Tissue Engineering Construct for Articular Cartilage Restoration with Stromal Cells from Synovium vs. Dental Pulp—A Pre-Clinical Study
Abstract
:1. Introduction
2. Methods
2.1. Experimental Design
2.2. Harvesting, Isolation, and Expansion of MSCs
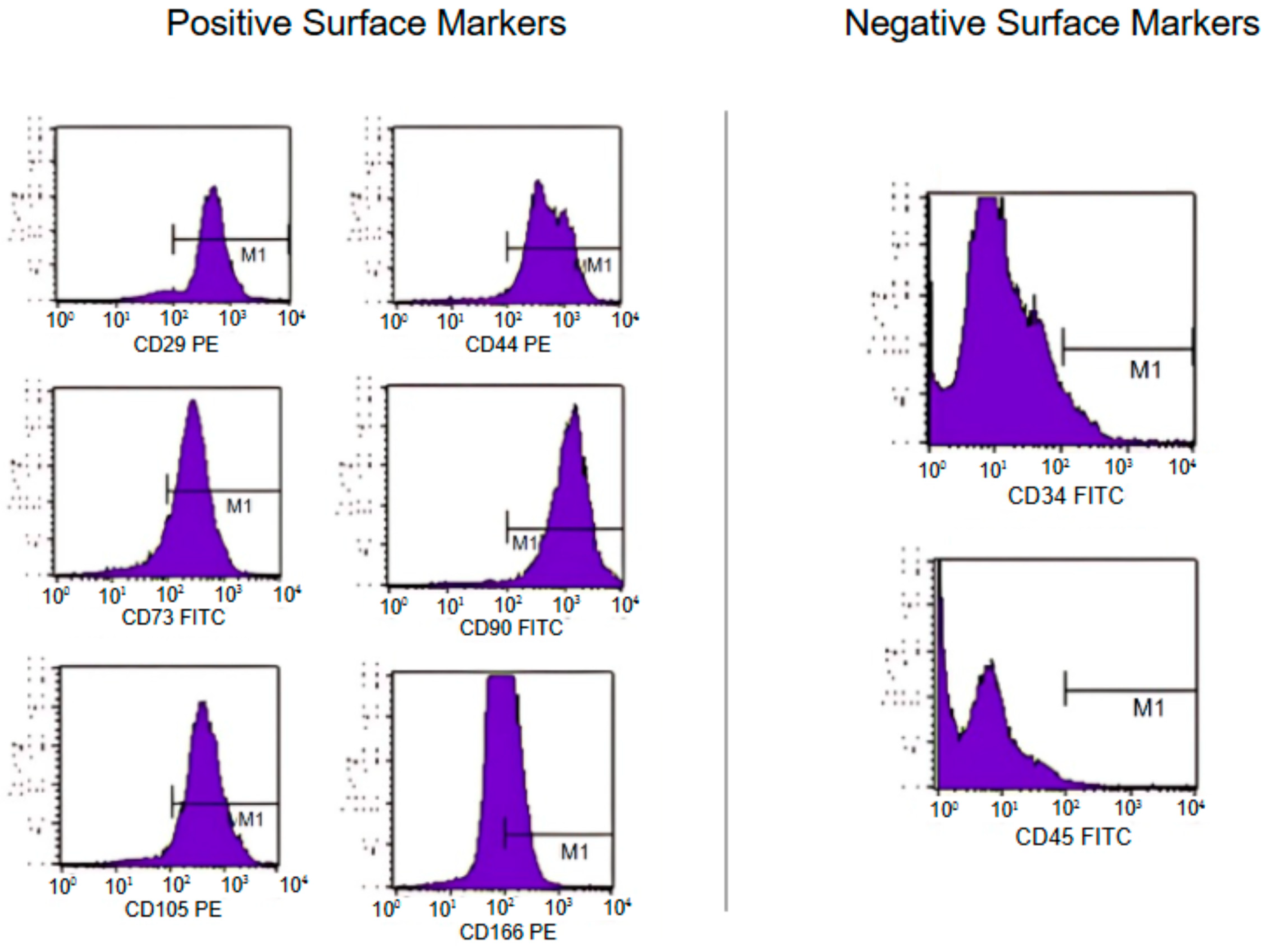
2.3. Characterization of MSCs
2.4. TEC Development
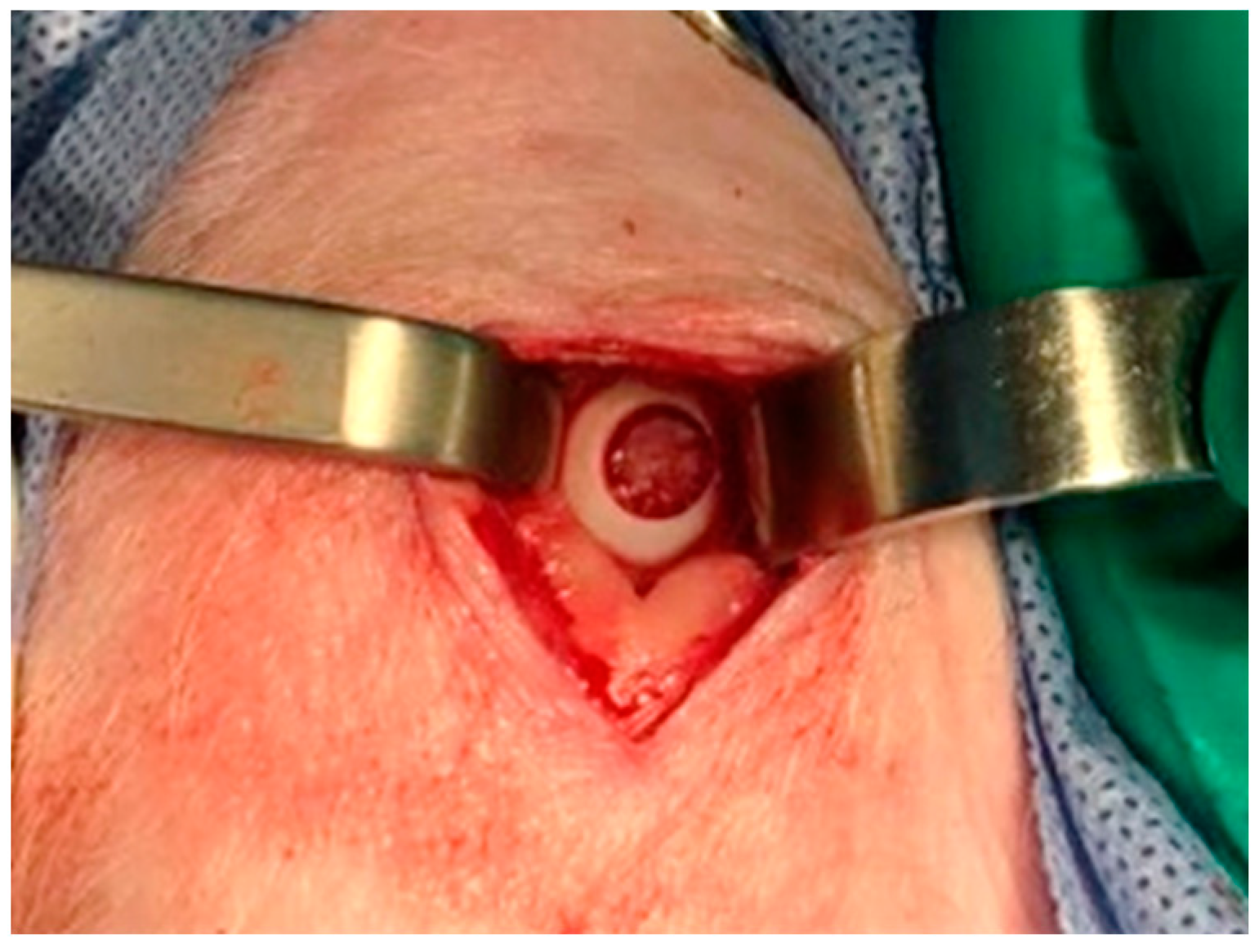
2.5. Animal Model and Surgical Technique
2.6. Evaluation Methods
2.6.1. Magnetic Resonance Imaging
2.6.2. Histological Evaluation
2.7. Statistical Analysis
3. Results
3.1. Characterization of MSC Strains
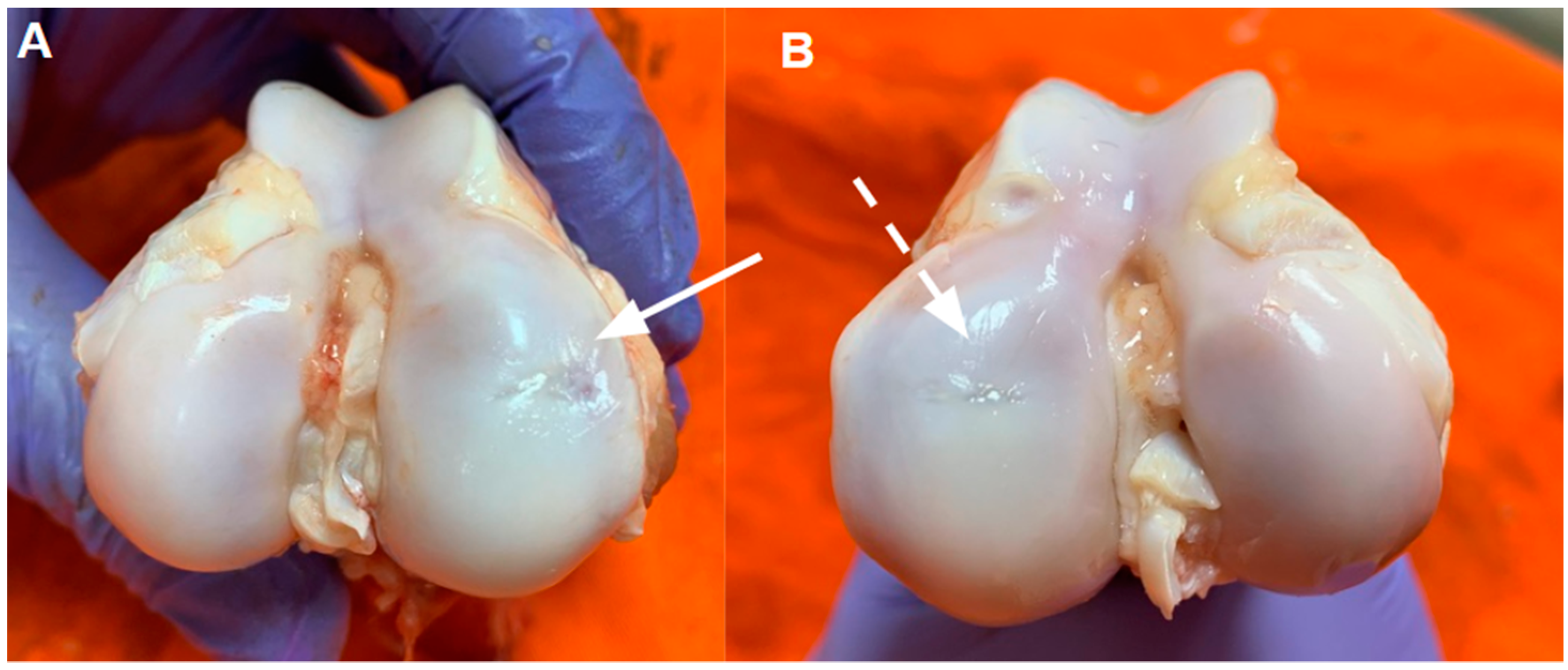
3.2. Macroscopic Characterization
3.3. Magnetic Resonance Imaging
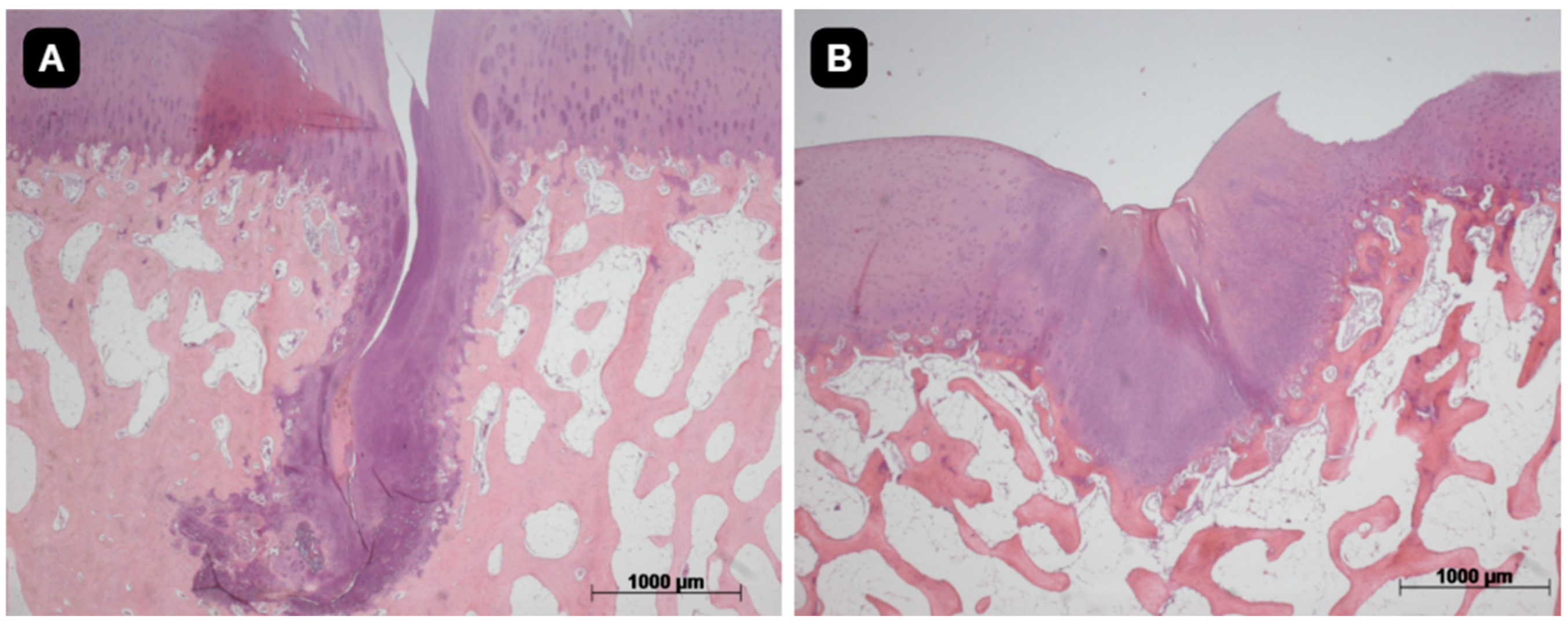
3.4. Histological Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gomoll, A.H.; Madry, H.; Knutsen, G.; van Dijk, N.; Seil, R.; Brittberg, M.; Kon, E. The subchondral bone in articular cartilage repair: Current problems in the surgical management. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 434. [Google Scholar] [CrossRef] [PubMed]
- Showery, J.E.; Kusnezov, N.A.; Dunn, J.C.; Bader, J.O.; Belmont, P.J.; Waterman, B.R. The rising incidence of degenerative and posttraumatic osteoarthritis of the knee in the United States military. J. Arthroplast. 2016, 31, 2108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shi, Y. Mesenchymal stem/stromal cells (MSCs): Origin, immune regulation, and clinical applications. Cell Mol. Immunol. 2023, 20, 555–557. [Google Scholar] [CrossRef]
- Ando, W.; Tateishi, K.; Katakai, D.; Hart, D.A.; Higuchi, C.; Nakata, K.; Hashimoto, J.; Fujie, H.; Shino, K.; Yoshikawa, H.; et al. In vitro generation of a scaffold-free tissue-engineered construct (TEC) derived from human synovial mesenchymal stem cells: Biological and mechanical properties and further chondrogenic potential. Tissue Eng. Part A 2008, 14, 2041. [Google Scholar] [CrossRef]
- Bueno, D.F. Bone Tissue Engineering with Dental Pulp Stem Cells for Alveolar Cleft Repair. Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT03766217?cond=Alveolar+Cleft+Repair&term=bueno&rank=1 (accessed on 24 November 2024).
- Fernandes, T.L.; Kimura, H.A.; Pinheiro, C.C.G.; Shimomura, K.; Nakamura, N.; Ferreira, J.R.; Gomoll, A.H.; Hernandez, A.J.; Bueno, D.F. Human synovial mesenchymal stem cells good manufacturing practices for articular cartilage regeneration. Tissue Eng. Part C Methods 2018, 24, 709. [Google Scholar] [CrossRef]
- Kubosch, E.J.; Lang, G.; Furst, D.; Kubosch, D.; Izadpanah, K.; Rolauffs, B.; Sudkamp, N.P.; Schmal, H. The potential for synovium-derived stem cells in cartilage repair. Curr. Stem Cell Res. Ther. 2018, 13, 174. [Google Scholar] [CrossRef]
- Pinheiro, C.C.; Leyendecker Junior, A.; Tanikawa, D.Y.; Ferreira, J.R.M.; Jarrahy, R.; Bueno, D.F. Is there a noninvasive source of MSCs isolated with GMP methods with better osteogenic potential? Stem. Cells Int. 2019, 2019, 1. [Google Scholar] [CrossRef]
- SantAnna, J.P.; Faria, R.R.; Assad, I.P.; Pinheiro, C.C.; Aiello, V.D.; Albuquerque-Neto, C.; Bortolussi, R.; Cestari, I.A.; Maizato, M.J.; Hernandez, A.J.; et al. Tissue Engineering and Cell Therapy for Cartilage Repair: Preclinical Evaluation Methods. Tissue Eng. Part C Methods 2022, 28, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. and NC3Rs Reporting Guidelines Working Group. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.M. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J. Bone Jt. Surg. 2010, 92, 2039. [Google Scholar] [CrossRef] [PubMed]
- Goebel, L.; Zurakowski, D.; Müller, A.; Pape, D.; Cucchiarini, M.; Madry, H. 2D and 3D MOCART scoring systems assessed by 9.4T high-field MRI correlate with elementary and complex histological scoring systems in a translational model of osteochondral repair. Osteoarthr. Cartil. 2014, 22, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Trattnig, S.; Domayer, S.; Welsch, G.W.; Mosher, T.; Eckstein, F. MR imaging of cartilage and its repair in the knee—A review. Eur. Radiol. 2009, 19, 1582–1594. [Google Scholar] [CrossRef]
- Domayer, S.E.; Kutscha-Lissberg, F.; Welsch, G.; Dorotka, R.; Nehrer, S.; Gäbler, C.; Mamisch, T.C.; Trattnig, S. T2 mapping in the knee after microfracture at 3.0 T: Correlation of global T2 values and clinical outcome—Preliminary results. Osteoarthr. Cartil. 2008, 16, 903–908. [Google Scholar] [CrossRef]
- White, L.M.; Sussman, M.S.; Hurtig, M.; Probyn, L.; Tomlinson, G.; Kandel, R. Cartilage T2 assessment: Differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology 2006, 241, 407–414. [Google Scholar] [CrossRef]
- Mainil-Varlet, P.; Van Damme, B.; Nesic, D.; Knutsen, G.; Kandel, R.; Roberts, S. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am. J. Sports Med. 2010, 38, 880–890. [Google Scholar] [CrossRef]
- Meng, X.; Ziadlou, R.; Grad, S.; Alini, M.; Wen, C.; Lai, Y.; Qin, L.; Zhao, Y.; Wang, X. Animal Models of Osteochondral Defect for Testing Biomaterials. Biochem. Res. Int. 2020, 2020, 9659412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mei, R.; Wan, Z.; Yang, C.; Shen, X.; Wang, R.; Zhang, H.; Yang, R.; Li, J.; Song, Y.; Su, H. Advances and clinical challenges of mesenchymal stem cell therapy. Front. Immunol. 2024, 15, 1421854. [Google Scholar] [CrossRef]
- Li, W.-J.; Chiang, H.; Kuo, T.-F.; Lee, H.-S.; Jiang, C.-C.; Tuan, R.S. Evaluation of articular cartilage repair using biodegradable nanofibrous scaffolds in a swine model: A pilot study. J. Tissue Eng. Regen. Med. 2009, 3, 1–7. [Google Scholar] [CrossRef]
- Shimomura, K.; Ando, W.; Tateishi, K.; Nansai, R.; Fujie, H.; Hart, D.A.; Kohda, H.; Kita, K.; Kanamoto, T.; Mae, T.; et al. The influence of skeletal maturity on allogenic synovial mesenchymal stem cell-based repair of cartilage in a large animal model. Biomaterials 2010, 31, 8004–8011. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, K.; Ando, W.; Hart, D.A.; Yonetani, Y.; Horibe, S.; Nakamura, N. Five-Year Outcomes After Implantation of a Scaffold-Free Tissue-Engineered Construct Generated from Autologous Synovial Mesenchymal Stromal Cells for Repair of Knee Chondral Lesions. Orthop. J. Sports Med. 2023, 11, 23259671231189474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shimomura, K.; Yasui, Y.; Koizumi, K.; Chijimatsu, R.; Hart, D.A.; Yonetani, Y.; Ando, W.; Nishii, T.; Kanamoto, T.; Horibe, S.; et al. First-in-Human Pilot Study of Implantation of a Scaffold-Free Tissue-Engineered Construct Generated from Autologous Synovial Mesenchymal Stem Cells for Repair of Knee Chondral Lesions. Am. J. Sports Med. 2018, 46, 2384–2393. [Google Scholar] [CrossRef] [PubMed]
- Crema, M.D.; Roemer, F.W.; Marra, M.D.; Burstein, D.; Gold, G.E.; Eckstein, F.; Baum, T.; Mosher, T.J.; Carrino, J.A.; Guermazi, A. Articular Cartilage in the Knee: Current MR imaging techniques and applications in clinical practice and research. Radiographics 2011, 31, 37–61. [Google Scholar] [CrossRef]
- Hayashi, D.; Li, X.; Murakami, A.M.; Roemer, F.W.; Trattnig, S.; Guermazi, A. Understanding Magnetic Resonance Imaging of Knee Cartilage Repair: A Focus on Clinical Relevance. Cartilage 2018, 9, 223–236. [Google Scholar] [CrossRef]
- Theruvath, A.J.; Mahmoud, E.E.; Wu, W.; Nejadnik, H.; Kiru, L.; Liang, T.; Felt, S.; Daldrup-Link, H.E. Ascorbic Acid and Iron Supplement Treatment Improves Stem Cell—Mediated Cartilage Regeneration in a Minipig Model. Am. J. Sports Med. 2021, 49, 1861–1870. [Google Scholar] [CrossRef]
- Ando, W.; Tateishi, K.; Hart, D.A.; Katakai, D.; Tanaka, Y.; Nakata, K.; Hashimoto, J.; Fujie, H.; Shino, K.; Yoshikawa, H.; et al. Cartilage repair using an in vitro generated scaffold-free tissue-engineered construct derived from porcine synovial mesenchymal stem cells. Biomaterials 2007, 28, 5462–5470. [Google Scholar] [CrossRef] [PubMed]
- Gardner, O.F.; Juneja, S.C.; Whetstone, H.; Nartiss, Y.; Sieker, J.T.; Veillette, C.; Keller, G.M.; Craft, A.M. Effective repair of articular cartilage using human pluripotent stem cell-derived tissue. Eur Cell Mater. 2019, 38, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Cao, R.; Wu, D.; Zhang, H.; Yang, F.; Wang, L. Dental Pulp Stem Cells for Bone Tissue Engineering: A Literature Review. Stem. Cells Int. 2023, 2023, 7357179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nowzari, F.; Zare, M.; Tanideh, N.; Meimandi-Parizi, A.; Kavousi, S.; Saneian, S.M.; Zare, S.; Koohi-Hosseinabadi, O.; Ghaemmaghami, P.; Dehghanian, A.; et al. Comparing the healing properties of intra-articular injection of human dental pulp stem cells and cell-free-secretome on induced knee osteoarthritis in male rats. Tissue Cell 2023, 82, 102055. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cui, S.; Zhou, Y.; Qiu, L. Dental Pulp Stem Cell-Derived Exosomes Alleviate Mice Knee Osteoarthritis by Inhibiting TRPV4-Mediated Osteoclast Activation. Int. J. Mol. Sci. 2023, 24, 4926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lo Monaco, M.; Gervois, P.; Beaumont, J.; Clegg, P.; Bronckaers, A.; Vandeweerd, J.M.; Lambrichts, I. Therapeutic Potential of Dental Pulp Stem Cells and Leukocyte- and Platelet-Rich Fibrin for Osteoarthritis. Cells 2020, 9, 980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, X.; Xu, L.; Xia, J.; Wen, C.; Liang, Y.; Zhang, Y. Harnessing knee joint resident mesenchymal stem cells in cartilage tissue engineering. Acta Biomater. 2023, 168, 372–387. [Google Scholar] [CrossRef] [PubMed]






| Histological Parameter | Score |
|---|---|
| 1. Tissue Morphology (viewed under polarized light) | 0%: Full thickness collagen fibers 100%: Normal cartilage birefringence |
| 2. Matrix staining (metachromasia) | 0%: No staining 100%: Full metachromasia |
| 3. Cell morphology | 0%: No round/oval cells 100%: Mostly round/oval cells |
| 4. Chondrocyte clustering (4 or more grouped cells) | 0%: Present 100%: Absent |
| 5. Surface architecture | 0%: Delamination, or major irregularity 100%: Smooth surface |
| 6. Basal integration | 0%: No integration 100%: Complete integration |
| 7. Formation of a tidemark | 0%: No calcification front 100%: Tidemark |
| 8. Subchondral bone abnormalities/marrow fibrosis | 0%: Abnormal 100%: Normal marrow |
| 9. Inflammation | 0%: Present 100%: Absent |
| 10. Abnormal calcification/ossification | 0%: Present 100%: Absent |
| 11. Vascularization (within the repaired tissue) | 0%: Present 100%: Absent |
| 12. Surface/superficial assessment | 0%: Total loss or complete disruption 100%: Resembles intact articular cartilage |
| 13. Mid/deep zone assessment | 0%: Fibrous tissue 100%: Normal hyaline cartilage |
| 14. Overall assessment | 0%: Bad (fibrous tissue) 100%: Good (hyaline cartilage) |
| Type | Surface Marker | Percentages |
|---|---|---|
| Positive | CD166 | 57.1% |
| Positive | CD105 | 95.1% |
| Positive | CD90 | 97.5% |
| Positive | CD73 | 83.3% |
| Positive | CD44 | 93.5% |
| Positive | CD29 | 93.0% |
| Negative | CD117 | 5.0% |
| Negative | CD31 | 4.8% |
| Negative | CD45 | 4.1% |
| Negative | CD34 | 4.8% |
| ICRS-2 Parameters | Dental Pulp | Synovial | Control | p |
|---|---|---|---|---|
| 1. Tissue morphology (viewed under polarized light) | 55.7 ± 14 | 67.1 ± 17.0 | 39.3 ± 24 | * p < 0.05 |
| 2. Matrix Staining (metachromasia) | 68.3 ± 20.4 | 86.7 ± 8.2 | 59.2 ± 26.4 | n.s. |
| 3. Cell Morphology | 43.3 ± 12.1 | 65.7 ± 25.1 | 47.9 ± 28.1 | n.s. |
| 4. Chondrocyte clustering | 78.3 ± 11.7 | 90.7 ± 11.7 | 85.8 ± 12.4 | n.s. |
| 5. Surface architecture | 65.7 ± 22.3 | 58.6 ± 25.5 | 43.8 ± 21 | n.s. |
| 6. Basal integration | 100 ± 0 | 92.9 ± 18.9 | 62.9 ± 43.4 | n.s. |
| 7. Formation of a tidemark | 55.8 ± 33.5 | 72.9 ± 34.5 | 60.8 ± 24.2 | n.s. |
| 8. Subchondral bone abnormalities/marrow fibrosis | 36.7 ± 40.3 | 61.4 ± 37.2 | 43.5 ± 26.9 | n.s. |
| 9. Inflammation | 83.3 ± 18.6 | 97.1 ± 7.6 | 88.8 ± 27.4 | n.s. |
| 10. Abnormal calcification/ossification | 84.3 ± 35.4 | 100 ± 0 | 98.5 ± 5.5 | n.s. |
| 11. Vascularization (within the repaired tissue) | 81.7 ± 21.4 | 91.4 ± 22.7 | 90.8 ± 27.5 | n.s. |
| 12. Surface/superficial assessment | 60 ± 30 | 67.1 ± 21.4 | 44.3 ± 22.7 | n.s. |
| 13. Mid/deep zone assessment | 35 ± 38.9 | 57.1 ± 33 | 40 ± 19.1 | n.s. |
| 14. Overall assessment | 54.3 ± 12.2 | 64.3 ± 19 | 42.1 ± 14.8 | * p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, T.L.; Santanna, J.P.C.; de Faria, R.R.; Pastore, E.R.; Bueno, D.F.; Hernandez, A.J. Tissue Engineering Construct for Articular Cartilage Restoration with Stromal Cells from Synovium vs. Dental Pulp—A Pre-Clinical Study. Pharmaceutics 2024, 16, 1558. https://doi.org/10.3390/pharmaceutics16121558
Fernandes TL, Santanna JPC, de Faria RR, Pastore ER, Bueno DF, Hernandez AJ. Tissue Engineering Construct for Articular Cartilage Restoration with Stromal Cells from Synovium vs. Dental Pulp—A Pre-Clinical Study. Pharmaceutics. 2024; 16(12):1558. https://doi.org/10.3390/pharmaceutics16121558
Chicago/Turabian StyleFernandes, Tiago Lazzaretti, João Paulo Cortez Santanna, Rafaella Rogatto de Faria, Enzo Radaic Pastore, Daniela Franco Bueno, and Arnaldo José Hernandez. 2024. "Tissue Engineering Construct for Articular Cartilage Restoration with Stromal Cells from Synovium vs. Dental Pulp—A Pre-Clinical Study" Pharmaceutics 16, no. 12: 1558. https://doi.org/10.3390/pharmaceutics16121558
APA StyleFernandes, T. L., Santanna, J. P. C., de Faria, R. R., Pastore, E. R., Bueno, D. F., & Hernandez, A. J. (2024). Tissue Engineering Construct for Articular Cartilage Restoration with Stromal Cells from Synovium vs. Dental Pulp—A Pre-Clinical Study. Pharmaceutics, 16(12), 1558. https://doi.org/10.3390/pharmaceutics16121558







