidic, basic and amphoteric drugs. Eur. J. Pharm. Sci. 2013, 48, 291–300. [Google Scholar] [CrossRef]
- Henriques, P.; Fortuna, A.; Doktorovová, S. Spray dried powders for nasal delivery: Process and formulation considerations. Eur. J. Pharm. Biopharm. 2022, 176, 1–20. [Google Scholar] [CrossRef]
- Gebhardt, U.; Knoch, A.; Rauchfuss, R.; Schneider, M. Stable Ketamine Solutions. Patent WO1994023711A1, 27 October 1994. [Google Scholar]
- Huang, Z.; Parikh, S.; Fish, W.P. Interactions between a poorly soluble cationic drug and sodium dodecyl sulfate in dissolution medium and their impact on in vitro dissolution behavior. Int. J. Pharm. 2018, 535, 350–359. [Google Scholar] [CrossRef]
- Mai, N.N.S.; Otsuka, Y.; Kawano, Y.; Hanawa, T. Preparation and characterization of solid dispersions composed of curcumin, hydroxypropyl cellulose and/or sodium dodecyl sulfate by grinding with vibrational ball milling. Pharmaceuticals 2020, 13, 383. [Google Scholar] [CrossRef] [PubMed]

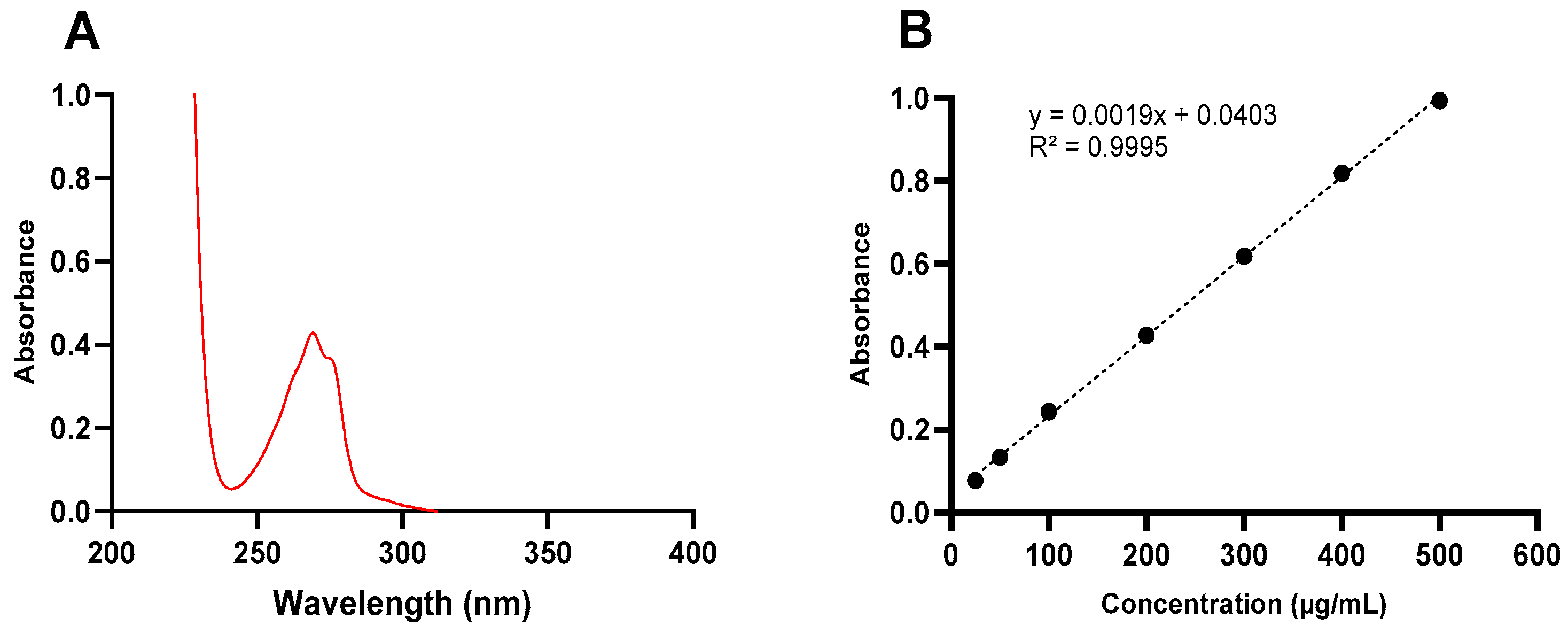
| Linear range (µg/mL) | 25–500 |
| Regression equation | Y = 0.0019x + 0.0403 |
| Standard error of slope | 1.9 × 10−5 |
| Standard error of intercept | 1.96 × 10−3 |
| R2 (mean ± SD) | 0.9995 ± 0.0074 |
| LOD (µg/mL) | 3.39 |
| LOQ (µg/mL) | 10.27 |
| Added Concentration (µg/mL) | Intra-Day (n = 12) | Inter-Day (n = 12) | |||||
|---|---|---|---|---|---|---|---|
| Concentration Found (Mean ± SD) | Precision (RSD, %) | Accuracy (RE, %) | Concentration Found (Mean ± SD) | Precision (RSD, %) | Accuracy (RE, %) | ||
| 25 | 25.51 ± 1.13 | 4.43 | 2.07 | 25.16 ± 1.08 | 4.32 | 0.66 | |
| 100 | 102.35 ± 2.36 | 2.31 | 2.35 | 100.56 ± 1.83 | 1.82 | 0.56 | |
| 250 | 256.61 ± 3.12 | 1.21 | 2.64 | 252.71 ± 1.33 | 0.52 | 1.08 | |
| Concentration (µg/mL) (Mean ± SD) | ||||
|---|---|---|---|---|
| 25 | 100 | 250 | ||
| Room temperature (25 °C) | Day 1 | 24.26 ± 3.71 | 100.64 ± 0.92 | 255.91 ± 0.30 |
| Day 2 | 24.46 ± 1.48 | 99.77 ± 0.97 | 253.10 ± 5.26 | |
| 1 week | 25.82 ± 0.90 | 103.89 ± 0.28 | 250.12 ± 0.30 | |
| Refrigerator (4 °C) | Day 1 | 24.15 ± 1.15 | 100.29 ± 0.79 | 255.38 ± 3.65 |
| Day 2 | 25.12 ± 0.90 | 97.00 ± 1.26 | 254.42 ± 3.58 | |
| 1 week | 25.21 ± 1.33 | 100.82 ± 2.57 | 254.50 ± 3.30 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idoudi, S.; Saleh, A.; Akkbik, M.; Amine, L.; Alansari, K.; Rachid, O.; Alkilany, A.M. Investigating Strategies to Enhance the Aqueous Solubility of Ketamine HCl for Intranasal Delivery. Pharmaceutics 2024, 16, 1502. https://doi.org/10.3390/pharmaceutics16121502
Idoudi S, Saleh A, Akkbik M, Amine L, Alansari K, Rachid O, Alkilany AM. Investigating Strategies to Enhance the Aqueous Solubility of Ketamine HCl for Intranasal Delivery. Pharmaceutics. 2024; 16(12):1502. https://doi.org/10.3390/pharmaceutics16121502
Chicago/Turabian StyleIdoudi, Sourour, Alaaeldin Saleh, Mohammed Akkbik, Leena Amine, Khalid Alansari, Ousama Rachid, and Alaaldin M. Alkilany. 2024. "Investigating Strategies to Enhance the Aqueous Solubility of Ketamine HCl for Intranasal Delivery" Pharmaceutics 16, no. 12: 1502. https://doi.org/10.3390/pharmaceutics16121502
APA StyleIdoudi, S., Saleh, A., Akkbik, M., Amine, L., Alansari, K., Rachid, O., & Alkilany, A. M. (2024). Investigating Strategies to Enhance the Aqueous Solubility of Ketamine HCl for Intranasal Delivery. Pharmaceutics, 16(12), 1502. https://doi.org/10.3390/pharmaceutics16121502







