Biocompatible Carbon Dots/Polyurethane Composites as Potential Agents for Combating Bacterial Biofilms: N-Doped Carbon Quantum Dots/Polyurethane and Gamma Ray-Modified Graphene Quantum Dots/Polyurethane Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of CAUR-CQDs, GQD50 Nanoparticles, and Polyurethane-Based Composite Films
2.2.1. Synthesis of CAUR-CQDs, GQD50 Nanoparticles and Corresponding Polyurethane Composite Films
2.2.2. Characterization of CAUR-CQDs, GQD50 Nanoparticles, and Corresponding Polyurethane Composite Films
2.3. Reactive Oxygen Production
2.4. Antibacterial Activity of Polyurethane Composite Films
2.5. Antibiofouling Activity of Polyurethane Composite Films
2.6. Biocompatibility Studies of Polyurethane Composite Films
2.6.1. Cell Culture
2.6.2. Cell Viability Assay
2.6.3. Hemolysis Assay
3. Results
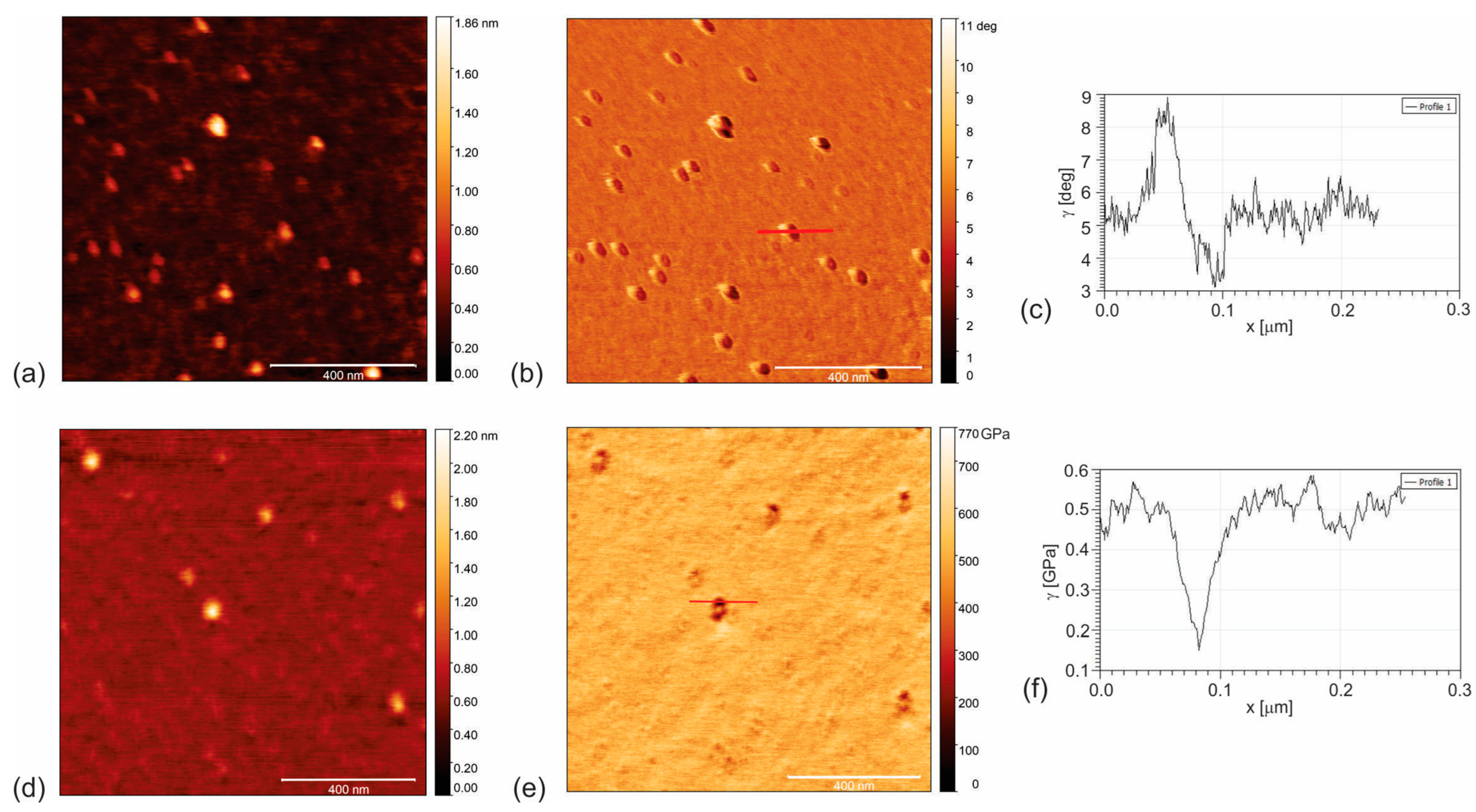
3.1. Surface Morphology of CAUR-CQDs and GQD50 Nanoparticles
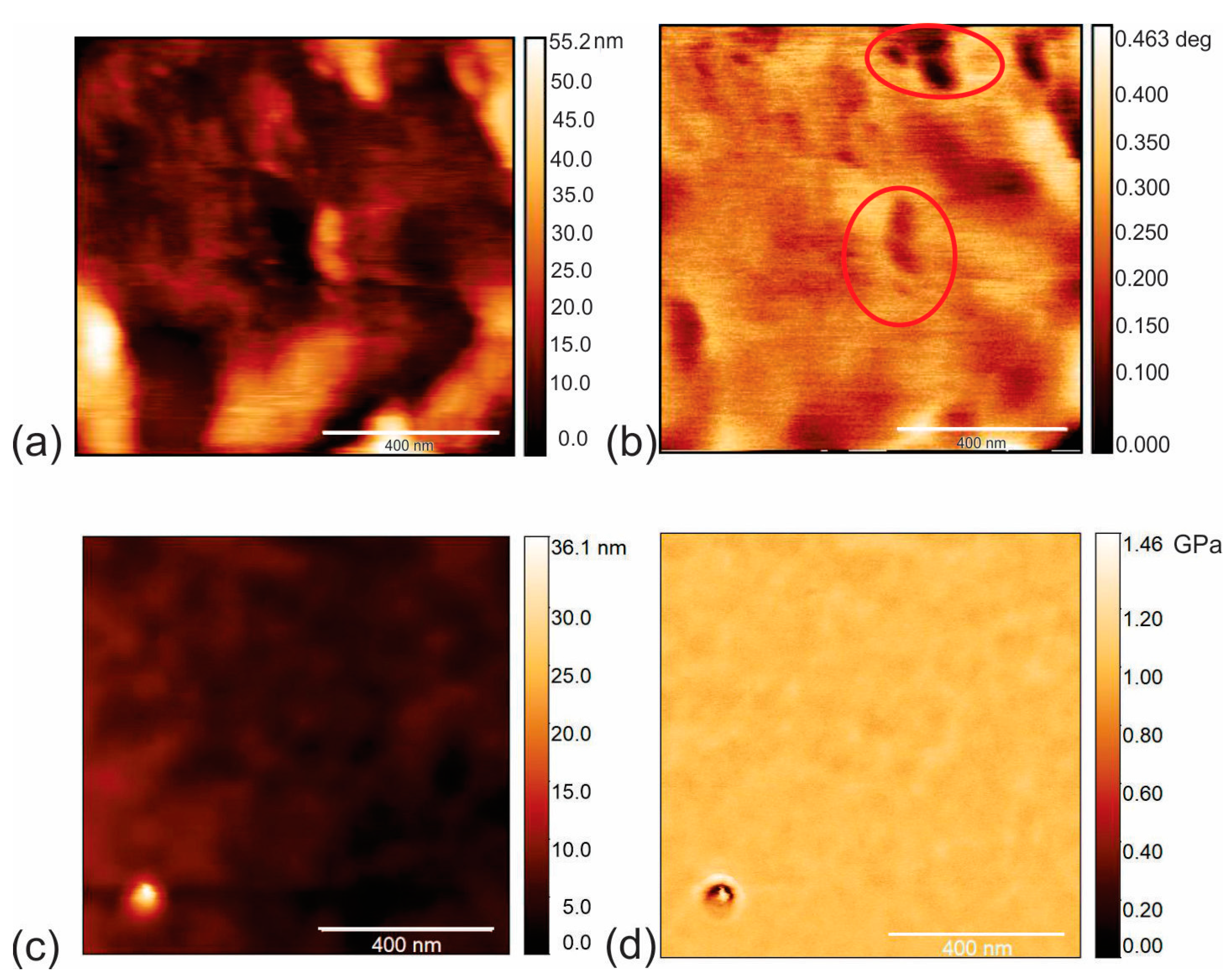
3.2. Surface Morphology of CAUR-CQDs/PU and GQD50/PU Composite Films
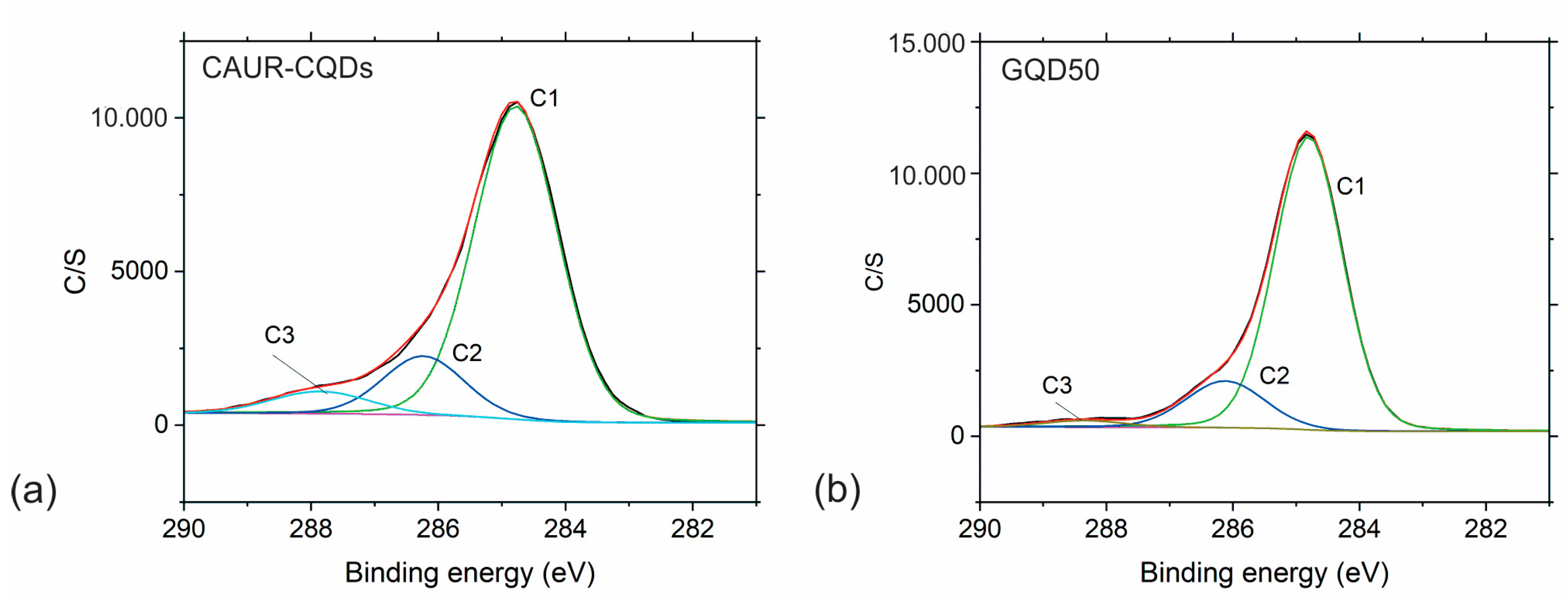
3.3. Chemical Composition
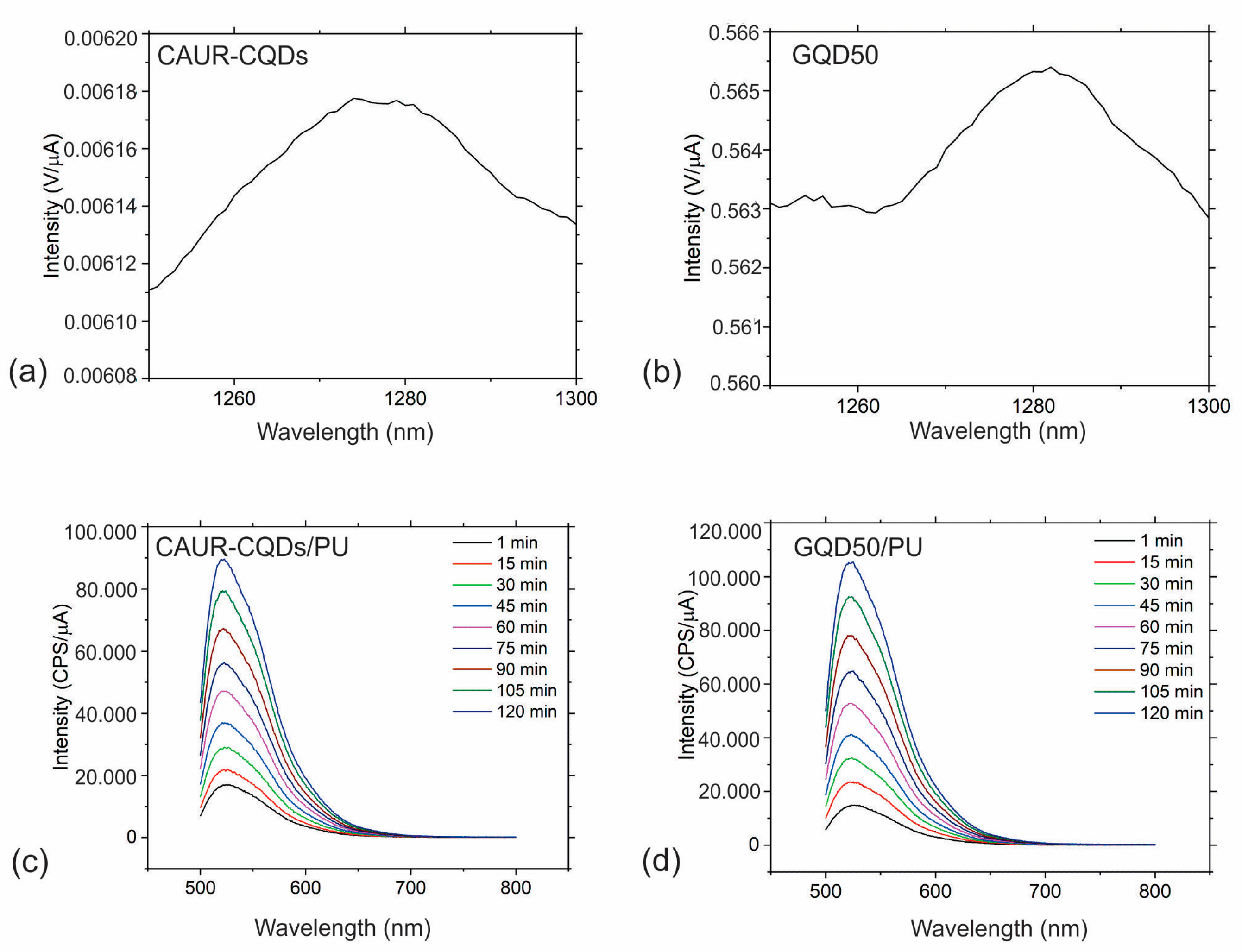
3.4. Photoluminescence of CAUR-CQDs and GQD50 Nanoparticles and Polymer Composite Films
3.5. ROS Production
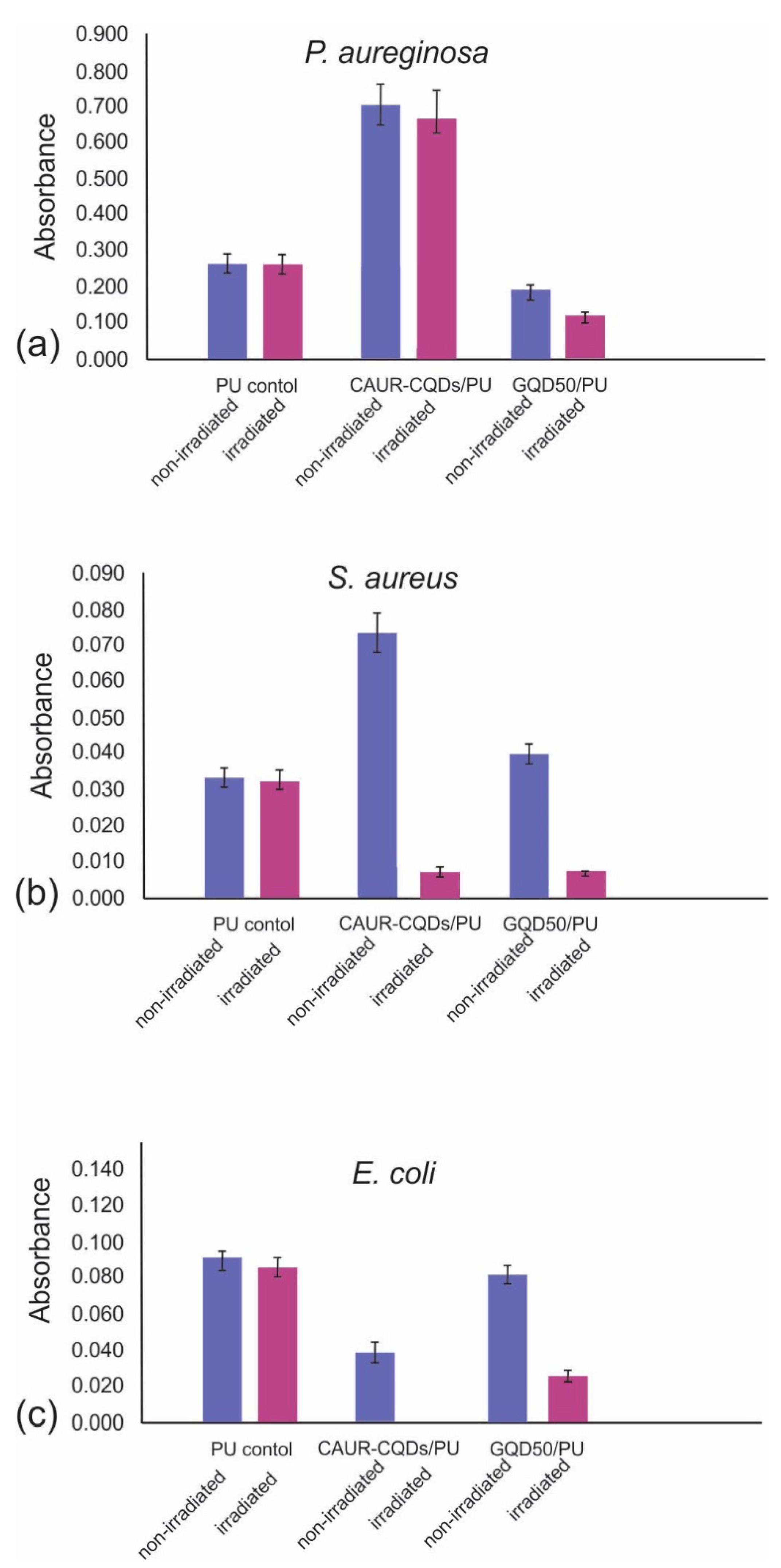
3.6. Antibacterial Activity of CAUR-CQDs/PU and GQD50/PU Composite Films
3.7. Antibiofouling Activity of CAUR-CQDs/PU and GQD50/PU Composite Films
3.8. Biocompatibility Studies
3.8.1. Cytotoxicity
3.8.2. Hemolysis Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Shree, P.; Singh, C.K.; Sodhi, K.K.; Surya, J.N.; Singh, D.K. Biofilms: Understanding the structure and contribution towards bacterial resistance in antibiotics. Med. Microecol. 2023, 16, 100084. [Google Scholar] [CrossRef]
- Moser, C.; Pedersen, H.T.; Lerche, C.J.; Kolpen, M.; Line, L.; Thomsen, K.; Høiby, N.; Jensen, P.O. Biofilms and host response—Helpful or harmful. APMIS 2017, 125, 320–338. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.G.; Yousef, A.E. Combating bacterial biofilms: Current and emerging antibiofilm strategies for treating persistent infections. Antibiotics 2023, 12, 1005. [Google Scholar] [CrossRef]
- Ciofu, O.; Moser, C.; Jensen, P.O.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef]
- Marković, Z.M.; Budimir Filimonović, M.; Milivojević, D.D.; Kovač, J.; Todorović Marković, B. Antibacterial and antibiofouling activities of carbon polymerized dots/polyurethane and C60/polyurethane composite films. J. Funct. Biomater. 2024, 15, 73. [Google Scholar] [CrossRef]
- Marković, Z.M.; Milivojević, D.D.; Kovač, J.; Todorović Marković, B.M. Phloroglucinol-based carbon quantum dots/polyurethane composite films: How structure of carbon quantum dots affects antibacterial and antibiofouling efficiency of composite films. Polymers 2024, 16, 1646. [Google Scholar] [CrossRef]
- Sehmi, S.; Noimark, S.; Weiner, J.; Allan, E.; MacRobert, A.J.; Parkin, I.P. Potent antibacterial activity of copper embedded into silicone and polyurethane. ACS Appl. Mater. Interfaces 2015, 7, 22807–22813. [Google Scholar] [CrossRef]
- McCoy, C.P.; O’Neil, E.J.; Cowley, J.F.; Carson, L.; De Baróid, A.T.; Gdowski, G.T.; Gorman, S.P.; Jones, D.S. Photodynamic antimicrobial polymers for infection control. PLoS ONE 2014, 9, e108500. [Google Scholar] [CrossRef]
- Walker, T.; Canales, M.; Noimark, S.; Page, K.; Parkin, I.; Faull, J.; Bhatti, M.; Ciric, A.L. Light activated antimicrobial surface is active against bacterial, viral and fungal organisms. Sci. Rep. 2017, 7, 15298. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Piccirillo, C.; Pratten, J.; Prokopovich, P.; Chrzanowski, W.; Parkin, I.P.; Wilson, M. The antimicrobial properties of light-activated polymers containing methylene blue and gold nanoparticles. Biomaterials 2009, 30, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, C.; Perni, S.; Gil-Thomas, J.; Prokopovich, P.; Wilson, M.; Pratten, J.; Parkin, I.P. Antimicrobial activity of methylene blue and toluidine blue O covalently bound to a modified silicone polymer surface. J. Mater. Chem. 2009, 19, 6167–6171. [Google Scholar] [CrossRef]
- Dong, X.; Liang, W.; Meziani, M.J.; Sun, Y.P.; Yang, L. Carbon dots as potent antimicrobial agents. Theranostics 2020, 10, 671–686. [Google Scholar] [CrossRef]
- Ullal, N.; Mehta, R.; Sunil, D. Separation and purification of fluorescent carbon dots—An unmet challenge. Analyst 2024, 149, 1680. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Stalikas, C. Antimicrobial properties of carbon quantum dots. In Micro and Nano Technologies, Nanotoxicity; Rajendran, S., Mukherjee, A., Nguyen, T.A., Godugu, C., Shukla, R.K., Eds.; Elsevier: New York, NY, USA, 2020; pp. 301–315. [Google Scholar] [CrossRef]
- Yu, M.; Li, P.; Huang, R.; Xu, C.; Zhang, S.; Wang, Y.; Gong, X.; Xing, X. Antibacterial and antibiofilm mechanisms of carbon dots: A review. J. Mater. Chem. B 2023, 11, 734–754. [Google Scholar] [CrossRef]
- Li, H.T.; He, X.D.; Kang, Z.H.; Huang, H.; Liu, Y.; Liu, J.L.; Lian, S.Y.; Tsang, C.H.A.; Yang, X.B.; Lee, S.T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Marković, Z.M.; Kováčová, M.; Jeremić, S.R.; Nagy, Š.; Milivojević, D.D.; Kubat, P.; Kleinová, A.; Budimir, M.D.; Mojsin, M.M.; Stevanović, M.J.; et al. Highly efficient antibacterial polymer composites based on hydrophobic riboflavin carbon polymerized dots. Nanomaterials 2022, 12, 4070. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: Open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–218. [Google Scholar] [CrossRef]
- AM-FM Viscoelastic Mapping Mode. Available online: https://afm.oxinst.com/assets/uploads/products/asylum/documents/AM-FM-Viscoelastic-Mapping-Mode-Application-Note.pdf (accessed on 12 September 2024).
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Physical Electronics Inc.: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Software.informer. Available online: https://multipak.software.informer.com (accessed on 9 September 2024).
- Lin, H.; Shen, Y.; Chen, D.; Lin, L.; Wilson, B.C.; Li, B.; Xie, S. Feasibility study on quantitative measurements of singlet oxygen generation using singlet oxygen sensor green. J. Fluoresc. 2013, 23, 41–47. [Google Scholar] [CrossRef]
- ISO 22196:2007; Plastics—Measurement of Antibacterial Activity on Plastics Surfaces. ISO Central Secretariat: Vernier, Geneva, 2007. Available online: https://www.iso.org/standard/40759.html (accessed on 16 April 2024).
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, R.; Li, X.; Wang, C.; Bao, H.; Wang, S.; Fang, J.; Huang, J.; Wang, C. Blood-compatible polyaniline coated electrospun polyurethane fiber scaffolds for enhanced adhesion and proliferation of human umbilical vein endothelial cells. Fibers Polym. 2019, 20, 250–260. [Google Scholar] [CrossRef]
- Karahaliloglu, Z. Electrospun PU-PEG and PU-PC hybrid scaffolds for vascular tissue engineering. Fibers Polym. 2017, 18, 2135–2145. [Google Scholar] [CrossRef]
- Bati, A.S.R.; Yu, L.P.; Tawfik, S.A.; Spencer, M.J.S.; Shaw, P.E.; Batmunkh, M.; Shapter, J.G. Electrically sorted single-walled carbon nanotubes-based electron transporting layers for perovskite solar cells. iScience 2019, 14, 100–112. [Google Scholar] [CrossRef]
- Miyashiroa, D.; Taira, H.; Hamano, R.; Reserva, R.L.; Umemura, K. Mechanical vibration of single-walled carbon nanotubes at different lengths and carbon nanobelts by modal analysis method. Compos. Part C Open Access. 2020, 2, 100028. [Google Scholar] [CrossRef]
- Khoei, A.R.; Khorrami, M.S. Mechanical properties of graphene oxide: A molecular dynamics study. Fuller. Nanotub. Carbon Nanostruct. 2016, 24, 594–603. [Google Scholar] [CrossRef]
- Kutluoglu, E.E.; Orhan, E.O.; Bayram, O.; Ocak, S.B. Gamma-ray irradiation effects on capacitance and conductance of graphene-based Schottky diode. Phys. B 2021, 621, 413306. [Google Scholar] [CrossRef]
- Infrared Spectroscopy Absorption Table. Available online: https://chem.libretexts.org/Ancillary_Materials/Reference/Reference_Tables/Spectroscopic_Reference_Tables/Infrared_Spectroscopy_Absorption_Table (accessed on 11 May 2024).
- Wang, B.; Lu, S. The light of carbon dots: From mechanism to applications. Matter 2022, 5, 110–149. [Google Scholar] [CrossRef]
- Wen, X.; Yu, P.; Toh, Y.R.; Hao, X.; Tang, J. Intrinsic and extrinsic fluorescence in carbon nanodots: Ultrafast time-resolved fluorescence and carrier dynamics. Adv. Opt. Mater. 2013, 1, 173–178. [Google Scholar] [CrossRef]
- Vaishampayan, A.; Grohmann, E. Antimicrobials functioning through ROS-mediated mechanisms: Current insights. Microorganisms 2022, 10, 61. [Google Scholar] [CrossRef]
- Kováčová, M.; Marković, Z.M.; Humpolíček, P.; Mičušík, M.; Švajdlenková, H.; Kleinová, A.; Danko, M.; Kubát, P.; Vajdák, J.; Capáková, Z.; et al. Carbon quantum dots modified polyurethane nanocomposites as effective photocatalytic and antibacterial agents. ACS Biomater. Sci. Eng. 2018, 4, 3983–3993. [Google Scholar] [CrossRef]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. Graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of surface properties, bacterial motility, and hydrodynamic conditions on bacterial surface sensing and their initial adhesion. Front. Bioeng. Biotechnol. 2021, 12, 643722. [Google Scholar] [CrossRef] [PubMed]
- Auer, G.K.; Weibel, D.B. Bacterial cell mechanics. Biochemistry 2017, 56, 3710–3724. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Lin, L.; Zheng, M.; Liu, W.; Lin, R. Antibacterial functionalized carbon dots and their application in bacterial infections and inflammation. J. Mater. Chem. B 2023, 11, 9386–9403. [Google Scholar] [CrossRef]
- Maisch, T.; Baier, J.; Franz, B.; Maier, M.; Landthaler, M.; Szeimies, R.; Bäumler, W. The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 7223–7228. [Google Scholar] [CrossRef]
- Herrling, M.P.; Lackner, S.; Nirschl, H.; Horn, H.; Guthausen, G. Recent NMR/MRI studies of biofilm structures and dynamics. Annu. Rep. NMR Spectrosc. 2019, 97, 163–213. [Google Scholar] [CrossRef]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaT cells as a reliable in vitro differentiation model to dissect the inflammatory/repair response of human keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef]
- Hanel, K.H.; Cornelissen, C.; Luscher, B.; Baron, J.M. Cytokines and the skin barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef]
- Chaguza, C.; Pöntinen, A.K.; Top, J.; Arredondo-Alonso, S.; Freitas, A.R.; Novais, C.; Torres, C.; Bentley, S.D.; Peixe, L.; Coque, T.M.; et al. The population-level impact of Enterococcus faecalis genetics on intestinal colonization and extraintestinal infection. Microbiol. Spectr. 2023, 11, e00201-23. [Google Scholar] [CrossRef]
- Satyaprakash, A.K.; Ravanfar, P.; Tyring, S.K. Skin and soft-tissue infections. In Antibiotic and Chemotherapy; Finch, R.G., Greenwood, D., Norrby, S.R., Whitley, R.J., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; pp. 617–632. [Google Scholar]




| Sample | C (at%) | O (at%) | N (at%) |
|---|---|---|---|
| CAUR-CQDs | 83 | 9.3 | 7.7 |
| GQD50 | 83.2 | 16.4 | 0.4 |
| Sample | C1 | C2 | C3 | O1 | O2 | O3 |
|---|---|---|---|---|---|---|
| CAUR-CQDs | 284.8 eV | 286.3 eV | 288.9 eV | 531.1 eV | 532.3 eV | 533.6 eV |
| Bond assignment | C-C | C-N | C=O | O=C-N | O=C | O-C |
| % of bonds | 79.3 | 14.5 | 6.3 | 59.8 | 30.7 | 9.6 |
| GQD50 | 284.8 | 286.1 | 288.4 | 531.1 | 532.4 | 533.5 |
| Bond assignment | C-C | C–O | C=O/O-C–O | C=O (aromatic) | O=C (aliphatic) | O-C |
| % of bonds | 82.7 | 15.3 | 2.1 | 23.9 | 58.6 | 17.6 |
| Bacterial Strains | RCAUR-CQDs/PU | RGQD50/PU |
|---|---|---|
| S. aureus | 5.2 | 0.13 |
| MRSA | 4.3 | 0.5 |
| E. faecalis | 4.7 | 1.12 |
| P. aeruginosa | 0.06 | 0.05 |
| K. pneumonie | 5.3 | 1.5 |
| L. monocytogenes | 0.06 | 1.04 |
| E. coli | 0.02 | 0.02 |
| A. baumanii | 4.9 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marković, Z.; Dorontić, S.; Jovanović, S.; Kovač, J.; Milivojević, D.; Marinković, D.; Mojsin, M.; Todorović Marković, B. Biocompatible Carbon Dots/Polyurethane Composites as Potential Agents for Combating Bacterial Biofilms: N-Doped Carbon Quantum Dots/Polyurethane and Gamma Ray-Modified Graphene Quantum Dots/Polyurethane Composites. Pharmaceutics 2024, 16, 1565. https://doi.org/10.3390/pharmaceutics16121565
Marković Z, Dorontić S, Jovanović S, Kovač J, Milivojević D, Marinković D, Mojsin M, Todorović Marković B. Biocompatible Carbon Dots/Polyurethane Composites as Potential Agents for Combating Bacterial Biofilms: N-Doped Carbon Quantum Dots/Polyurethane and Gamma Ray-Modified Graphene Quantum Dots/Polyurethane Composites. Pharmaceutics. 2024; 16(12):1565. https://doi.org/10.3390/pharmaceutics16121565
Chicago/Turabian StyleMarković, Zoran, Sladjana Dorontić, Svetlana Jovanović, Janez Kovač, Dušan Milivojević, Dragana Marinković, Marija Mojsin, and Biljana Todorović Marković. 2024. "Biocompatible Carbon Dots/Polyurethane Composites as Potential Agents for Combating Bacterial Biofilms: N-Doped Carbon Quantum Dots/Polyurethane and Gamma Ray-Modified Graphene Quantum Dots/Polyurethane Composites" Pharmaceutics 16, no. 12: 1565. https://doi.org/10.3390/pharmaceutics16121565
APA StyleMarković, Z., Dorontić, S., Jovanović, S., Kovač, J., Milivojević, D., Marinković, D., Mojsin, M., & Todorović Marković, B. (2024). Biocompatible Carbon Dots/Polyurethane Composites as Potential Agents for Combating Bacterial Biofilms: N-Doped Carbon Quantum Dots/Polyurethane and Gamma Ray-Modified Graphene Quantum Dots/Polyurethane Composites. Pharmaceutics, 16(12), 1565. https://doi.org/10.3390/pharmaceutics16121565








