Pharmacogenetics of Neoadjuvant MAP Chemotherapy in Localized Osteosarcoma: A Study Based on Data from the GEIS-33 Protocol
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients’ Characteristics
2.3. Outcome Measures
2.4. SNPs Selection and Genotyping
2.5. Immunohistochemical Studies
2.6. Statistical Analysis
3. Results
3.1. Clinical Results
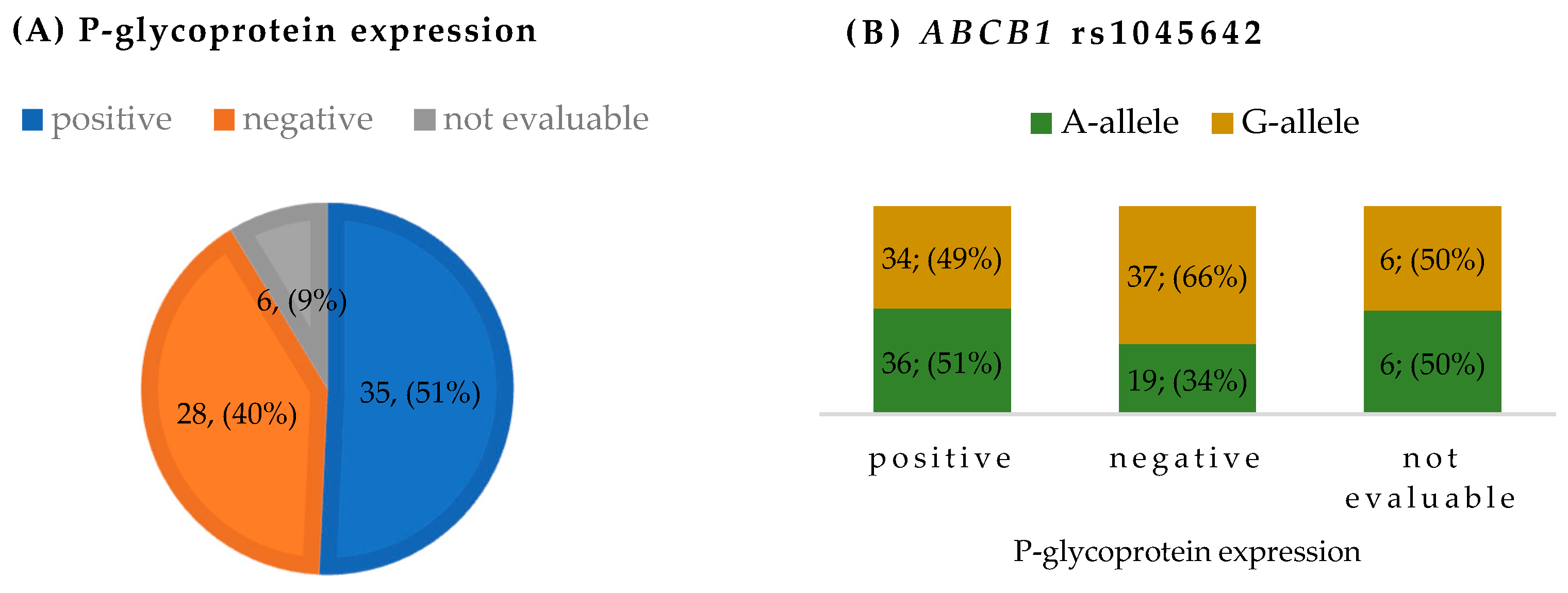
3.2. P-Glycoprotein Expression and ABCB1 Genetic Variants
3.3. Association Analyses and Pathological Response
3.4. Association Analyses and Toxicity
3.5. Survival Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bacci, G.; Briccoli, A.; Ferrari, S.; Longhi, A.; Mercuri, M.; Capanna, R.; Donati, D.; Lari, S.; Forni, C.; DePaolis, M. Neoadjuvant Chemotherapy for Osteosarcoma of the Extremity: Long-Term Results of the Rizzoli’s 4th Protocol. Eur. J. Cancer 2001, 37, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Smeland, S.; Mercuri, M.; Bertoni, F.; Longhi, A.; Ruggieri, P.; Alvegard, T.A.; Picci, P.; Capanna, R.; Bernini, G.; et al. Neoadjuvant Chemotherapy with High-Dose Ifosfamide, High-Dose Methotrexate, Cisplatin, and Doxorubicin for Patients with Localized Osteosarcoma of the Extremity: A Joint Study by the Italian and Scandinavian Sarcoma Groups. J. Clin. Oncol. 2005, 23, 8845–8852. [Google Scholar] [CrossRef] [PubMed]
- Meyers, P.A.; Schwartz, C.L.; Krailo, M.D.; Healey, J.H.; Bernstein, M.L.; Betcher, D.; Ferguson, W.S.; Gebhardt, M.C.; Goorin, A.M.; Harris, M.; et al. Osteosarcoma: The Addition of Muramyl Tripeptide to Chemotherapy Improves Overall Survival—A Report from the Children’s Oncology Group. J. Clin. Oncol. 2008, 26, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Provisor, A.J.; Ettinger, L.J.; Nachman, J.B.; Krailo, M.D.; Makley, J.T.; Yunis, E.J.; Huvos, A.G.; Betcher, D.L.; Baum, E.S.; Kisker, C.T.; et al. Treatment of Nonmetastatic Osteosarcoma of the Extremity with Preoperative and Postoperative Chemotherapy: A Report from the Children’s Cancer Group. J. Clin. Oncol. 1997, 15, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Bacci, G.; Mercuri, M.; Longhi, A.; Ferrari, S.; Bertoni, F.; Versari, M.; Picci, P. Grade of Chemotherapy-Induced Necrosis as a Predictor of Local and Systemic Control in 881 Patients with Non-Metastatic Osteosarcoma of the Extremities Treated with Neoadjuvant Chemotherapy in a Single Institution. Eur. J. Cancer 2005, 41, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, G.U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic Factors in High-Grade Osteosarcoma of the Extremities or Trunk: An Analysis of 1,702 Patients Treated on Neoadjuvant Cooperative Osteosarcoma Study Group Protocols. J. Clin. Oncol. 2002, 20, 776–790. [Google Scholar] [CrossRef]
- Whelan, J.S.; Bielack, S.S.; Marina, N.; Smeland, S.; Jovic, G.; Hook, J.M.; Krailo, M.; Anninga, J.; Butterfass-Bahloul, T.; Böhling, T.; et al. EURAMOS-1, an International Randomised Study for Osteosarcoma: Results from Pre-Randomisation Treatment. Ann. Oncol. 2015, 26, 407–414. [Google Scholar] [CrossRef]
- Marina, N.M.; Smeland, S.; Bielack, S.S.; Bernstein, M.; Jovic, G.; Krailo, M.D.; Hook, J.M.; Arndt, C.; van den Berg, H.; Brennan, B.; et al. Comparison of MAPIE versus MAP in Patients with a Poor Response to Preoperative Chemotherapy for Newly Diagnosed High-Grade Osteosarcoma (EURAMOS-1): An Open-Label, International, Randomised Controlled Trial. Lancet Oncol. 2016, 17, 1396–1408. [Google Scholar] [CrossRef]
- Janeway, K.A.; Grier, H.E. Sequelae of Osteosarcoma Medical Therapy: A Review of Rare Acute Toxicities and Late Effects. Lancet Oncol. 2010, 11, 670–678. [Google Scholar] [CrossRef]
- Chan, E.S.L.; Cronstein, B.N. Mechanisms of Action of Methotrexate. Bull. Hosp. Joint Dis. 2013, 71, S5–S8. [Google Scholar]
- Rabik, C.A.; Dolan, M.E. Molecular Mechanisms of Resistance and Toxicity Associated with Platinating Agents. Cancer Treat. Rev. 2007, 33, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Zamble, D.B.; Mu, D.; Reardon, J.T.; Sancar, A.; Lippard, S.J. Repair of Cisplatin-DNA Adducts by the Mammalian Excision Nuclease. Biochemistry 1996, 35, 10004–10013. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.; Reindl, K.M. Glutathione S-Transferases in Cancer. Antioxidants 2021, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Caronia, D.; Patiño-García, A.; Milne, R.L.; Zalacain-Díez, M.; Pita, G.; Alonso, M.R.; Moreno, L.T.; Sierrasesumaga-Ariznabarreta, L.; Benítez, J.; Gonzáles-Neira, A. Common Variations in ERCC2 Are Associated with Response to Cisplatin Chemotherapy and Clinical Outcome in Osteosarcoma Patients. Pharmacogenomics J. 2009, 9, 347–353. [Google Scholar] [CrossRef]
- Jabeen, S.; Holmboe, L.; Alnæs, G.I.G.; Andersen, A.M.; Hall, K.S.; Kristensen, V.N. Impact of Genetic Variants of RFC1, DHFR and MTHFR in Osteosarcoma Patients Treated with High-Dose Methotrexate. Pharmacogenomics J. 2015, 15, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, M.; Arany, A.; Semsei, A.F.; Csordas, K.; Eipel, O.; Gezsi, A.; Kutszegi, N.; Csoka, M.; Muller, J.; Erdelyi, D.J.; et al. Pharmacogenetic Analysis of High-Dose Methotrexate Treatment in Children with Osteosarcoma. Oncotarget 2017, 8, 9388–9398. [Google Scholar] [CrossRef]
- Liu, Z.F.; Asila, A.L.J.; Aikenmu, K.; Zhao, J.; Meng, Q.C.; Fang, R. Influence of ERCC2 Gene Polymorphisms on the Treatment Outcome of Osteosarcoma. Genet. Mol. Res. 2015, 14, 12967–12972. [Google Scholar] [CrossRef]
- Ji, W.P.; He, N. Bin Investigation on the DNA Repaired Gene Polymorphisms and Response to Chemotherapy and Overall Survival of Osteosarcoma. Int. J. Clin. Exp. Pathol. 2015, 8, 894–899. [Google Scholar]
- Caronia, D.; Patiño-Garcia, A.; Peréz-Martínez, A.; Pita, G.; Moreno, L.T.; Zalacain-Díez, M.; Molina, B.; Colmenero, I.; Sierrasesúmaga, L.; Benítez, J.; et al. Effect of ABCB1 and ABCC3 Polymorphisms on Osteosarcoma Survival after Chemotherapy: A Pharmacogenetic Study. PLoS ONE 2011, 6, e26091. [Google Scholar] [CrossRef]
- Windsor, R.E.; Strauss, S.J.; Kallis, C.; Wood, N.E.; Whelan, J.S. Germline Genetic Polymorphisms May Influence Chemotherapy Response and Disease Outcome in Osteosarcoma: A Pilot Study. Cancer 2012, 118, 1856–1867. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Biason, P.; Iacoboni, E.; Gagno, S.; Fanelli, M.; Tavanti, E.; Vella, S.; Ferrari, S.; Roli, A.; Roncato, R.; et al. Candidate Germline Polymorphisms of Genes Belonging to the Pathways of Four Drugs Used in Osteosarcoma Standard Chemotherapy Associated with Risk, Survival and Toxicity in Non-Metastatic High-Grade Osteosarcoma. Oncotarget 2016, 7, 61970–61987. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneshwar, K.; Harris, M.; Gusev, Y.; Madhavan, S.; Iyer, R.; Vilboux, T.; Deeken, J.; Yang, E.; Shankar, S. Genome Sequencing Analysis of Blood Cells Identifies Germline Haplotypes Strongly Associated with Drug Resistance in Osteosarcoma Patients. BMC Cancer 2019, 19, 357. [Google Scholar] [CrossRef] [PubMed]
- Hurkmans, E.G.E.; Klumpers, M.J.; Vermeulen, S.H.; Hagleitner, M.M.; Flucke, U.; Schreuder, H.W.B.; Gelderblom, H.; Bras, J.; Guchelaar, H.J.; Coenen, M.J.H.; et al. Analysis of Drug Metabolizing Gene Panel in Osteosarcoma Patients Identifies Association Between Variants in SULT1E1, CYP2B6 and CYP4F8 and Methotrexate Levels and Toxicities. Front. Pharmacol. 2020, 11, 1241. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0; U.S. Department of Health and Human Services: Washington, DC, USA, 2009.
- Xie, L.; Guo, W.; Yang, Y.; Ji, T.; Xu, J. More Severe Toxicity of Genetic Polymorphisms on MTHFR Activity in Osteosarcoma Patients Treated with High-Dose Methotrexate. Oncotarget 2018, 9, 11465–11476. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Patrizio, M.P.; Luppi, S.; Serra, M. Pharmacogenomics and Pharmacogenetics in Osteosarcoma: Translational Studies and Clinical Impact. Int. J. Mol. Sci. 2020, 21, 4659. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Z.; Yang, Z.; Feng, C.; Zhou, X.; Tu, C.; Li, Z. MTHFR Polymorphism Is Associated With Severe Methotrexate-Induced Toxicity in Osteosarcoma Treatment. Front. Oncol. 2021, 11, 781386. [Google Scholar] [CrossRef]
- Biason, P.; Hattinger, C.M.; Innocenti, F.; Talamini, R.; Alberghini, M.; Scotlandi, K.; Zanusso, C.; Serra, M.; Toffoli, G. Nucleotide Excision Repair Gene Variants and Association with Survival in Osteosarcoma Patients Treated with Neoadjuvant Chemotherapy. Pharmacogenomics J. 2012, 12, 476–483. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Casotti, C.; Patrizio, M.P.; Luppi, S.; Fantoni, L.; Scotlandi, K.; Ibrahim, T.; Serra, M. Pharmacogenomic Profiling of Cisplatin-Resistant and -Sensitive Human Osteosarcoma Cell Lines by Multimodal Targeted Next Generation Sequencing. Int. J. Mol. Sci. 2022, 23, 11787. [Google Scholar] [CrossRef]
- Hurkmans, E.G.E.; Brand, A.C.A.M.; Verdonschot, J.A.J.; te Loo, D.M.W.M.; Coenen, M.J.H. Pharmacogenetics of Chemotherapy Treatment Response and -Toxicities in Patients with Osteosarcoma: A Systematic Review. BMC Cancer 2022, 22, 1326. [Google Scholar] [CrossRef]
- Liu, B.; Liu, G.; Liu, B.; Guo, Y.; Peng, N.; Li, T. Correlation between Gene Polymorphism and Adverse Reactions of High-Dose Methotrexate in Osteosarcoma Patients: A Systematic Review and Meta-Analysis. World J. Surg. Oncol. 2024, 22, 19. [Google Scholar] [CrossRef]
- Auton, A.; Abecasis, G.R.; Altshuler, D.M.; Durbin, R.M.; Bentley, D.R.; Chakravarti, A.; Clark, A.G.; Donnelly, P.; Eichler, E.E.; Flicek, P.; et al. A Global Reference for Human Genetic Variation. Nature 2015, 526, 68–74. [Google Scholar] [PubMed]
- Serra, M.; Scotlandi, K.; Reverter-Branchat, G.; Ferrari, S.; Manara, M.C.; Benini, S.; Incaprera, M.; Bertoni, F.; Mercuri, M.; Briccoli, A.; et al. Value of P-Glycoprotein and Clinicopathologic Factors as the Basis for New Treatment Strategies in High-Grade Osteosarcoma of the Extremities. J. Clin. Oncol. 2003, 21, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Hattinger, C.M. The Pharmacogenomics of Osteosarcoma. Pharmacogenomics J. 2017, 17, 11–20. [Google Scholar] [CrossRef]
- Palmerini, E.; Meazza, C.; Tamburini, A.; Bisogno, G.; Ferraresi, V.; Asaftei, S.D.; Milano, G.M.; Coccoli, L.; Manzitti, C.; Luksch, R.; et al. Phase 2 Study for Nonmetastatic Extremity High-Grade Osteosarcoma in Pediatric and Adolescent and Young Adult Patients with a Risk-Adapted Strategy Based on ABCB1/P-Glycoprotein Expression: An Italian Sarcoma Group Trial (ISG/OS-2). Cancer 2022, 128, 1958–1966. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Ding, F.; Liu, M.; Ma, D.Z.; Zheng, C.K.; Kan, W.S. Association between P-Glycoprotein Expression and Response to Chemotherapy in Patients with Osteosarcoma: A Systematic and Meta-Analysis. J. Cancer Res. Ther. 2014, 10, C206–C209. [Google Scholar] [CrossRef]
- Schwartz, C.L.; Gorlick, R.; Teot, L.; Krailo, M.; Chen, Z.; Goorin, A.; Grier, H.E.; Bernstein, M.L.; Meyers, P. Multiple Drug Resistance in Osteogenic Sarcoma: INT0133 from the Children’s Oncology Group. J. Clin. Oncol. 2007, 25, 2057–2062. [Google Scholar] [CrossRef]
- Laechelt, S.; Turrini, E.; Ruehmkorf, A.; Siegmund, W.; Cascorbi, I.; Haenisch, S. Impact of ABCC2 Haplotypes on Transcriptional and Posttranscriptional Gene Regulation and Function. Pharmacogenomics J. 2011, 11, 25–34. [Google Scholar] [CrossRef]
- Haenisch, S.; May, K.; Wegner, D.; Caliebe, A.; Cascorbi, I.; Siegmund, W. Influence of Genetic Polymorphisms on Intestinal Expression and Rifampicin-Type Induction of ABCC2 and on Bioavailability of Talinolol. Pharmacogenet. Genomics 2008, 18, 357–365. [Google Scholar] [CrossRef]
- Ogasawara, K.; Chitnis, S.D.; Gohh, R.Y.; Christians, U.; Akhlaghi, F. Multidrug Resistance-Associated Protein 2 (MRP2/ABCC2) Haplotypes Significantly Affect the Pharmacokinetics of Tacrolimus in Kidney Transplant Recipients. Clin. Pharmacokinet. 2013, 52, 751–762. [Google Scholar] [CrossRef]
- Benhamou, S.; Sarasin, A. ERCC2/XPD Gene Polymorphisms and Lung Cancer: A HuGE Review. Am. J. Epidemiol. 2005, 161, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Goričar, K.; Kovač, V.; Jazbec, J.; Zakotnik, B.; Lamovec, J.; Dolžan, V. Genetic Variability of DNA Repair Mechanisms and Glutathione-S-Transferase Genes Influences Treatment Outcome in Osteosarcoma. Cancer Epidemiol. 2015, 39, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, T.S.; Thorn, C.F.; Yang, J.J.; Ulrich, C.M.; French, D.; Zaza, G.; Dunnenberger, H.M.; Marsh, S.; McLeod, H.L.; Giacomini, K.; et al. PharmGKB Summary: Methotrexate Pathway. Pharmacogenet. Genomics 2011, 21, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.L.; Gottesman, M.M. A Synonymous Polymorphism in a Common MDR1 (ABCB1) Haplotype Shapes Protein Function. Biochim. Biophys. Acta 2009, 1794, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug Resistance in Cancer: Role of ATP-Dependent Transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Boyle, A.P.; Hong, E.L.; Hariharan, M.; Cheng, Y.; Schaub, M.A.; Kasowski, M.; Karczewski, K.J.; Park, J.; Hitz, B.C.; Weng, S.; et al. Annotation of Functional Variation in Personal Genomes Using RegulomeDB. Genome Res. 2012, 22, 1790–1797. [Google Scholar] [CrossRef]
- Lang, T.; Hitzi, M.; Burk, O.; Mornhinweg, E.; Keil, A.; Kerb, R.; Klein, K.; Zanger, U.M.; Eichelbaum, M.; Fromm, M.F. Genetic Polymorphisms in the Multidrug Resistance-Associated Protein 3 (ABCC3, MRP3) Gene and Relationship to Its MRNA and Protein Expression in Human Liver. Pharmacogenetics 2004, 14, 155–164. [Google Scholar] [CrossRef]
| Characteristic | n (%) |
|---|---|
| Age at diagnosis (years), median (range) | 14 (4–32) |
| Sex | |
| Female | 32 (46.4) |
| Male | 37 (53.6) |
| Primary tumor site | |
| Femur | 45 (65.2) |
| Tibia/fibula | 12 (17.4) |
| Humerus/radius | 8 (11.6) |
| Other | 4 (5.8) |
| Surgical margins | |
| Wide | 44 (63.8) |
| Radical | 7 (10.1) |
| Marginal | 15 (21.7) |
| Not available | 3 (4.3) |
| Pathological response | |
| ≥90 | 26 (37.7) |
| <90 | 40 (58) |
| Not available | 3 (4.3) |
| Death (Yes) | 14 (21.2) |
| Progression (Yes) | 16 (23.9) |
| Gene Symbol | Reference SNP | Molecular Consequence | Identifiers |
|---|---|---|---|
| ERCC1 | rs11615 | Synonymous | NM_001983.4:c.354T>C (p.Asn118=) |
| ERCC2 | rs13181 | Missense | NM_000400.4:c.2251A>C (p.Lys751Gln) |
| ERCC2 | rs1799793 | Missense | NM_000400.4:c.934G>A (p.Asp312Asn) |
| GSTP1 | rs1695 | Missense | NM_000852.4:c.313A>G (p.Ile105Val) |
| ABCC3 | rs4793665 | 2KB upstream | NC_000017.11:g.50634726C>T |
| ABCB1 | rs1045642 | Synonymous | NM_001348944.2:c.3435T>C (p.Ile1145=) |
| ABCB1 | rs2032582 | Missense | NM_001348944.2:c.2677T>G (p.Ser893Ala); c.2677T>A (p.Ser893Thr) |
| ABCB1 | rs1128503 | Synonymous | NM_001348944.2:c.1236T>C (p.Gly412=) |
| ABCC2 | rs2273697 | Missense | NM_000392.5:c.1249G>A (p.Val417Ile) |
| ABCC2 | rs3740066 | synonymous | NM_000392.5:c.3972C>T (p.Ile1324=) |
| MTHFR | rs1801133 | Missense | NM_005957.4:c.665C>T (p.Ala222Val) a |
| MTHFR | rs1801131 | Missense | NM_005957.4:c.1286A>C (p.Glu429Ala) a |
| SLC19A1 | rs1051266 | Missense | NM_194255.4(SLC19A1):c.80A>G (p.His27Arg) |
| Pathological Response (n = 66) | |||||
|---|---|---|---|---|---|
| Gene | Allele | Poor Responders Frequency | Good Responders Frequency | p-Value | OR |
| MTHFR | rs1801131-G | 0.35 | 0.33 | 0.78 | 1.11 |
| MTHFR | rs1801133-A | 0.31 | 0.33 | 0.86 | 0.94 |
| SLC19A1 | rs1051266-A | 0.43 | 0.48 | 0.53 | 0.80 |
| ABCB1 | rs1045642-A | 0.48 | 0.37 | 0.21 | 1.57 |
| ABCB1 | rs2032582-A | 0.41 | 0.25 | 0.06 | 2.11 |
| ABCB1 | rs1128503-A | 0.4 | 0.33 | 0.40 | 1.37 |
| ABCC2 | rs2273697-A | 0.24 | 0.1 | 0.04 | 2.93 |
| ABCC2 | rs3740066-T | 0.5 | 0.35 | 0.08 | 1.89 |
| ABCC3 | rs4793665-C | 0.45 | 0.33 | 0.16 | 1.68 |
| ERCC1 | rs11615-G | 0.45 | 0.33 | 0.16 | 1.68 |
| ERCC2 | rs13181-G | 0.39 | 0.31 | 0.35 | 1.42 |
| ERCC2 | rs1799793-A | 0.39 | 0.21 | 0.047 | 2.24 |
| GSTP1 | rs1695-G | 0.43 | 0.48 | 0.53 | 0.80 |
| High-Dose Methotrexate Hepatotoxicity (n = 58) | |||||
|---|---|---|---|---|---|
| Gene | Allele | Grade 3–4 Frequency | Grade 0–2 Frequency | p-Value | OR |
| MTHFR | rs1801131-G | 0.43 | 0.26 | 0.07 | 2.13 |
| MTHFR | rs1801133-A | 0.24 | 0.41 | 0.05 | 0.46 |
| SLC19A1 | rs1051266-A | 0.46 | 0.41 | 0.64 | 1.2 |
| ABCB1 | rs1045642-A | 0.44 | 0.46 | 0.88 | 0.95 |
| ABCB1 | rs2032582-A | 0.33 | 0.43 | 0.25 | 0.64 |
| ABCB1 | rs1128503-G | 0.7 | 0.46 | 0.009 | 2.78 |
| ABCC2 | rs2273697-A | 0.21 | 0.13 | 0.25 | 1.82 |
| ABCC2 | rs3740066-T | 0.41 | 0.43 | 0.83 | 0.92 |
| ABCC3 | rs4793665-T | 0.69 | 0.5 | 0.04 | 2.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, J.; Arranz, M.J.; Martin-Broto, J.; Bautista, F.; Martínez-García, J.; Martínez-Trufero, J.; Vidal-Insua, Y.; Echebarria-Barona, A.; Díaz-Beveridge, R.; Valverde, C.; et al. Pharmacogenetics of Neoadjuvant MAP Chemotherapy in Localized Osteosarcoma: A Study Based on Data from the GEIS-33 Protocol. Pharmaceutics 2024, 16, 1585. https://doi.org/10.3390/pharmaceutics16121585
Salazar J, Arranz MJ, Martin-Broto J, Bautista F, Martínez-García J, Martínez-Trufero J, Vidal-Insua Y, Echebarria-Barona A, Díaz-Beveridge R, Valverde C, et al. Pharmacogenetics of Neoadjuvant MAP Chemotherapy in Localized Osteosarcoma: A Study Based on Data from the GEIS-33 Protocol. Pharmaceutics. 2024; 16(12):1585. https://doi.org/10.3390/pharmaceutics16121585
Chicago/Turabian StyleSalazar, Juliana, María J. Arranz, Javier Martin-Broto, Francisco Bautista, Jerónimo Martínez-García, Javier Martínez-Trufero, Yolanda Vidal-Insua, Aizpea Echebarria-Barona, Roberto Díaz-Beveridge, Claudia Valverde, and et al. 2024. "Pharmacogenetics of Neoadjuvant MAP Chemotherapy in Localized Osteosarcoma: A Study Based on Data from the GEIS-33 Protocol" Pharmaceutics 16, no. 12: 1585. https://doi.org/10.3390/pharmaceutics16121585
APA StyleSalazar, J., Arranz, M. J., Martin-Broto, J., Bautista, F., Martínez-García, J., Martínez-Trufero, J., Vidal-Insua, Y., Echebarria-Barona, A., Díaz-Beveridge, R., Valverde, C., Luna, P., Vaz-Salgado, M. A., Blay, P., Álvarez, R., & Sebio, A. (2024). Pharmacogenetics of Neoadjuvant MAP Chemotherapy in Localized Osteosarcoma: A Study Based on Data from the GEIS-33 Protocol. Pharmaceutics, 16(12), 1585. https://doi.org/10.3390/pharmaceutics16121585









