Investigation of Spray Drying Parameters to Formulate Novel Spray-Dried Proliposome Powder Formulations Followed by Their Aerosolization Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Spray Drying of Mannitol, Trehalose, and LMH Carriers
2.3. Spray Drying Proliposome (SDP) Formulations
2.4. Surface Morphology of Coarse Carriers, SD Formulations, and SDP Formulations via Scanning Electron Microscopy (SEM)
2.5. Powder X-Ray Diffraction (PXRD) Studies
2.6. Moisture Analysis via Thermogravimetric Analysis (TGA)
2.7. In Vitro Performance of SDP Formulations Using a Next-Generation Impactor (NGI)
2.8. Separation and Entrapment Efficiency of BDP
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of Inlet Temperature on the Physicochemical Properties of Spray-Dried (SD) Formulations
3.1.1. Effect of Inlet Temperatures on Particle Morphology and Particle Size
3.1.2. Effect of Inlet Temperatures on Production Yield, Moisture Content, and Particle Crystallinity
3.2. Effect of Airflow Rates on the Physicochemical Properties of Spray-Dried Formulations
3.2.1. Effect Airflow Rates on Particle Morphology and Particle Size
3.2.2. Effect Airflow Rates on Production Yield, Moisture Content, and Particle Crystallinity
3.3. Effect of Pump Feed Rates on the Physicochemical Properties of Spray-Dried (SD) Formulations
3.3.1. Effect of Pump Feed Rates on Particle Morphology and Particle Size
3.3.2. Effect of Pump Feed Rates on Production Yield, Moisture Content, and Particle Crystallinity
3.4. Optimized Spray-Dried Proliposome (SDP) Formulation Using DMPC as a Phospholipid
3.4.1. Morphology of Spray-Dried Proliposome (SDP) Formulations
3.4.2. Production Yield, Moisture Content, and Particle Size Analysis of SDP Formulations
3.4.3. PXRD of SDP Formulations
3.4.4. Deposition of SDP Formulations Using NGI
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, I.; Yousaf, S.; Subramanian, S.; Alhnan, M.A.; Ahmed, W.; Elhissi, A. Proliposome Powders for the Generation of Liposomes: The Influence of Carbohydrate Carrier and Separation Conditions on Crystallinity and Entrapment of a Model Antiasthma Steroid. AAPS PharmSciTech 2018, 19, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Al-Hasani, A.; Khan, M.H.; Khan, A.N.; Alam, F.-E.; Sadozai, S.K.; Elhissi, A.; Khan, J.; Yousaf, S. Impact of dispersion media and carrier type on spray-dried proliposome powder formulations loaded with beclomethasone dipropionate for their pulmonary drug delivery via a next generation impactor. PLoS ONE 2023, 18, e0281860. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, L.; Pfutzner, A.; Heise, T. Alternative routes of administration as an approach to improve insulin therapy: Update on dermal, oral, nasal and pulmonary insulin delivery. Curr. Pharm. Des. 2001, 7, 1327–1351. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, S.; Yousaf, S.; Khan, I. A review on the synthesis of bio-based surfactants using green chemistry principles. DARU J. Pharm. Sci. 2022, 30, 407–426. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Saimi, N.I.; Salim, N.; Ahmad, N.; Abdulmalek, E.; Abdul Rahman, M.B. Aerosolized Niosome Formulation Containing Gemcitabine and Cisplatin for Lung Cancer Treatment: Optimization, Characterization and In Vitro Evaluation. Pharmaceutics 2021, 13, 59. [Google Scholar] [CrossRef]
- Najlah, M.; Hidayat, K.; Omer, H.K.; Mwesigwa, E.; Ahmed, W.; AlObaidy, K.G.; Phoenix, D.A.; Elhissi, A. A facile approach to manufacturing non-ionic surfactant nanodipsersions using proniosome technology and high-pressure homogenization. J. Liposome Res. 2015, 25, 32–37. [Google Scholar] [CrossRef]
- Lewis, P.O.; Khan, I.; Patel, P. Successful stepdown treatment of pulmonary histoplasmosis with thrice-weekly liposomal amphotericin B in a hospital-associated, outpatient infusion centre: A case report. J. Clin. Pharm. Ther. 2018, 43, 269–272. [Google Scholar] [CrossRef]
- Elhissi, A. Liposomes for Pulmonary Drug Delivery: The Role of Formulation and Inhalation Device Design. Curr. Pharm. Des. 2017, 23, 362–372. [Google Scholar] [CrossRef]
- Wang, J.L.; Hanafy, M.S.; Xu, H.; Leal, J.; Zhai, Y.; Ghosh, D.; Williams Iii, R.O.; David Charles Smyth, H.; Cui, Z. Aerosolizable siRNA-encapsulated solid lipid nanoparticles prepared by thin-film freeze-drying for potential pulmonary delivery. Int. J. Pharm. 2021, 596, 120215. [Google Scholar] [CrossRef]
- Huang, Z.; Kłodzińska, S.N.; Wan, F.; Nielsen, H.M. Nanoparticle-mediated pulmonary drug delivery: State of the art towards efficient treatment of recalcitrant respiratory tract bacterial infections. Drug Deliv. Transl. Res. 2021, 11, 1634–1654. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, K.; Li, J.; Liao, J.; Ma, L. Engineering of hybrid anticancer drug-loaded polymeric nanoparticles delivery system for the treatment and care of lung cancer therapy. Drug Deliv. 2021, 28, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.P.; Gaspar, M.M.; Eleutério, C.V.; Grenha, A.; Blanco, M.; Gonçalves, L.M.D.; Taboada, P.; Almeida, A.J.; Remuñán-López, C. Microencapsulated Solid Lipid Nanoparticles as a Hybrid Platform for Pulmonary Antibiotic Delivery. Mol. Pharm. 2017, 14, 2977–2990. [Google Scholar] [CrossRef]
- Khan, I.; Hussein, S.; Houacine, C.; Khan Sadozai, S.; Islam, Y.; Bnyan, R.; Elhissi, A.; Yousaf, S. Fabrication, characterization and optimization of nanostructured lipid carrier formulations using Beclomethasone dipropionate for pulmonary drug delivery via medical nebulizers. Int. J. Pharm. 2021, 598, 120376. [Google Scholar] [CrossRef]
- Ansam, M.; Yousaf, S.; Bnyan, R.; Khan, I. Anti-aging Liposomal Formulation. Mini Review. Novel Approaches Drug Designing Dev. 2018, 3, 66–68. [Google Scholar]
- Hunt, C.A.; Tsang, S. α-Tocopherol retards autoxidation and prolongs the shelf-life of liposomes. Int. J. Pharm. 1981, 8, 101–110. [Google Scholar] [CrossRef]
- Wong, M.; Thompson, T.E. Aggregation of dipalmitoylphosphatidylcholine vesicles. Biochemistry 1982, 21, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Payne, N.I.; Timmins, P.; Ambrose, C.V.; Ward, M.D.; Ridgway, F. Proliposomes: A novel solution to an old problem. J. Pharm. Sci. 1986, 75, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gupta, R.B.; Betageri, G.V. Formulation, characterization, and in vitro release of glyburide from proliposomal beads. Drug Deliv. 2001, 8, 25–27. [Google Scholar] [CrossRef]
- Alves, G.P.; Santana, M.H.A. Phospholipid dry powders produced by spray drying processing: Structural, thermodynamic and physical properties. Powder Technol. 2004, 145, 139–148. [Google Scholar] [CrossRef]
- Apostolou, M.; Fatokun, A.A.; Assi, S.; Khan, I. Targeted Lipid-Based Drug Delivery Systems for Lung Cancer Therapy. Appl. Sci. 2024, 14, 6759. [Google Scholar] [CrossRef]
- Newman, S.P. AEROSOLS. In Encyclopedia of Respiratory Medicine; Laurent, G.J., Shapiro, S.D., Eds.; Academic Press: Oxford, UK, 2006; pp. 58–64. [Google Scholar] [CrossRef]
- Littringer, E.M.; Mescher, A.; Eckhard, S.; Schröttner, H.; Langes, C.; Fries, M.; Griesser, U.; Walzel, P.; Urbanetz, N.A. Spray Drying of Mannitol as a Drug Carrier—The Impact of Process Parameters on Product Properties. Dry. Technol. 2012, 30, 114–124. [Google Scholar] [CrossRef]
- Wang, W.; Dufour, C.; Zhou, W. Impacts of spray-drying conditions on the physicochemical properties of soy sauce powders using maltodextrin as auxiliary drying carrier. CyTA-J. Food 2015, 13, 548–555. [Google Scholar] [CrossRef]
- Pikal, M.J.; Lukes, A.L.; Lang, J.E.; Gaines, K. Quantitative crystallinity determinations for β-lactam antibiotics by solution calorimetry: Correlations with stability. J. Pharm. Sci. 1978, 67, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Sussich, F.; Urbani, R.; Princivalle, F.; Cesàro, A. Polymorphic Amorphous and Crystalline Forms of Trehalose. J. Am. Chem. Soc. 1998, 120, 7893–7899. [Google Scholar] [CrossRef]
- Lechanteur, A.; Evrard, B. Influence of Composition and Spray-Drying Process Parameters on Carrier-Free DPI Properties and Behaviors in the Lung: A review. Pharmaceutics 2020, 12, 55. [Google Scholar] [CrossRef]
- Omer, H.K.; Hussein, N.R.; Ferraz, A.; Najlah, M.; Ahmed, W.; Taylor, K.M.G.; Elhissi, A.M.A. Spray-Dried Proliposome Microparticles for High-Performance Aerosol Delivery Using a Monodose Powder Inhaler. AAPS PharmSciTech 2018, 19, 2434–2448. [Google Scholar] [CrossRef]
- Ghandi, A.; Powell, I.B.; Chen, X.D.; Adhikari, B. The Effect of Dryer Inlet and Outlet Air Temperatures and Protectant Solids on the Survival of Lactococcus lactis during Spray Drying. Dry. Technol. 2012, 30, 1649–1657. [Google Scholar] [CrossRef]
- Maury, M.; Murphy, K.; Kumar, S.; Shi, L.; Lee, G. Effects of process variables on the powder yield of spray-dried trehalose on a laboratory spray-dryer. Eur. J. Pharm. Biopharm. 2005, 59, 565–573. [Google Scholar] [CrossRef]
- Zhang, T.; Youan, B.-B.C. Analysis of process parameters affecting spray-dried oily core nanocapsules using factorial design. AAPS PharmSciTech 2010, 11, 1422–1431. [Google Scholar] [CrossRef]
- Mah, P.T.; O’Connell, P.; Focaroli, S.; Lundy, R.; O’Mahony, T.F.; Hastedt, J.E.; Gitlin, I.; Oscarson, S.; Fahy, J.V.; Healy, A.M. The use of hydrophobic amino acids in protecting spray dried trehalose formulations against moisture-induced changes. Eur. J. Pharm. Biopharm. 2019, 144, 139–153. [Google Scholar] [CrossRef]
- Peng, Y.; Gardner, D.J. Spray-drying cellulose nanofibrils: Effect of drying process parameters on particle morphology and size distribution. Wood Fiber Sci. 2012, 44, 448–461. [Google Scholar]
- Broadhead, J.; Rouan, S.K.; Hau, I.; Rhodes, C.T. The effect of process and formulation variables on the properties of spray-dried beta-galactosidase. J. Pharm. Pharmacol. 1994, 46, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Daniher, D.I.; Zhu, J. Dry powder platform for pulmonary drug delivery. Particuology 2008, 6, 225–238. [Google Scholar] [CrossRef]
- de Boer, A.H.; Gjaltema, D.; Hagedoorn, P.; Frijlink, H.W. Can ‘extrafine’ dry powder aerosols improve lung deposition? Eur. J. Pharm. Biopharm. 2015, 96, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Byron, P.R. Prediction of drug residence times in regions of the human respiratory tract following aerosol inhalation. J. Pharm. Sci. 1986, 75, 433–438. [Google Scholar] [CrossRef] [PubMed]
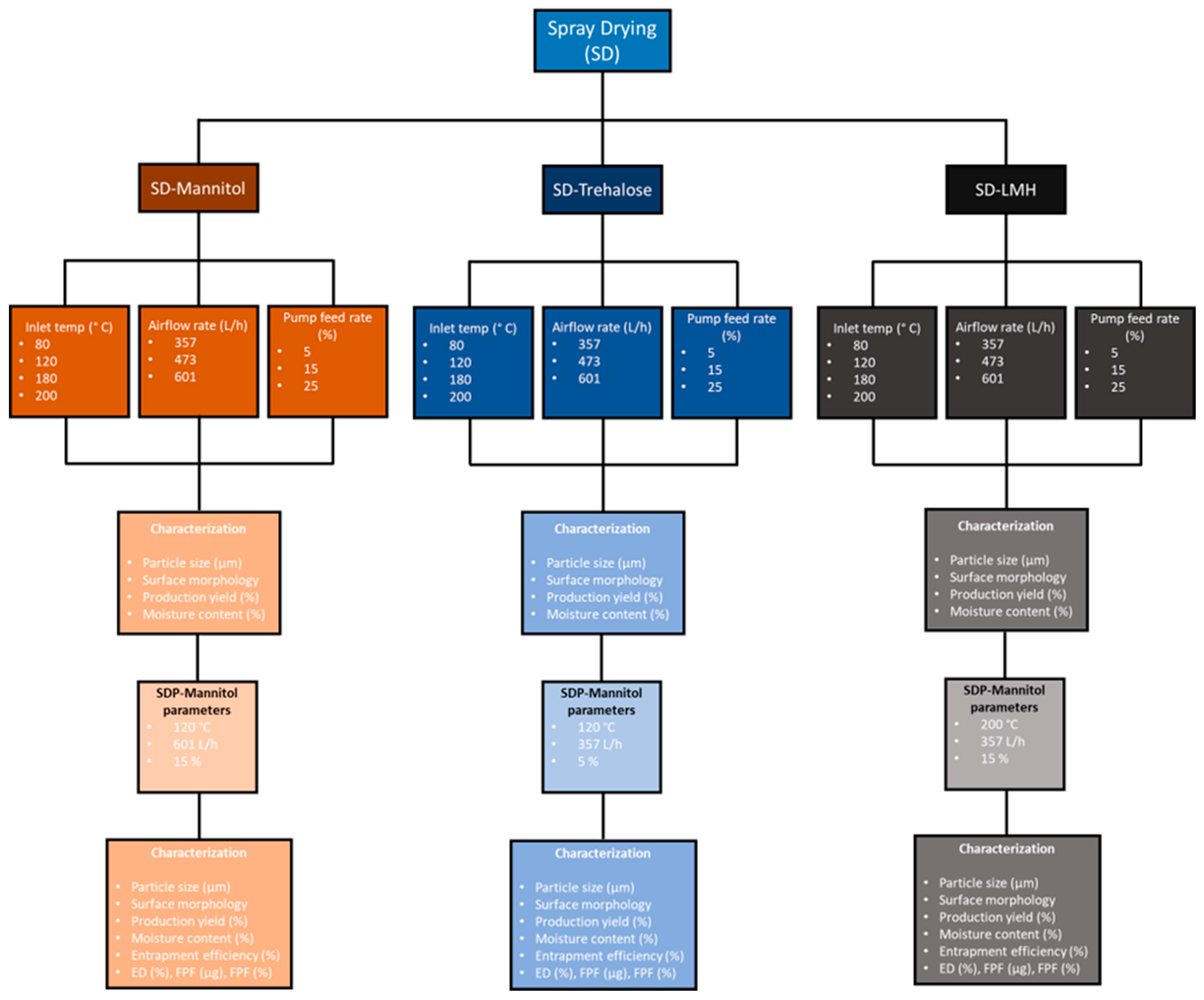








| Formulation | Carbohydrate Carrier | Inlet Temperature (°C) | Outlet Temperature (°C) | Airflow Rate (L/h) | Pump Feed Rate (%) | Aspirator (%) |
|---|---|---|---|---|---|---|
| F1 | Mannitol | 80 | 35 | 357 | 15 | 100 |
| F2 | Mannitol | 120 | 54 | 357 | 15 | 100 |
| F3 | Mannitol | 160 | 73 | 357 | 15 | 100 |
| F4 | Mannitol | 200 | 85 | 357 | 15 | 100 |
| F5 | Mannitol | 120 | 52 | 473 | 15 | 100 |
| F6 | Mannitol | 120 | 51 | 601 | 15 | 100 |
| F7 | Mannitol | 120 | 75 | 357 | 5 | 100 |
| F8 | Mannitol | 120 | 46 | 357 | 25 | 100 |
| F9 | Trehalose | 80 | 40 | 357 | 15 | 100 |
| F10 | Trehalose | 120 | 50 | 357 | 15 | 100 |
| F11 | Trehalose | 160 | 61 | 357 | 15 | 100 |
| F12 | Trehalose | 200 | 65 | 357 | 15 | 100 |
| F13 | Trehalose | 120 | 44 | 473 | 15 | 100 |
| F14 | Trehalose | 120 | 40 | 601 | 15 | 100 |
| F15 | Trehalose | 120 | 65 | 357 | 5 | 100 |
| F16 | Trehalose | 120 | 48 | 357 | 25 | 100 |
| F17 | LMH | 80 | 35 | 357 | 15 | 100 |
| F18 | LMH | 120 | 53 | 357 | 15 | 100 |
| F19 | LMH | 160 | 73 | 357 | 15 | 100 |
| F20 | LMH | 200 | 84 | 357 | 15 | 100 |
| F21 | LMH | 200 | 82 | 473 | 15 | 100 |
| F22 | LMH | 200 | 79 | 601 | 15 | 100 |
| F23 | LMH | 200 | 87 | 357 | 5 | 100 |
| F24 | LMH | 200 | 80 | 357 | 25 | 100 |
| Formulations | SD Conditions Inlet Temp (°C), Airflow Rate (L/h), Pump Feed Rate (%) | Production Yield (%) | Particle Size (µm) | Moisture (%) |
|---|---|---|---|---|
| Mannitol | 0.09 ± 0.01 | |||
| F1 | 80, 357, 15 | 38.17 ± 2.61 | 5.64 ± 3.61 | 0.33 ± 0.03 |
| F2 | 120, 357, 15 | 60.73 ± 4.12 | 4.58 ± 0.91 | 0.22 ± 0.03 |
| F3 | 160, 357, 15 | 63.64 ± 2.35 | 5.69 ± 2.72 | 0.16 ± 0.02 |
| F4 | 200, 357, 15 | 55.57 ± 6.64 | 7.82 ± 2.12 | 0.14 ± 0.02 |
| Trehalose | 9.74 ± 1.43 | |||
| F9 | 80, 357, 15 | 14.63 ± 3.32 | Fused particles | 7.22 ± 1.13 |
| F10 | 120, 357, 15 | 58.28 ± 4.61 | 4.74 ± 0.95 | 5.12 ± 0.62 |
| F11 | 160, 357, 15 | 58.94 ± 4.26 | 6.95 ± 1.46 | 3.54 ± 0.47 |
| F12 | 200, 357, 15 | 74.37 ± 4.33 | 8.27 ± 2.23 | 1.75 ± 0.85 |
| LMH | 5.09 ± 1.25 | |||
| F17 | 80, 357, 15 | 27.26 ± 2.72 | Fused particles | 4.92 ± 0.84 |
| F18 | 120, 357, 15 | 52.68 ± 1.46 | Fused particles | 4.49 ± 0.92 |
| F19 | 160, 357, 15 | 65.34 ± 2.35 | 6.92 ± 1.52 | 3.82 ± 0.88 |
| F20 | 200, 357, 15 | 70.03 ± 4.29 | 5.16 ± 1.23 | 1.99 ± 0.77 |
| Formulations | SD Conditions Inlet Temp (°C), Airflow Rate (L/h), Pump Feed Rate (%) | Production Yield (%) | Particle Size (µm) | Moisture (%) |
|---|---|---|---|---|
| Mannitol | 0.09 ± 0.01 | |||
| F2 | 120, 357, 15 | 60.73 ± 4.12 | 4.58 ± 0.91 | 0.22 ± 0.03 |
| F5 | 120, 473, 15 | 84.57 ± 4.53 | 4.29 ± 0.51 | 0.24 ± 0.02 |
| F6 | 120, 601, 15 | 86.01 ± 3.25 | 2.96 ± 0.45 | 0.25 ± 0.03 |
| Trehalose | 9.74 ± 1.43 | |||
| F10 | 120, 357, 15 | 58.28 ± 4.61 | 4.74 ± 0.95 | 5.12 ± 0.62 |
| F13 | 120, 473, 15 | 64.55 ± 4.34 | Fused particles | 5.22 ± 0.81 |
| F14 | 120, 601, 15 | 60.72 ± 4.96 | Fused particles | 5.24 ± 1.01 |
| LMH | 5.09 ± 1.25 | |||
| F20 | 200, 357, 15 | 70.03 ± 4.29 | 5.16 ± 1.23 | 1.99 ± 0.77 |
| F21 | 200, 473, 15 | 73.54 ± 7.61 | Fused particles | 4.05 ± 0.79 |
| F22 | 200, 601, 15 | 72.65 ± 6.73 | Fused particles | 4.13 ± 0.87 |
| Formulations | SD Conditions Inlet Temp (°C), Airflow Rate (L/h), Pump Feed Rate (%) | Production Yield (%) | Particle Size (µm) | Moisture (%) |
|---|---|---|---|---|
| Mannitol | 0.09 ± 0.01 | |||
| F6 | 120, 601, 15 | 86.01 ± 3.25 | 2.96 ± 1.42 | 0.25 ± 0.03 |
| F7 | 120, 601, 5 | 88.41 ± 0.64 | 3.17 ± 1.41 | 0.20 ± 0.02 |
| F8 | 120, 601, 25 | 78.41 ± 3.02 | 4.07 ± 1.59 | 0.28 ± 0.03 |
| Trehalose | 9.74 ± 1.43 | |||
| F10 | 120, 357, 15 | 58.28 ± 4.61 | 4.74 ± 0.95 | 5.12 ± 0.62 |
| F15 | 120, 357, 5 | 72.55 ± 5.42 | 4.55 ± 0.46 | 3.76 ± 0.75 |
| F16 | 120, 357, 25 | 23.71 ± 4.67 | 4.89 ± 0.32 | 5.54 ± 0.84 |
| LMH | 5.09 ± 1.25 | |||
| F20 | 200, 357, 15 | 70.03 ± 4.29 | 5.16 ± 1.32 | 1.99 ± 0.77 |
| F23 | 200, 357, 5 | 73.69 ± 2.48 | 7.48 ± 3.04 | 1.55 ± 0.31 |
| F24 | 200, 357, 25 | 56.17 ± 3.98 | 7.76 ± 3.18 | 3.38 ± 0.67 |
| Formulation | SDP-Mannitol | SDP-Trehalose | SDP-LMH |
|---|---|---|---|
| Inlet temp (°C) | 120 | 120 | 200 |
| Outlet temp (°C) | 57 | 74 | 98 |
| Airflow rate (L/h) | 601 | 357 | 357 |
| Pump feed rate (%) | 15 | 5 | 15 |
| Production yield (%) | 82.45 ± 4.58 | 69.54 ± 3.23 | 77.33 ± 3.75 |
| Moisture content (%) | 0.22 ± 0.01 | 2.65 ± 0.06 | 1.52 ± 0.04 |
| Particle size via SEM (µm) | 2.64 ± 1.51 | 4.83 ± 2.26 | 4.19 ± 2.11 |
| Entrapment efficiency (%) | 98.51 ± 2.17 | 94.89 ± 4.26 | 96.06 ± 4.67 |
| Formulations | SDP-Mannitol | SDP-Trehalose | SDP-LMH |
|---|---|---|---|
| ED (%) | 96.28 ± 3.14 | 89.63 ± 2.21 | 97.32 ± 3.35 |
| FPD (µg) | 285.81 ± 8.42 | 153.71 ± 6.53 | 211.19 ± 7.74 |
| FPF (%) | 56.84 ± 4.52 | 23.76 ± 4.42 | 44.27 ± 4.77 |
| RF (%) | 86.44 ± 4.55 | 52.92 ± 5.03 | 77.68 ± 4.19 |
| MMAD (µm) | 2.89 ± 0.13 | 4.98 ± 0.12 | 4.02 ± 0.14 |
| GSD | 2.44 ± 0.03 | 1.84 ± 0.02 | 1.96 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, I.; Edes, K.; Alsaadi, I.; Al-Khaial, M.Q.; Bnyan, R.; Khan, S.A.; Sadozai, S.K.; Khan, W.; Yousaf, S. Investigation of Spray Drying Parameters to Formulate Novel Spray-Dried Proliposome Powder Formulations Followed by Their Aerosolization Performance. Pharmaceutics 2024, 16, 1541. https://doi.org/10.3390/pharmaceutics16121541
Khan I, Edes K, Alsaadi I, Al-Khaial MQ, Bnyan R, Khan SA, Sadozai SK, Khan W, Yousaf S. Investigation of Spray Drying Parameters to Formulate Novel Spray-Dried Proliposome Powder Formulations Followed by Their Aerosolization Performance. Pharmaceutics. 2024; 16(12):1541. https://doi.org/10.3390/pharmaceutics16121541
Chicago/Turabian StyleKhan, Iftikhar, Kaylome Edes, Ismail Alsaadi, Mohammed Q. Al-Khaial, Ruba Bnyan, Saeed A. Khan, Sajid K. Sadozai, Wasiq Khan, and Sakib Yousaf. 2024. "Investigation of Spray Drying Parameters to Formulate Novel Spray-Dried Proliposome Powder Formulations Followed by Their Aerosolization Performance" Pharmaceutics 16, no. 12: 1541. https://doi.org/10.3390/pharmaceutics16121541
APA StyleKhan, I., Edes, K., Alsaadi, I., Al-Khaial, M. Q., Bnyan, R., Khan, S. A., Sadozai, S. K., Khan, W., & Yousaf, S. (2024). Investigation of Spray Drying Parameters to Formulate Novel Spray-Dried Proliposome Powder Formulations Followed by Their Aerosolization Performance. Pharmaceutics, 16(12), 1541. https://doi.org/10.3390/pharmaceutics16121541







