Development and Optimization of Andrographis paniculata Extract-Loaded Self-Microemulsifying Drug Delivery System Using Experimental Design Model
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Methods
2.2.1. Andrographolide Solubility Study
2.2.2. HPLC Condition
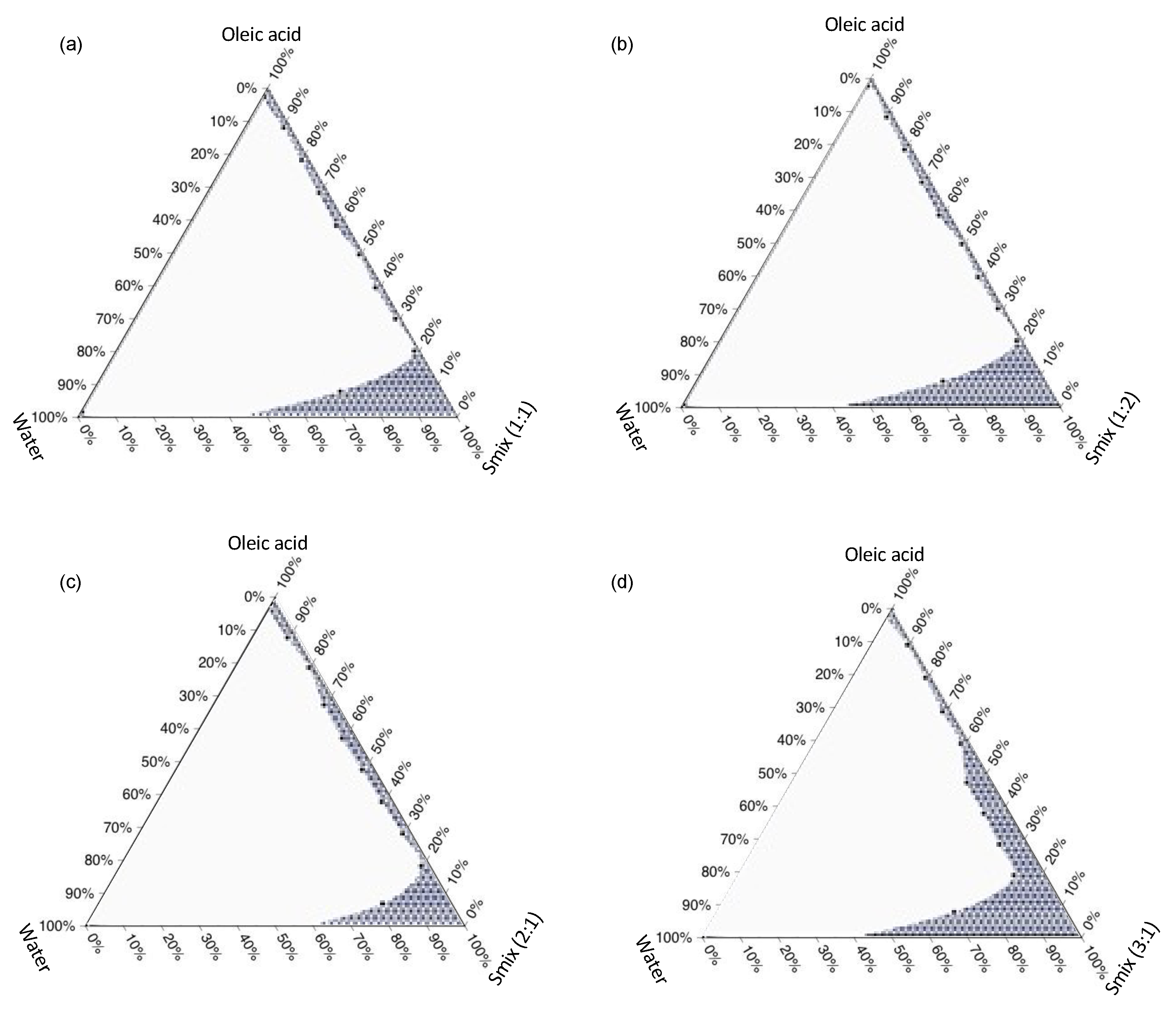
2.2.3. Construction of Ternary Phase Diagram
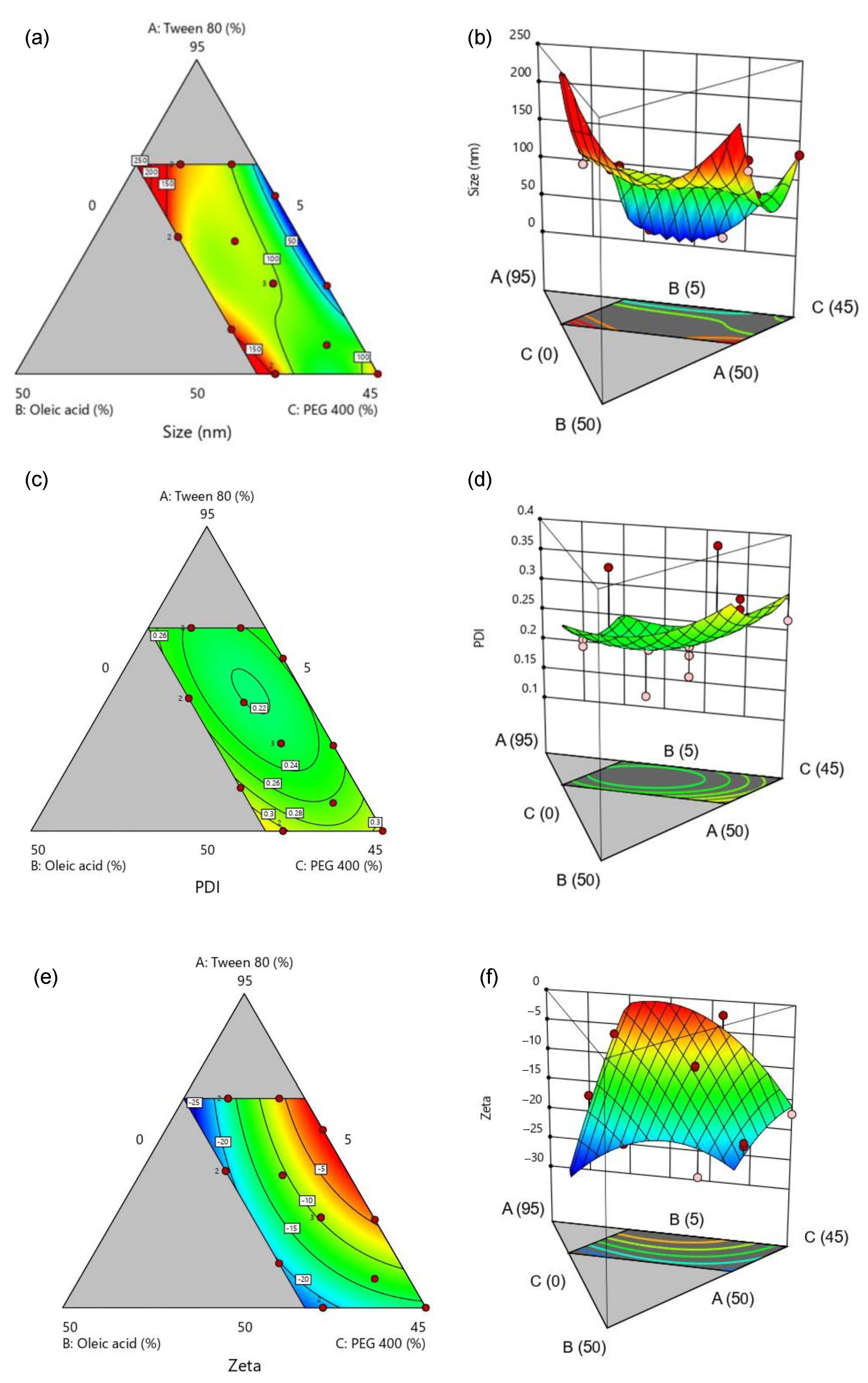
2.2.4. Experimental Design
2.2.5. Characterizations of SMEDDS
Droplet Size, PDI, and Zeta Potential
Physical Stability of the SMEDDS
2.2.6. Formulation of AGP-SMEDDS Soft Capsules
2.2.7. Assay and Dissolution
2.2.8. Uniformity of Dosage Unit
2.2.9. Stability Study
2.2.10. Ex Vivo Membrane Permeation Study
2.2.11. Statistical Analysis
3. Results and Discussion
3.1. Andrographolide Solubility Study
3.2. Ternary Phase Diagram
3.3. Optimization of SMEDDS
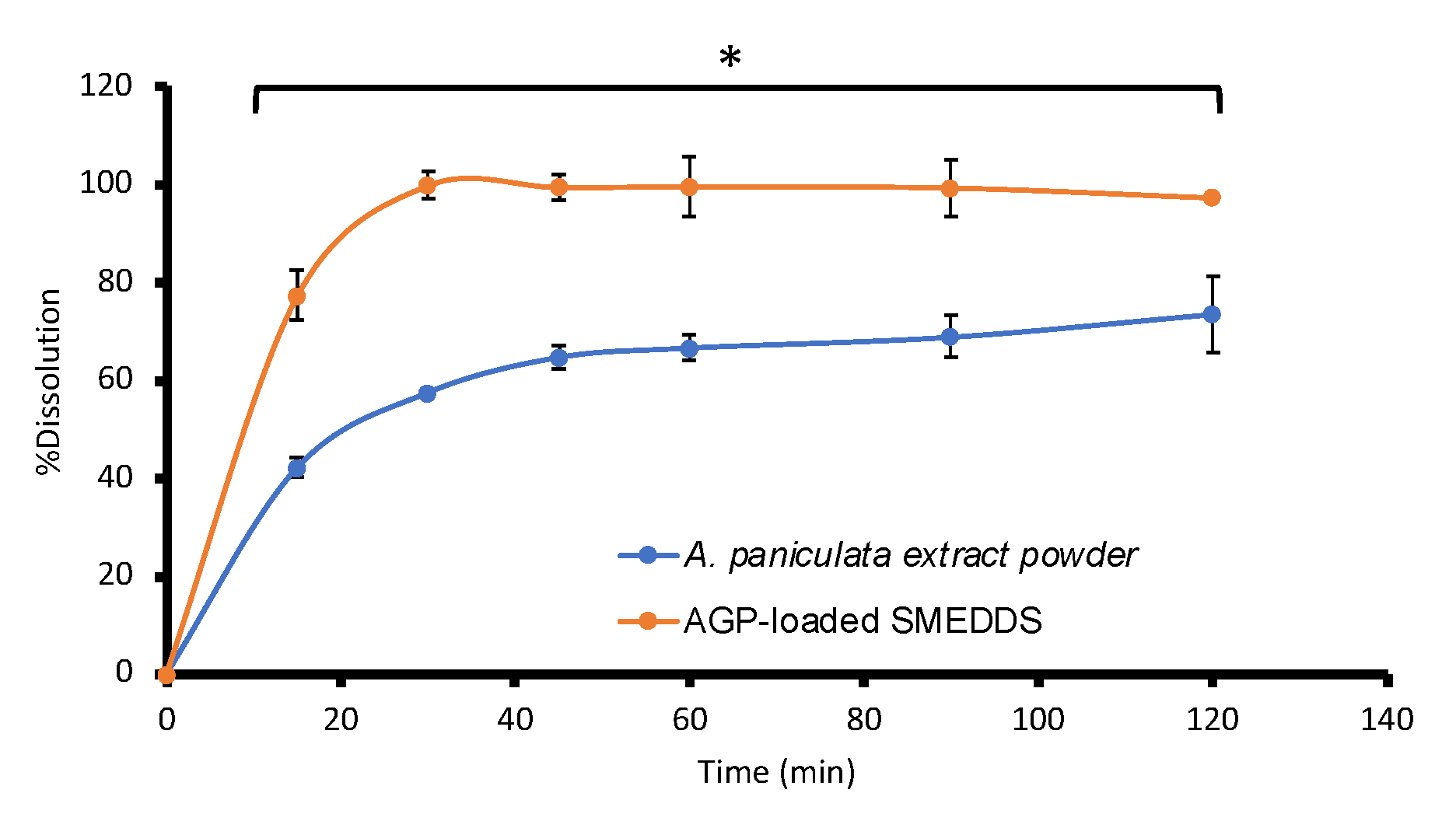
3.4. AGP Assay, Uniformity of Dosage Unit, Dissolution, and Stability Studies
3.5. Ex Vivo Membrane Permeation Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayakumar, T.; Hsieh, C.Y.; Lee, J.J.; Sheu, J.R. Experimental and Clinical Pharmacology of Andrographis paniculata and Its Major Bioactive Phytoconstituent Andrographolide. Evid. Based Complement. Altern. Med. 2013, 2013, 846740. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Wei, A.; Zhou, Q.; Yuan, M.; Lei, K.; Liu, Y.; Song, J.; Guo, L.; Ye, Q. Andrographolide: A review of its pharmacology, pharmacokinetics, toxicity and clinical trials and pharmaceutical researches. Phytother. Res. 2022, 36, 336–364. [Google Scholar] [CrossRef] [PubMed]
- Iruretagoyena, M.I.; Tobar, J.A.; Gonzalez, P.A.; Sepulveda, S.E.; Figueroa, C.A.; Burgos, R.A.; Hancke, J.L.; Kalergis, A.M. Andrographolide interferes with T cell activation and reduces experimental autoimmune encephalomyelitis in the mouse. J. Pharmacol. Exp. Ther. 2005, 312, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Urbi, Z.; Sule, A.; Hafizur Rahman, K.M. Andrographis paniculata (Burm. f.) Wall. ex Nees: A review of ethnobotany, phytochemistry, and pharmacology. Sci. World J. 2014, 2014, 274905. [Google Scholar] [CrossRef] [PubMed]
- Intharuksa, A.; Arunotayanun, W.; Yooin, W.; Sirisa-Ard, P. A Comprehensive Review of Andrographis paniculata (Burm. f.) Nees and Its Constituents as Potential Lead Compounds for COVID-19 Drug Discovery. Molecules 2022, 27, 4479. [Google Scholar] [CrossRef] [PubMed]
- Sa-ngiamsuntorn, K.; Suksatu, A.; Pewkliang, Y.; Thongsri, P.; Kanjanasirirat, P.; Manopwisedjaroen, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Pitiporn, S.; Chaopreecha, J.; et al. Anti-SARS-CoV-2 Activity of Andrographis paniculata Extract and Its Major Component Andrographolide in Human Lung Epithelial Cells and Cytotoxicity Evaluation in Major Organ Cell Representatives. J. Nat. Prod. 2021, 84, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Ratiani, L.; Pachkoria, E.; Mamageishvili, N.; Shengelia, R.; Hovhannisyan, A.; Panossian, A. Efficacy of Kan Jang® in Patients with Mild COVID-19: Interim Analysis of a Randomized, Quadruple-Blind, Placebo-Controlled Trial. Pharmaceuticals 2022, 15, 1013. [Google Scholar] [CrossRef] [PubMed]
- Loureiro Damasceno, J.P.; Silva da Rosa, H.; Silva de Araújo, L.; Jacometti Cardoso Furtado, N.A. Andrographis paniculata Formulations: Impact on Diterpene Lactone Oral Bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2022, 47, 19–30. [Google Scholar] [CrossRef]
- Pandey, A.; Gulati, S.; Gupta, A.; Tripathi, Y. Variation in andrographolide content among different accessions of Andrographis paniculata. Pharma Innov. J. 2019, 8, 140–144. [Google Scholar]
- Chao, W.W.; Lin, B.F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin. Med. 2010, 5, 17. [Google Scholar] [CrossRef]
- Narimanyan, M.; Jamalyan, K.; Balyan, A.; Barth, A.; Palm, S.; Wikman, G.; Panossian, A. Early intervention with Kan Jang(R) to treat upper-respiratory tract infections: A randomized, quadruple-blind study. J. Tradit. Complement. Med. 2021, 11, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Hancke, J.; Burgos, R.; Caceres, D.; Wikman, G. A double-blind study with a new monodrug Kan Jang: Decrease of symptoms and improvement in the recovery from common colds. Phytother. Res. 1995, 9, 559–562. [Google Scholar] [CrossRef]
- Raman, S.; Murugaiyah, V.; Parumasivam, T. Andrographis paniculata Dosage Forms and Advances in Nanoparticulate Delivery Systems: An Overview. Molecules 2022, 27, 6164. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Rao, C.H. Andrographolide: Its pharmacology, natural bioavailability and current approaches to increase its content in andrographispaniculata. Int. J. Complement. Altern. Med. 2018, 11, 355–360. [Google Scholar] [CrossRef]
- Oseni, B.A.; Azubuike, C.P.; Okubanjo, O.O.; Igwilo, C.I.; Panyam, J. Encapsulation of Andrographolide in poly(lactide-co-glycolide) Nanoparticles: Formulation Optimization and in vitro Efficacy Studies. Front. Bioeng. Biotechnol. 2021, 9, 639409. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Gulati, M.; Jha, N.K.; Disouza, J.; Patravale, V.; Dua, K.; Singh, S.K. Recent advances in developing polymeric micelles for treating cancer: Breakthroughs and bottlenecks in their clinical translation. Drug Discov. Today 2022, 27, 1495–1512. [Google Scholar] [CrossRef] [PubMed]
- Jamaludin, R.; Mohd Daud, N.; Raja Sulong, R.S.; Yaakob, H.; Abdul Aziz, A.; Khamis, S.; Md Salleh, L. Andrographis paniculata-loaded niosome for wound healing application: Characterisation and in vivo analyses. J. Drug Deliv. Sci. Technol. 2021, 63, 102427. [Google Scholar] [CrossRef]
- Li, M.; Zhang, T.; Zhu, L.; Wang, R.; Jin, Y. Liposomal andrographolide dry powder inhalers for treatment of bacterial pneumonia via anti-inflammatory pathway. Int. J. Pharm. 2017, 528, 163–171. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, S.; Ahmad, F.; Rathaur, S. Antifilarial efficacy of green silver nanoparticles synthesized using Andrographis paniculata. J. Drug Deliv. Sci. Technol. 2020, 56, 101557. [Google Scholar] [CrossRef]
- Karthik, K.; Shashank, M.; Revathi, V.; Tatarchuk, T. Facile microwave-assisted green synthesis of NiO nanoparticles from Andrographis paniculata leaf extract and evaluation of their photocatalytic and anticancer activities. Mol. Cryst. Liq. 2018, 673, 70–80. [Google Scholar] [CrossRef]
- Sanati, P.; Chua, L.S.; Nasiri, R.; Hashemi, S.-S. Nanoencapsulation of Andrographolide Rich Extract for the Inhibition of Cervical and Neuroblastoma Cancer Cells. J. Biomed. Nanotechnol. 2020, 16, 1370–1380. [Google Scholar] [CrossRef]
- Asasutjarit, R.; Sooksai, N.; Fristiohady, A.; Lairungruang, K.; Ng, S.-F.; Fuongfuchat, A. Optimization of Production Parameters for Andrographolide-Loaded Nanoemulsion Preparation by Microfluidization and Evaluations of Its Bioactivities in Skin Cancer Cells and UVB Radiation-Exposed Skin. Pharmaceutics 2021, 13, 1290. [Google Scholar] [CrossRef]
- Syukri, Y.; Nugroho, B.H.; Sirin, M. Determination of andrographolide content in self nano emulsifying drug delivery system (SNEDDS) for in vitro diffusion study using validated HPLC. AIP Conf. Proc. 2020, 2229, 030016. [Google Scholar] [CrossRef]
- Kulsirirat, T.; Sathirakul, K.; Kamei, N.; Takeda-Morishita, M. The in vitro and in vivo study of novel formulation of andrographolide PLGA nanoparticle embedded into gelatin-based hydrogel to prolong delivery and extend residence time in joint. Int. J. Pharm. 2021, 602, 120618. [Google Scholar] [CrossRef] [PubMed]
- Kansom, T.; Sajomsang, W.; Saeeng, R.; Rojanarata, T.; Ngawhirunpat, T.; Patrojanasophon, P.; Opanasopit, P. Fabrication and characterization of andrographolide analogue (3A.1) nanosuspensions stabilized by amphiphilic chitosan derivatives for colorectal cancer therapy. J. Drug Deliv. Sci. Technol. 2019, 54, 101287. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Si, Y.; Xu, H. Andrographolide and its derivatives: Current achievements and future perspectives. Eur. J. Med. Chem. 2021, 224, 113710. [Google Scholar] [CrossRef]
- Mahato, R. Chapter 2—Multifunctional Micro- and Nanoparticles. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Mitra, A.K., Cholkar, K., Mandal, A., Eds.; Elsevier: Boston, MA, USA, 2017; pp. 21–43. [Google Scholar]
- Akula, S.; Gurram, A.K.; Devireddy, S.R. Self-Microemulsifying Drug Delivery Systems: An Attractive Strategy for Enhanced Therapeutic Profile. Int. Sch. Res. Not. 2014, 2014, 964051. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.S.; Delmarre, D.; Gao, D. Stable drug encapsulation in micelles and microemulsions. Int. J. Pharm. 2007, 345, 9–25. [Google Scholar] [CrossRef]
- Neslihan Gursoy, R.; Benita, S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharmacother. 2004, 58, 173–182. [Google Scholar] [CrossRef]
- Shah, N.H.; Carvajal, M.T.; Patel, C.I.; Infeld, M.H.; Malick, A.W. Self-emulsifying drug delivery systems (SEDDS) with polyglycolyzed glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs. Int. J. Pharm. 1994, 106, 15–23. [Google Scholar] [CrossRef]
- Rini, S.; Muhammad Taufiq, F.; Aqlyna, F. The Importance of Microemulsion for the Surfactant Injection Process in Enhanced Oil Recovery. In Surfactants and Detergents; Ashim Kumar, D., Ed.; IntechOpen: Rijeka, Croatia, 2021; Chapter 2. [Google Scholar]
- Sunaina; Sethi, V.; Mehta, S.K.; Ganguli, A.K.; Vaidya, S. Understanding the role of co-surfactants in microemulsions on the growth of copper oxalate using SAXS. Phys. Chem. Chem. Phys. 2019, 21, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Fawzia, H.; Mona, E.-M.; Makar, S. Ocular drug deliver and the importance of microemulsion as a potential delivery system. Int. J. Pharm. Chem. Sci. 2012, 1, 723–737. [Google Scholar]
- Vaidya, S.; Ganguli, A.K. 2.01—Microemulsion Methods for Synthesis of Nanostructured Materials. In Comprehensive Nanoscience and Nanotechnology, 2nd ed.; Andrews, D.L., Lipson, R.H., Nann, T., Eds.; Academic Press: Oxford, UK, 2019; pp. 1–12. [Google Scholar]
- Shah, A.V.; Desai, H.H.; Thool, P.; Dalrymple, D.; Serajuddin, A.T.M. Development of self-microemulsifying drug delivery system for oral delivery of poorly water-soluble nutraceuticals. Drug Dev. Ind. Pharm. 2018, 44, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Subongkot, T.; Ngawhirunpat, T.; Opanasopit, P. Development of Ultradeformable Liposomes with Fatty Acids for Enhanced Dermal Rosmarinic Acid Delivery. Pharmaceutics 2021, 13, 404. [Google Scholar] [CrossRef] [PubMed]
- Suriyaamporn, P.; Opanasopit, P.; Rangsimawong, W.; Ngawhirunpat, T. Optimal Design of Novel Microemulsions-Based Two-Layered Dissolving Microneedles for Delivering Fluconazole in Treatment of Fungal Eye Infection. Pharmaceutics 2022, 14, 472. [Google Scholar] [CrossRef] [PubMed]
- Rathore, C.; Hemrajani, C.; Sharma, A.K.; Gupta, P.K.; Jha, N.K.; Aljabali, A.A.A.; Gupta, G.; Singh, S.K.; Yang, J.-C.; Dwivedi, R.P.; et al. Self-nanoemulsifying drug delivery system (SNEDDS) mediated improved oral bioavailability of thymoquinone: Optimization, characterization, pharmacokinetic, and hepatotoxicity studies. Drug Deliv. Transl. Res. 2023, 13, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.; Spierings, E.L.H.; Kunkel, T. Celecoxib Oral Solution and the Benefits of Self-Microemulsifying Drug Delivery Systems (SMEDDS) Technology: A Narrative Review. Pain Ther. 2023, 12, 1109–1119. [Google Scholar] [CrossRef]
- Kriegel, C.; Festag, M.; Kishore, R.S.K.; Roethlisberger, D.; Schmitt, G. Pediatric Safety of Polysorbates in Drug Formulations. Children 2019, 7, 1. [Google Scholar] [CrossRef]
- Ma, B.-L.; Yang, Y.; Dai, Y.; Li, Q.; Lin, G.; Ma, Y.-M. Polyethylene glycol 400 (PEG400) affects the systemic exposure of oral drugs based on multiple mechanisms: Taking berberine as an example. RSC Adv. 2017, 7, 2435–2442. [Google Scholar] [CrossRef]
- Oleic acid [MAK Value Documentation, 2002]. In The MAK-Collection for Occupational Health and Safety; Wiley: Hoboken, NJ, USA, 2012; pp. 246–266.
- Huang, Y.; Zhang, S.; Shen, H.; Li, J.; Gao, C. Controlled Release of the Nimodipine-Loaded Self-Microemulsion Osmotic Pump Capsules: Development and Characterization. AAPS PharmSciTech 2018, 19, 1308–1319. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.M.; Yang, T.L.; Putri, A.D.; Chen, C.T. Application of Design of Experiments in the Development of Self-Microemulsifying Drug Delivery Systems. Pharmaceuticals 2023, 16, 283. [Google Scholar] [CrossRef] [PubMed]
- Kosinski, T.M.; Brown, M.C.; Valdovinos, K.; Zavala, P.J. Acquisition and Retention of Sterile Compounding Accuracy Skills. Am. J. Pharm. Educ. 2017, 81, 115. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeia (USP-NF). USP Monographs, Reserpine Tablets; United States Pharmacopeia: Rockville, MD, USA, 2023. [Google Scholar]
- United States Pharmacopeia (USP-NF). USP Monographs Dietary Supplement Monographs, Sennosides Tablets; United States Pharmacopeia: Rockville, MD, USA, 2023. [Google Scholar]
- United States Pharmacopeia (USP-NF). Dietary Supplement Monographs, Powdered Andrographis Extract; United States Pharmacopeia: Rockville, MD, USA, 2023. [Google Scholar]
- Zhang, Y.; He, L.; Yue, S.; Huang, Q.; Zhang, Y.; Yang, J. Characterization and evaluation of a self-microemulsifying drug delivery system containing tectorigenin, an isoflavone with low aqueous solubility and poor permeability. Drug Deliv. 2017, 24, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Kallakunta, V.R.; Bandari, S.; Jukanti, R.; Veerareddy, P.R. Oral self emulsifying powder of lercanidipine hydrochloride: Formulation and evaluation. Powder Technol. 2012, 221, 375–382. [Google Scholar] [CrossRef]
- Kim, D.S.; Cho, J.H.; Park, J.H.; Kim, J.S.; Song, E.S.; Kwon, J.; Giri, B.R.; Jin, S.G.; Kim, K.S.; Choi, H.G.; et al. Self-microemulsifying drug delivery system (SMEDDS) for improved oral delivery and photostability of methotrexate. Int. J. Nanomed. 2019, 14, 4949–4960. [Google Scholar] [CrossRef] [PubMed]
- Dholakiya, A.; Dudhat, K.; Patel, J.; Mori, D. An integrated QbD based approach of SMEDDS and liquisolid compacts to simultaneously improve the solubility and processability of hydrochlorthiazide. J. Drug Deliv. Sci. Technol. 2021, 61, 102162. [Google Scholar] [CrossRef]
- Sha, X.; Yan, G.; Wu, Y.; Li, J.; Fang, X. Effect of self-microemulsifying drug delivery systems containing Labrasol on tight junctions in Caco-2 cells. Eur. J. Pharm. Sci. 2005, 24, 477–486. [Google Scholar] [CrossRef]
- Subudhi, B.B.; Mandal, S. Self-Microemulsifying Drug Delivery System: Formulation and Study Intestinal Permeability of Ibuprofen in Rats. J. Pharm. 2013, 2013, 328769. [Google Scholar] [CrossRef]
- Metry, M.; Krug, S.A.; Karra, V.K.; Ekins, S.; Hoag, S.W.; Kane, M.A.; Fink, J.C.; Polli, J.E. Lack of an Effect of Polysorbate 80 on Intestinal Drug Permeability in Humans. Pharm. Res. 2022, 39, 1881–1890. [Google Scholar] [CrossRef]
- Dimitrijevic, D.; Shaw, A.J.; Florence, A.T. Effects of some non-ionic surfactants on transepithelial permeability in Caco-2 cells. J. Pharm. Pharmacol. 2000, 52, 157–162. [Google Scholar] [CrossRef]
- Padula, C.; Pescina, S.; Nicoli, S.; Santi, P. New Insights on the Mechanism of Fatty Acids as Buccal Permeation Enhancers. Pharmaceutics 2018, 10, 201. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, Z.; Liu, W.; Hou, Z.; Zhang, D.; Huang, L.; Zhang, Y. Oleic acid as a protein ligand improving intestinal absorption and ocular benefit of fucoxanthin in water through protein-based encapsulation. Food Funct. 2019, 10, 4381–4395. [Google Scholar] [CrossRef]


| Oil | Surfactant | Co-Surfactant |
|---|---|---|
| Coconut oil Maize oil Olive oil Soybean oil Sunflower oil Oleic acid | Cremophor RH40 Transcutol® P Labrasol® Tween® 20 Tween® 80 | Ethanol PEG400 Propylene glycol Glycerin |
| Oil | Surfactant | Co-Surfactant | |||
|---|---|---|---|---|---|
| Solvent | Solubility (mg/mL) | Solvent | Solubility (mg/mL) | Solvent | Solubility (mg/mL) |
| Coconut oil | 0.16 ± 0.02 | Chromophore RH40 | 25.24 ± 2.96 | Ethanol | 26.33 ± 1.07 |
| Corn oil | 0.10 ± 0.01 | Translutol® P | 27.08 ± 1.69 | PEG 400 | 54.76 ± 4.92 * |
| Olive oil | 0.22 ± 0.03 | Labrasol® | 24.73 ± 0.75 | Propylene glycol | 33.24 ± 3.16 |
| Soybean oil | 0.32 ± 0.02 | Tween® 20 | 29.20 ± 0.94 | Glycerin | 6.86 ± 1.41 |
| Sunflower oil | 0.16 ± 0.01 | Tween® 80 | 50.14 ± 4.23 * | ||
| Oleic acid | 1.64 ± 0.02 * | ||||
| RUN | X1 Tween® 80 | X2 Oleic Acid | X3 PEG 400 | Y1 Droplet Size (nm) | Y2 PDI | Y3 Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| 1 | 63 | 11.5 | 25.5 | 105.40 | 0.1770 | −9.30 |
| 2 | 80 | 14.5 | 5.5 | 136.50 | 0.2310 | −14.60 |
| 3 | 56.4 | 20 | 23.6 | 135.20 | 0.2730 | −24.90 |
| 4 | 69.6 | 20 | 10.4 | 129.60 | 0.2710 | −20.60 |
| 5 | 80 | 8 | 12 | 99.90 | 0.3360 | −5.80 |
| 6 | 54.1 | 9.3 | 36.6 | 85.30 | 0.2760 | −13.70 |
| 7 | 80 | 14.5 | 5.5 | 118.20 | 0.2200 | −19.60 |
| 8 | 69 | 13.3 | 17.7 | 97.00 | 0.2200 | −12.30 |
| 9 | 63 | 11.5 | 25.5 | 104.90 | 0.2260 | −9.50 |
| 10 | 50 | 17.8 | 32.2 | 147.60 | 0.3240 | −19.90 |
| 11 | 50 | 18 | 32 | 132.00 | 0.2650 | −17.40 |
| 12 | 75.5 | 5 | 19.5 | 16.50 | 0.1180 | −1.50 |
| 13 | 50 | 17.8 | 32.2 | 134.00 | 0.3080 | −19.30 |
| 14 | 62.6 | 5 | 32.4 | 15.40 | 0.3760 | −2.30 |
| 15 | 69.6 | 20 | 10.4 | 132.20 | 0.2520 | −18.40 |
| 16 | 63 | 11.5 | 25.5 | 106.80 | 0.2120 | −9.20 |
| Factors | Goal | Lower Limit | Upper Limit | Solution | Desirability | Results | p-Value |
|---|---|---|---|---|---|---|---|
| Tween® 80 (%wt) | is in range | 50 | 80 | 68.998 | 1.000 | ||
| Oleic acid (%wt) | is in range | 5 | 20 | 13.257 | |||
| PEG 400 (%wt) | is in range | 0 | 45 | 17.745 | |||
| Size (nm) | is in range | 80 | 115 | 101.68 ± 21.53 | 97.1 ± 0.76 | 0.75 | |
| PDI | none | ||||||
| Zeta potential (mV) | is in range | −24.9 | −10 | −10.76 ± 2.38 | −9.0 ± 0.95 | 0.30 |
| J (µg·cm−2·h−1) | Kp (×10−3 cm−2·h−1) | Q24/A (µg·cm−2) | |
|---|---|---|---|
| A. paniculata extract | 19.90 ± 3.84 | 0.99 ± 0.19 | 447.34 ± 77.14 |
| AGP-SMEDDS | 35.49 ± 4.12 * | 2.00 ± 0.23 * | 827.87 ± 63.77 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pornpitchanarong, C.; Akkaramongkolporn, P.; Nattapulwat, N.; Opanasopit, P.; Patrojanasophon, P. Development and Optimization of Andrographis paniculata Extract-Loaded Self-Microemulsifying Drug Delivery System Using Experimental Design Model. Pharmaceutics 2024, 16, 166. https://doi.org/10.3390/pharmaceutics16020166
Pornpitchanarong C, Akkaramongkolporn P, Nattapulwat N, Opanasopit P, Patrojanasophon P. Development and Optimization of Andrographis paniculata Extract-Loaded Self-Microemulsifying Drug Delivery System Using Experimental Design Model. Pharmaceutics. 2024; 16(2):166. https://doi.org/10.3390/pharmaceutics16020166
Chicago/Turabian StylePornpitchanarong, Chaiyakarn, Prasert Akkaramongkolporn, Nattawat Nattapulwat, Praneet Opanasopit, and Prasopchai Patrojanasophon. 2024. "Development and Optimization of Andrographis paniculata Extract-Loaded Self-Microemulsifying Drug Delivery System Using Experimental Design Model" Pharmaceutics 16, no. 2: 166. https://doi.org/10.3390/pharmaceutics16020166





