Innovative Multilayer Electrospun Patches for the Slow Release of Natural Oily Extracts as Dressings to Boost Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines
2.3. Solutions Preparation and Electrospinning Process
2.4. Morphology Characterization of Membranes
2.5. Chemical Characterization of Membranes
2.6. Wettability Test and Contact Angles Measurements
2.7. In Vitro Oily Extract Release Analysis by LC/HESI/MS
2.8. Viability Assay
2.9. Fluorescent Stainings to Analyse Cell
2.10. Wound Healing Assay (or Scratch Test)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Membrane Production
3.2. Membrane Characterization
3.2.1. Morphological Analysis
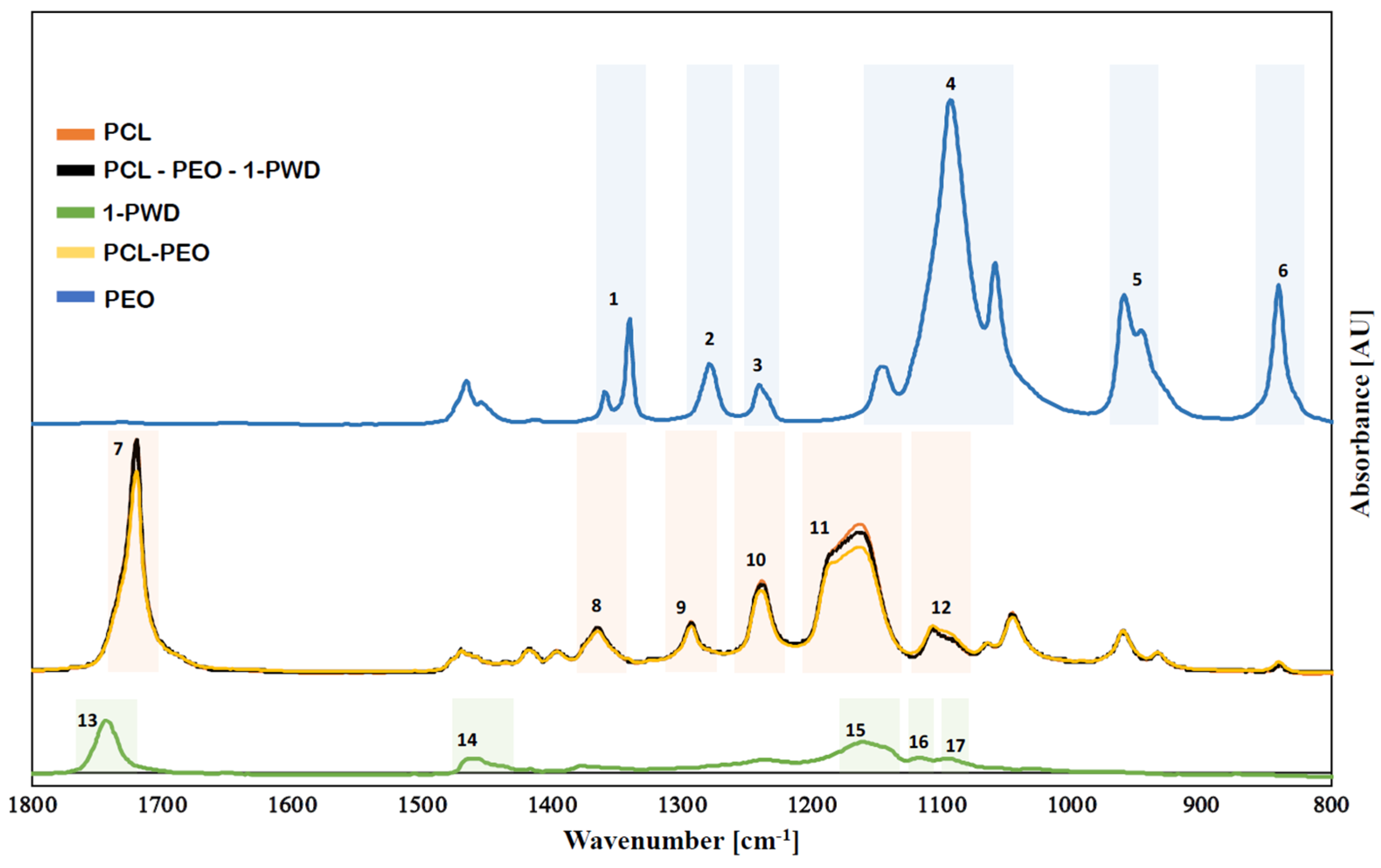
3.2.2. Chemical Structure of Membranes
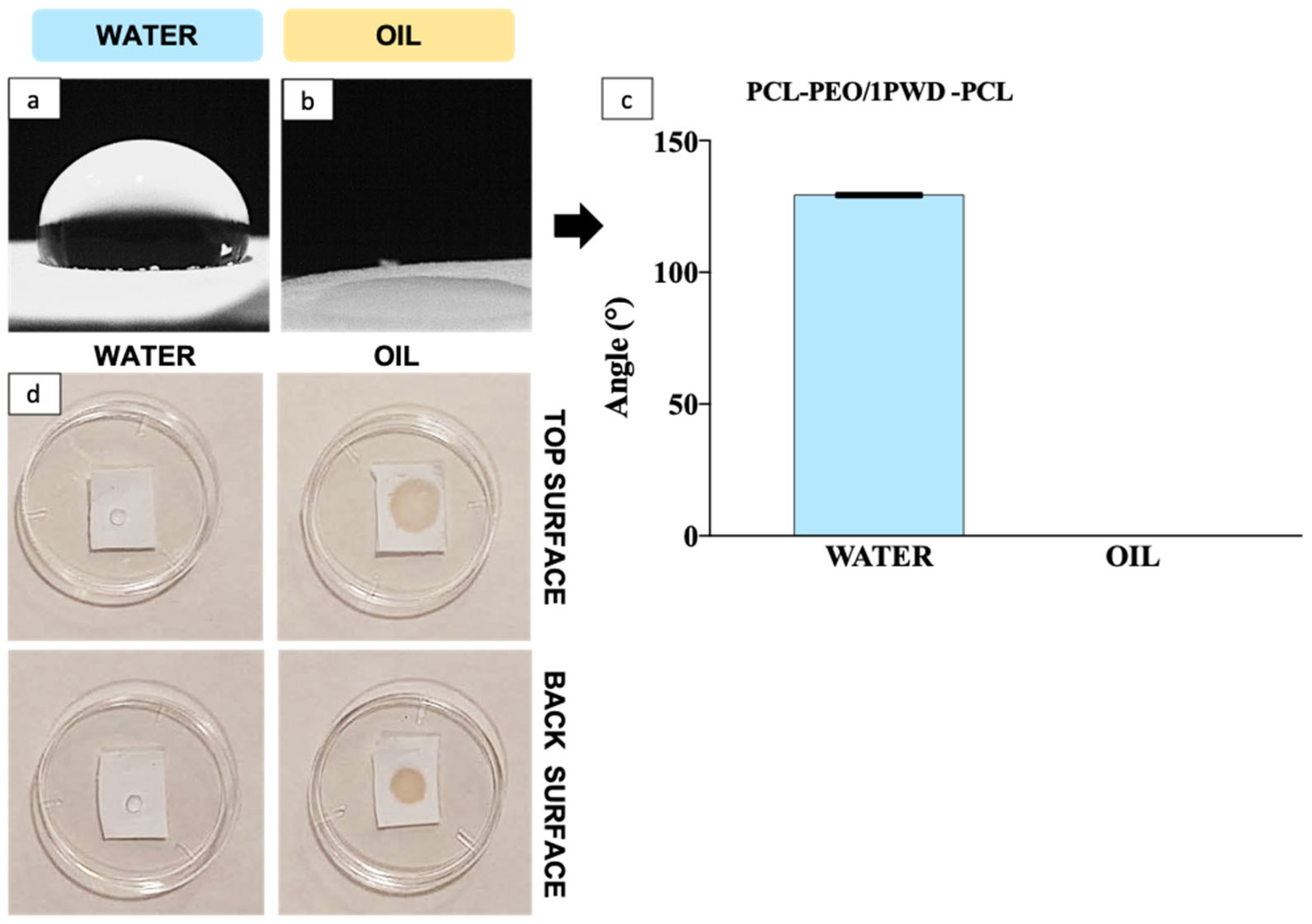
3.2.3. Hydrophilicity
3.3. Biological Tests
3.3.1. Cell Viability and Biocompatibility
3.3.2. Cell Adhesion
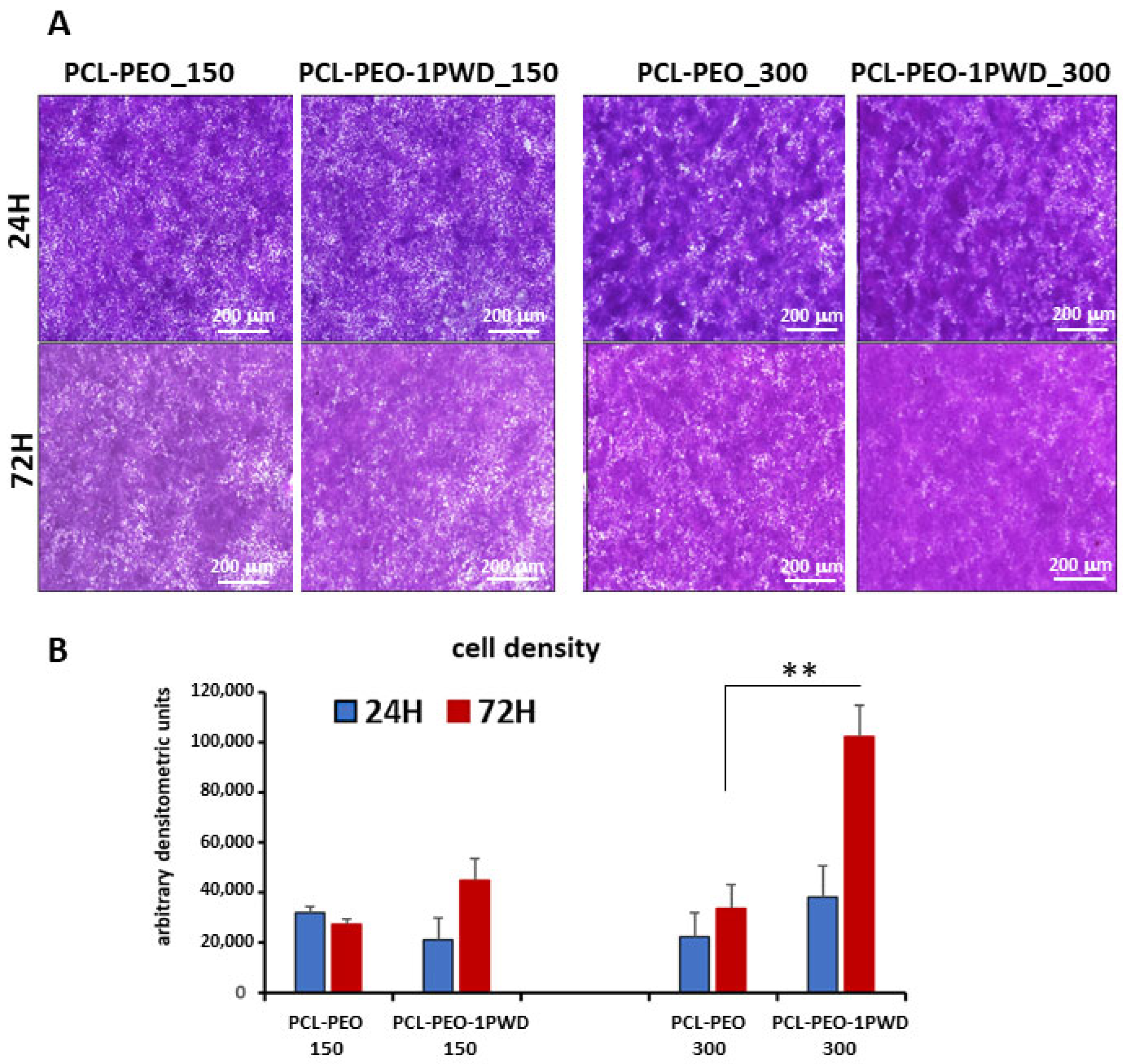
3.3.3. Proliferation Test
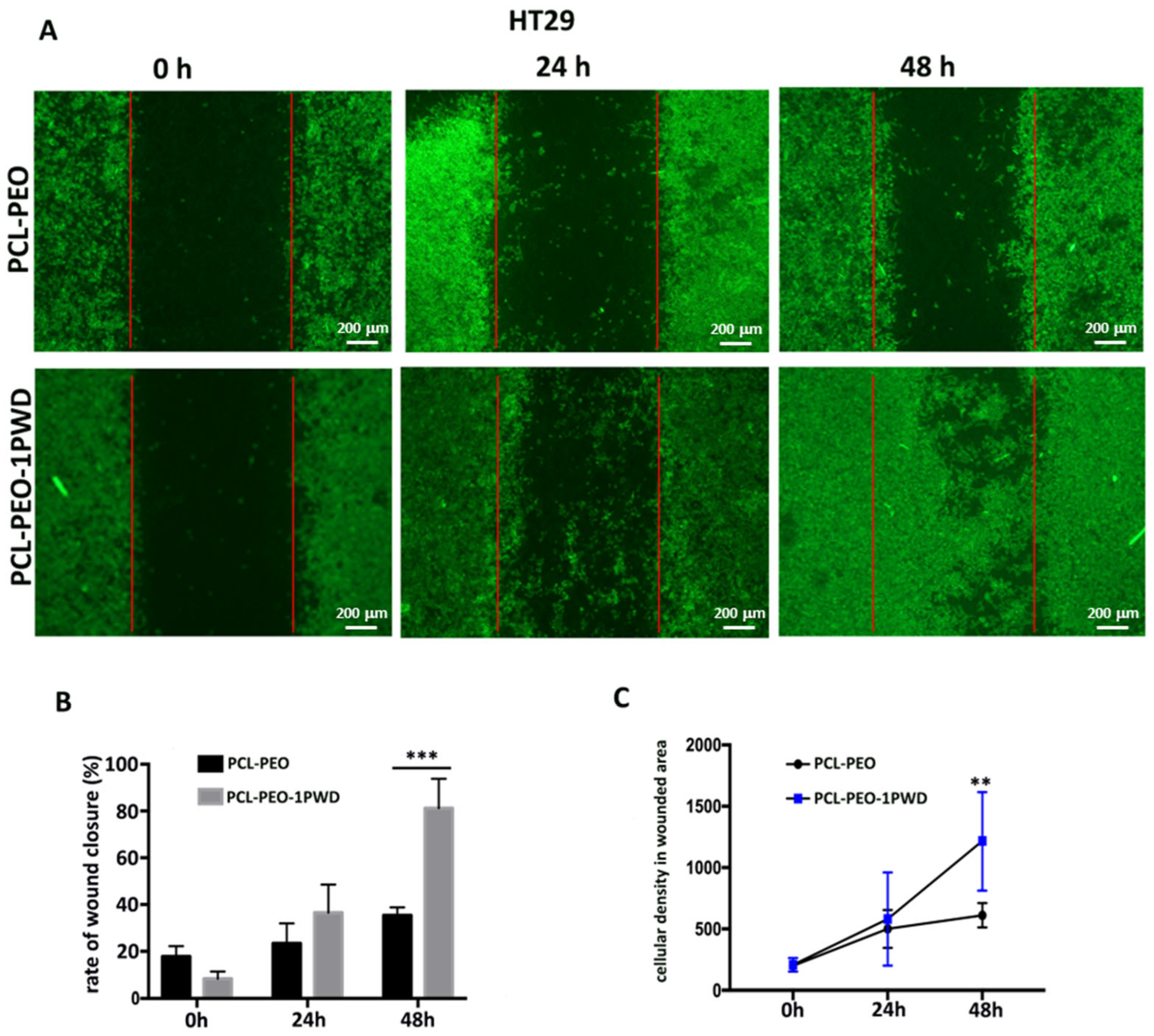
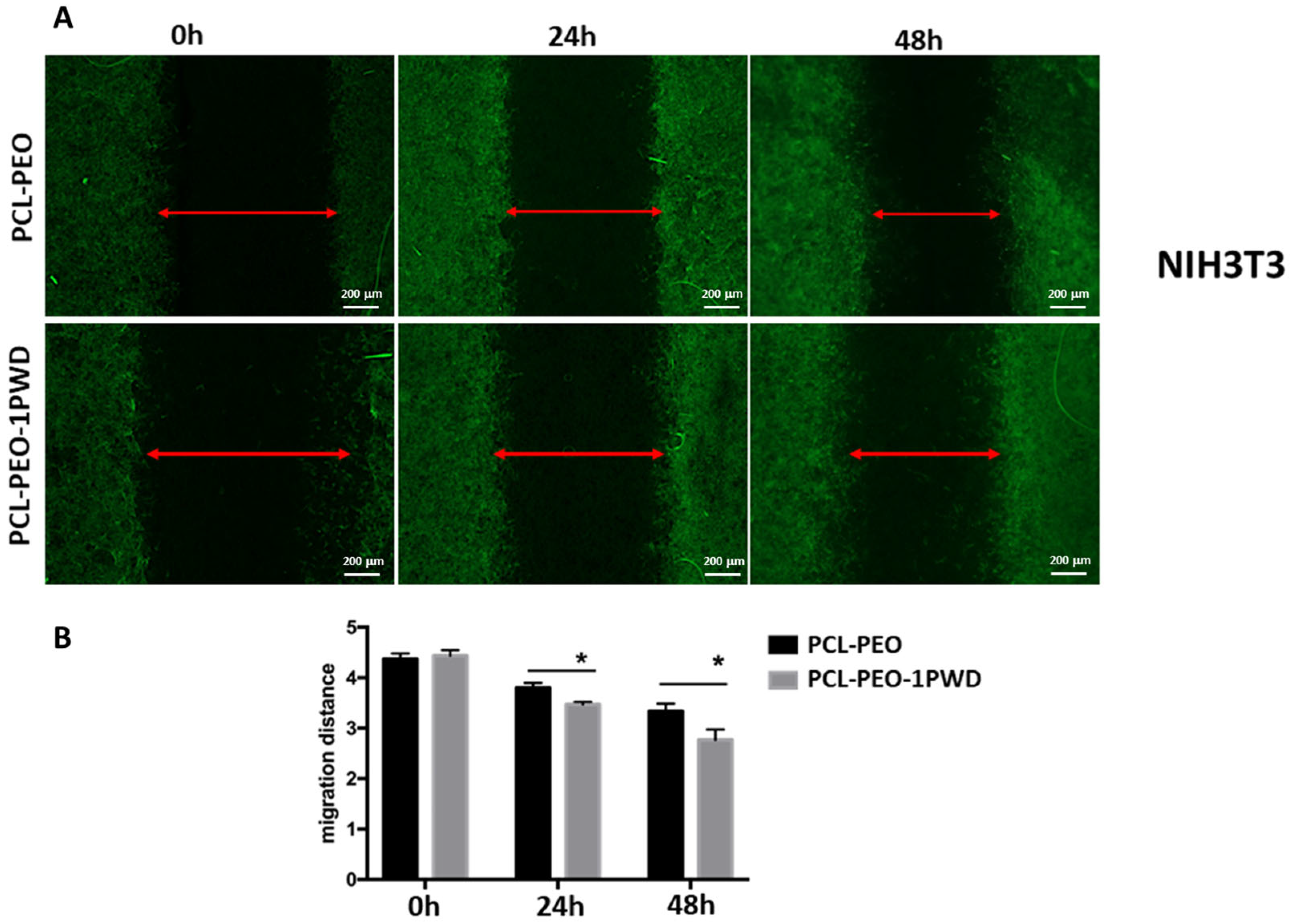
3.3.4. Cell Single Layer Migration Assay (Wound Closure Assay)
3.4. Controlled Release of 1-PWD© Extracts from the Electrospun System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akombaetwa, N.; Bwanga, A.; Makoni, P.A.; Witika, B.A. Applications of electrospun drug-eluting nanofibers in wound healing: Current and future perspectives. Polymers 2022, 14, 2931. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Sill, T.J.; Von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Reis, R.; Neves, N. Electrospinning: Processing technique for tissue engineering scaffolding. Int. Mater. Rev. 2008, 53, 257–274. [Google Scholar] [CrossRef]
- Carotenuto, F.; Fiaschini, N.; Di Nardo, P.; Rinaldi, A. Towards a Material-by-Design Approach to Electrospun Scaffolds for Tissue Engineering Based on Statistical Design of Experiments (DOE). Materials 2023, 16, 1539. [Google Scholar] [CrossRef]
- Fiaschini, N.; Giuliani, C.; Vitali, R.; Tammaro, L.; Valerini, D.; Rinaldi, A. Design and Manufacturing of Antibacterial Electrospun Polysulfone Membranes Functionalized by Ag Nanocoating via Magnetron Sputtering. Nanomaterials 2022, 12, 3962. [Google Scholar] [CrossRef]
- Seyedmahmoud, R.; Rainer, A.; Mozetic, P.; Maria Giannitelli, S.; Trombetta, M.; Traversa, E.; Licoccia, S.; Rinaldi, A. A primer of statistical methods for correlating parameters and properties of electrospun poly (l-lactide) scaffolds for tissue engineering—PART 1: Design of experiments. J. Biomed. Mater. Res. Part A 2015, 103, 91–102. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, H.; Yu, F.; Pei, Y.; Luo, X. Superclear, porous cellulose membranes with chitosan-coated nanofibers for visualized cutaneous wound healing dressing. ACS Appl. Mater. Interfaces 2020, 12, 24370–24379. [Google Scholar] [CrossRef]
- Cheng, L.; Sun, X.; Chen, L.; Zhang, L.; Wang, F.; Zhang, Y.; Pan, G.; Zhang, Y.; Zhang, L.; Cui, W. Nano-in-micro electronspun membrane: Merging nanocarriers and microfibrous scaffold for long-term scar inhibition. Chem. Eng. J. 2020, 397, 125405. [Google Scholar] [CrossRef]
- Stie, M.B.; Corezzi, M.; Juncos Bombin, A.D.; Ajalloueian, F.; Attrill, E.; Pagliara, S.; Jacobsen, J.; Chronakis, I.S.; Nielsen, H.M.; Fodera, V. Waterborne electrospinning of α-lactalbumin generates tunable and biocompatible nanofibers for drug delivery. ACS Appl. Nano Mater. 2020, 3, 1910–1921. [Google Scholar] [CrossRef]
- Abdullah, T.; Gauthaman, K.; Mostafavi, A.; Alshahrie, A.; Salah, N.; Morganti, P.; Chianese, A.; Tamayol, A.; Memic, A. Author correction: Sustainable drug release from polycaprolactone coated chitin-lignin gel fibrous scaffolds. Sci. Rep. 2022, 12, 15051. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.; Yadav, B.K.N. Recent patents on polymeric electrospun nanofibers and their applications in drug delivery. Recent Pat. Nanotechnol. 2018, 12, 174–179. [Google Scholar] [CrossRef]
- Abedalwafa, M.; Wang, F.; Wang, L.; Li, C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: A review. Rev. Adv. Mater. Sci 2013, 34, 123–140. [Google Scholar]
- Dimitrov, I.; Tsvetanov, C. 4.21—High-Molecular-Weight Poly (ethylene oxide). Polym. Sci. A Compr. Ref. 2012, 4, 551–569. [Google Scholar] [CrossRef]
- Garcia-Salinas, S.; Evangelopoulos, M.; Gamez-Herrera, E.; Arruebo, M.; Irusta, S.; Taraballi, F.; Mendoza, G.; Tasciotti, E. Electrospun anti-inflammatory patch loaded with essential oils for wound healing. Int. J. Pharm. 2020, 577, 119067. [Google Scholar] [CrossRef]
- Eğri, Ö.; Erdemir, N. Production of Hypericum perforatum oil-loaded membranes for wound dressing material and in vitro tests. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1404–1415. [Google Scholar] [CrossRef]
- Unalan, I.; Endlein, S.J.; Slavik, B.; Buettner, A.; Goldmann, W.H.; Detsch, R.; Boccaccini, A.R. Evaluation of electrospun poly (ε-caprolactone)/gelatin nanofiber mats containing clove essential oil for antibacterial wound dressing. Pharmaceutics 2019, 11, 570. [Google Scholar] [CrossRef]
- Mohamadi, P.S.; Hivechi, A.; Bahrami, H.; Hemmatinegad, N.; Milan, P.B. Antibacterial and biological properties of coconut oil loaded poly (ε-caprolactone)/gelatin electrospun membranes. J. Ind. Text. 2022, 51, 906S–930S. [Google Scholar] [CrossRef]
- Stoyanova, N.; Nachev, N.; Spasova, M. Innovative Bioactive Nanofibrous Materials Combining Medicinal and Aromatic Plant Extracts and Electrospinning Method. Membranes 2023, 13, 840. [Google Scholar] [CrossRef]
- Mainetti, S.; Carnevali, F. An experience with paediatric burn wounds treated with a plant-derived wound therapeutic. J. Wound Care 2013, 22, 681–689. [Google Scholar] [CrossRef]
- Carnevali, F.; Franchini, D.; Otranto, D.; Giangaspero, A.; Di Bello, A.; Ciccarelli, S.; Szpila, K.; Valastro, C.; van der Esch, A.S. A formulation of neem and hypericum oily extract for the treatment of the wound myiasis by Wohlfahrtia magnifica in domestic animals. Parasitol. Res. 2019, 118, 2361–2367. [Google Scholar] [CrossRef]
- Läuchli, S.; Hafner, J.; Wehrmann, C.; French, L.; Hunziker, T. Post-surgical scalp wounds with exposed bone treated with a plant-derived wound therapeutic. J. Wound Care 2012, 21, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Mori, C.L.D.O.; Passos, N.A.d.; Oliveira, J.E.; Altoé, T.F.; Mori, F.A.; Mattoso, L.H.C.; Scolforo, J.R.; Tonoli, G.H.D. Nanostructured polylactic acid/candeia essential oil mats obtained by electrospinning. J. Nanomater. 2015, 16, 33. [Google Scholar] [CrossRef]
- Stefanović, I.S.; Džunuzović, J.V.; Džunuzović, E.S.; Dapčević, A.; Šešlija, S.I.; Balanč, B.D.; Dobrzyńska-Mizera, M. Composition-property relationship of polyurethane networks based on polycaprolactone diol. Polym. Bull. 2021, 78, 7103–7128. [Google Scholar] [CrossRef]
- Bergeron, C.; Perrier, E.; Potier, A.; Delmas, G. A study of the deformation, Network, and aging of polyethylene oxide films by infrared spectroscopy and calorimetric measurements. Int. J. Spectrosc. 2012, 2012, 432046. [Google Scholar] [CrossRef]
- Andreu, V.; Mendoza, G.; Arruebo, M.; Irusta, S. Smart dressings based on nanostructured fibers containing natural origin antimicrobial, anti-inflammatory, and regenerative compounds. Materials 2015, 8, 5154–5193. [Google Scholar] [CrossRef] [PubMed]
- Bindschadler, M.; McGrath, J.L. Sheet migration by wounded monolayers as an emergent property of single-cell dynamics. J. Cell Sci. 2007, 120, 876–884. [Google Scholar] [CrossRef]
- Friedl, P.; Gilmour, D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2009, 10, 445–457. [Google Scholar] [CrossRef]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef]
- Liao, G.; Nagasaki, T.; Gundersen, G.G. Low concentrations of nocodazole interfere with fibroblast locomotion without significantly affecting microtubule level: Implications for the role of dynamic microtubules in cell locomotion. J. Cell Sci. 1995, 108, 3473–3483. [Google Scholar] [CrossRef]
- Haldar, S.; Mulani, F.A.; Aarthy, T.; Dandekar, D.S.; Thulasiram, H.V. Expedient preparative isolation and tandem mass spectrometric characterization of C-seco triterpenoids from Neem oil. J. Chromatogr. A 2014, 1366, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Barrek, S.; Paisse, O.; Grenier-Loustalot, M.-F. Analysis of neem oils by LC–MS and degradation kinetics of azadirachtin-A in a controlled environment: Characterization of degradation products by HPLC–MS–MS. Anal. Bioanal. Chem. 2004, 378, 753–763. [Google Scholar] [CrossRef] [PubMed]
- García-Moreno, P.J.; Guadix, A.; Guadix, E.M.; Jacobsen, C. Physical and oxidative stability of fish oil-in-water emulsions stabilized with fish protein hydrolysates. Food Chem. 2016, 203, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-C.; Siedlecki, C.A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef]





| Metabolite | 24 h | 48 h | 72 h |
|---|---|---|---|
| Salannin | 4.19 ± 0.133 | 3.94 ± 0.18 | 3.57 ± 0.568 |
| Nimbin | 1.64 ± 0.241 | 1.00 ± 0.072 | 0.82 ± 0.052 |
| Deacetyl-Nimbin | 0.55 ± 0.031 | 0.53 ± 0.187 | 0.17 ± 0.049 |
| Deacetyl-Salannin | 0.72 ± 0.132 | 0.82 ± 0.108 | 0.69 ± 0.039 |
| Azadirachtin I | 0.36 ± 0.079 | 0.15 ± 0.024 | 0.31 ± 0.096 |
| Azadirachtin H | 0.07 ± 0.002 | 0.09 ± 0.035 | 0.49 ± 0.152 |
| Azadirachtin D | - | - | 0.15 ± 0.029 |
| Azadirachtin A | 0.09 ± 0.021 | 0.143 ± 0.044 | 0.63 ± 0.146 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiaschini, N.; Carnevali, F.; Van der Esch, S.A.; Vitali, R.; Mancuso, M.; Sulli, M.; Diretto, G.; Negroni, A.; Rinaldi, A. Innovative Multilayer Electrospun Patches for the Slow Release of Natural Oily Extracts as Dressings to Boost Wound Healing. Pharmaceutics 2024, 16, 159. https://doi.org/10.3390/pharmaceutics16020159
Fiaschini N, Carnevali F, Van der Esch SA, Vitali R, Mancuso M, Sulli M, Diretto G, Negroni A, Rinaldi A. Innovative Multilayer Electrospun Patches for the Slow Release of Natural Oily Extracts as Dressings to Boost Wound Healing. Pharmaceutics. 2024; 16(2):159. https://doi.org/10.3390/pharmaceutics16020159
Chicago/Turabian StyleFiaschini, Noemi, Fiorella Carnevali, Stephen Andrew Van der Esch, Roberta Vitali, Mariateresa Mancuso, Maria Sulli, Gianfranco Diretto, Anna Negroni, and Antonio Rinaldi. 2024. "Innovative Multilayer Electrospun Patches for the Slow Release of Natural Oily Extracts as Dressings to Boost Wound Healing" Pharmaceutics 16, no. 2: 159. https://doi.org/10.3390/pharmaceutics16020159
APA StyleFiaschini, N., Carnevali, F., Van der Esch, S. A., Vitali, R., Mancuso, M., Sulli, M., Diretto, G., Negroni, A., & Rinaldi, A. (2024). Innovative Multilayer Electrospun Patches for the Slow Release of Natural Oily Extracts as Dressings to Boost Wound Healing. Pharmaceutics, 16(2), 159. https://doi.org/10.3390/pharmaceutics16020159












